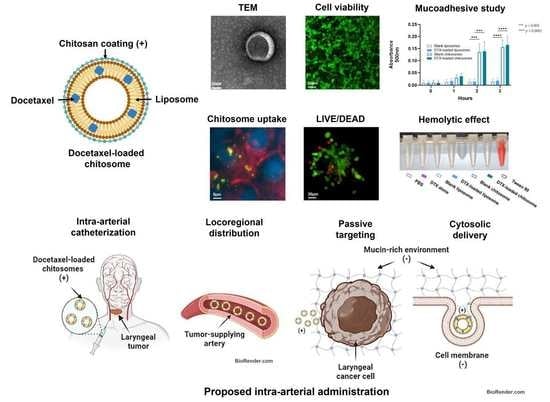
Chitosomes Loaded with Docetaxel as a Promising Drug Delivery System to Laryngeal Cancer Cells: An In Vitro Cytotoxic Study
Abstract
1. Introduction
2. Results and Discussion
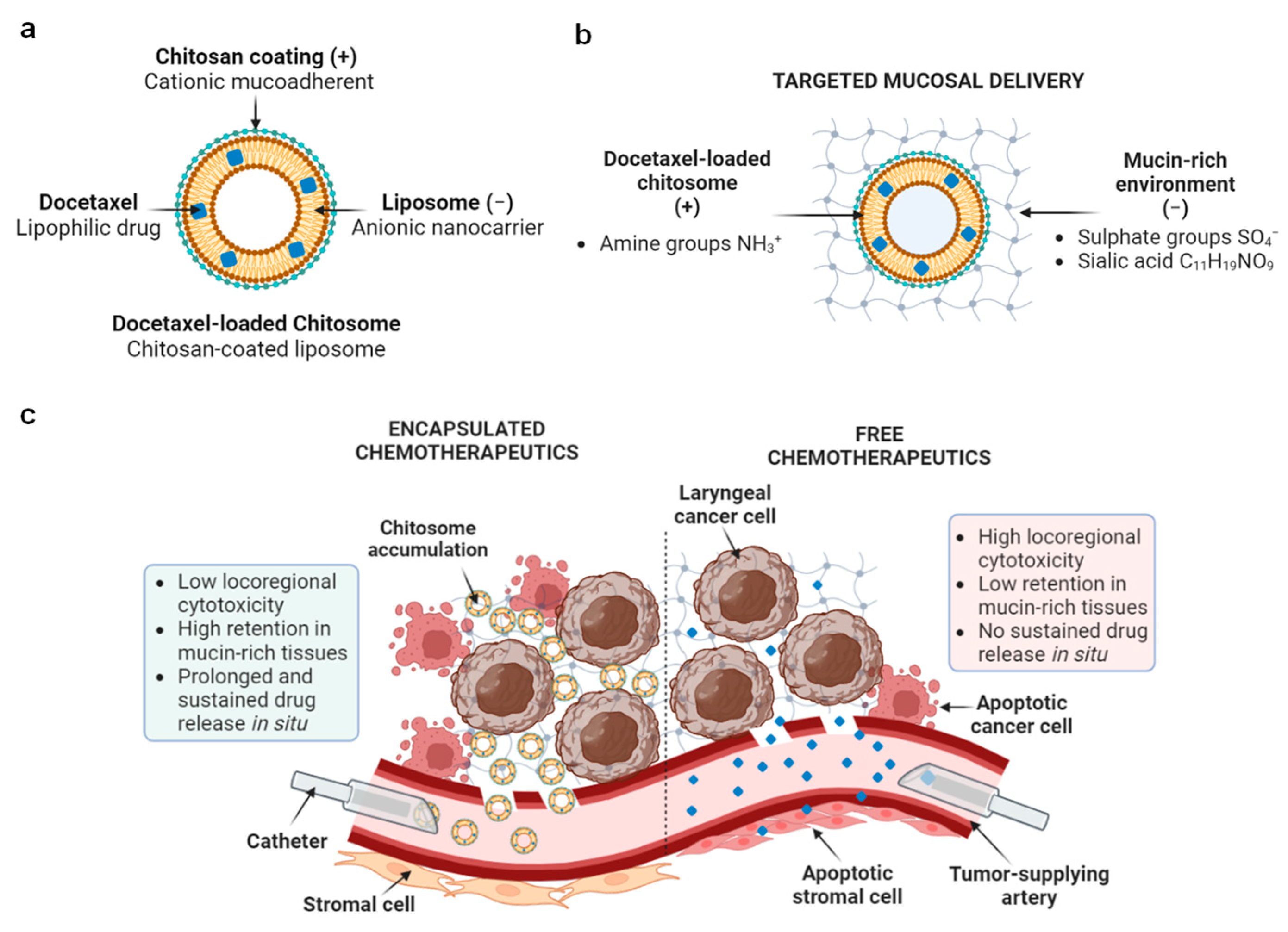
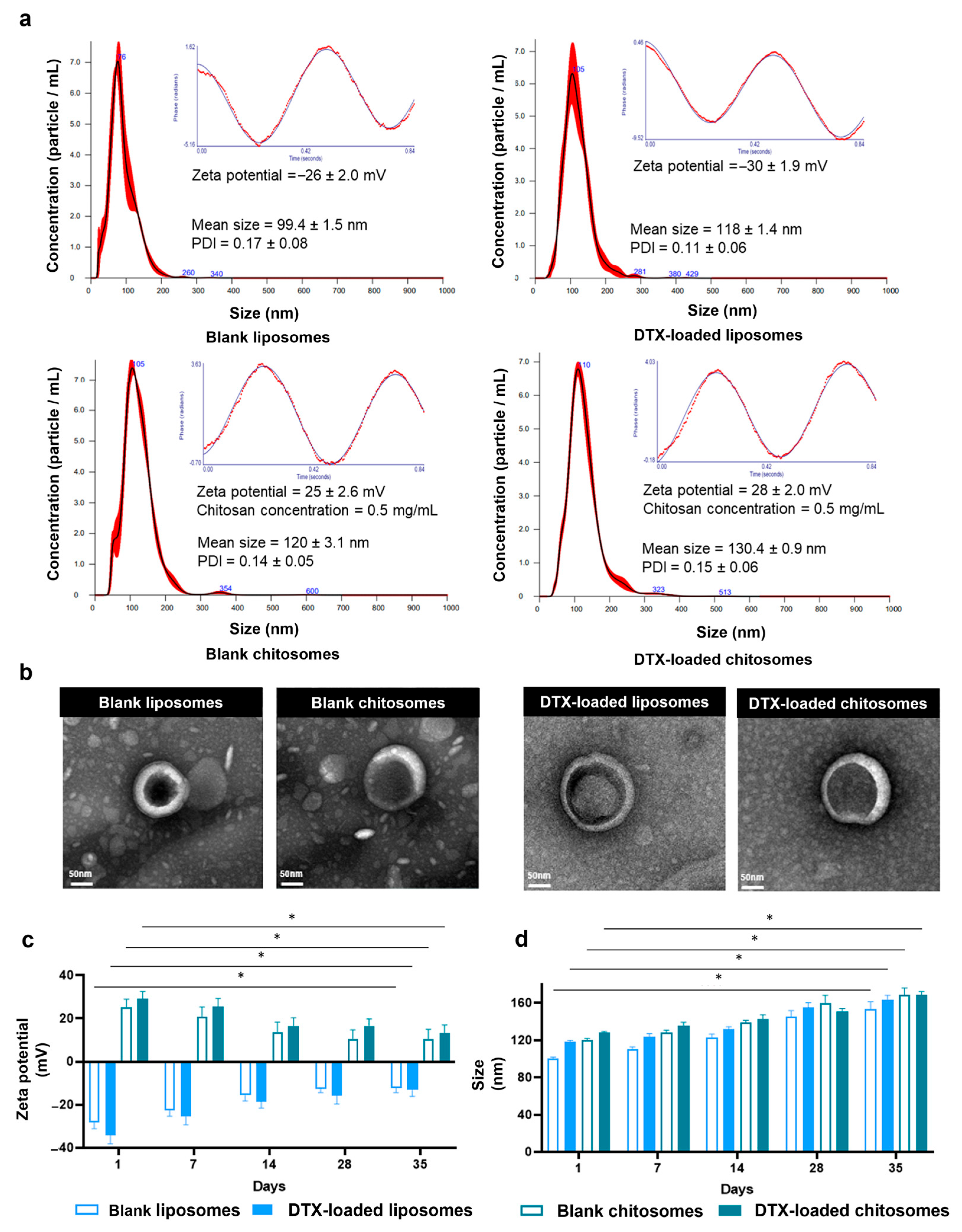
2.1. DTX-Loaded Chitosomes Possessed the Expected Physical Properties
Size and Surface Charge Analysis
2.2. Addition of a Chitosan Coating Showed Improved Mucoadhesiveness and Drug Release Profile of Liposomes
2.2.1. FTIR Spectroscopy
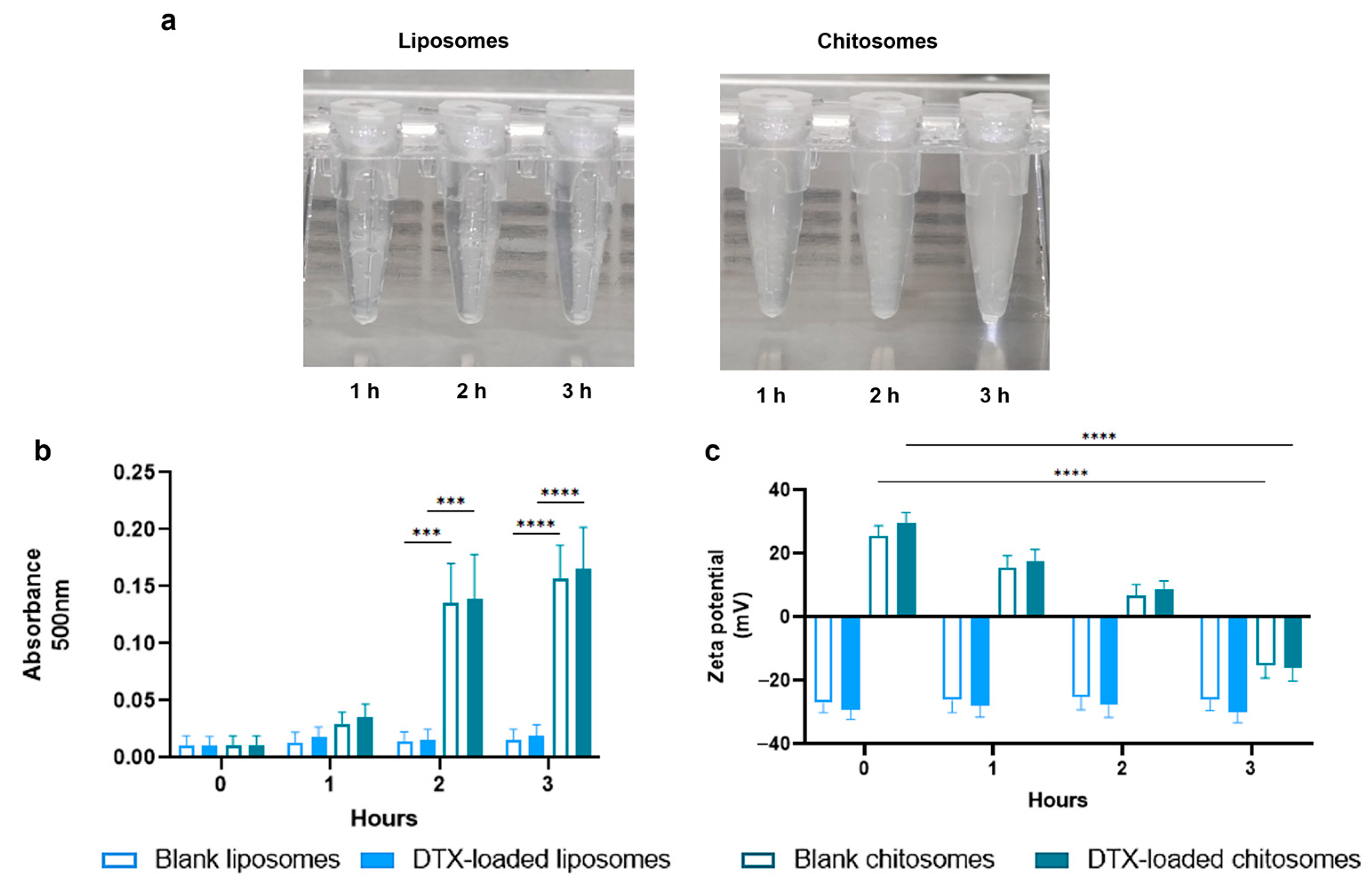
2.2.2. Mucoadhesive Studies
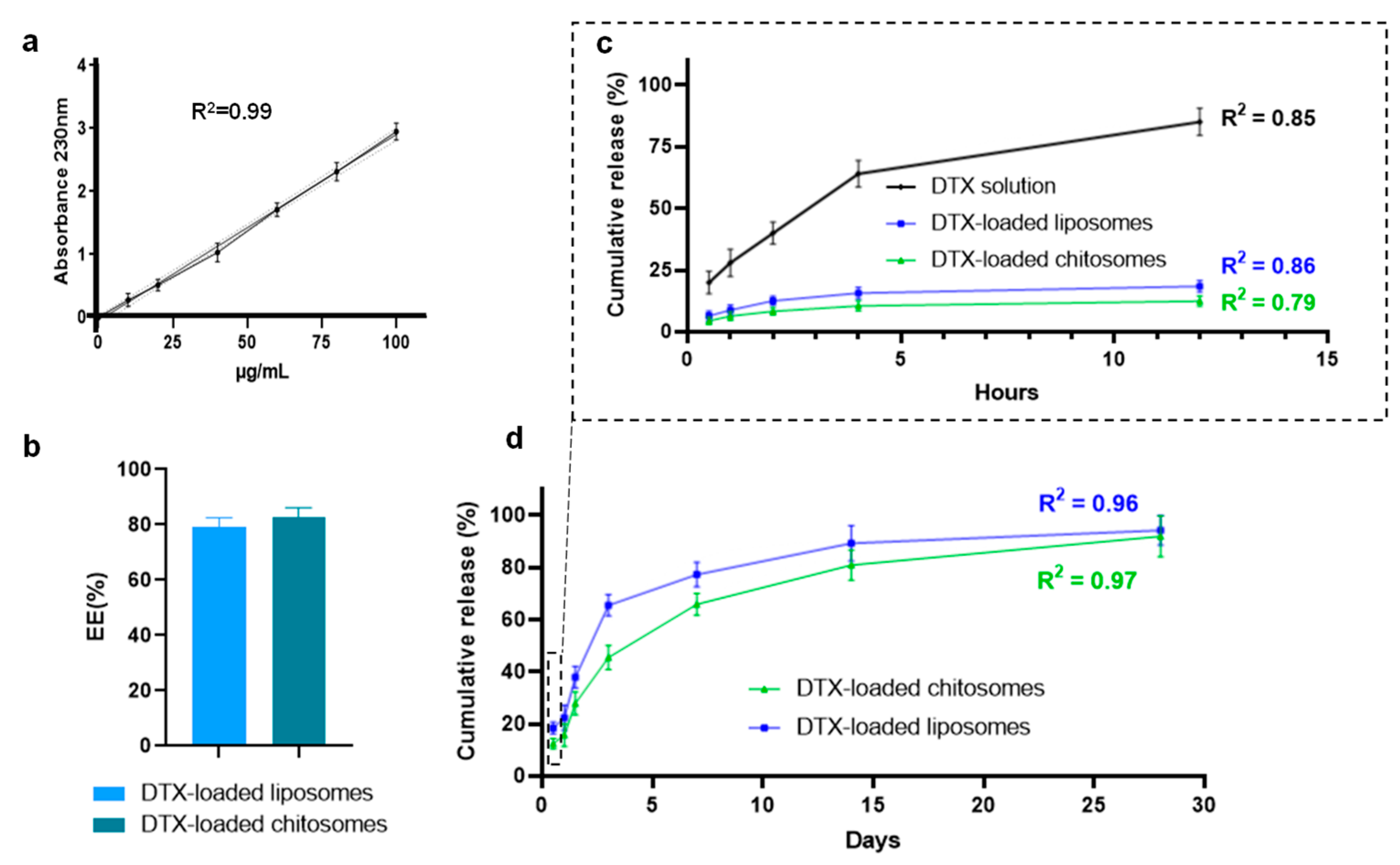
2.2.3. DTX Entrapment and Release Studies
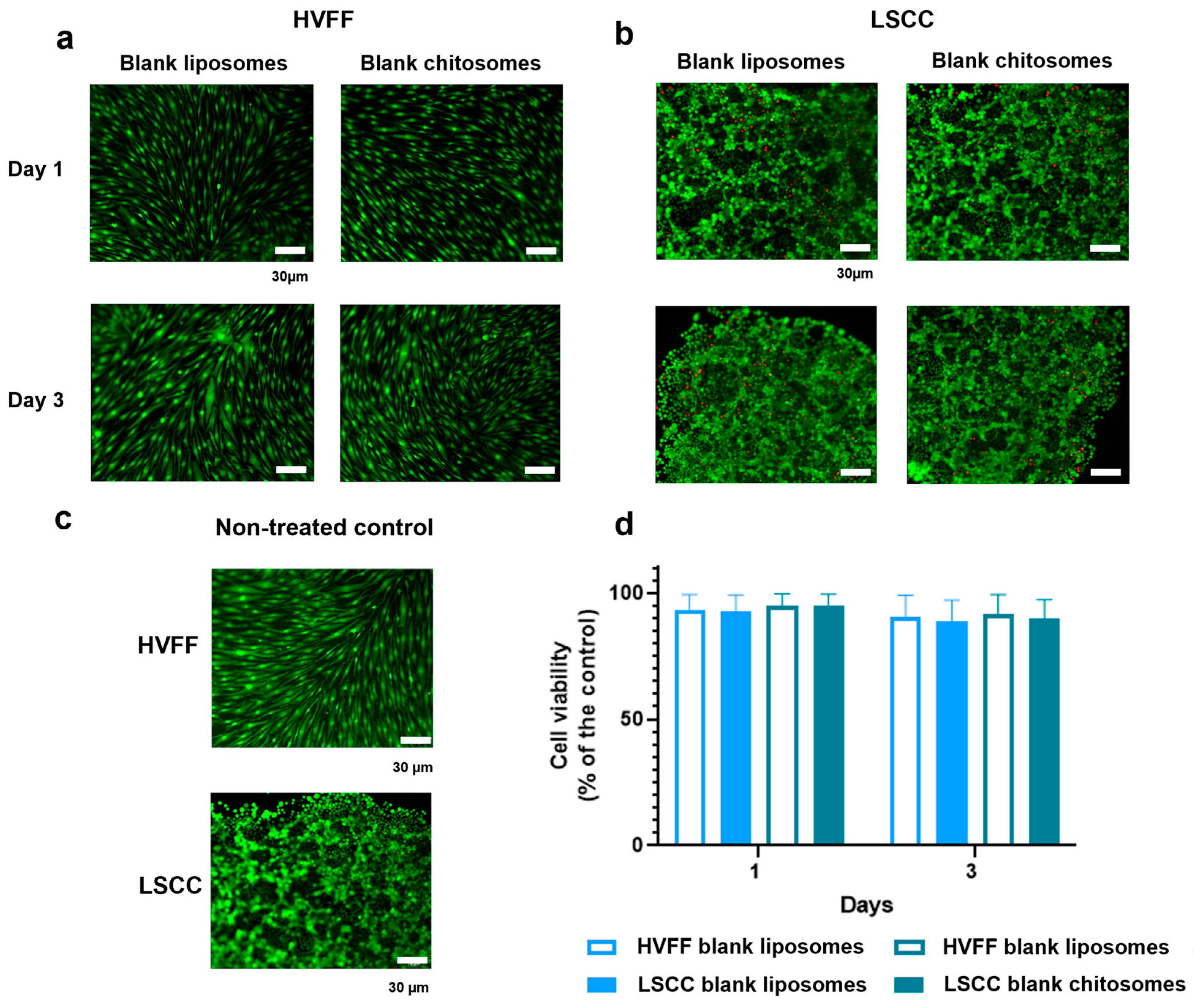
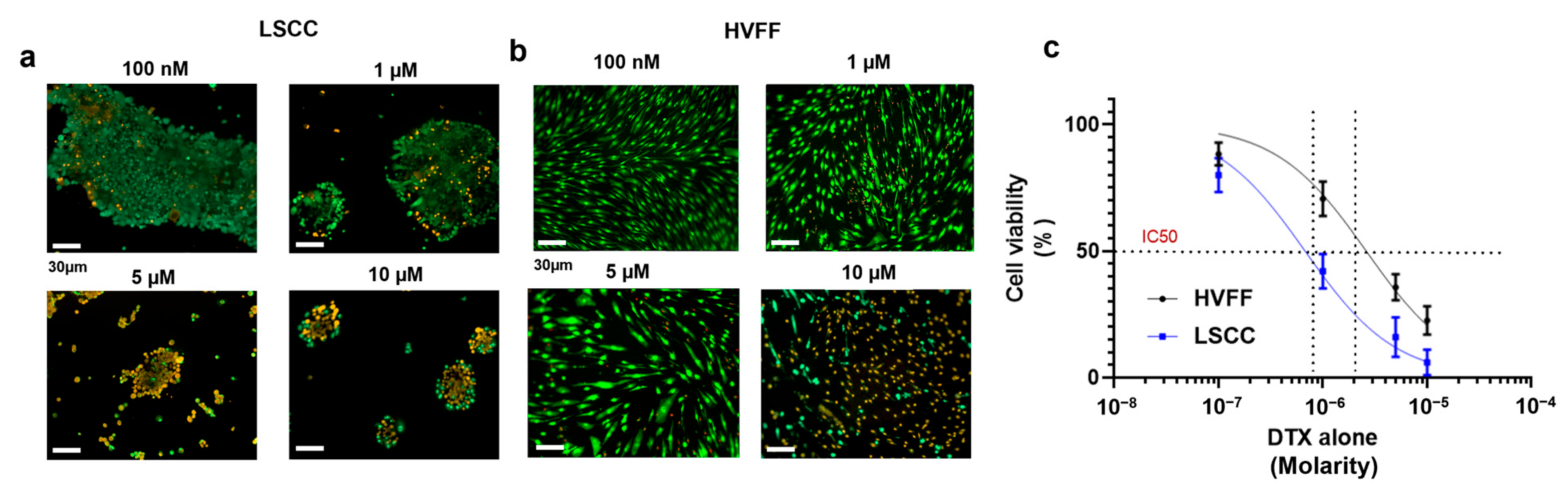
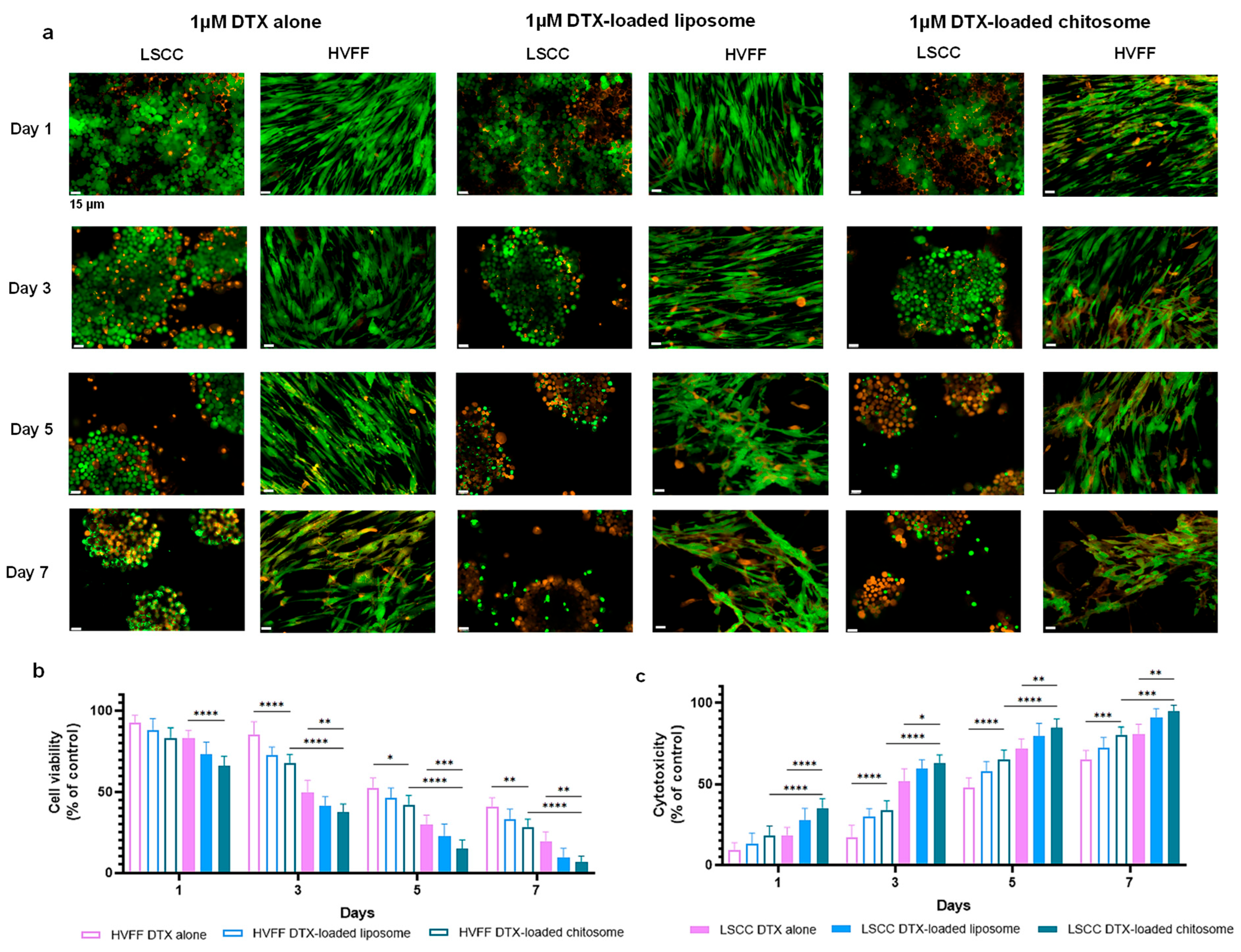
2.3. Docetaxel-Loaded Chitosomes Showed Higher Toxicity to Cancer Cells Than Healthy Stromal and Blood Cells
2.3.1. HVFF and LSCC Viability
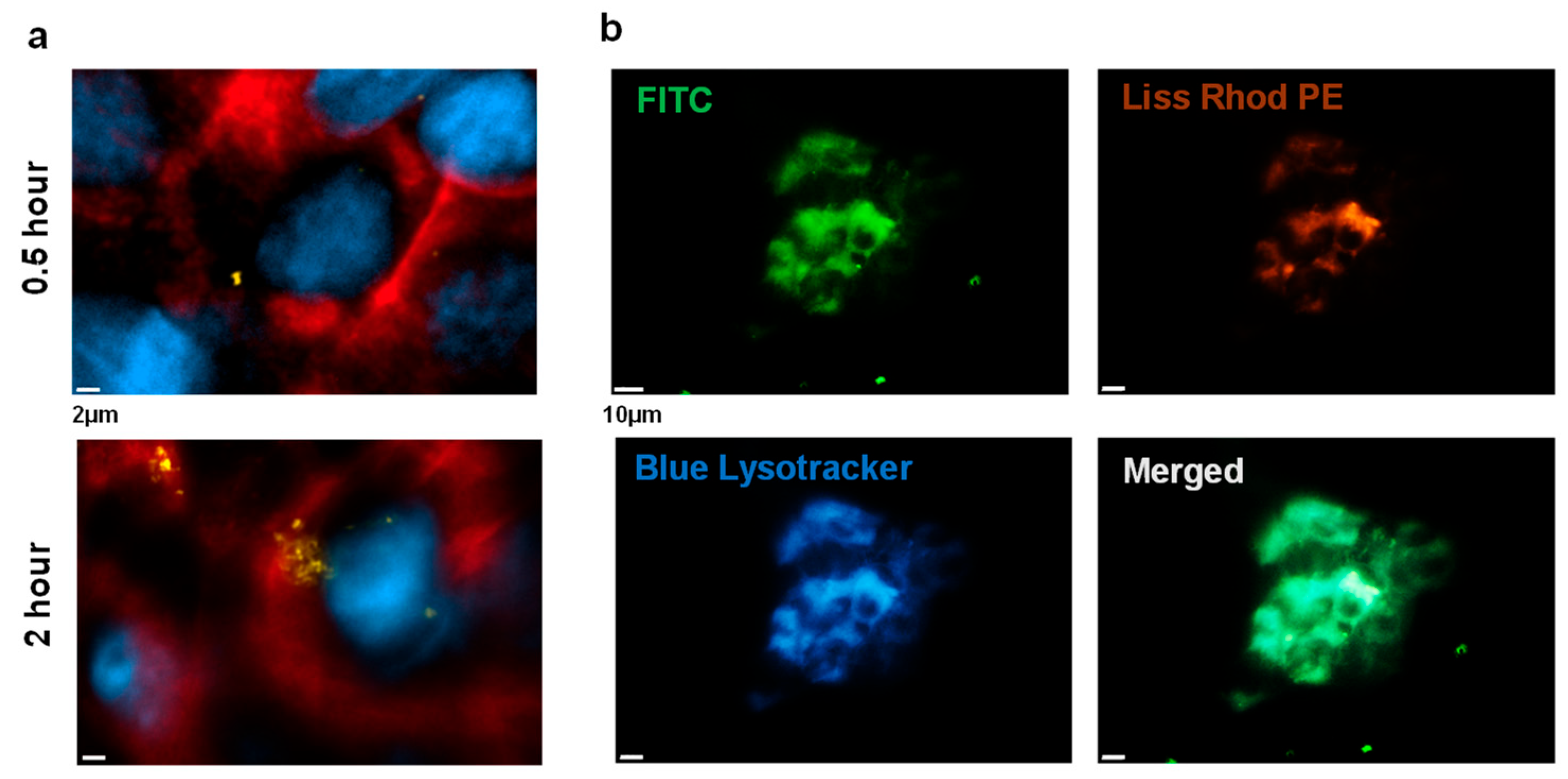
2.3.2. Chitosome Uptake by LSCCs
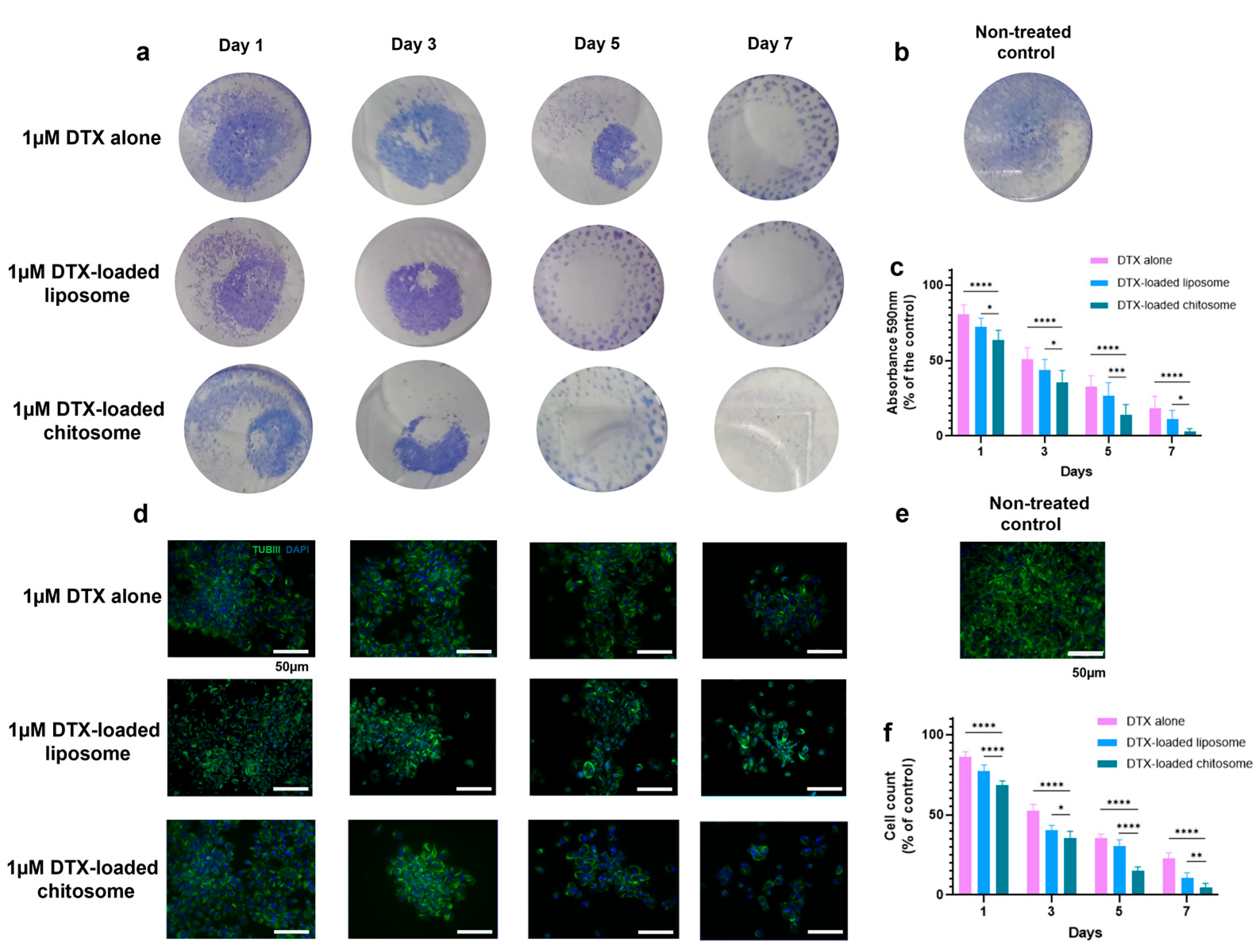
2.3.3. Docetaxel-Loaded Chitosomes Effectively Reduced LSCC and HVFF Colony Formation
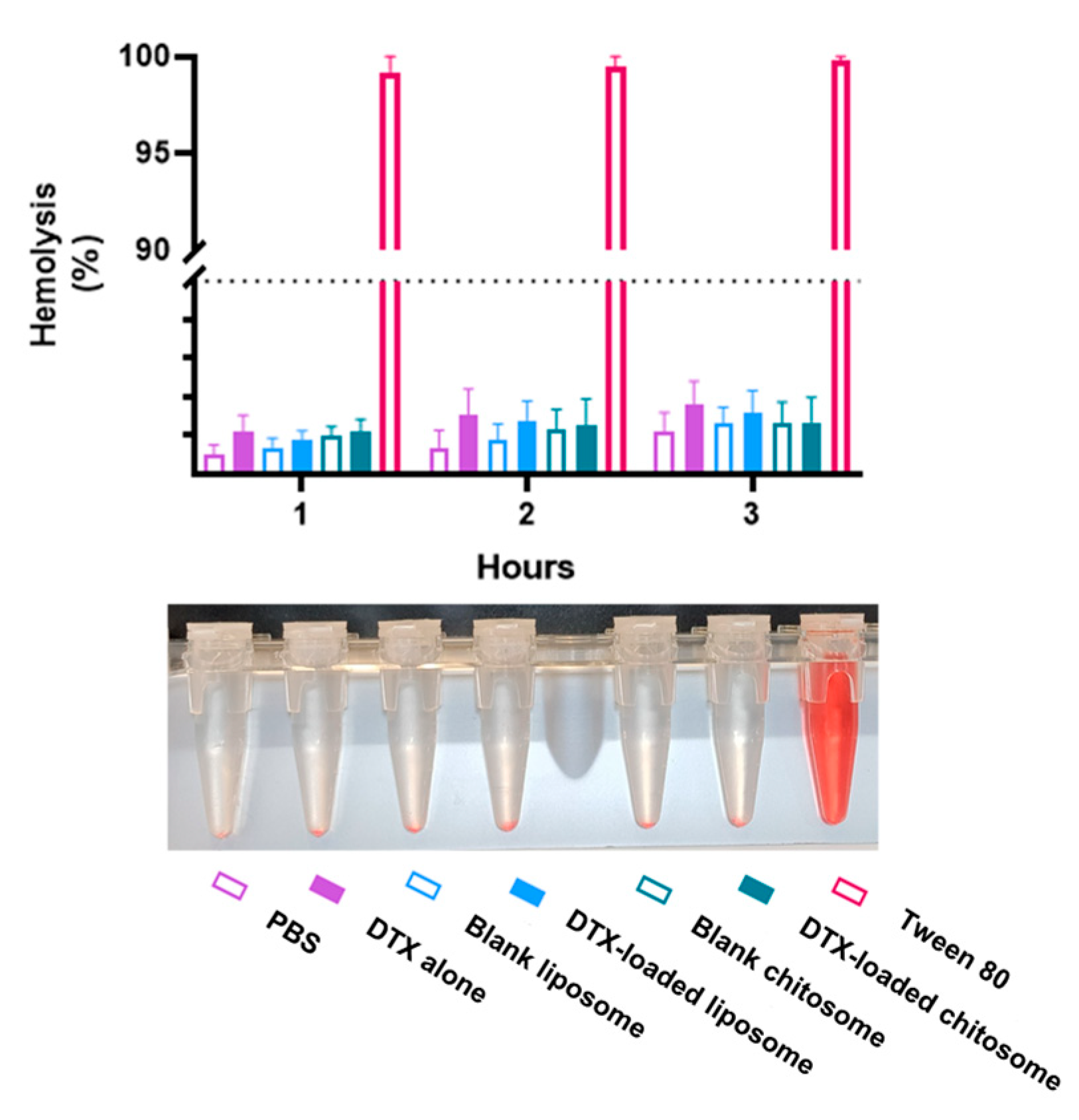
2.3.4. Chitosomes Did Not Induce Hemolysis of Human Red Blood Cells
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Liposomes
3.3. Fabrication of Chitosomes
3.4. Size and Zeta Potential Analyses of Chitosomes
3.5. Transmission Electron Microscopy of Chitosomes
3.6. FTIR Characterization of Chitosomes
3.7. Mucoadhesive Behavior of Chitosomes
3.8. Drug Entrapment Efficiency and Release of Chitosomes
3.9. HVFFs and LSCCs Culture Protocol
3.10. HVFF and LSCC Viability Analyses for Liposomes and Chitosomes
3.11. Chitosome Uptake by LSCCs
3.12. DTX-Loaded Chitosome Effect on LSCCs
3.13. Hemolytic Effect of Chitosomes
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moya-Garcia, C.R.; Okuyama, H.; Sadeghi, N.; Li, J.; Tabrizian, M.; Li-Jessen, N.Y.K. In vitro models for head and neck cancer: Current status and future perspective. Front. Oncol. 2022, 12, 3691. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Ghi, M.G.; Franzese, C.; Codecà, C.; Gau, M.; Fayette, J. The Slippery Role of Induction Chemotherapy in Head and Neck Cancer: Myth and Reality. Front. Oncol. 