ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration
Abstract
1. Introduction
2. Results
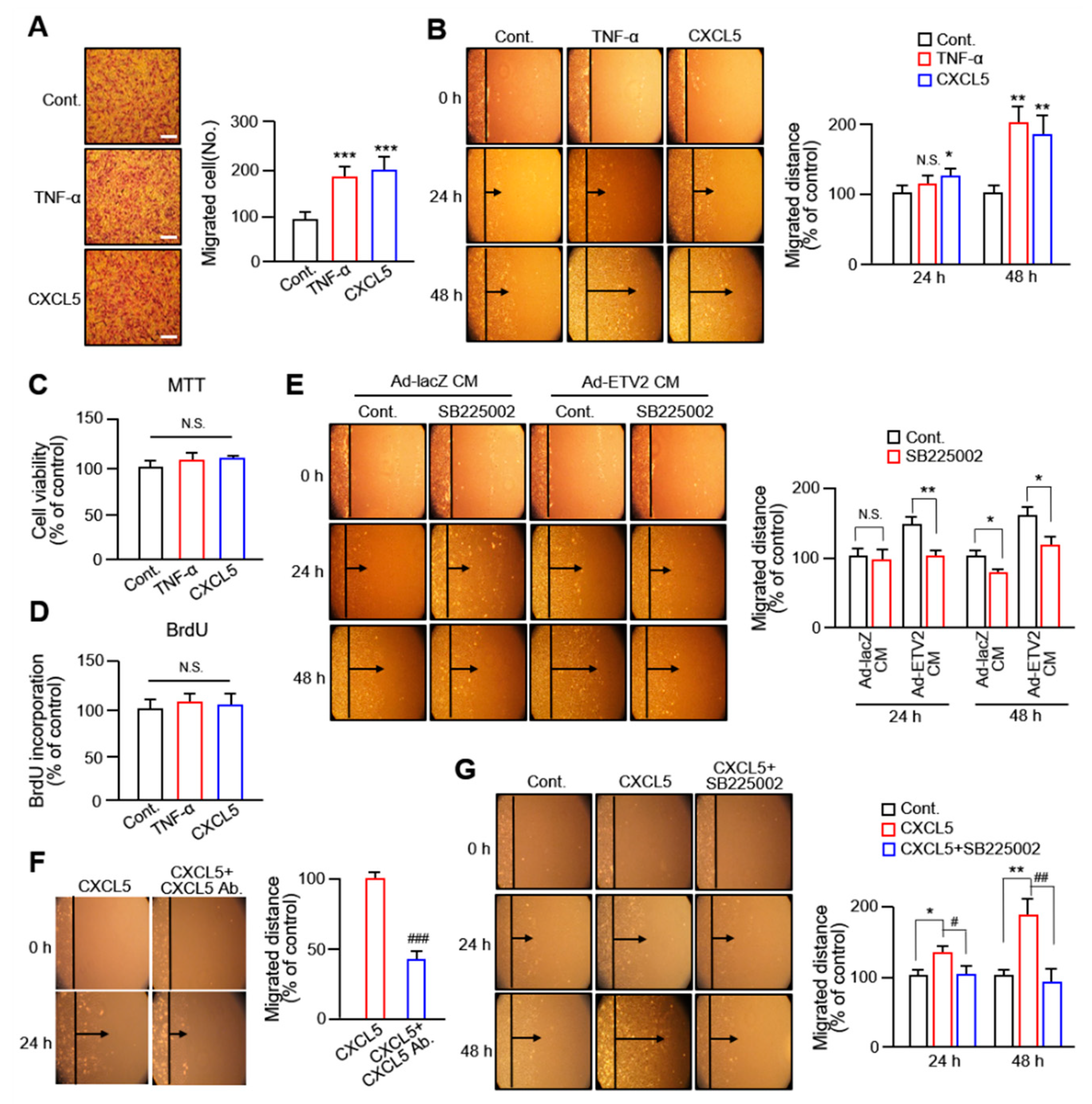
2.1. Conditioned Medium from ETV2-Transduced Human Umbilical ECs Promotes VSMC Migration
2.2. CXCL5 Secreted from ECs Facilitates VSMC Migration via the CXC Motif Chemokine Receptor 2 Axis
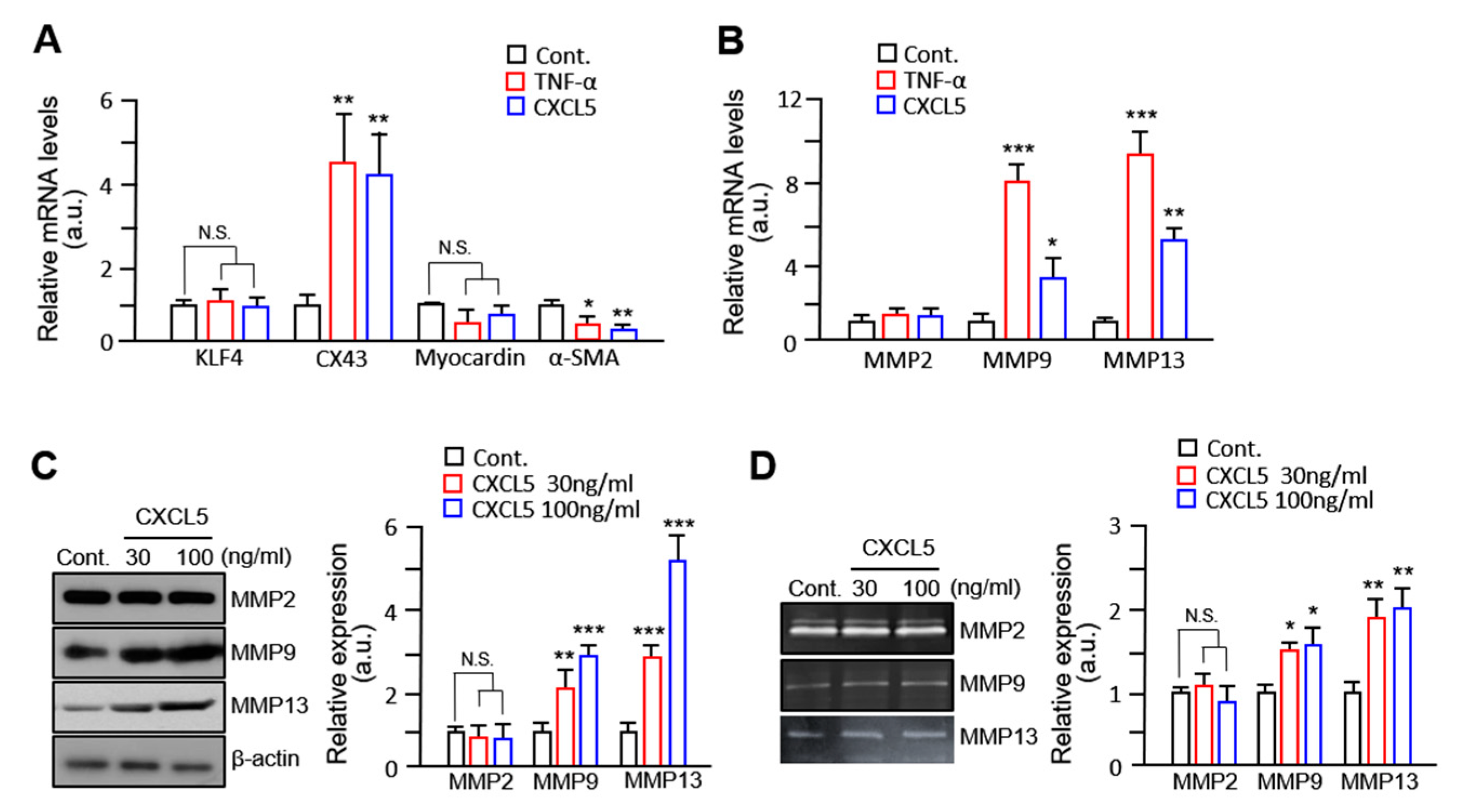
2.3. VSMC Migration by CXCL5 Is Mediated by MMP9/MMP13 but Not MMP2
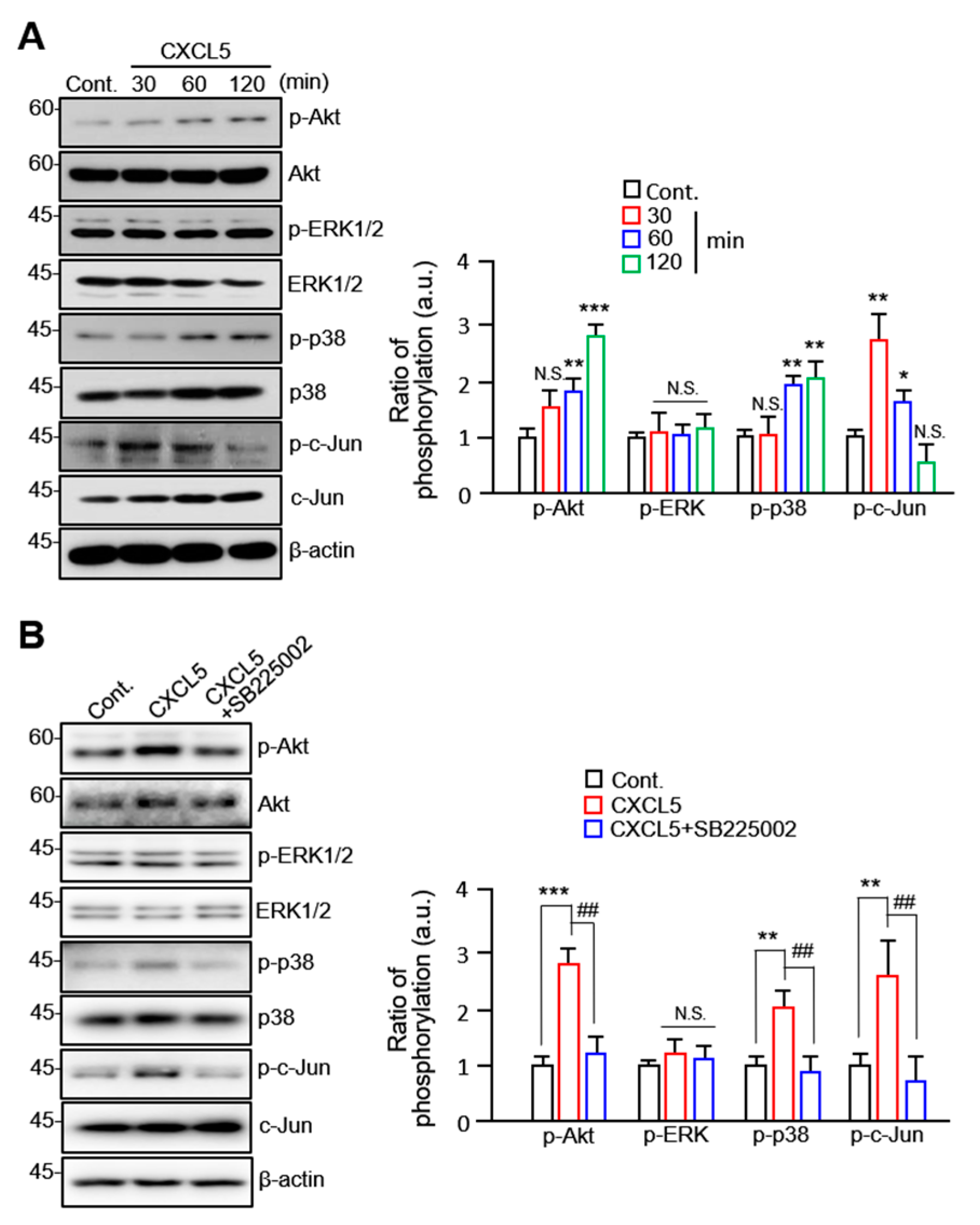
2.4. CXCL5 Stimulates VSMC Migration in an Akt/p38-c-Jun-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures
4.3. Construction of Adenoviral Vector
4.4. Cytokine Antibody Array
4.5. RNA Isolation and Quantitative RT-qPCR
4.6. MTT Cell Viability Assay
4.7. BrdU Proliferation Assay
4.8. Cell Migration Assay
4.9. Gelatin and Collagen Zymography
4.10. Western Blot Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Endothelial-Vascular Smooth Muscle Cells Interactions in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Latsios, G.; Vogiatzi, G.; Tousoulis, D. Chapter 1.3—Atherosclerotic Plaque; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Agnelli, G.; Belch, J.J.F.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Heiss, G.; Shahar, E.; Earp, M.J.; Toole, J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2004, 160, 259–269. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Méndez-Barbero, N.; Gutiérrez-Muñoz, C.; Blanco-Colio, L.M. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int. J. Mol. Sci. 2021, 22, 7284. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Tomaszewski, K.A.; Niedzwiedzki, L.; Walocha, J.A.; Niedzwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Amanso, A.M.; Griendling, K.K. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front. Biosci. (Schol. Ed.) 2012, 4, 1044–1064. [Google Scholar] [PubMed]
