Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics
Abstract
1. Introduction
2. Collagen
2.1. Collagen Structure and Biosynthesis Process
2.2. Collagen Turnover and Degradation
2.3. Classification of Collagen Types
2.4. Types of Collagens in Articular Cartilage
3. Role of Collagen in Cartilage Damage
3.1. Collagen Is the Main Component of the Cartilage Matrix
3.2. Collagen Is a Major Target of Cartilage Degeneration
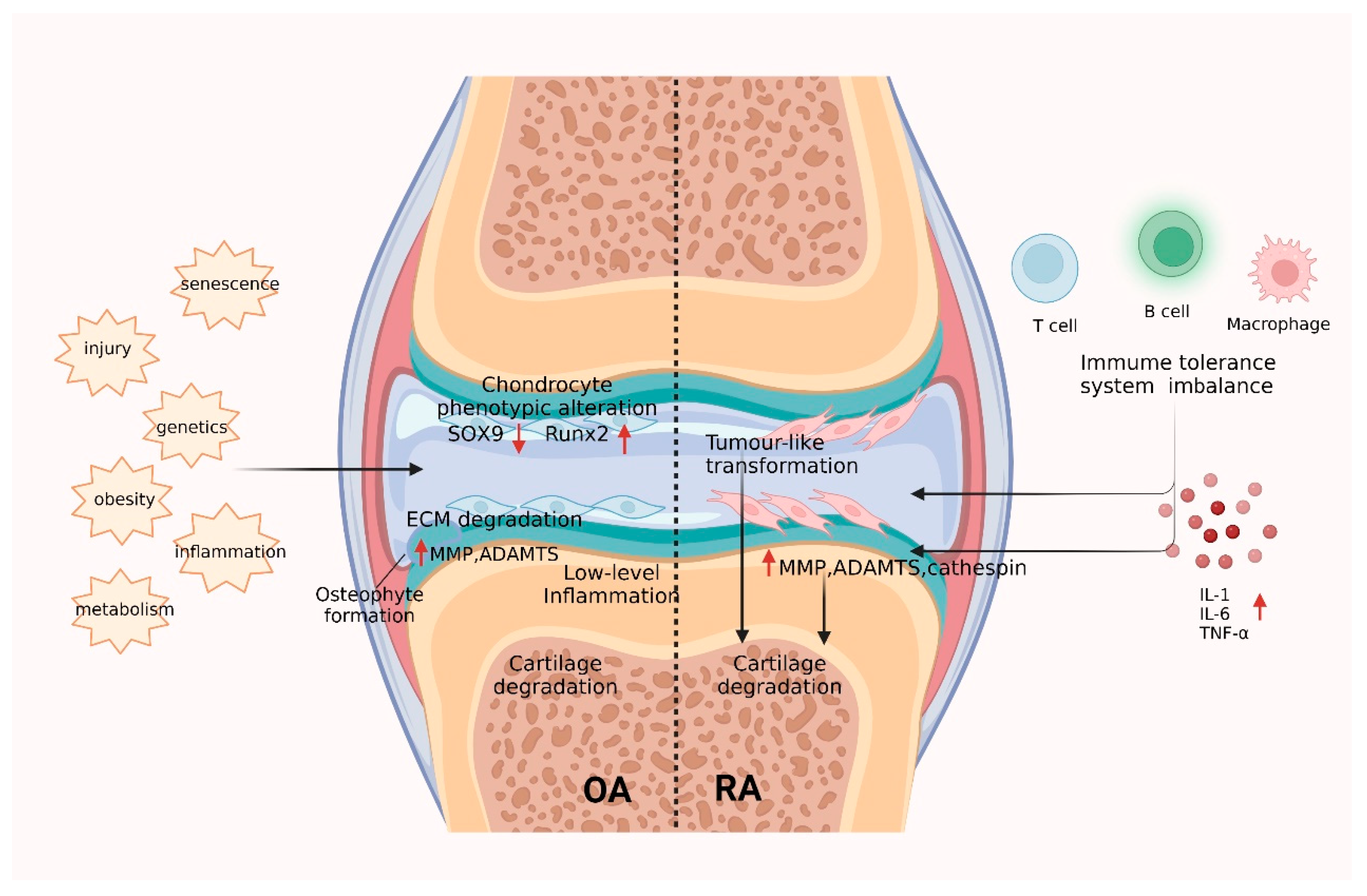
3.2.1. Mechanisms of Collagen in OA Cartilage Injury
3.2.2. Mechanisms of Collagen in RA Cartilage Injury
4. Collagen Metabolites as Biochemical Markers for OA and RA
5. Collagen as a Tool for Cartilage Repair
5.1. Collagen Derivatives for Cartilage Repair
5.2. Collagen Matrices as Vehicles for Direct Drug Delivery
5.3. Collagen Matrices as Vehicles for Cell Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, E.T.; Oecal, S.; Mörgelin, M.; Schmid, P.W.N.; Buchner, J.; Baumann, U.; Gebauer, J.M. Collagen's primary structure determines collagen:HSP47 complex stoichiometry. J. Biol. Chem. 2021, 297, 101169. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, C.; Sun, Y.; Francis, M.; Ryu, M.S.; Grider, A.; Ye, K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthr. Cartil. 2021, 29, 1029–1035. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, X.; Huang, C.; Tang, Y.; Zhou, Q.; Chen, W. LncRNAs and Rheumatoid Arthritis: From Identifying Mechanisms to Clinical Investigation. Front. Immunol. 2021, 12, 807738. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernandez, V.; Granados, M.M.; Dominguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Onnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Engstroem, A.; Sharma, N.; Karsdal, M.A. Blood and urinary collagen markers in osteoarthritis: Markers of tissue turnover and disease activity. Expert Rev. Mol. Diagn. 2020, 20, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Guo, W.; Tian, G.; Luo, X.; Peng, L.; Liu, S.; Sui, X.; Guo, Q.; Li, X. Clinical Application Status of Articular Cartilage Regeneration Techniques: Tissue-Engineered Cartilage Brings New Hope. Stem Cells Int. 2020, 2020, 5690252. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
- Kirkness, M.W.H.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Quality Control of Procollagen in Cells. Annu. Rev. Biochem. 2021, 90, 631–658. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Sivan, S.S.; Wachtel, E.; Tsitron, E.; Sakkee, N.; van der Ham, F.; Degroot, J.; Roberts, S.; Maroudas, A. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J. Biol. Chem. 2008, 283, 8796–8801. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Møller, M.B.; Krogsgaard, M.R.; Grum-Schwensen, T.; Petersen, M.M.; Kjaer, M. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci. Transl. Med. 2016, 8, 346ra390. [Google Scholar] [CrossRef]
- Onursal, C.; Dick, E.; Angelidis, I.; Schiller, H.B.; Staab-Weijnitz, C.A. Collagen Biosynthesis, Processing, and Maturation in Lung Ageing. Front. Med. 2021, 8, 593874. [Google Scholar] [CrossRef] [PubMed]
- Manka, S.W.; Carafoli, F.; Visse, R.; Bihan, D.; Raynal, N.; Farndale, R.W.; Murphy, G.; Enghild, J.J.; Hohenester, E.; Nagase, H. Structural insights into triple-helical collagen cleavage by matrix metalloproteinase 1. Proc. Natl. Acad. Sci. USA 2012, 109, 12461–12466. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Afratis, N.A.; Selman, M.; Pardo, A.; Sagi, I. Emerging insights into the role of matrix metalloproteases as therapeutic targets in fibrosis. Matrix Biol. 2018, 68–69, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Aguda, A.H.; Panwar, P.; Du, X.; Nguyen, N.T.; Brayer, G.D.; Brömme, D. Structural basis of collagen fiber degradation by cathepsin K. Proc. Natl. Acad. Sci. USA 2014, 111, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar] [CrossRef]
