Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantification of Hispolon by HPLC
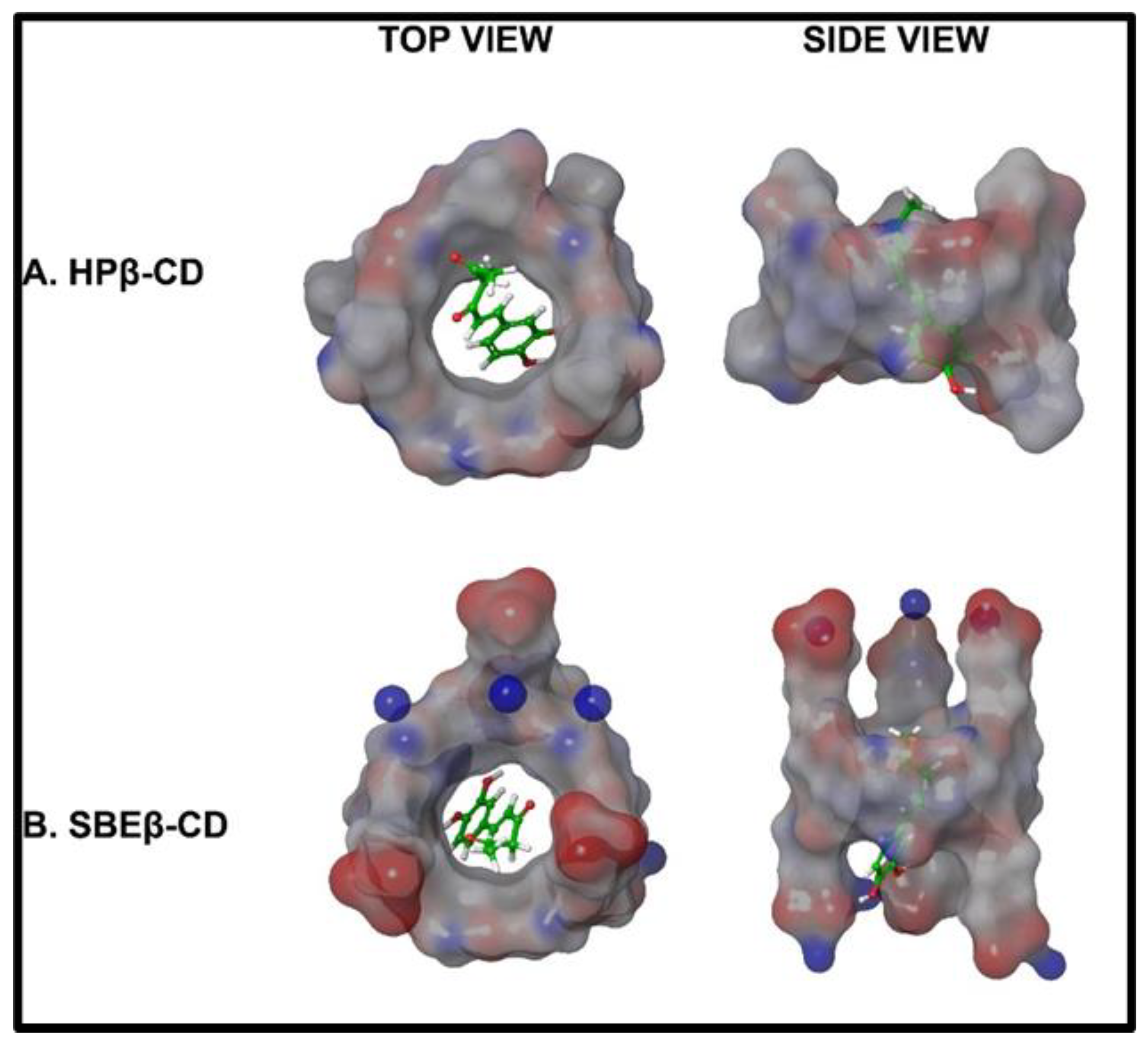
2.2. Cyclodextrin for Inclusion Complexation by In-Silico Molecular Modeling and Phase Solubility Studies
2.3. Complex Preparation and Solid-State Characterization Studies
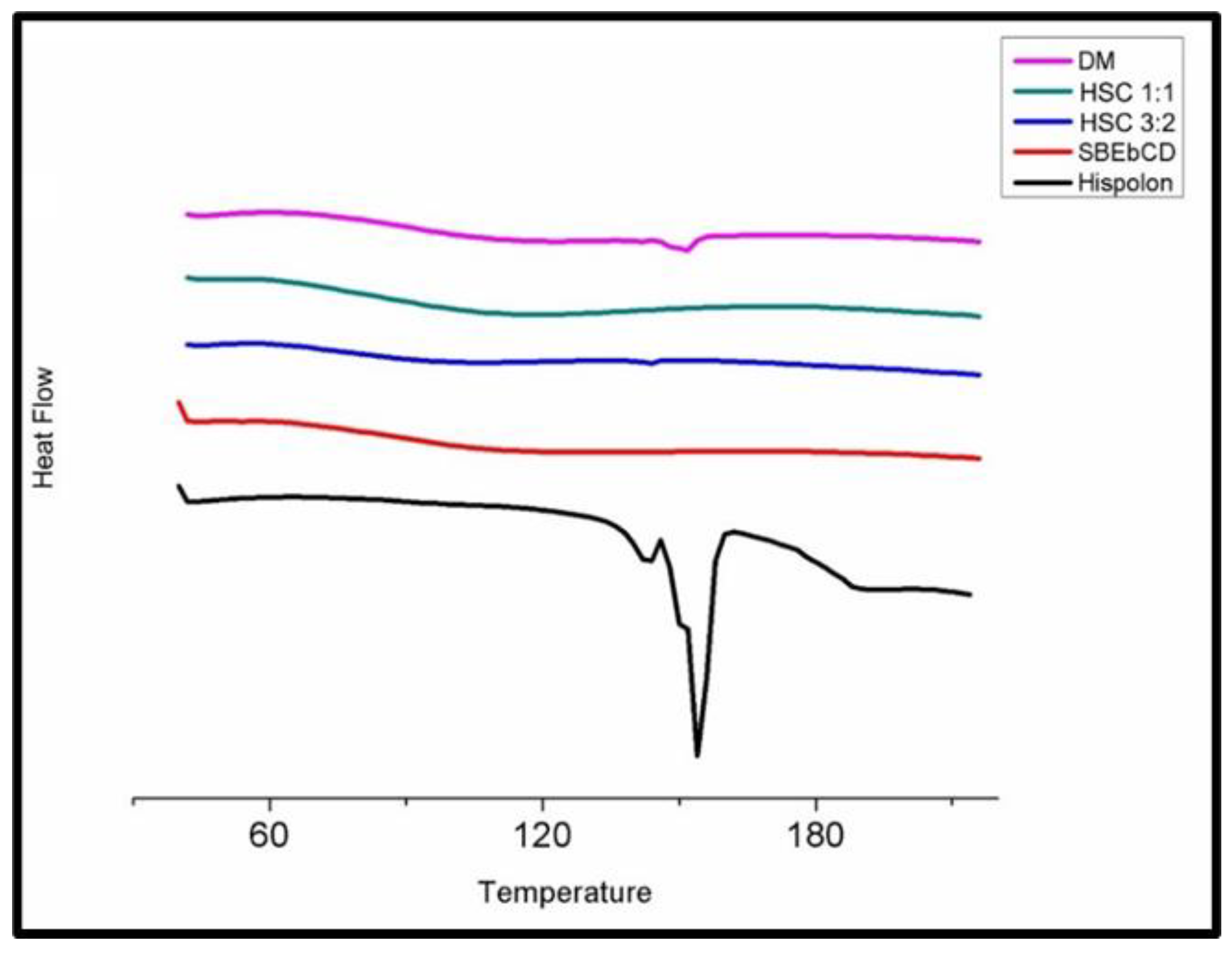
2.3.1. DSC (Differential Scanning Calorimetry)
2.3.2. FTIR (Fourier Transform Infrared Spectroscopy)
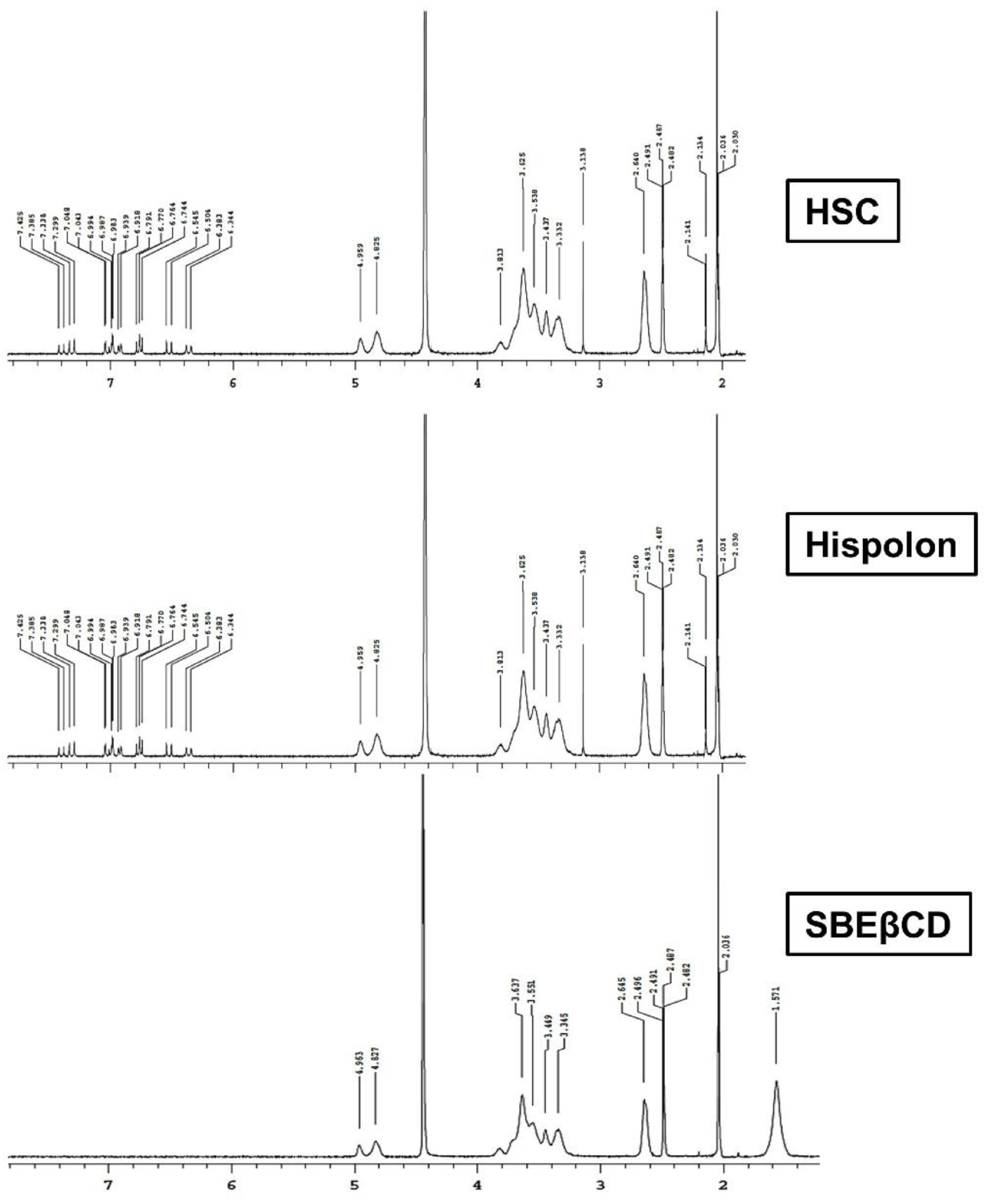
2.3.3. Proton NMR
2.3.4. SEM (Scanning Electron Microscopy)
2.4. Optimization of Liposomal Formulations and Physiochemical Characterization
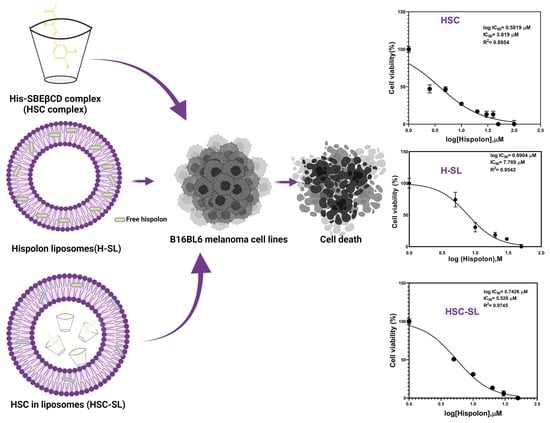
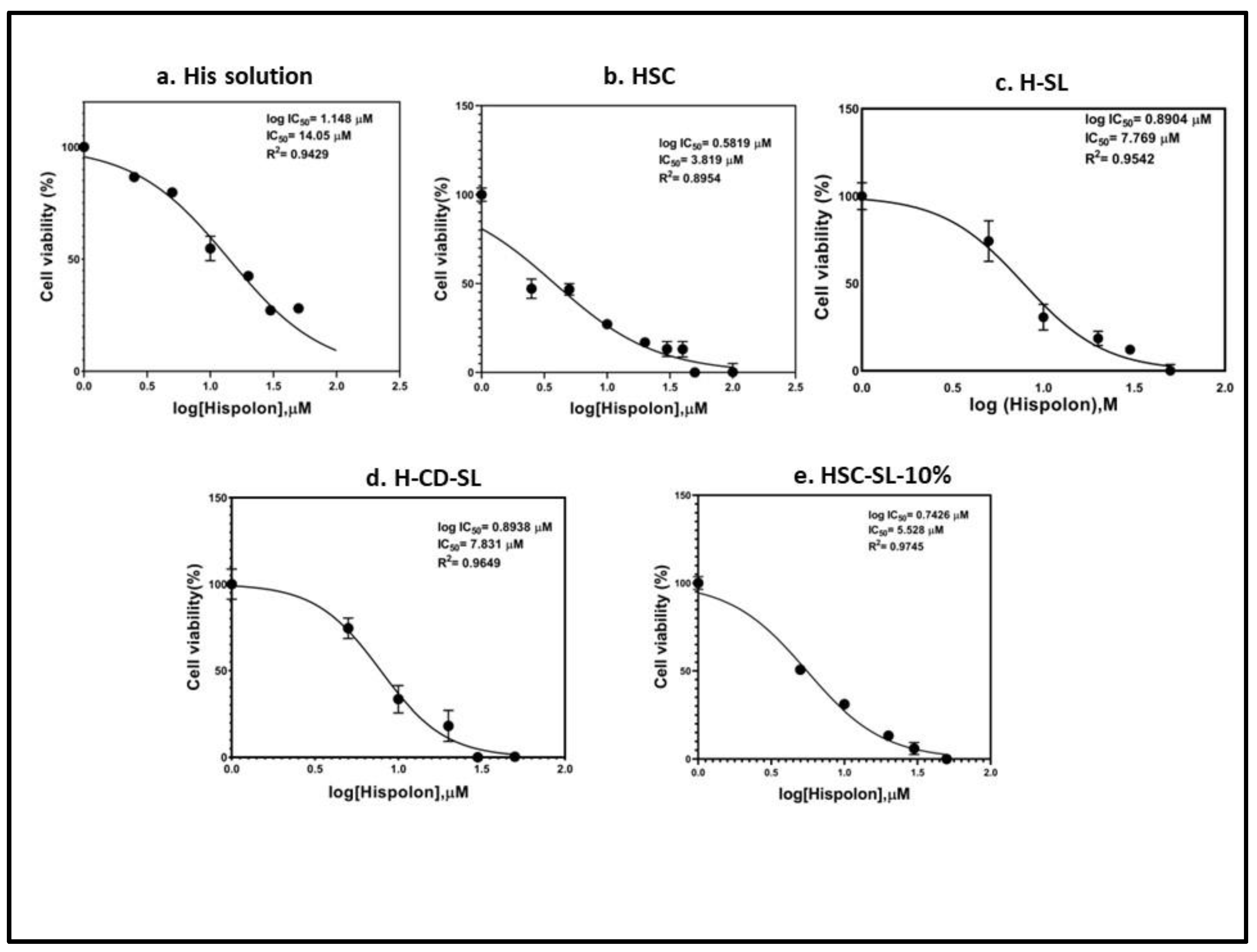
2.5. Cytotoxicity Studies of Solid HSC
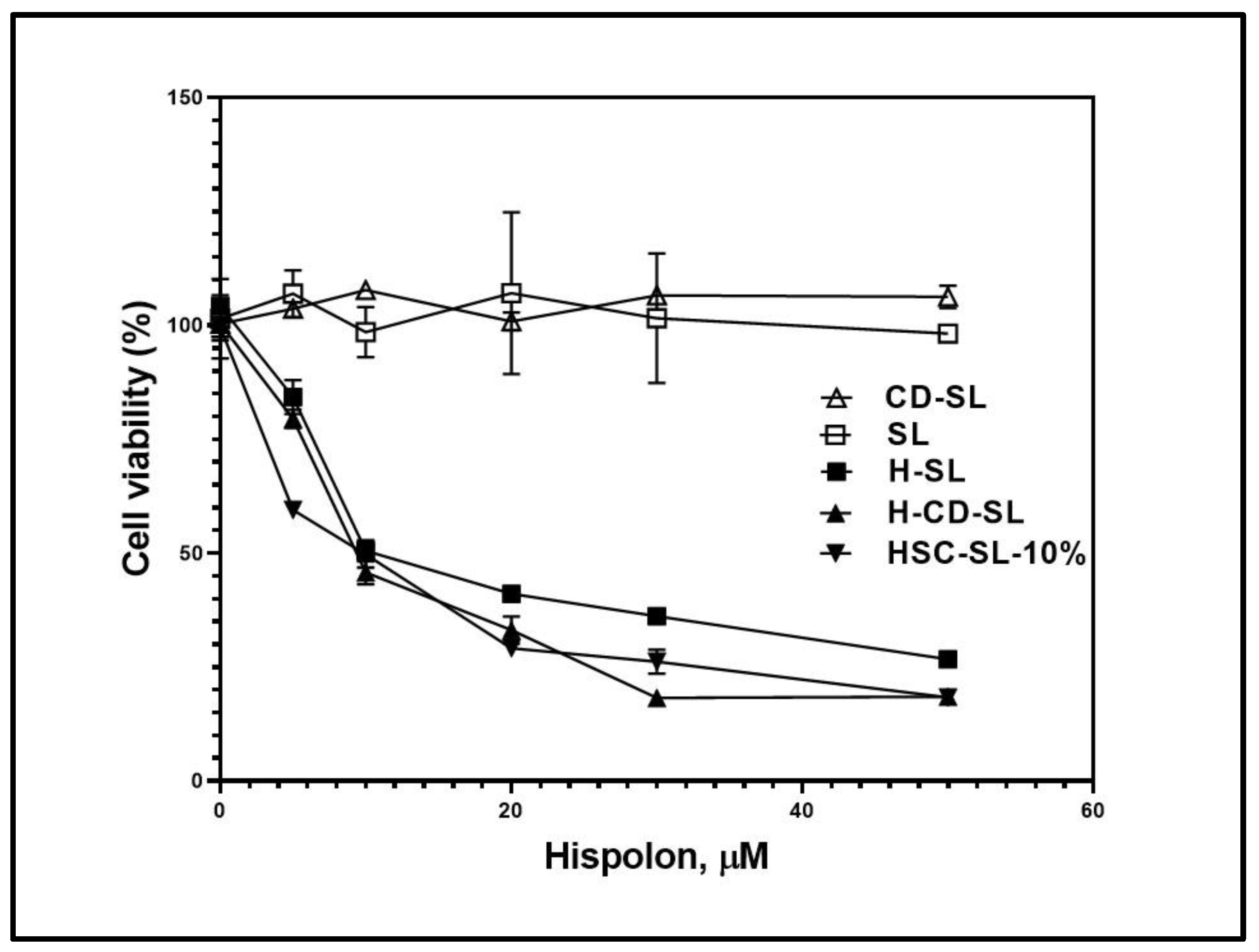
2.6. Comparison of Cytotoxicity Studies of Various Liposomal Formulations
3. Materials and Methods
3.1. Materials
3.2. Cell Lines
3.3. HPLC Method
3.4. Phase Solubility Studies
3.5. In Silico Molecular Docking Studies
3.6. Continuous Variation Method (Job’s Plot)
3.7. Preparation of Hispolon-SBEβCD Complex (HSC)
3.7.1. Dry Mixture (DM)
3.7.2. Freeze Dried Hispolon-SBEβCD Complex (HSC)
3.8. Determination of Hispolon Content in HSC Complex
3.9. Solid-State Characterization
3.9.1. DSC
3.9.2. FTIR
3.9.3. 1H-NMR
3.9.4. SEM
3.10. Preparation of Liposomal Formulations Incorporating Cyclodextrins and HSC Inclusion Complexes
3.10.1. Formulation of Blank Sterically Stabilized Liposomes (SL)
3.10.2. Formulation of Hispolon Loaded Sterically Stabilized Liposomes (H-SL)
3.10.3. Formulation of Hispolon-SBEβCD Liposomes with In-Process Complexation (H-CD-SL)
3.10.4. Formulation of Hispolon Complex in Liposomes (HSC-SL)
3.10.5. Determination of Phospholipid Concentration
3.10.6. Physiochemical Characterization of Liposomes
Particle Size of Liposomes