- Chakraborty, R.; Chatterjee, P.; Dave, J.M.; Ostriker, A.C.; Greif, D.M.; Rzucidlo, E.M.; Martin, K.A. Targeting smooth muscle cell phenotypic switching in vascular disease. JVS Vasc. Sci. 2021, 2, 79–94. [Google Scholar] [CrossRef]
- Rzucidlo, E.M.; Martin, K.A.; Powell, R.J. Regulation of vascular smooth muscle cell differentiation. J Vasc. Surg. 2007, 45 (Suppl. A), A25–A32. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B. We have contact: Endothelial cell-smooth muscle cell interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef]
- Gilbertson-Beadling, S.K.; Fisher, C. A potential role for N-cadherin in mediating endothelial cell-smooth muscle cell interactions in the rat vasculature. Lab. Investig. 1993, 69, 203–209. [Google Scholar]
- Isakson, B.E.; Damon, D.N.; Day, K.H.; Liao, Y.; Duling, B.R. Connexin40 and connexin43 in mouse aortic endothelium: Evidence for coordinated regulation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1199–H1205. [Google Scholar] [CrossRef] [PubMed]
- Lutter, S.; Xie, S.; Tatin, F.; Makinen, T. Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J. Cell Biol. 2012, 197, 837–849. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef]
- Hafiane, A.; Daskalopoulou, S.S. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism 2018, 85, 213–222. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Schoppet, M.; Schurgers, L.J.; Reynolds, J.L.; McNair, R.; Heiss, A.; Jahnen-Dechent, W.; Hackeng, T.M.; Schlieper, G.; Harrison, P.; et al. Prothrombin Loading of Vascular Smooth Muscle Cell-Derived Exosomes Regulates Coagulation and Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e22–e32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.S.; Nguyen, P.; Wang, K.C.; Weiss, A.; Kuo, Y.C.; Chiu, J.J.; Shyy, J.Y.; Chien, S. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: Role of shear stress. Circ. Res. 2013, 113, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.X.; Jiang, J.; Jiang, X.H.; Wang, X.D.; Ji, S.Y.; Han, Y.; Long, D.K.; Shen, B.R.; Yan, Z.Q.; Chien, S.; et al. PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1908–1913. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.H.; Park, B.-W.; Kim, R.; Hoang, A.D.; Woo, S.-K.; Xiong, W.; Lee, Y.J.; Ban, K.; Park, H.-J. In vivo transduction of ETV2 improves cardiac function and induces vascular regeneration following myocardial infarction. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar]
- Park, C.; Lee, T.J.; Bhang, S.H.; Liu, F.; Nakamura, R.; Oladipupo, S.S.; Pitha-Rowe, I.; Capoccia, B.; Choi, H.S.; Kim, T.M.; et al. Injury-Mediated Vascular Regeneration Requires Endothelial ER71/ETV2. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.F.; Zahradka, P. Vascular smooth muscle cell motility: From migration to invasion. Exp. Clin. Cardiol. 2010, 15, e75–e85. [Google Scholar] [PubMed]
- Lee, D.H.; Kim, T.M.; Kim, J.K.; Park, C. ETV2/ER71 Transcription Factor as a Therapeutic Vehicle for Cardiovascular Disease. Theranostics 2019, 9, 5694–5705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Li, N.; Wu, T.; Zheng, X.; Heng, B.C.; Zou, D.; Xu, J. Upregulation of ETV2 Expression Promotes Endothelial Differentiation of Human Dental Pulp Stem Cells. Cell Transplant. 2021, 30, 963689720978739. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, Y.; Xu, J. Activation of CXCL5-CXCR2 axis promotes proliferation and accelerates G1 to S phase transition of papillary thyroid carcinoma cells and activates JNK and p38 pathways. Cancer Biol. Ther. 2019, 20, 608–616. [Google Scholar] [CrossRef]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lin, C.; Zhou, H.J.; Min, W. Smooth muscle cell differentiation: Mechanisms and models for vascular diseases. Front. Biol. 2017, 12, 392–405. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Fan, L.J.; Zhang, X.Y.; Li, J.P.; Yu, C.X.; Bao, L.Y.; Cao, D.S.; Xing, W.B.; Liao, X.H.; et al. Myocardin inhibited the gap protein connexin 43 via promoted miR-206 to regulate vascular smooth muscle cell phenotypic switch. Gene 2017, 616, 22–30. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Chapter Eight-Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. In Advances in Pharmacology; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 81, pp. 241–330. [Google Scholar]
- Hirase, T.; Node, K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am. J. Physiol. -Heart Circ. Physiol. 2012, 302, H499–H505. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zou, M.-H. Molecular Insights and Therapeutic Targets for Diabetic Endothelial Dysfunction. Circulation 2009, 120, 1266–1286. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, D.H.; Kim, J.K.; Choi, H.S.; Dwivedi, B.; Rupji, M.; Kowalski, J.; Green, S.J.; Song, H.; Park, W.J.; et al. ETV2/ER71 regulates the generation of FLK1+ cells from mouse embryonic stem cells through miR-126-MAPK signaling. Stem Cell Res. Ther. 2019, 10, 328. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Sun, M.; Deng, X.; Wu, X.; Ma, Y.; Li, M.; Shuoa, S.M.; You, Q.; Miao, L. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun. 2020, 40, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, D.; Li, X.; Ma, S.; Zhang, C.; Wang, J.; Li, Y.; Liang, L.; Zhang, P.; Qu, Y.; et al. CXCL5, the upregulated chemokine in patients with uterine cervix cancer, in vivo and in vitro contributes to oncogenic potential of Hela uterine cervix cancer cells. Biomed. Pharmacother. 2018, 107, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ou, B.; Han, D.; Wang, P.; Zong, Y.; Zhu, C.; Liu, D.; Zheng, M.; Sun, J.; Feng, H.; et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol. Cancer 2017, 16, 70. [Google Scholar] [CrossRef]
- Chen, C.; Xu, Z.-Q.; Zong, Y.-P.; Ou, B.-C.; Shen, X.-H.; Feng, H.; Zheng, M.-H.; Zhao, J.-K.; Lu, A.-G. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NF-κB pathway in colorectal cancer. Cell Death Dis. 2019, 10, 178. [Google Scholar] [CrossRef]