- Jaalinoja, J.; Ylostalo, J.; Beckett, W.; Hulmes, D.J.; Ala-Kokko, L. Trimerization of collagen IX alpha-chains does not require the presence of the COL1 and NC1 domains. Biochem. J. 2008, 409, 545–554. [Google Scholar] [CrossRef]
- Deng, H.; Huang, X.; Yuan, L. Molecular genetics of the COL2A1-related disorders. Mutat. Res. Rev. Mutat. Res. 2016, 768, 1–13. [Google Scholar] [CrossRef]
- Mak, K.M.; Png, C.Y.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. 2016, 299, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kirchner, M. Collagen Mimetic Peptides. Bioengineering 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Oudart, J.B.; Monboisse, J.C.; Maquart, F.X.; Brassart, B.; Brassart-Pasco, S.; Ramont, L. Type XIX collagen: A new partner in the interactions between tumor cells and their microenvironment. Matrix Biol. 2017, 57–58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Momota, R.; Narasaki, M.; Komiyama, T.; Naito, I.; Ninomiya, Y.; Ohtsuka, A. Drosophila type XV/XVIII collagen mutants manifest integrin mediated mitochondrial dysfunction, which is improved by cyclosporin A and losartan. Int. J. Biochem. Cell Biol. 2013, 45, 1003–1011. [Google Scholar] [CrossRef]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef]
- Wang, T.; Hauswirth, A.G.; Tong, A.; Dickman, D.K.; Davis, G.W. Endostatin is a trans-synaptic signal for homeostatic synaptic plasticity. Neuron 2014, 83, 616–629. [Google Scholar] [CrossRef]
- Wakabayashi, T. Transmembrane Collagens in Neuromuscular Development and Disorders. Front. Mol. Neurosci. 2020, 13, 635375. [Google Scholar] [CrossRef]
- Heikkinen, A.; Tu, H.; Pihlajaniemi, T. Collagen XIII: A type II transmembrane protein with relevance to musculoskeletal tissues, microvessels and inflammation. Int. J. Biochem. Cell Biol. 2012, 44, 714–717. [Google Scholar] [CrossRef]
- Villone, D.; Fritsch, A.; Koch, M.; Bruckner-Tuderman, L.; Hansen, U.; Bruckner, P. Supramolecular interactions in the dermo-epidermal junction zone: Anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J. Biol. Chem. 2008, 283, 24506–24513. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic Structure, Physiology, and Biochemistry of Connective Tissues and Extracellular Matrix Collagens. Adv. Exp. Med. Biol. 2021, 1348, 5–43. [Google Scholar] [CrossRef] [PubMed]
- Godwin, A.R.F.; Starborg, T.; Sherratt, M.J.; Roseman, A.M.; Baldock, C. Defining the hierarchical organisation of collagen VI microfibrils at nanometre to micrometre length scales. Acta Biomater. 2017, 52, 21–32. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, S. Salicin inhibits AGE-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes: Therapeutic potential in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef]
- Veidal, S.S.; Larsen, D.V.; Chen, X.; Sun, S.; Zheng, Q.; Bay-Jensen, A.C.; Leeming, D.J.; Nawrocki, A.; Larsen, M.R.; Schett, G.; et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin. Biochem. 2012, 45, 541–546. [Google Scholar] [CrossRef]
- Lam, N.P.; Li, Y.; Waldman, A.B.; Brussiau, J.; Lee, P.L.; Olsen, B.R.; Xu, L. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch. Oral Biol. 2007, 52, 579–584. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Duan, L.; Li, X.; Zhang, Y.; Zhou, Q. The effects of velvet antler polypeptides on the phenotype and related biological indicators of osteoarthritic rabbit chondrocytes. Acta Biochim. Pol. 2011, 58, 297–302. [Google Scholar] [CrossRef]