Zeta Potential of Liposomes
Osmololality of Liposomes
Recovery and Yield of the Process
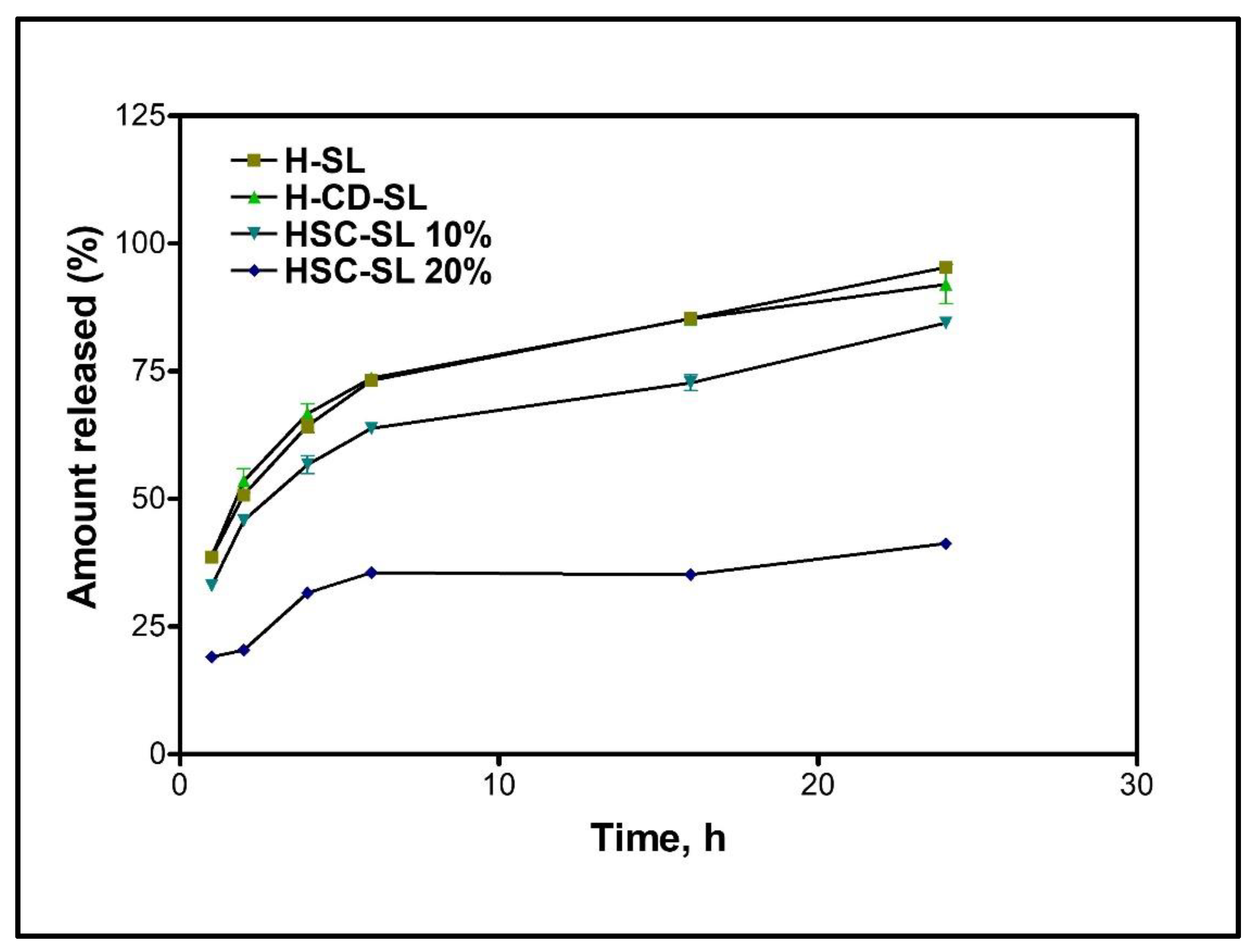
3.11. In Vitro Release of Hispolon from Liposomal Formulations
3.12. Cytotoxicity Studies of Hispolon and Solid HSC
3.13. Cytotoxicity Studies of Various Liposomal Formulations
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development–Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef]
- Toopmuang, P.; Khamchum, C.; Punsuvon, V. Detection and confirmation of hispolon in the mushroom Phellinus linteus. J. Sci. Asia 2014, 40, 141–144. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lien, H.-M.; Chang, H.-Y.; Huang, C.-L.; Liu, J.-J.; Chang, Y.-C.; Chen, C.-C.; Lai, C.-H. Biological evaluation of Phellinus linteus-fermented broths as anti-inflammatory agents. J. Biosci. Bioeng. 2014, 118, 88–93. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Ramachandra, M.; Sethuramu, K.; Subbaraju, G.V. Synthesis and antioxidant activity of hispolon, a yellow pigment from Inonotus hispidius. ChemInform 2002, 33. [Google Scholar] [CrossRef]
- Al Saqr, A.; Majrashi, M.; Alrbyawi, H.; Govindarajulu, M.; Fujihashi, A.; Gottumukkala, S.; Poudel, I.; Arnold, R.D.; Babu, R.J.; Dhanasekaran, M. Elucidating the anti-melanoma effect and mechanisms of Hispolon. Life Sci. 2020, 256, 117702. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rahman, M.; Alharbi, K.S.; Altowayan, W.M.; Ali, A.M.A.; Almalki, W.H.; Barkat, M.A.; Singh, T.; Nasar, S.; Akhter, M.H. Hispolon-Loaded Liquid Crystalline Nanoparticles: Development, Stability, In Vitro Delivery Profile, and Assessment of Hepatoprotective Activity in Hepatocellular Carcinoma. ACS Omega 2022, 7, 9452–9464. [Google Scholar] [CrossRef]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, Ş.