The Role of Integrin Receptor’s α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen
Abstract
1. Introduction
2. Results
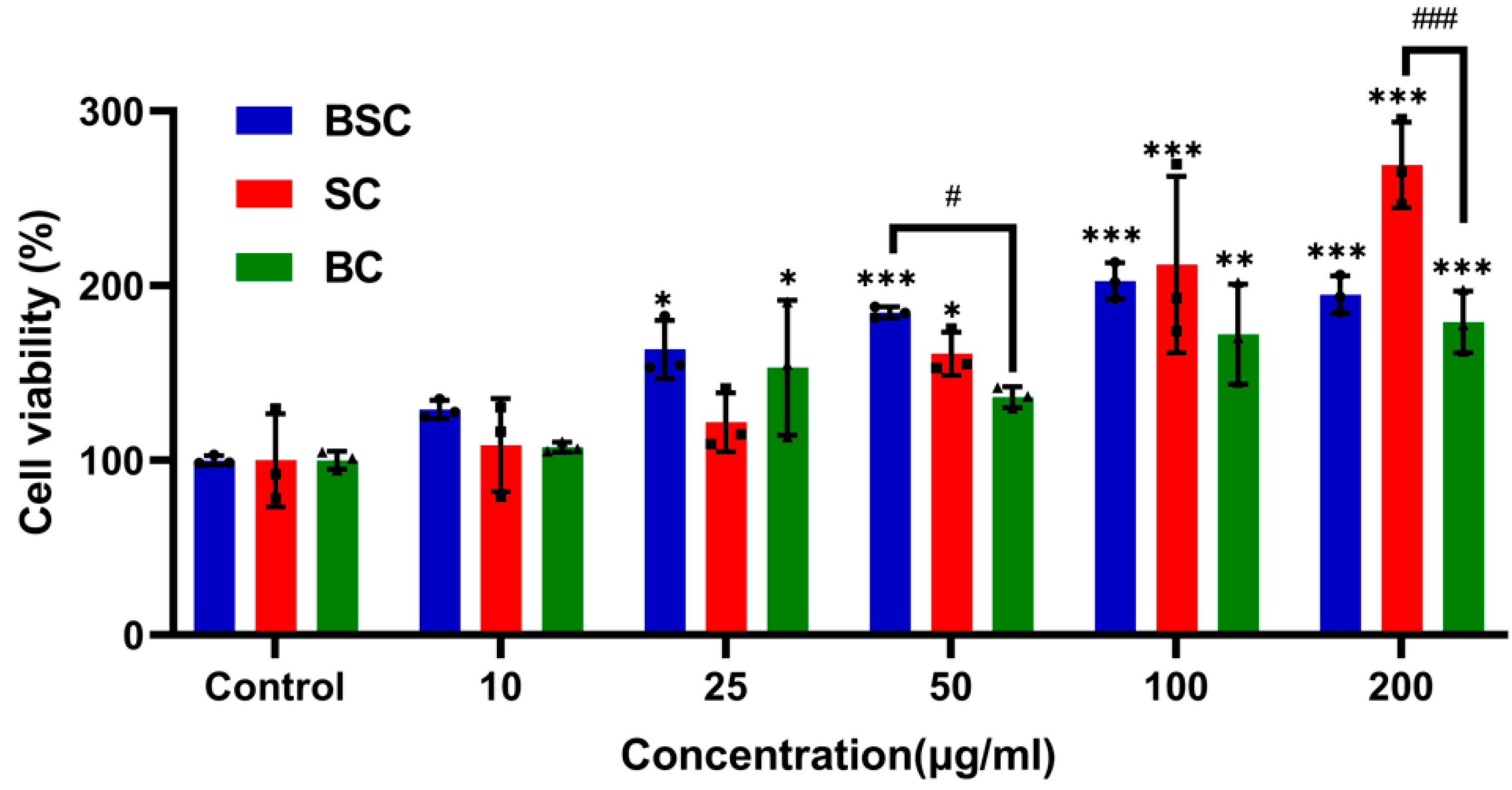
2.1. Cell Proliferation and Viability
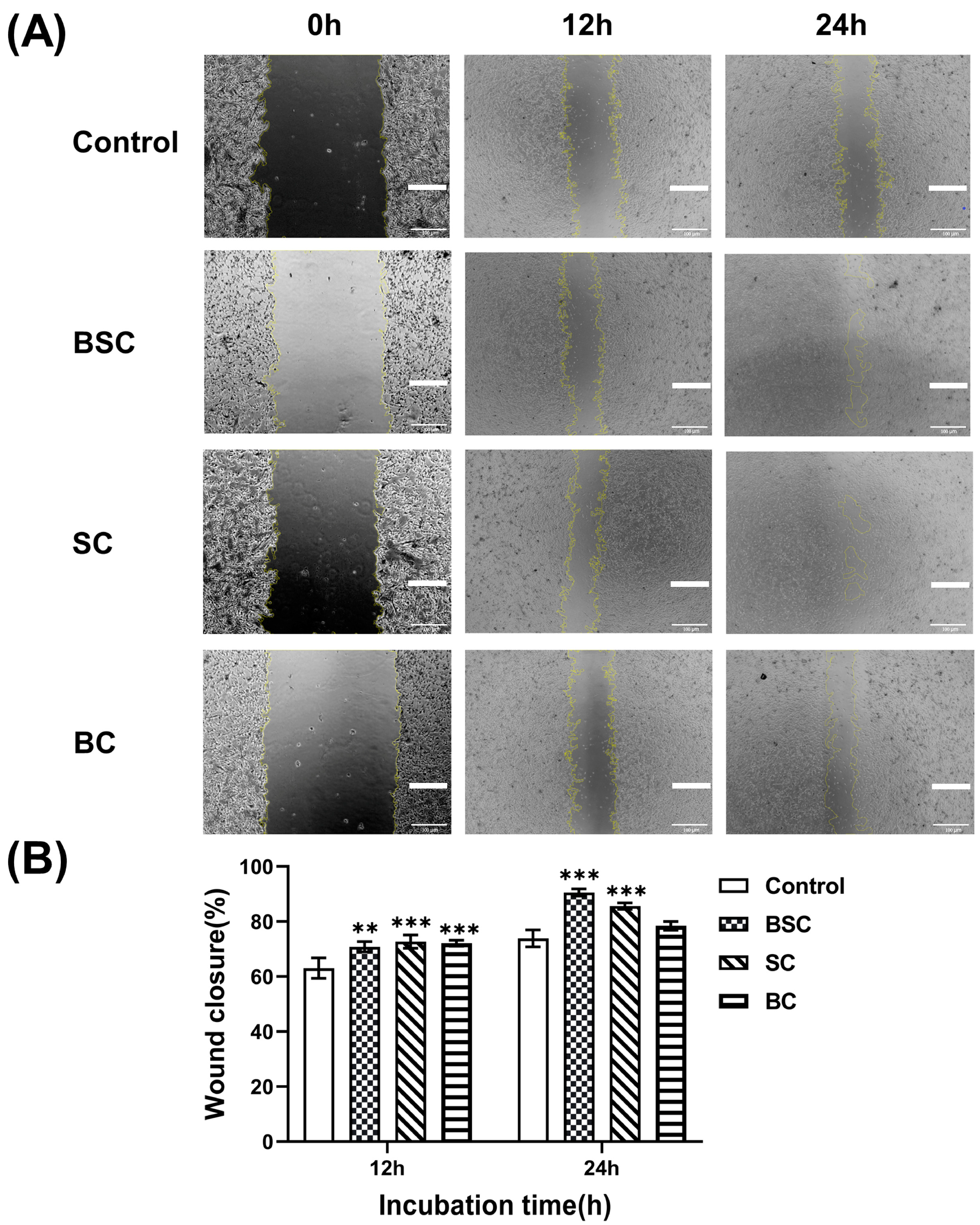
2.2. Cell Scratch Wound Healing
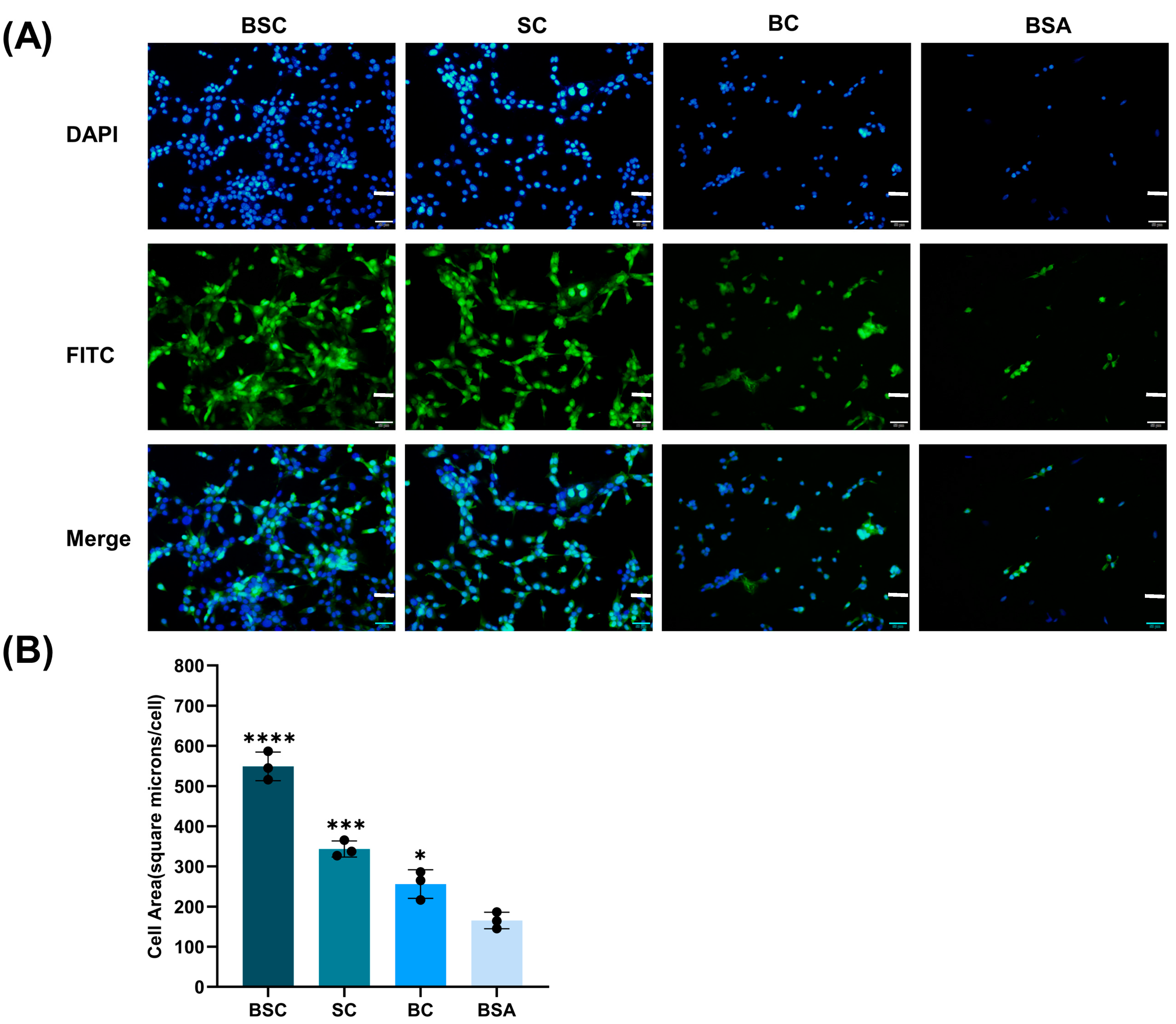
2.3. Cell Adhesion and Spreading
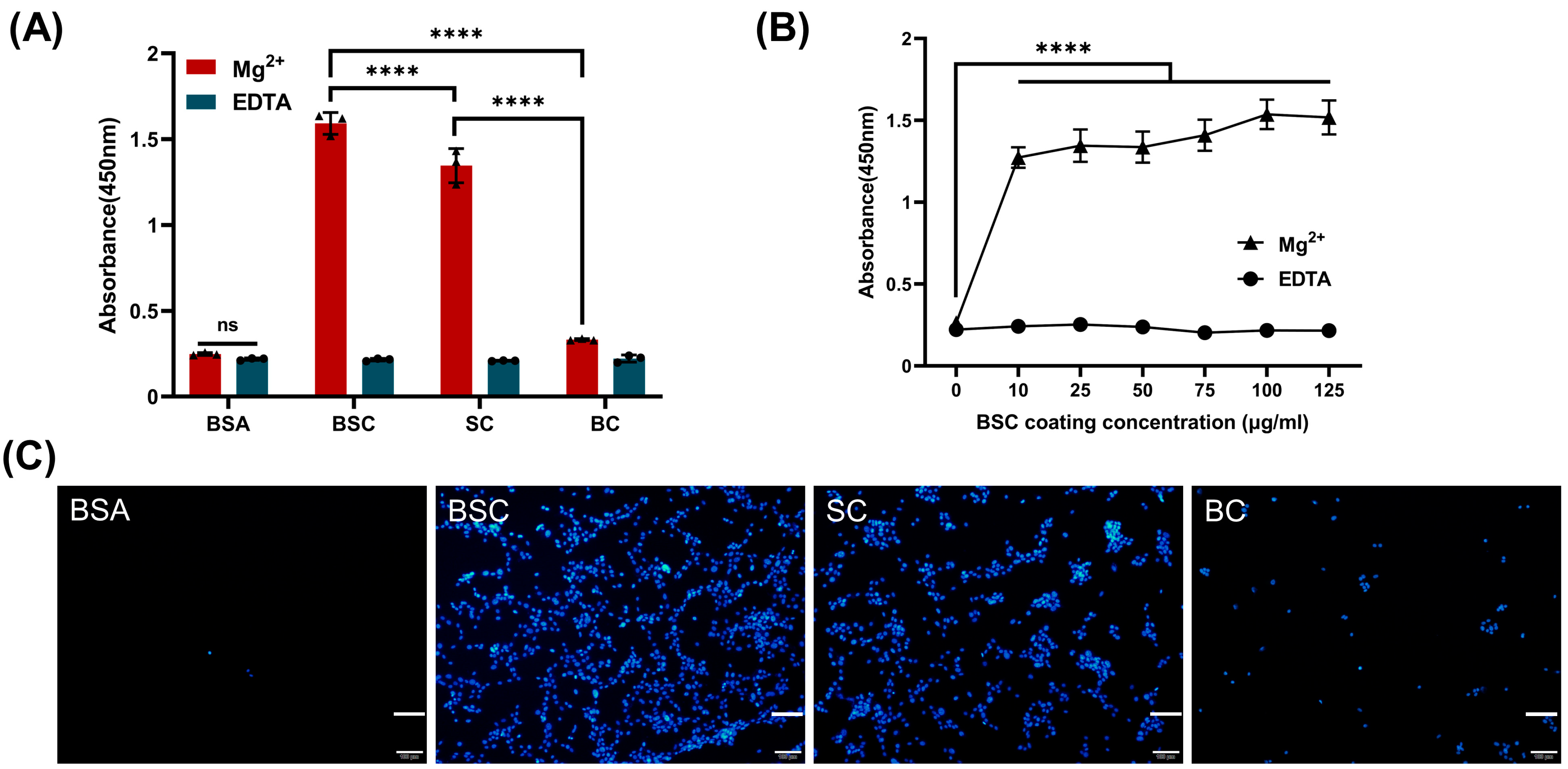
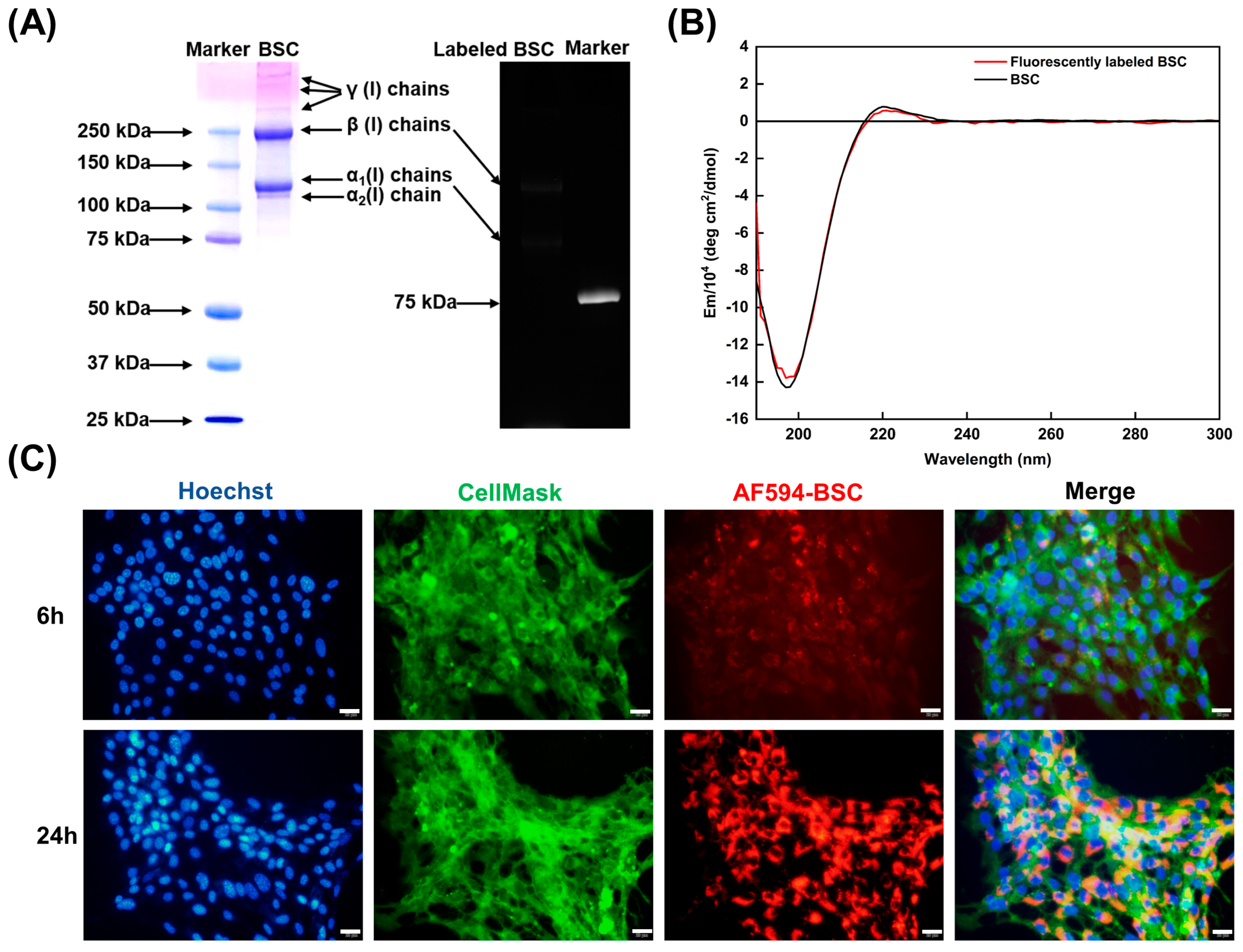
2.4. Effect of BSC in the Extracellular Matrix
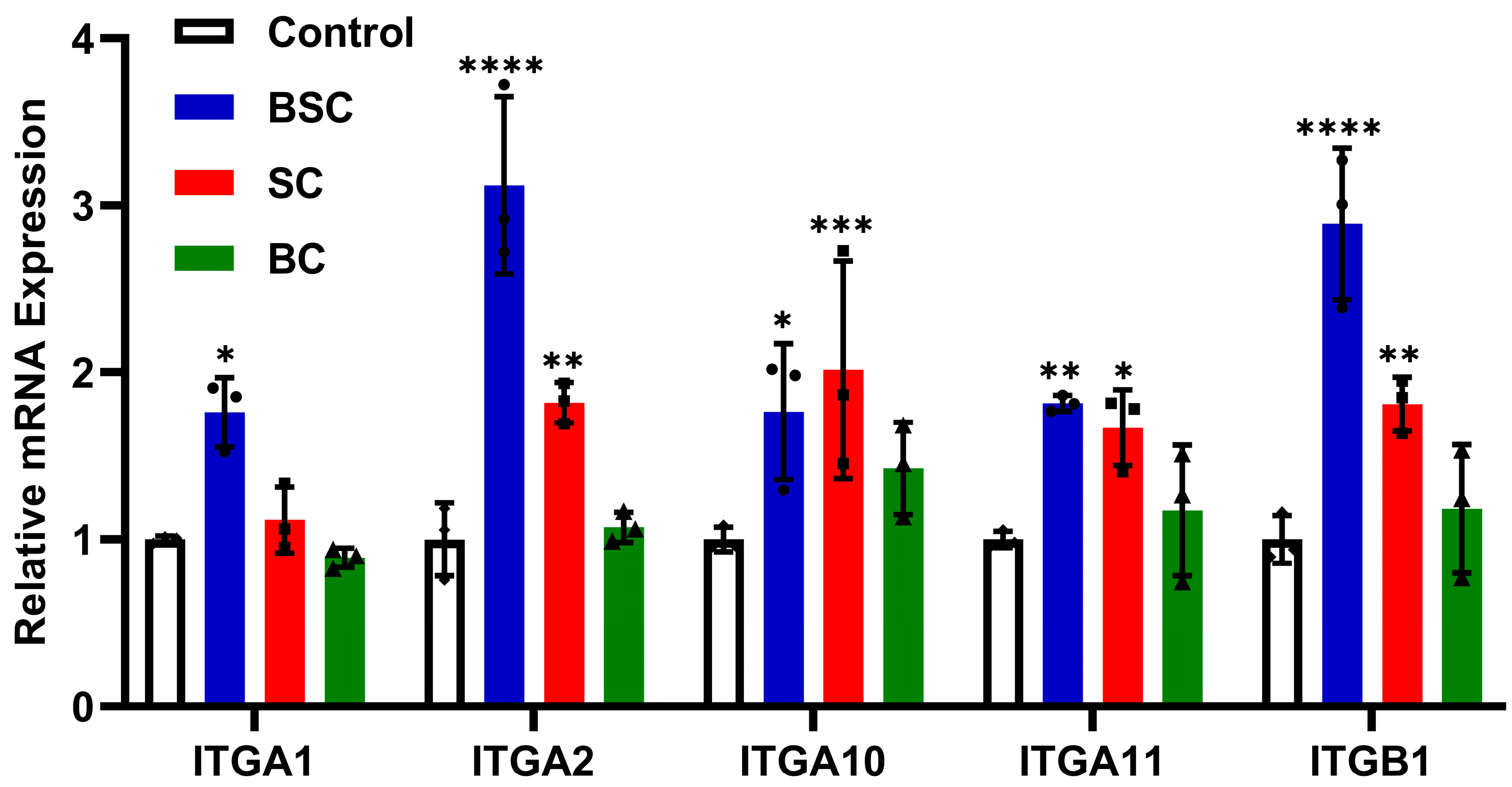
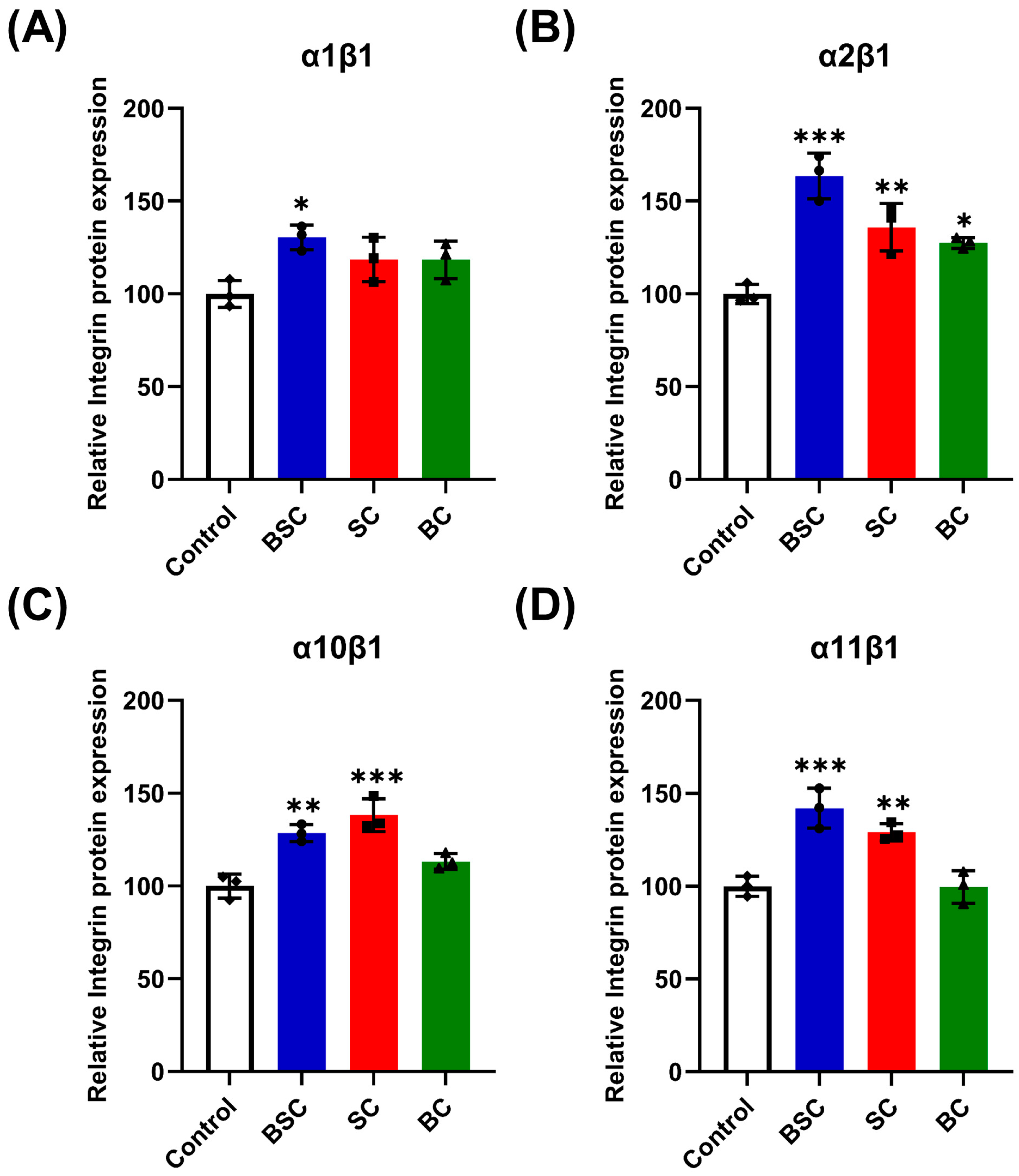
2.5. Effect of Marine Collagen on MSCs Integrin Expression
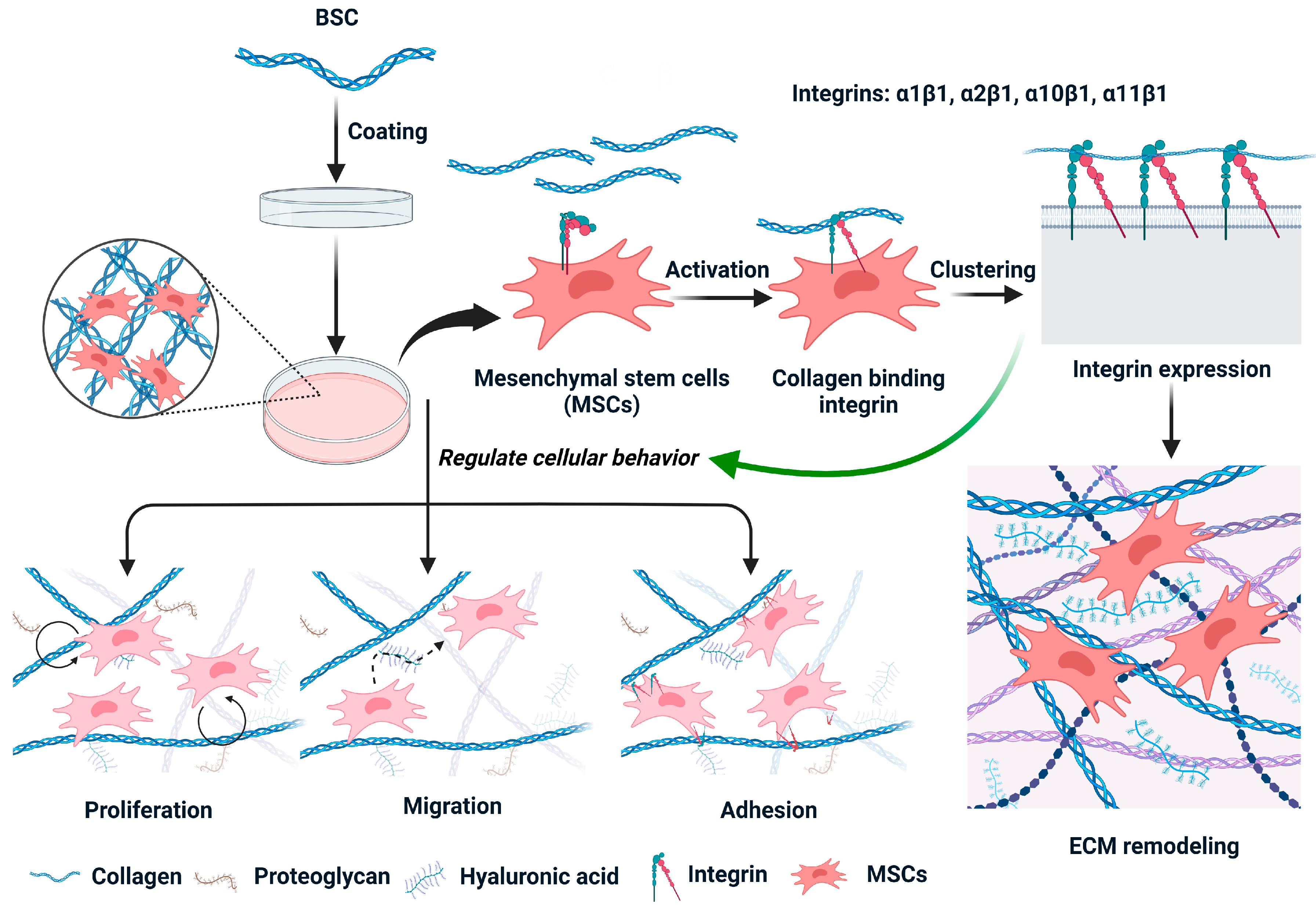
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation of Collagen-Coated Plates
4.3. Mouse Bone Marrow-Derived MSCs Culture
4.4. Cell Proliferation Assay
4.5. In Vitro Scratch Assay
4.6. Cell Adhesion Analysis
4.7. Cell Spreading Analysis
4.8. ECM Remodeling Tests
4.8.1. Characterization of Functionalized BSC Molecules
4.8.2. Cell Culture Experiments
4.9. Quantitative Real-Time Polymerase Chain Reaction(qRT-PCR)