- Brown, D.J.; Bishop, P.; Hamdi, H.; Kenney, M.C. Cleavage of structural components of mammalian vitreous by endogenous matrix metalloproteinase-2. Curr. Eye Res. 1996, 15, 435–445. [Google Scholar] [CrossRef]
- Varma, S.; Orgel, J.P.; Schieber, J.D. Nanomechanics of Type I Collagen. Biophys. J. 2016, 111, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Guterman-Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G.; Prockop, D.J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum. Mutat. 1997, 9, 300–315. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Chichester, C.O. Review: Collagen markers in early arthritic diseases. Clin. Chim. Acta 2006, 365, 68–77. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta1-SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- Wang, C.; Brisson, B.K.; Terajima, M.; Li, Q.; Hoxha, K.; Han, B.; Goldberg, A.M.; Sherry Liu, X.; Marcolongo, M.S.; Enomoto-Iwamoto, M.; et al. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. 2020, 85–86, 47–67. [Google Scholar] [CrossRef]
- Hosseininia, S.; Weis, M.A.; Rai, J.; Kim, L.; Funk, S.; Dahlberg, L.E.; Eyre, D.R. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthr. Cartil. 2016, 24, 1029–1035. [Google Scholar] [CrossRef]
- Volk, S.W.; Shah, S.R.; Cohen, A.J.; Wang, Y.; Brisson, B.K.; Vogel, L.K.; Hankenson, K.D.; Adams, S.L. Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcif. Tissue Int. 2014, 94, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ge, G. Complexity of type IV collagens: From network assembly to function. Biol Chem. 2019, 400, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Foldager, C.B.; Toh, W.S.; Christensen, B.B.; Lind, M.; Gomoll, A.H.; Spector, M. Collagen Type IV and Laminin Expressions during Cartilage Repair and in Late Clinically Failed Repair Tissues from Human Subjects. Cartilage 2016, 7, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mundel, T.M.; Kalluri, R. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007, 74, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Carter, B.G.; Eyre, D.R. Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J. Biol. Chem. 2009, 284, 5539–5545. [Google Scholar] [CrossRef]
- Sun, M.; Connizzo, B.K.; Adams, S.M.; Freedman, B.R.; Wenstrup, R.J.; Soslowsky, L.J.; Birk, D.E. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype. Am. J. Pathol. 2015, 185, 1436–1447. [Google Scholar] [CrossRef]
- Brindo da Cruz, I.C.; Velosa, A.P.P.; Carrasco, S.; Dos Santos Filho, A.; Tomaz de Miranda, J.; Pompeu, E.; Fernandes, T.L.; Bueno, D.F.; Fanelli, C.; Goldenstein-Schainberg, C.; et al. Post-Adipose-Derived Stem Cells (ADSC) Stimulated by Collagen Type V (Col V) Mitigate the Progression of Osteoarthritic Rabbit Articular Cartilage. Front. Cell Dev. Biol. 2021, 9, 606890. [Google Scholar] [CrossRef]
- Aigner, T.; Hambach, L.; Soder, S.; Schlotzer-Schrehardt, U.; Poschl, E. The C5 domain of Col6A3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem. Biophys. Res. Commun. 2002, 290, 743–748. [Google Scholar] [CrossRef]
- Söder, S.; Hambach, L.; Lissner, R.; Kirchner, T.; Aigner, T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthr. Cartil. 2002, 10, 464–470. [Google Scholar] [CrossRef]
- Theocharidis, G.; Drymoussi, Z.; Kao, A.P.; Barber, A.H.; Lee, D.A.; Braun, K.M.; Connelly, J.T. Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. J. Investig. Dermatol. 2016, 136, 74–83. [Google Scholar] [CrossRef]