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ. Res. 2020, 190, 110017. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Lu, T.-L.; Huang, G.-J.; Wang, H.-J.; Chen, J.-L.; Hsu, H.-P.; Lu, T.-J. Hispolon promotes MDM2 downregulation through chaperone-mediated autophagy. Biochem. Biophys. Res. Commun. 2010, 398, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Li, L.; Wu, B.; Chen, S.-F.; Zhou, H.; Wang, Y.; Li, Y.-Q. Hispolon induces apoptosis in human gastric cancer cells through a ROS-mediated mitochondrial pathway. Free Radic. Biol. Med. 2008, 45, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Aldawsari, M.F.; Alrbyawi, H.; Poudel, I.; Annaji, M.; Mulabagal, V.; Ramani, M.V.; Gottumukkala, S.; Tiwari, A.K.; Dhanasekaran, M. Co-delivery of hispolon and doxorubicin liposomes improves efficacy against melanoma cells. AAPS PharmSciTech 2020, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.; Auezova, L.; Danjou, P.-E.; Fourmentin, S.; Greige-Gerges, H. First evaluation of drug-in-cyclodextrin-in-liposomes as an encapsulating system for nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef]
- Souri, J.; Almasi, H.; Hamishehkar, H.; Amjadi, S. Sodium caseinate-coated and β-cyclodextrin/vitamin E inclusion complex-loaded nanoliposomes: A novel stabilized nanocarrier. LWT 2021, 151, 112174. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Ferreira, L.; Peixoto, D.; Silva, F.; Soares, M.J.; Zeinali, M.; Zafar, H.; Mascarenhas-Melo, F.; Raza, F.; Mazzola, P.G. Cyclodextrins as an encapsulation molecular strategy for volatile organic compounds–pharmaceutical applications. Colloids Surf. B Biointerfaces 2022, 218, 112758. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-cyclodextrin-in-liposomes as a carrier system for volatile essential oil components: Application to anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C.; Yang, X.