- Gao, Y.; Guan, Z.; Chen, J.; Xie, H.; Yang, Z.; Fan, J.; Wang, X.; Li, L. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int. J. Oncol. 2015, 47, 690–700. [Google Scholar] [CrossRef]
- Sapharikas, E.; Lokajczyk, A.; Fischer, A.M.; Boisson-Vidal, C. Fucoidan Stimulates Monocyte Migration via ERK/p38 Signaling Pathways and MMP9 Secretion. Mar. Drugs 2015, 13, 4156–4170. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Parks, W.C. Role of matrix metalloproteinases in epithelial migration. J. Cell. Biochem. 2009, 108, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wang, P.H.; Lu, Y.T.; Yang, J.S.; Yang, S.F.; Ho, Y.T.; Lin, C.W.; Hsin, C.H. Morusin Suppresses Cancer Cell Invasion and MMP-2 Expression through ERK Signaling in Human Nasopharyngeal Carcinoma. Molecules 2020, 25, 4851. [Google Scholar] [CrossRef]
- Husain, S.; Jafri, F.; Crosson, C.E. Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells: A PKC- and ERK-dependent process. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Lee, R.H.; Kim, M.S.; Lewis, C.A.; Park, C. ETV2/ER71, the key factor leading the paths to vascular regeneration and angiogenic reprogramming. Stem Cell Res. Ther. 2023, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.H.; Fuster, V.; Badimon, L.; Badimon, J.; Taubman, M.B.; Chesebro, J.H. Syndromes of accelerated atherosclerosis: Role of vascular injury and smooth muscle cell proliferation. J. Am. Coll. Cardiol. 1990, 15, 1667–1687. [Google Scholar] [CrossRef]
- Kwartler, C.S.; Zhou, P.; Kuang, S.-Q.; Duan, X.-Y.; Gong, L.; Milewicz, D.M. Vascular Smooth Muscle Cell Isolation and Culture from Mouse Aorta. Bio-Protocol 2016, 6, e2045. [Google Scholar] [CrossRef]