- Zelenski, N.A.; Leddy, H.A.; Sanchez-Adams, J.; Zhang, J.; Bonaldo, P.; Liedtke, W.; Guilak, F. Type VI Collagen Regulates Pericellular Matrix Properties, Chondrocyte Swelling, and Mechanotransduction in Mouse Articular Cartilage. Arthritis Rheumatol. 2015, 67, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- De Palma, S.; Leone, R.; Grumati, P.; Vasso, M.; Polishchuk, R.; Capitanio, D.; Braghetta, P.; Bernardi, P.; Bonaldo, P.; Gelfi, C. Changes in muscle cell metabolism and mechanotransduction are associated with myopathic phenotype in a mouse model of collagen VI deficiency. PLoS ONE 2013, 8, e56716. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.; Nandakumar, K.S.; Holmdahl, R. Type IX collagen deficiency enhances the binding of cartilage-specific antibodies and arthritis severity. Arthritis Res. Ther. 2006, 8, R102. [Google Scholar] [CrossRef] [PubMed]
- Brachvogel, B.; Zaucke, F.; Dave, K.; Norris, E.L.; Stermann, J.; Dayakli, M.; Koch, M.; Gorman, J.J.; Bateman, J.F.; Wilson, R. Comparative proteomic analysis of normal and collagen IX null mouse cartilage reveals altered extracellular matrix composition and novel components of the collagen IX interactome. J. Biol. Chem. 2013, 288, 13481–13492. [Google Scholar] [CrossRef]
- He, Y.; Manon-Jensen, T.; Arendt-Nielsen, L.; Petersen, K.K.; Christiansen, T.; Samuels, J.; Abramson, S.; Karsdal, M.A.; Attur, M.; Bay-Jensen, A.C. Potential diagnostic value of a type X collagen neo-epitope biomarker for knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 611–620. [Google Scholar] [CrossRef]
- Shen, G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofacial Res. 2005, 8, 11–17. [Google Scholar] [CrossRef]
- McAlinden, A.; Traeger, G.; Hansen, U.; Weis, M.A.; Ravindran, S.; Wirthlin, L.; Eyre, D.R.; Fernandes, R.J. Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform. Matrix Biol. 2014, 34, 105–113. [Google Scholar] [CrossRef]
- Li, A.; Wei, Y.; Hung, C.; Vunjak-Novakovic, G. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials 2018, 173, 47–57. [Google Scholar] [CrossRef]
- Lawrence, E.A.; Kague, E.; Aggleton, J.A.; Harniman, R.L.; Roddy, K.A.; Hammond, C.L. The mechanical impact of col11a2 loss on joints; col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170335. [Google Scholar] [CrossRef]
- Gregory, K.E.; Keene, D.R.; Tufa, S.F.; Lunstrum, G.P.; Morris, N.P. Developmental distribution of collagen type XII in cartilage: Association with articular cartilage and the growth plate. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 2005–2016. [Google Scholar] [CrossRef]
- Chiquet, M.; Birk, D.E.; Bonnemann, C.G.; Koch, M. Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol. 2014, 53, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Izu, Y.; Ezura, Y.; Koch, M.; Birk, D.E.; Noda, M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016, 364, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, H.L.; Meng, X.; Zhang, G.; Veit, G.; Sun, M.; Klement, J.F.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Type XIV Collagen Regulates Fibrillogenesis: Premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 2009, 284, 8427–8438. [Google Scholar] [CrossRef]
- Kassner, A.; Hansen, U.; Miosge, N.; Reinhardt, D.P.; Aigner, T.; Bruckner-Tuderman, L.; Bruckner, P.; Grassel, S. Discrete integration of collagen XVI into tissue-specific collagen fibrils or beaded microfibrils. Matrix Biol. 2003, 22, 131–143. [Google Scholar] [CrossRef]
- Grässel, S.; Bauer, R.J. Collagen XVI in health and disease. Matrix Biol. 2013, 32, 64–73. [Google Scholar] [CrossRef]