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Remenar, E.; Van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, J.C.; Loaiza-Bonilla, A.; Talebi, T.N.; Warsch, S.; Fernandez, G.; Raez, L.E.; Santos, E.S. Concurrent chemoradiotherapy versus induction chemotherapy followed by chemoradiotherapy (sequential approach) in the management of head and neck cancer. Expert Rev. Anticancer Ther. 2013, 13, 1065–1072. [Google Scholar] [CrossRef]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of taxane resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Bredell, M.G.; Ernst, J.; El-Kochairi, I.; Dahlem, Y.; Ikenberg, K.; Schumann, D.M. Current relevance of hypoxia in head and neck cancer. Oncotarget 2016, 7, 50781–50804. [Google Scholar] [CrossRef]
- Doktorova, H.; Hrabeta, J.; Khalil, M.A.; Eckschlager, T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed Pap. 2015, 159, 166–177. [Google Scholar] [CrossRef]
- Sadeghi, N.; Khalife, S.; Mascarella, M.A.; Ramanakumar, A.V.; Richardson, K.; Joshi, A.S.; Bouganim, N.; Taheri, R.; Fuson, A.; Siegel, R. Pathologic response to neoadjuvant chemotherapy in HPV-associated oropharynx cancer. Head Neck 2020, 42, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ye, L.; Zhu, Y.; Ding, H.; Wang, S.; Ying, H.; Wu, C.; Zhou, L.; Wang, X.; Tian, S. Induction chemotherapy of modified docetaxel, cisplatin, 5-fluorouracil for laryngeal preservation in locally advanced hypopharyngeal squamous cell carcinoma. Head Neck 2022, 44, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Aigner, K.R.; Selak, E.; Aigner, K. Short-term intra-arterial infusion chemotherapy for head and neck cancer patients maintaining quality of life. J. Cancer Res. Clin. Oncol. 2019, 145, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.H.; Chung, M.J.; Hong, R.L.; Tseng, T.Y.; Tai, H.C.; Cheng, N.C.; Hsieh, J.H.; Horng, S.Y.; Lai, H.S. The impacts of intra-arterial chemotherapy on head and neck microvascular reconstruction. J. Formos. Med. Assoc. 2020, 119, 1524–1531. [Google Scholar] [CrossRef]
- Heianna, J.; Makino, W.; Hirakawa, H.; Agena, S.; Tomita, H.; Ariga, T.; Ishikawa, K.; Takehara, S.; Maemoto, H.; Murayama, S. Therapeutic efficacy of selective intra-arterial chemoradiotherapy with docetaxel and nedaplatin for fixed bulky nodal disease in head and neck cancer of unknown primary. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3105–3113. [Google Scholar] [CrossRef]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Bao, W.; Tian, F.; Lyu, C.; Liu, B.; Li, B.; Zhang, L.; Liu, X.; Li, F.; Li, D.; Gao, X.; et al. Experimental and theoretical explorations of nanocarriers’ multistep delivery performance for rational design and anticancer prediction. Sci. Adv. 2021, 7, eaba2458. [Google Scholar] [CrossRef]