-X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, X.; Zhang, W.; Lin, C.; Chen, F.; Yang, X.; Pan, W. Drug-in-cyclodextrin-in-liposomes: A novel drug delivery system for flurbiprofen. Int. J. Pharm. 2015, 492, 40–45. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Mura, P. Preparation and characterisation of liposomes encapsulating ketoprofen–cyclodextrin complexes for transdermal drug delivery. Int. J. Pharm. 2005, 298, 55–67. [Google Scholar] [CrossRef]
- McCormack, B.; Gregoriadis, G. Drugs-in-cyclodextrins-in liposomes: A novel concept in drug delivery. Int. J. Pharm. 1994, 112, 249–258. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin–drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-cyclodextrin-in-liposome: The effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, S.; Gu, W.; Peng, P.; Dong, J.; Xu, F.; Yang, X.; Xiong, Z.; Yang, X. Characterization of 9-nitrocamptothecin-in-cyclodextrin-in-liposomes modified with transferrin for the treating of tumor. Int. J. Pharm. 2015, 490, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Jain, A.; Bhola, M. Multivesicular liposomes bearing celecoxib-β-cyclodextrin complex for transdermal delivery. Drug Deliv. 2007, 14, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rescifina, A.; Surdo, E.; Cardile, V.; Avola, R.; Eleonora Graziano, A.C.; Stancanelli, R.; Tommasini, S.; Pistarà, V.; Ventura, C.A. Gemcitabine anticancer activity enhancement by water soluble celecoxib/sulfobutyl ether-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2019, 206, 792–800. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Belgamwar, V.S. Inclusion complex of chrysin with sulfobutyl ether-β-cyclodextrin (Captisol®): Preparation, characterization, molecular modelling and in vitro anticancer activity. J. Mol. Struct. 2017, 1128, 563–571. [Google Scholar] [CrossRef]