- Zwolanek, D.; Veit, G.; Eble, J.A.; Gullberg, D.; Ruggiero, F.; Heino, J.; Meier, M.; Stetefeld, J.; Koch, M. Collagen XXII binds to collagen-binding integrins via the novel motifs GLQGER and GFKGER. Biochem. J. 2014, 459, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Bruckner-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.; Moss, J.B.; Pace, J.M.; Bridgewater, L.C. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol. 2005, 24, 177–184. [Google Scholar] [CrossRef]
- Hjorten, R.; Hansen, U.; Underwood, R.A.; Telfer, H.E.; Fernandes, R.J.; Krakow, D.; Sebald, E.; Wachsmann-Hogiu, S.; Bruckner, P.; Jacquet, R.; et al. Type XXVII collagen at the transition of cartilage to bone during skeletogenesis. Bone 2007, 41, 535–542. [Google Scholar] [CrossRef]
- Mayo, J.L.; Holden, D.N.; Barrow, J.R.; Bridgewater, L.C. The transcription factor Lc-Maf participates in Col27a1 regulation during chondrocyte maturation. Exp. Cell Res. 2009, 315, 2293–2300. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef]
- Guilak, F.; Alexopoulos, L.G.; Upton, M.L.; Youn, I.; Choi, J.B.; Cao, L.; Setton, L.A.; Haider, M.A. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann. N. Y. Acad. Sci. 2006, 1068, 498–512. [Google Scholar] [CrossRef]
- Okada, Y. Matrix-degrading metalloproteinases and their roles in joint destruction. Mod. Rheumatol. 2000, 10, 121–128. [Google Scholar] [CrossRef]
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—Two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef]
- Li, N.G.; Shi, Z.H.; Tang, Y.P.; Wang, Z.J.; Song, S.L.; Qian, L.H.; Qian, D.W.; Duan, J.A. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr. Med. Chem. 2011, 18, 977–1001. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Moncada-Pazos, A.; Obaya, A.J.; Viloria, C.G.; López-Otín, C.; Cal, S. The nutraceutical flavonoid luteolin inhibits ADAMTS-4 and ADAMTS-5 aggrecanase activities. J. Mol. Med. 2011, 89, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Cobo-Molinos, J.; Antich, C.; López-Ruiz, E. Osteoarthritis: Trauma vs Disease. Adv. Exp. Med. Biol. 2018, 1059, 63–83. [Google Scholar] [CrossRef]
- Sherwood, J.C.; Bertrand, J.; Eldridge, S.E.; Dell'Accio, F. Cellular and molecular mechanisms of cartilage damage and repair. Drug Discov. Today 2014, 19, 1172–1177. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, Y.J.; Kim, T.K.; Chang, C.B.; Chung, J.H.; Shin, K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res. Ther. 2010, 12, R231. [Google Scholar] [CrossRef]
- Challa, T.D.; Rais, Y.; Ornan, E.M. Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways. Mol. Cell. Endocrinol. 2010, 323, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Vuolteenaho, K.; Nieminen, R.; Moilanen, T.; Moilanen, E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 2011, 29, 57–64. [Google Scholar] [PubMed]
- Scotece, M.; Mobasheri, A. Leptin in osteoarthritis: Focus on articular cartilage and chondrocytes. Life Sci. 2015, 140, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Lefèvre, S.; Schwarz, M.; Meier, F.M.P.; Zimmermann-Geller, B.; Tarner, I.H.; Rickert, M.; Steinmeyer, J.; Sauerbier, M.; Rehart, S.; Müller-Ladner, U.; et al. Disease-Specific Effects of Matrix and Growth Factors on Adhesion and Migration of Rheumatoid Synovial Fibroblasts. J. Immunol. 2017, 198, 4588–4595. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, D.; Yang, H.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Fibroblast-like synoviocytes in rheumatoid arthritis: Surface markers and phenotypes. Int. Immunopharmacol. 2021, 93, 107392. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef]