- Wu, Q.; Qu, M.; Kim, H.J.; Zhou, X.; Jiang, X.; Chen, Y.; Zhu, J.; Ren, L.; Wolter, T.; Kang, H.; et al. A Shear-Thinning Biomaterial-Mediated Immune Checkpoint Blockade. ACS Appl. Mater. Interfaces 2022, 14, 35309–35318. [Google Scholar] [CrossRef]
- Coburn, P.T.; Li, X.; Li, J.; Kishimoto, Y.; Li-Jessen, N.Y.K. Progress in Vocal Fold Regenerative Biomaterials: An Immunological Perspective. Adv. NanoBiomed Res. 2022, 2, 2100119. [Google Scholar] [CrossRef]
- Li-Jessen, N.Y.K.; Ridgway, C. Neurologic and Neurodegenerative Diseases of the Larynx; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–40. [Google Scholar]
- Coburn, P.T.; Herbay, A.C.; Berrini, M.; Li-Jessen, N.Y.K. An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications. J. Biomed. Mater. Res.-Part A 2021, 109, 1337–1352. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-loaded chitosomes for treatment of malignant pleural mesothelioma—A rare thoracic cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Alomrani, A.; Badran, M.; Harisa, G.I.; ALshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi. Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Egbu, R.; Jannati, G.; Al-Jamal, W.T. Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int. J. Pharm. 2016, 514, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Paun, R.A.; Dumut, D.C.; Centorame, A.; Thuraisingam, T.; Hajduch, M.; Mistrik, M.; Dzubak, P.; De Sanctis, J.B.; Radzioch, D.; Tabrizian, M. One-Step Synthesis of Nanoliposomal Copper Diethyldithiocarbamate and Its Assessment for Cancer Therapy. Pharmaceutics 2022, 14, 640. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Soltani, M.; Momeni, M.M.; Rahmim, A. Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework. Front. Oncol. 2021, 11, 655781. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Stevanović, M. Biomedical Applications of Nanostructured Polymeric Materials. In Nanostructured Polymer Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popović, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, H.; Huang, T.; Zhang, C.; Wu, J.; Luo, S. Simultaneous delivery of anti-miRNA and docetaxel with supramolecular self-assembled “chitosome” for improving chemosensitivity of triple negative breast cancer cells. Drug Deliv. Transl. Res. 