- Yakavets, I.; Lassalle, H.-P.; Scheglmann, D.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition. Nanomaterials 2018, 8, 847. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Elbatanony, R.S.; Shukla, S.K.; Kulkarni, N.S.; Kanabar, D.D.; Chauhan, G.; Ayehunie, S.; Chen, Z.-S.; Muth, A.; Gupta, V. Bypassing P-glycoprotein mediated efflux of afatinib by cyclodextrin complexation–Evaluation of intestinal absorption and anti-cancer activity. J. Mol. Liq. 2021, 327, 114866. [Google Scholar] [CrossRef]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Iacobazzi, R.M.; Fanizza, E.; Laquintana, V.; Perrone, M.; Maggi, V.; Franco, M. A new complex of curcumin with sulfobutylether-β-cyclodextrin: Characterization studies and in vitro evaluation of cytotoxic and antioxidant activity on HepG-2 cells. J. Pharm. Sci. 2014, 103, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Shelley, H.; Grant, M.; Smith, F.T.; Abarca, E.M.; Jayachandra Babu, R. Improved Ocular Delivery of Nepafenac by Cyclodextrin Complexation. AAPS PharmSciTech 2018, 19, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin complexation for enhanced stability and non-invasive pulmonary delivery of resveratrol—Applications in non-small cell lung cancer treatment. Aaps Pharmscitech 2020, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.-X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Nabih Maria, D.; Mishra, S.R.; Wang, L.; Helmy Abd-Elgawad, A.-E.; Abd-Elazeem Soliman, O.; Salah El-Dahan, M.; Jablonski, M.M. Water-soluble complex of curcumin with cyclodextrins: Enhanced physical properties for ocular drug delivery. Curr. Drug Deliv. 2017, 14, 875–886. [Google Scholar]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocoll. 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 2007, 338, 35–42. [Google Scholar] [CrossRef]