- Ju, S.; Lim, L.; Jiao, H.-Y.; Choi, S.; Jun, J.Y.; Ki, Y.-J.; Choi, D.-H.; Lee, J.Y.; Song, H. Oxygenated polycyclic aromatic hydrocarbons from ambient particulate matter induce electrophysiological instability in cardiomyocytes. Part. Fibre Toxicol. 2020, 17, 25. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| ETV2 | ATTCAGCTGTGGCAGTTCCT | CCGAAGCGGTACGTGTACTT |
| CXCL1 | GCGCCCAAACCGAAGTCATA | ATGGGGGATGCAGGATTGAG |
| CXCL5 | CGGGAAGGAAATTTGTCTTGA | AGCTTAAGCGGCAAACATAGG |
| IL-6 | GCACTGGCAGAAAACAACCT | TCAAACTCCAAAAGACCAGTGA |
| MCP-1 | TTCCCCTAGCTTTCCCCAGA | TCCCAGGGGTAGAACTGTGG |
| Angiogenin | TTCCTGACCCAGCACTATGATG | CGTCTCCTCATGATGCTTTCAC |
| Il-8 | CTCTTGGCAGCCTTCCTGATT | ACTCTCAATCACTCTCAGTTCT |
| KLF4 | TCAAGAGCTCATGCCACCGG | CTCGCCTGTGTGAGTTCGCA |
| CX43 | GGCCTTCCTGCTCATCCA | GGGATCTCTCTTGCAGGTGTAGA |
| Myocardin | CAGAAAGTGACAAGAACGATACAG | TGAAGCAGCCGAGCATAGG |
| α-SMA | AACTGGTATTGTGCTGGACTCTGG | CACGGACGATCTCACGCTCAG |
| MMP2 | GCGATGGCAAGGTGTGGTGT | GTACCAGTGTCAGTATCAGC |
| MMP9 | AGGCGCCGTGGTCCCCACTTACTT | GCAGGGTTTGCCGTCTCCGTTGCC |
| MMP13 | TCGCATTGTGAGAGTCATGCCAACA | TGTGGTTCCAGCCACGCATAGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Chu, B.; Choi, D.-H.; Lim, L.; Song, H. ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration. Int. J. Mol. Sci. 2023, 24, 9904. https://doi.org/10.3390/ijms24129904
Sun N, Chu B, Choi D-H, Lim L, Song H. ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration. International Journal of Molecular Sciences. 2023; 24(12):9904. https://doi.org/10.3390/ijms24129904
Chicago/Turabian StyleSun, Ningning, Beyongsam Chu, Dong-Hyun Choi, Leejin Lim, and Heesang Song. 2023. "ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration" International Journal of Molecular Sciences 24, no. 12: 9904. https://doi.org/10.3390/ijms24129904
APA StyleSun, N., Chu, B., Choi, D.-H., Lim, L., & Song, H. (2023). ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration. International Journal of Molecular Sciences, 24(12), 9904. https://doi.org/10.3390/ijms24129904