2020, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Megahed, M.A.; El-sawy, H.S.; Reda, A.M.; Abd-Allah, F.I.; Abu, S.K.; Lila, A.E.; Ismael, H.R.; El-Say, K.M. Effect of nanovesicular surface-functionalization via chitosan and / or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 2022, 307, 120908. [Google Scholar] [CrossRef]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Molecular design of layer-by-layer functionalized liposomes for oral drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 43341–43351. [Google Scholar] [CrossRef]
- AlQahtani, S.A.; Harisa, G.I.; Alomrani, A.H.; Alanazi, F.K.; Badran, M.M. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf. B Biointerfaces 2021, 197, 111380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Swami, R.; Jeengar, M.K.; Khan, W.; Sistla, R. p-Aminophenyl-α-d-mannopyranoside engineered lipidic nanoparticles for effective delivery of docetaxel to brain. Chem. Phys. Lipids 2015, 188, 1–9. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Ying, J.N.S.; Ling, J.F.S.; Ting, J.; Ting, J.S.S.; Hwen, I.K.Z.; Suen, H.W.; Kamar, H.S.S.; Gorain, B.; et al. Mucoadhesive Nanocarriers as a Promising Strategy to Enhance Intracellular Delivery against Oral Cavity Carcinoma. Pharmaceutics 2022, 14, 795. [Google Scholar] [CrossRef]
- Collado-González, M.; Espinosa, Y.G.; Goycoolea, F.M. Interaction between Chitosan and Mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef]
- Alshraim, M.O.; Sangi, S.; Harisa, G.I.; Alomrani, A.H.; Yusuf, O.; Badran, M.M. Chitosan-coated flexible liposomes magnify the anticancer activity and bioavailability of docetaxel: Impact on composition. Molecules 2019, 24, 250. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Cai, Q.; Fan, S.; Xu, Q.; Zang, J.; Yang, H.; Yu, W.; Li, Z.; Zhang, Z. Acetic acid transporter-mediated, oral, multifunctional polymer liposomes for oral delivery of docetaxel. Colloids Surf. B Biointerfaces 2021, 198, 111499. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Akhter, S.; Ahmad, I.; Hafeez, Z.; Rizvi, M.M.A.; Jain, G.K.; Ahmad, F.J. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of Docetaxel and Thymoquinone by Chitosan grafted lipid nanocapsules: Formulation optimization, in vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 186, 110603. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Souto, G.D.; Pereira, G.G.; Bianchera, A.; Fasiolo, L.T.; Colombo, G.; Marques, M.; Pohlmann, A.R.; et al. Chitosan-coated nanoparticles: Effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of low molecular weight chitosan and chitooligosaccharides (COS): A review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Sohail, M.F.; Khurshid, Z.; Ammar, A.; Saeed, A.M.; Shazia, F.; Gul, S.; Zia, M. Synthesis, Characterization, and In Vivo Distribution of 99mTc Radiolabelled Docetaxel Loaded Folic Acid-Thiolated Chitosan Enveloped Liposomes. Bionanoscience 2023, 13, 134–144. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Assadpour, S.; Shiran, M.R.; Akhtari, J. Chitosan coating of anionic liposomes containing sumatriptan succinate: A candidate for nasal administration. Nanomed. J. 2021, 8, 132–139. [Google Scholar]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef]
- Rumengan, I.F.M.; Suryanto, E.; Modaso, R.; Wullur, S.; Tallei, T.E.; Limbong, D. Structural Characteristics of Chitin and Chitosan Isolated from the Biomass of Cultivated Rotifer, Brachionus rotundiformis. Int. J. Fish. Aquat. Sci. 2014, 3, 12–18. [Google Scholar]
- Alinaghi, A.; Rouini, M.R.; Johari Daha, F.; Moghimi, H.R. The influence of lipid composition and surface charge on biodistribution of intact liposomes releasing from hydrogel-embedded vesicles. Int. J. Pharm. 2014, 459, 30–39. [Google Scholar] [CrossRef]
- Yang, M.; Ding, Y.; Zhang, L.; Qian, X.; Jiang, X.; Liu, B. Novel thermosensitive polymeric micelles for docetaxel delivery. J. Biomed. Mater. Res. Part A 2007, 81, 847–857. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Naidu, V.G.M.; Sistla, R. Peptide conjugated polymeric nanoparticles as a carrier for targeted delivery of docetaxel. Colloids Surf. B Biointerfaces 2014, 117, 166–173. [Google Scholar] [CrossRef]
- Altman, K.W.; Kinoshita, Y.; Tan, M.; Burstein, D.; Radosevich, J.A. Western blot confirmation of the H+/K+-ATPase proton pump in the human larynx and submandibular gland. Otolaryngol.-Head Neck Surg. 2011, 145, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, E.; Fang, J.Y.; Tahara, K. Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm. 2021, 593, 120148. [Google Scholar] [CrossRef]
- Chatzitaki, A.T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.V.; Price, M.R.; Segal-Eiras, A. Detection and isolation of MUC1 Mucin from larynx squamous cell carcinoma. Pathol. Oncol. Res. 2000, 6, 93–99. [Google Scholar] [CrossRef]