- Huang, Z.; London, E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin–lipid interaction. Langmuir 2013, 29, 14631–14638. [Google Scholar] [CrossRef]
- Fatouros, D.; Hatzidimitriou, K.; Antimisiaris, S. Liposomes encapsulating prednisolone and prednisolone–cyclodextrin complexes: Comparison of membrane integrity and drug release. Eur. J. Pharm. Sci. 2001, 13, 287–296. [Google Scholar] [CrossRef]
- Lin, E.-Y.; Chen, Y.-S.; Li, Y.-S.; Chen, S.-R.; Lee, C.-H.; Huang, M.-H.; Chuang, H.-M.; Harn, H.-J.; Yang, H.-H.; Lin, S.-Z. Liposome consolidated with cyclodextrin provides prolonged drug retention resulting in increased drug bioavailability in brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, J.; Wang, F.; Liang, W. Preparation, characterization and pharmacokinetics of liposomes-encapsulated cyclodextrins inclusion complexes for hydrophobic drugs. Drug Deliv. 2007, 14, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Koh, E.M.; Kim, Y.N.; Song, J.; Song, C.H.; Jung, K.J. Immunomodulatory Effect of Hispolon on LPS-Induced RAW264. 7 Cells and Mitogen/Alloantigen-Stimulated Spleen Lymphocytes of Mice. Pharmaceutics 2022, 14, 1423. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Cannavà, C.; Cristiano, M.C.; Fresta, M.; Majolino, D.; Paolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B Biointerfaces 2014, 115, 22–28. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Qiu, N.; Cheng, X.; Wang, G.; Wang, W.; Wen, J.; Zhang, Y.; Song, H.; Ma, L.; Wei, Y.; Peng, A. Inclusion complex of barbigerone with hydroxypropyl-β-cyclodextrin: Preparation and in vitro evaluation. Carbohydr. Polym. 2014, 101, 623–630. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Connors, K.; Higuchi, T. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Schmidt, A.K.; Cottaz, S.; Driguez, H.; Schulz, G.E. Structure of cyclodextrin glycosyltransferase complexed with a derivative of its main product β-cyclodextrin. Biochemistry 1998, 37, 5909–5915. [Google Scholar] [CrossRef]