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, Y.Z.; Li, R.H.; Bai, H.T.; Zhu, Z.Q.; Zhu, L.W.; Zhu, C.Y.; Che, Z.J.; Liu, H.; Wang, J.C.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.Y.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.M.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.X.; Li, S.D.; Hong, P.Z. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Alves, A.L.; Costa-Gouveia, J.; de Castro, J.V.; Sotelo, C.G.; Vazquez, J.A.; Perez-Martin, R.I.; Torrado, E.; Neves, N.; Reis, R.L.; Castro, A.G.; et al. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomater. 2022, 141, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.J.; Gregory, C.A.; Alge, D.L. Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels. Acta Biomater. 2020, 101, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Heilshorn, S.C.; Blau, H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 2018, 557, 335–342. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.Y.; Gao, X.L.; Gao, Y.; Li, Y.; Deng, M.Y.; Tan, J.L.; Gou, J.; Liu, C.; Dou, C.; Li, Z.L.; et al. Multiple integrin ligands provide a highly adhesive and osteoinductive surface that improves selective cell retention technology. Acta Biomater. 2019, 85, 106–116. [Google Scholar] [CrossRef]
- Razafiarison, T.; Silvan, U.; Meier, D.; Snedeker, J.G. Surface-Driven Collagen Self-Assembly Affects Early Osteogenic Stem Cell Signaling. Adv. Healthc. Mater. 2016, 5, 1481–1492. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef]
- Diogo, G.S.; Carneiro, F.; Freitas-Ribeiro, S.; Sotelo, C.G.; Perez-Martin, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Prionace glauca skin collagen bioengineered constructs as a promising approach to trigger cartilage regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 111587. [Google Scholar] [CrossRef]
- Muthukumar, T.; Prabu, P.; Ghosh, K.; Sastry, T.P. Fish scale collagen sponge incorporated with Macrotyloma uniflorum plant extract as a possible wound/burn dressing material. Colloids Surf. B-Biointerfaces 2014, 113, 207–212. [Google Scholar] [CrossRef]