- Utispan, K.; Koontongkaew, S. Mucin 1 regulates the hypoxia response in head and neck cancer cells. J. Pharmacol. Sci. 2021, 147, 331–339. [Google Scholar] [CrossRef]
- Lu, H.; Liang, D.; Zhu, Y.; Xu, W.; Zhou, K.; Liu, L.; Liu, S.; Yang, W. Prognostic and clinicopathological significance of MUC expression in head and neck cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 96359–963772. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707106/pdf/oncotarget-08-96359.pdf (accessed on 1 June 2023). [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Li, Y.; Xia, H.; Cheng, Y.; Liu, C.; Xia, Y.; Wang, Y.; Yue, Y.; Cheng, X.; et al. The Fabrication of Docetaxel-Containing Emulsion for Drug Release Kinetics and Lipid Peroxidation. Pharmaceutics 2022, 14, 1993. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.H.; Kim, J.S. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Arch. Pharm. Res. 2018, 41, 765–775. [Google Scholar] [CrossRef]
- Avanti Polar Lipids. Phase Transition Temperature for Glycerophospholipids [Internet]; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; p. 2. Available online: https://avantilipids.com/wp-content/uploads/2015/11/Phase_Transition_Temps_for_Glycerophospholipids_Table.pdf (accessed on 25 April 2023).
- Li, N.; Fu, T.; Fei, W.; Han, T.; Gu, X.; Hou, Y.; Liu, Y.; Yang, J. Vitamin E D-alpha-tocopheryl polyethylene glycol 1000 succinate-conjugated liposomal docetaxel reverses multidrug resistance in breast cancer cells. J. Pharm. Pharmacol. 2019, 71, 1243–1254. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Giordani, B.; Paulsen, M.H.; Vanić, Ž.; Flaten, G.E.; Vitali, B.; Basnet, P.; Bayer, A.; Strøm, M.B.; Škalko-Basnet. Tailored anti-biofilm activity—Liposomal delivery for mimic of small antimicrobial peptide. Biomater. Adv. 2023, 145, 213238. [Google Scholar] [CrossRef]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Heidari, H.R.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug targeting strategies based on charge dependent uptake of nanoparticles into cancer cells. J. Pharm. Pharm. Sci. 2019, 22, 191–220. [Google Scholar] [CrossRef]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines 2022, 13, 1623. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Zhong, J.T.; Shen, L.F.; Dai, L.B.; Zhou, S.H.; Fan, J.; Yao, H.T.; Lu, Z.J. Effect of Glut-1 and HIF-1α double knockout by CRISPR/CAS9 on radiosensitivity in laryngeal carcinoma via the PI3K/Akt/mTOR pathway. J. Cell. Mol. Med. 2022, 26, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, J.; Sun, Z.; Wang, W. Oxidation and Reduction Dual-Responsive Polymeric Prodrug Micelles Co-delivery Precisely Prescribed Paclitaxel and Honokiol for Laryngeal Carcinoma Combination Therapy. Front. Pharmacol. 2022, 13, 934632. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Accolla, M.L.; Cilurzo, F.; Cristiano, M.C.; Cosco, D.; Castelli, F.; Sarpietro, M.G.; Fresta, M.; Celia, C. Interaction between PEG lipid and DSPE/DSPC phospholipids: An insight of PEGylation degree and kinetics of de-PEGylation. Colloids Surf. B Biointerfaces 2017, 155, 266–275. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Rezayat, S.M.; Faridi-Majidi, R.; Sadri, K.; Jaafari, M.R. Optimization of Docetaxel Loading Conditions in Liposomes: Proposing potential products for metastatic breast carcinoma chemotherapy. Sci. Rep. 2020, 10, 5569. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.Y.; Lu, X.; Mou, Z.Z.; Lin, G.M. Docetaxel-loaded liposomes: Preparation, pH sensitivity, Pharmacokinetics, and tissue distribution. J. Zhejiang Univ. Sci. B 2012, 13, 981–989. [Google Scholar] [CrossRef]
- Jahan, K.; Mekhail, M.; Tabrizian, M. One-step fabrication of apatite-chitosan scaffold as a potential injectable construct for bone tissue engineering. Carbohydr. Polym. 2019, 203, 60–70. [Google Scholar] [CrossRef]
- Carey, T.E.; Bradford, C.R.; Schwartz, D.R.; Hsu, S.; Baker, S.R.; Van Dyke, D.L.; Hsu, S.; Baker, S.R. Characterization of Human Laryngeal Primary and Metastatic Squamous Cell Carcinoma Cell Lines UM-SCC-17A and UM-SCC-17B. Cancer Res. 1989, 49, 6098–6107. [Google Scholar]
- Brenner, J.C.; Graham, M.P.; Kumar, B.; Saunders, L.; Kupfer, R.; Lyons, R.H.; Bradford, C.R.; Carey, T.E. Genotyping of 73 um-scc Head and Neck Squamous Cell Carcinoma Cell Lines. Head Neck 2010, 32, 417–426. [Google Scholar] [CrossRef]
- Chen, X.; Thibeault, S.L. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng.-Part C Methods 2009, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.; Dybdal-Hargreaves, N.F.; Mooberry, S.L. Breast Cancer Cell Lines Exhibit Differential Sensitivities to Microtubule-targeting Drugs Independent of Doubling Time. Anticancer Res. 2015, 35, 5845–5850. [Google Scholar] [PubMed]