- Sathigari, S.; Chadha, G.; Lee, Y.H.P.; Wright, N.; Parsons, D.L.; Rangari, V.K.; Fasina, O.; Babu, R.J. Physicochemical Characterization of Efavirenz–Cyclodextrin Inclusion Complexes. AAPS PharmSciTech 2009, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Alrbyawi, H.; Poudel, I.; Arnold, R.D.; Babu, R.J. Co-delivery of doxorubicin and ceramide in a liposomal formulation enhances cytotoxicity in murine B16BL6 melanoma cell lines. Aaps Pharmscitech 2019, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
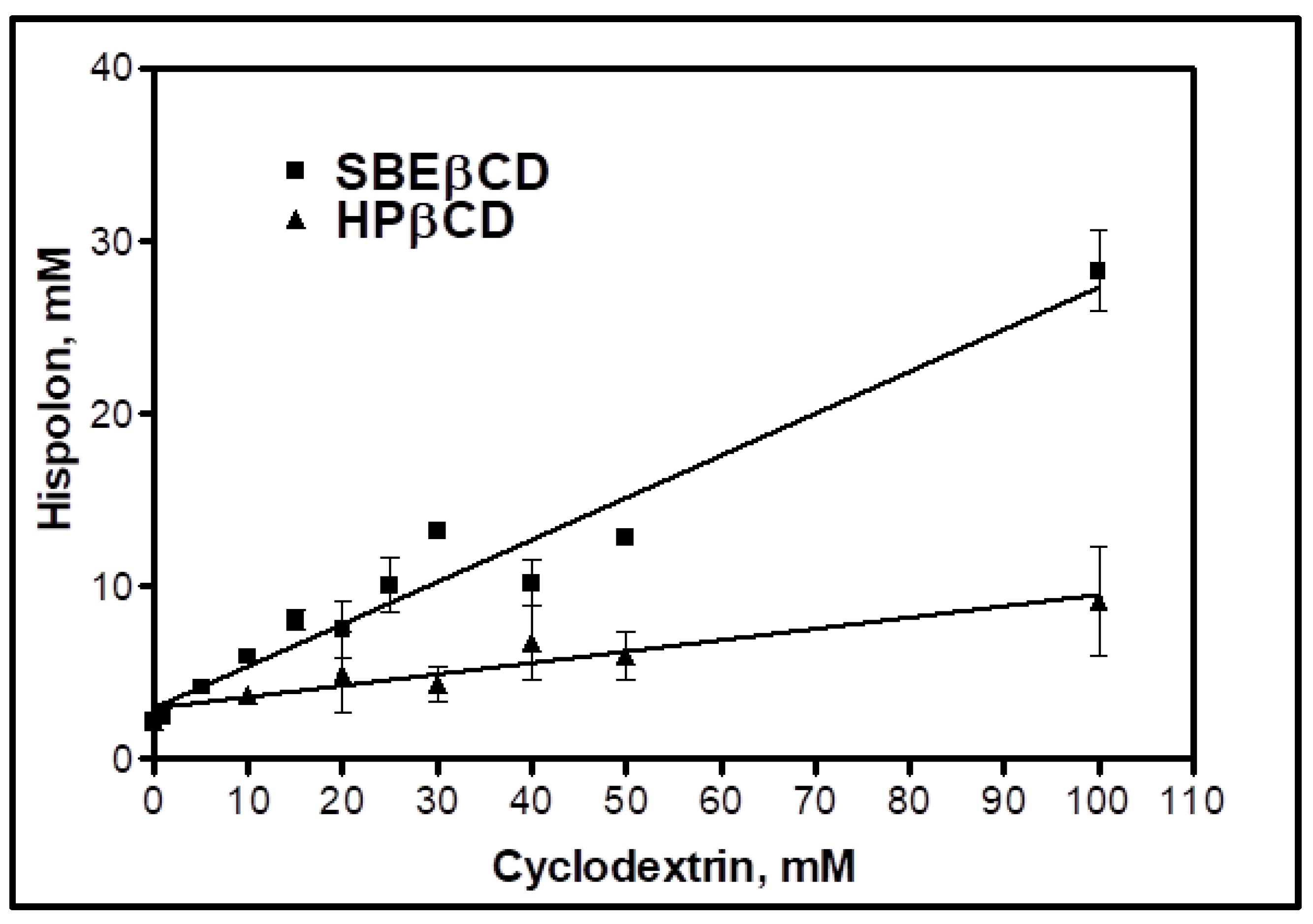



| Cyclodextrins | CD: Drug (Molar Ratio) | Stability Constant (Ks) | Complexation Efficiency (CE) | |
|---|---|---|---|---|
| SBEβCD | 1:1 | 340 M−1 | 0.47 | −14.3 KJ/mol |
| HPβCD | 1:1 | 38.7 M−1 | 0.08 | −8.96 KJ/mol |
| Hispolon Energy Minimization Level | Cyclodextrin Type | Energy | Docking Score |
|---|---|---|---|
| Level 1 | β-CD | 16.286 | −4.189 |
| Level 2 | β-CD | 19.297 | −4.473 |
| Level 1 | HP-β-CD | 16.286 | −4.953 |
| Level 2 | HP-β-CD | 19.297 | −4.305 |
| Level 1 | SBE-β-CD | 16.286 | −4.049 |
| Level 2 | SBE-β-CD | 19.297 | −3.826 |
| Composition | SL | H-SL | CD-SL | H-CD-SL | HSC-SL-10% |
|---|---|---|---|---|---|
| Hispolon | - | 1.000 | - | 1.000 | 1.000 (complex) |
| DSPC | 6.003 | 6.003 | 6.003 | 6.003 | 6.003 |
| Cholesterol | 3.335 | 3.335 | 3.335 | 3.335 | 3.335 |
| DSPE-mPEG | 0.667 | 0.667 | 0.667 | 0.667 | 0.667 |
| SBEβCD | - | - | 1.000 | 1.000 | 1.000 (complex) |
| His:Lipid | - | 1:10 | - | 1:10 | 1:10 |
| Liposome Batch | Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Osmolality (mOsm/kg) | Drug Loading % | Encapsulation Efficiency % |