- Fang, Q.; Ou, J.; Nandakumar, K.S. Autoantibodies as Diagnostic Markers and Mediator of Joint Inflammation in Arthritis. Mediat. Inflamm. 2019, 2019, 6363086. [Google Scholar] [CrossRef]
- Su, J.; Krock, E.; Barde, S.; Delaney, A.; Ribeiro, J.; Kato, J.; Agalave, N.; Wigerblad, G.; Matteo, R.; Sabbadini, R.; et al. Pain-like behavior in the collagen antibody-induced arthritis model is regulated by lysophosphatidic acid and activation of satellite glia cells. Brain Behav. Immun. 2022, 101, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foss, C.A.; Pomper, M.G.; Yu, S.M. Imaging denatured collagen strands in vivo and ex vivo via photo-triggered hybridization of caged collagen mimetic peptides. J. Vis. Exp. 2014, 83, e51052. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lambert, C.; Henrotin, Y. Coll2-1 and Coll2-1NO2 as exemplars of collagen extracellular matrix turnover—Biomarkers to facilitate the treatment of osteoarthritis? Expert Rev. Mol. Diagn. 2019, 19, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.C.; Chapurlat, R.; Garnero, P. Soluble biological markers in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x211040300. [Google Scholar] [CrossRef]
- Maijer, K.I.; Gudmann, N.S.; Karsdal, M.A.; Gerlag, D.M.; Tak, P.P.; Bay-Jensen, A.C. Neo-Epitopes—Fragments of Cartilage and Connective Tissue Degradation in Early Rheumatoid Arthritis and Unclassified Arthritis. PLoS ONE 2016, 11, e0149329. [Google Scholar] [CrossRef] [PubMed]
- Deberg, M.; Labasse, A.; Christgau, S.; Cloos, P.; Bang Henriksen, D.; Chapelle, J.P.; Zegels, B.; Reginster, J.Y.; Henrotin, Y. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2005, 13, 258–265. [Google Scholar] [CrossRef]
- Luo, Y.; He, Y.; Reker, D.; Gudmann, N.S.; Henriksen, K.; Simonsen, O.; Ladel, C.; Michaelis, M.; Mobasheri, A.; Karsdal, M.; et al. A Novel High Sensitivity Type II Collagen Blood-Based Biomarker, PRO-C2, for Assessment of Cartilage Formation. Int. J. Mol. Sci. 2018, 19, 3485. [Google Scholar] [CrossRef]
- Valdes, A.M.; Meulenbelt, I.; Chassaing, E.; Arden, N.K.; Bierma-Zeinstra, S.; Hart, D.; Hofman, A.; Karsdal, M.; Kloppenburg, M.; Kroon, H.M.; et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthr. Cartil. 2014, 22, 683–689. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Mobasheri, A.; Thudium, C.S.; Kraus, V.B.; Karsdal, M.A. Blood and urine biomarkers in osteoarthritis—An update on cartilage associated type II collagen and aggrecan markers. Curr. Opin. Rheumatol. 2022, 34, 54–60. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, B.; Sun, J.; Yin, M. C-Terminal Cross-Linked Telopeptides of Type II Collagen as Biomarker for Radiological Knee Osteoarthritis: A Meta-Analysis. Cartilage 2020, 11, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Husakova, M.; Bay-Jensen, A.C.; Forejtova, S.; Zegzulkova, K.; Tomcik, M.; Gregova, M.; Bubova, K.; Horinkova, J.; Gatterova, J.; Pavelka, K.; et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci. Rep. 2019, 9, 11218. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.C.; Kjelgaard-Petersen, C.F.; Petersen, K.K.; Arendt-Nielsen, L.; Quasnichka, H.L.; Mobasheri, A.; Karsdal, M.A.; Leeming, D.J. Aggrecanase degradation of type III collagen is associated with clinical knee pain. Clin. Biochem. 2018, 58, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sand, J.M.; Larsen, L.; Hogaboam, C.; Martinez, F.; Han, M.; Rossel Larsen, M.; Nawrocki, A.; Zheng, Q.; Karsdal, M.A.; Leeming, D.J. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis--validation of two novel biomarker assays. PLoS ONE 2013, 8, e84934. [Google Scholar] [CrossRef]