- Ge, B.L.; Hou, C.Y.; Bao, B.; Pan, Z.L.; de Val, J.; Elango, J.; Wu, W.H. Comparison of Physicochemical and Structural Properties of Acid-Soluble and Pepsin-Soluble Collagens from Blacktip Reef Shark Skin. Mar. Drugs 2022, 20, 376. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin-collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Khatayevich, D.; Gungormus, M.; Yazici, H.; So, C.; Cetinel, S.; Ma, H.; Jen, A.; Tamerler, C.; Sarikaya, M. Biofunctionalization of materials for implants using engineered peptides. Acta Biomater. 2010, 6, 4634–4641. [Google Scholar] [CrossRef]
- Veleva, A.N.; Heath, D.E.; Cooper, S.L.; Patterson, C. Selective endothelial cell attachment to peptide-modified terpolymers. Biomaterials 2008, 29, 3656–3661. [Google Scholar] [CrossRef]
- Karimi, F.; O'Connor, A.J.; Qiao, G.G.; Heath, D.E. Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration. Adv. Healthc. Mater. 2018, 7, 1701324. [Google Scholar] [CrossRef]
- Elango, J.; Lee, J.W.; Wang, S.J.; Henrotin, Y.; de Val, J.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W.H. Evaluation of Differentiated Bone Cells Proliferation by Blue Shark Skin Collagen via Biochemical for Bone Tissue Engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J. Potential Application of Hydrolyzed Fish Collagen for Inducing the Multidirectional Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Biomacromolecules 2014, 15, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; Assoian, R.K. Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 2001, 114, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Inj. Int. J. Care Inj. 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfi, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Dhavalikar, P.; Robinson, A.; Lan, Z.Y.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A.; et al. Review of Integrin-Targeting Biomaterials in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 2000795. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; Garcia, A.J.; Guldberg, R.E. Nanofiber Orientation and Surface Functionalization Modulate Human Mesenchymal Stem Cell Behavior In Vitro. Tissue Eng. Part A 2014, 20, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Her, G.J.; Wu, H.C.; Chen, M.H.; Chen, M.Y.; Chang, S.C.; Wang, T.W. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater. 2013, 9, 5170–5180. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Asanuma-Date, K.; Arisaka, F.; Hattori, S.; Ogawa, H. Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration. Glycobiology 2007, 17, 784–794. [Google Scholar] [CrossRef]
- Erat, M.C.; Sladek, B.; Campbell, I.D.; Vakonakis, I. Structural Analysis of Collagen Type I Interactions with Human Fibronectin Reveals a Cooperative Binding Mode. J. Biol. Chem. 2013, 288, 17441–17450. [Google Scholar] [CrossRef]
- Davidenko, N.; Hamaia, S.; Bax, D.V.; Malcor, J.D.; Schuster, C.F.; Gullberg, D.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Selecting the correct cellular model for assessing of the biological response of collagen-based biomaterials. Acta Biomater. 2018, 65, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Hamaia, S.; Farndale, R.W. Integrin Recognition Motifs in the Human Collagens. In I Domain Integrins; Gullberg, D., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 127–142. [Google Scholar]