|---|---|---|---|---|---|---|
| SL | 117 ± 5 | 0.129 | −28.5 ± 7.4 | - | - | - |
| H-SL | 130 ± 8 | 0.160 | −26.4 ± 8.0 | 385 ± 17 | 2.62 | 80.0 ± 0.86 |
| CD-SL | 124 ± 6 | 0.194 | −42.9 ± 13.6 | - | - | - |
| H-CD-SL | 126 ± 5 | 0.135 | −27.8 ± 7.7 | 341± 3 | 3.29 | 91.2 ± 0.14 |
| HSC-SL-10% | 126 ± 10 | 0.129 | −31.4 ± 6.6 | 344 ± 7 | 3.28 | 92.2 ± 0.03 |
| HSC-SL-20% | 114 ± 7.3 | 0.219 | −34.4 ± 6.8 | 341 ± 5 | 5.67 | 83.1 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, I.; Annaji, M.; Wibowo, F.S.; Arnold, R.D.; Fasina, O.; Via, B.; Rangari, V.; Peresin, M.S.; Smith, F.; Dhanasekaran, M.; et al. Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 14487. https://doi.org/10.3390/ijms232214487
Poudel I, Annaji M, Wibowo FS, Arnold RD, Fasina O, Via B, Rangari V, Peresin MS, Smith F, Dhanasekaran M, et al. Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines. International Journal of Molecular Sciences. 2022; 23(22):14487. https://doi.org/10.3390/ijms232214487
Chicago/Turabian StylePoudel, Ishwor, Manjusha Annaji, Fajar Setyo Wibowo, Robert D. Arnold, Oladiran Fasina, Brian Via, Vijaya Rangari, Maria Soledad Peresin, Forrest Smith, Muralikrishnan Dhanasekaran, and et al. 2022. "Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines" International Journal of Molecular Sciences 23, no. 22: 14487. https://doi.org/10.3390/ijms232214487
APA StylePoudel, I., Annaji, M., Wibowo, F. S., Arnold, R. D., Fasina, O., Via, B., Rangari, V., Peresin, M. S., Smith, F., Dhanasekaran, M., Tiwari, A. K., & Babu, R. J. (2022). Hispolon Cyclodextrin Complexes and Their Inclusion in Liposomes for Enhanced Delivery in Melanoma Cell Lines. International Journal of Molecular Sciences, 23(22), 14487. https://doi.org/10.3390/ijms232214487