- Angius, F.; Floris, A. Liposomes and MTT cell viability assay: An incompatible affair. Toxicol. Vitr. 2015, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Yitayew, M.Y.; Tabrizian, M. Hollow microcapsules through layer-by-layer self-assembly of Chitosan/Alginate on E. coli. MRS Adv. 2020, 5, 2401–2407. [Google Scholar] [CrossRef]
- Soleimani, M.; Somma, A.; Kaoud, T.; Goyal, R.; Bustamante, J.; Wylie, D.C.; Holay, N.; Looney, A.; Giri, U.; Triplett, T.; et al. Covalent JNK Inhibitor, JNK-IN-8, Suppresses Tumor Growth in Triple-Negative Breast Cancer by Activating TFEB- and TFE3-Mediated Lysosome Biogenesis and Autophagy. Mol. Cancer Ther. 2022, 21, 1547–1560. [Google Scholar] [CrossRef]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Groll, J.; Schmid, O. Real-Time Measurement of Cell Mechanics as a Clinically Relevant Readout of an In Vitro Lung Fibrosis Model Established on a Bioinspired Basement Membrane. Adv. Mater. 2022, 34, 2205083. [Google Scholar] [CrossRef]
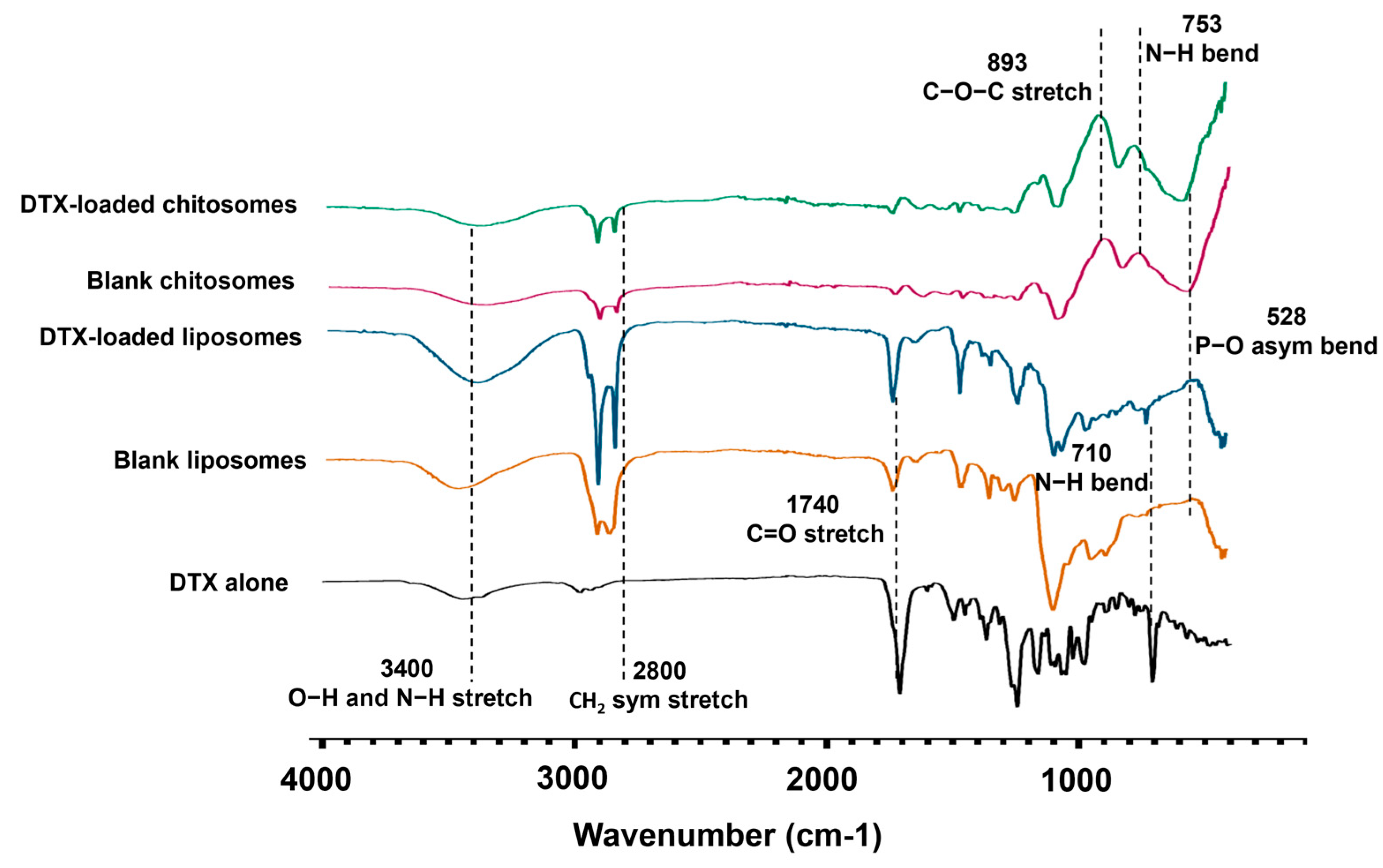

| Wavenumber (cm−1) | Vibrational Mode | Biomolecular Attributions |
|---|---|---|
| 528 | P-O asymmetrical bending | Phospholipids (PO4−3 molecule) |
| 710 | Benzamide N-H bending | Docetaxel |
| 753 | N-H bending | Chitosan |
| 893 | Glycosidic C-O-C stretching | Chitosan |
| 1740 | C=O stretching | Lipids |
| 2800 | CH2 stretching | Lipids |
| 3400 | O-H and N-H stretching | Lipids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Garcia, C.R.; Li-Jessen, N.Y.K.; Tabrizian, M. Chitosomes Loaded with Docetaxel as a Promising Drug Delivery System to Laryngeal Cancer Cells: An In Vitro Cytotoxic Study. Int. J. Mol. Sci. 2023, 24, 9902. https://doi.org/10.3390/ijms24129902
Moya-Garcia CR, Li-Jessen NYK, Tabrizian M. Chitosomes Loaded with Docetaxel as a Promising Drug Delivery System to Laryngeal Cancer Cells: An In Vitro Cytotoxic Study. International Journal of Molecular Sciences. 2023; 24(12):9902. https://doi.org/10.3390/ijms24129902
Chicago/Turabian StyleMoya-Garcia, Christian R., Nicole Y. K. Li-Jessen, and Maryam Tabrizian. 2023. "Chitosomes Loaded with Docetaxel as a Promising Drug Delivery System to Laryngeal Cancer Cells: An In Vitro Cytotoxic Study" International Journal of Molecular Sciences 24, no. 12: 9902. https://doi.org/10.3390/ijms24129902
APA StyleMoya-Garcia, C. R., Li-Jessen, N. Y. K., & Tabrizian, M. (2023). Chitosomes Loaded with Docetaxel as a Promising Drug Delivery System to Laryngeal Cancer Cells: An In Vitro Cytotoxic Study. International Journal of Molecular Sciences, 24(12), 9902. https://doi.org/10.3390/ijms24129902