- Gudmann, N.S.; Junker, P.; Juhl, P.; Thudium, C.S.; Siebuhr, A.S.; Byrjalsen, I.; Karsdal, M.A.; Bay-Jensen, A.C. Type IV collagen metabolism is associated with disease activity, radiographic progression and response to tocilizumab in rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 829–835. [Google Scholar]
- Jahr, H.; Matta, C.; Mobasheri, A. Physicochemical and biomechanical stimuli in cell-based articular cartilage repair. Curr. Rheumatol. Rep. 2015, 17, 22. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G.; Negru, P.A.; Marcu, M.F.; Andronie-Cioara, F.L. In-depth bibliometric analysis and current scientific mapping research in the context of rheumatoid arthritis pharmacotherapy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 154, 113614. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G.; Tit, D.M.; Behl, T.; Uivaraseanu, B.; Marcu, M.F. Highlighting the Benefits of Rehabilitation Treatments in Hip Osteoarthritis. Medicina 2022, 58, 494. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. Anti-inflammatory strategies in cartilage repair. Tissue Eng. Part B Rev. 2014, 20, 655–668. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O'Brien, F.J.; Lopez-Noriega, A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J. Control. Release 2015, 207, 112–119. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Mobasheri, A.; Mahmoudian, A.; Kalvaityte, U.; Uzieliene, I.; Larder, C.E.; Iskandar, M.M.; Kubow, S.; Hamdan, P.C.; de Almeida, C.S., Jr.; Favazzo, L.J.; et al. A White Paper on Collagen Hydrolyzates and Ultrahydrolyzates: Potential Supplements to Support Joint Health in Osteoarthritis? Curr. Rheumatol. Rep. 2021, 23, 78. [Google Scholar] [CrossRef]
- Barati, M.; Jabbari, M.; Navekar, R.; Farahmand, F.; Zeinalian, R.; Salehi-Sahlabadi, A.; Abbaszadeh, N.; Mokari-Yamchi, A.; Davoodi, S.H. Collagen supplementation for skin health: A mechanistic systematic review. J. Cosmet. Dermatol. 2020, 19, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- De Silva, V.; El-Metwally, A.; Ernst, E.; Lewith, G.; Macfarlane, G.J. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: A systematic review. Rheumatology 2011, 50, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, X.Y.; Wang, H.K.; Jia, J.F.; Zheng, Z.H.; Ding, J.; Fan, C.M. Oral administration of type-II collagen peptide 250–270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin. Immunol. 2007, 122, 75–84. [Google Scholar] [CrossRef]
- Park, K.S.; Park, M.J.; Cho, M.L.; Kwok, S.K.; Ju, J.H.; Ko, H.J.; Park, S.H.; Kim, H.Y. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 581–589. [Google Scholar] [CrossRef]
- Honvo, G.; Lengelé, L.; Charles, A.; Reginster, J.Y.; Bruyère, O. Role of Collagen Derivatives in Osteoarthritis and Cartilage Repair: A Systematic Scoping Review With Evidence Mapping. Rheumatol. Ther. 2020, 7, 703–740. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef]
- Kilmer, C.E.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef]
- Pal, P.; Nguyen, Q.C.; Benton, A.H.; Marquart, M.E.; Janorkar, A.V. Drug-Loaded Elastin-Like Polypeptide-Collagen Hydrogels with High Modulus for Bone Tissue Engineering. Macromol. Biosci. 2019, 19, e1900142. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Patel, R.; Yadav, U.C.S.; Vasita, R. Dual drug-delivering polycaprolactone-collagen scaffold to induce early osteogenic differentiation and coupled angiogenesis. Biomed. Mater. 2020, 15, 045008. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Noriega, A.; Ruiz-Hernandez, E.; Quinlan, E.; Storm, G.; Hennink, W.E.; O'Brien, F.J. Thermally triggered release of a pro-osteogenic peptide from a functionalized collagen-based scaffold using thermosensitive liposomes. J. Control. Release 2014, 187, 158–166. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Nanomedical approaches in the realm of rheumatoid arthritis. Ageing Res. Rev. 2023, 87, 101927. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, X.; Sang, W.; Wang, C.; Lu, H.; Xu, Y.; Zhong, Y.; Zhu, L.; He, C.; Ma, J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021, 6, 2372–2389. [Google Scholar] [CrossRef]
- Li, Y.Y.; Cheng, H.W.; Cheung, K.M.; Chan, D.; Chan, B.P. Mesenchymal stem cell-collagen microspheres for articular cartilage repair: Cell density and differentiation status. Acta Biomater. 2014, 10, 1919–1929. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Rocke, J.P.; De Bari, C. Cell-based approaches to joint surface repair: A research perspective. Osteoarthr. Cartil. 2013, 21, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Hui, T.Y.; Yeung, C.W.; Li, J.; Mo, I.; Chan, G.C. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials 2007, 28, 4652–4666. [Google Scholar] [CrossRef] [PubMed]

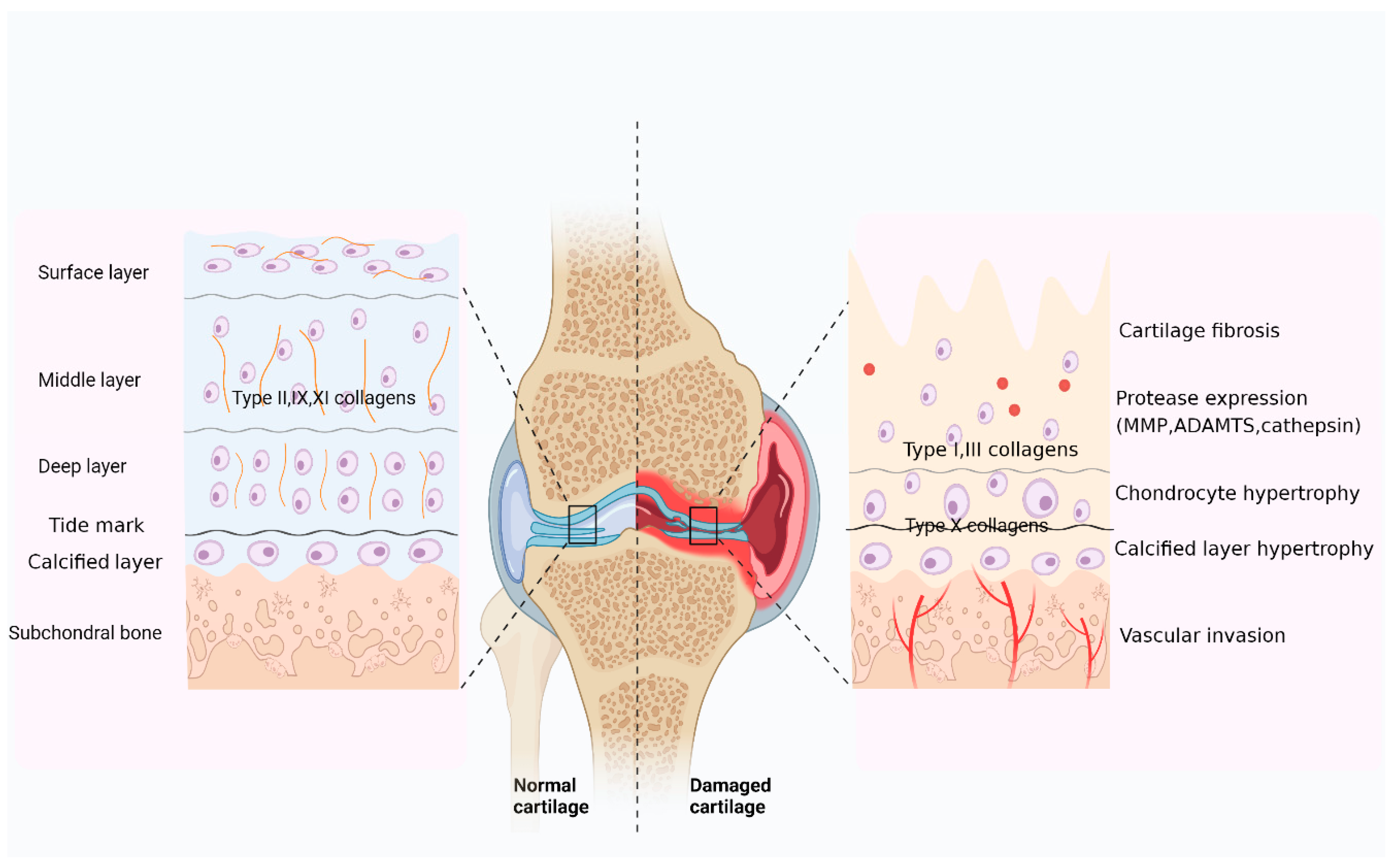
| Collagen Type | Molecular Composition | Classification | Distribution in Articular Cartilage | Susceptible to Proteinases |
|---|---|---|---|---|
| Type I | [α1(I)]2α2(I) | Fibril-forming collagens | Fibrocartilage, Elastic cartilage | MMP-2 [44] |
| Type II | [α1(II)]3 | Fibril-forming collagens | ECM of all zones | MMP-1,3, 13 [45] |
| Type III | [α1(III)]3 | Fibril-forming collagens | PCM | MMP-3 [46] |
| Type IV | [α1(IV)]2α2(IV) α3(IV)α4(IV)α5(IV) [α5(IV)]2α6(IV) | Network-forming collagens | PCM | MMP-2, 9 [44] |
| Type V | α1(V)2α2(V) | Fibril-forming collagens | PCM | MMP-2, 9 [47] |
| Type VI | α1(VI)α2(V)α3(V) α1(VI)α2(V)α4(V) α1(VI)α2(V)α5(V) α1(VI)α2(V)α6(V) | Beaded Filament-Forming Collagen | PCM | MMP-2, 9 [3] |
| Type IX | α1(IX)α2(IX)α3(IX) | FACIT | Growth-plate cartilage | MMP-3, 13 [48] |
| Type X | [α1(X)]3 | Network-forming collagens | Calcified zone and hypertrophic cartilage | MMP-1, 3, 13 [49] |
| Type XI | α1(XI)α2(XI)α3(XI) | Fibril-forming collagens | Articular cartilage | MMP-2 [50] |
| Type XII | α1[XII]3 | FACIT | Cartilage with more organized fibril orientation | NA |
| Type XIV | α1[XIV]3 | FACIT | Uniformly throughout the articular cartilage | MMP-13 |
| Type XVI | [α1(XVI)]3 | FACIT | Territorial matrix of chondrocytes | NA |
| Type XXII | [α1(XXII)]3 | FACIT | Articular surface of joint cartilage | NA |
| Type XXVII | [α1(XXVIII)]3 | Fibril-forming collagens | Proliferative Zone chondrocytes | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J. Mol. Sci. 2023, 24, 9841. https://doi.org/10.3390/ijms24129841
Ouyang Z, Dong L, Yao F, Wang K, Chen Y, Li S, Zhou R, Zhao Y, Hu W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. International Journal of Molecular Sciences. 2023; 24(12):9841. https://doi.org/10.3390/ijms24129841
Chicago/Turabian StyleOuyang, Ziwei, Lei Dong, Feng Yao, Ke Wang, Yong Chen, Shufang Li, Renpeng Zhou, Yingjie Zhao, and Wei Hu. 2023. "Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics" International Journal of Molecular Sciences 24, no. 12: 9841. https://doi.org/10.3390/ijms24129841
APA StyleOuyang, Z., Dong, L., Yao, F., Wang, K., Chen, Y., Li, S., Zhou, R., Zhao, Y., & Hu, W. (2023). Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. International Journal of Molecular Sciences, 24(12), 9841. https://doi.org/10.3390/ijms24129841