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in collagen type I of an integrin alpha(2)beta(1)-binding site containing an essential GER sequence. J. Biol. Chem. 1998, 273, 33287–33294. [Google Scholar] [CrossRef] [PubMed]
- Hamaia, S.W.; Pugh, N.; Raynal, N.; Nemoz, B.; Stone, R.; Gullberg, D.; Bihan, D.; Farndale, R.W. Mapping of Potent and Specific Binding Motifs, GLOGEN and GVOGEA, for Integrin alpha 1 beta 1 Using Collagen Toolkits II and III. J. Biol. Chem. 2012, 287, 26019–26028. [Google Scholar] [CrossRef]
- Siljander, P.R.M.; Hamaia, S.; Peachey, A.R.; Slatter, D.A.; Smethurst, P.A.; Ouwehand, W.H.; Knight, C.G.; Farndale, R.W. Integrin activation state determines selectivity for novel recognition sites in fibrillar collagens. J. Biol. Chem. 2004, 279, 47763–47772. [Google Scholar] [CrossRef]
- Farndale, R.W.; Silijander, P.R.M.; Onley, D.J.; Sundaresan, P.; Knight, C.G.; Barnes, M.J. Collagen-platelet interactions: Recognition and signalling. In Proteases and the Regulation of Biological Processes; Saklatvala, J., Nagase, H., Salvesen, G., Eds.; Biochemical Society Symposia; Portland Communications: London, UK, 2003; Volume 70, pp. 81–94. [Google Scholar]
- Krahn, K.N.; Bouten, C.V.C.; van Tuijl, S.; van Zandvoort, M.; Merkx, M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal. Biochem. 2006, 350, 177–185. [Google Scholar] [CrossRef]
- Siadat, S.M.; Silverman, A.A.; Susilo, M.E.; Paten, J.A.; DiMarzio, C.A.; Ruberti, J.W. Development of Fluorescently Labeled, Functional Type I Collagen Molecules. Macromol. Biosci. 2022, 22, 2100144. [Google Scholar] [CrossRef]
- Li, S.H.; Van den Diepstraten, C.; D'Souza, S.J.; Chan, B.M.C.; Pickering, J.G. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha 2 beta 1 integrin, RhoA, and fibronectin polymerization. Am. J. Pathol. 2003, 163, 1045–1056. [Google Scholar] [CrossRef]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef]
- Velling, T.; Risteli, J.; Wennerberg, K.; Mosher, D.F.; Johansson, S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha(11)beta(1) and alpha(2)beta(1). J. Biol. Chem. 2002, 277, 37377–37381. [Google Scholar] [CrossRef]
- Gallorini, M.; Carradori, S. Understanding collagen interactions and their targeted regulation by novel drugs. Expert Opin. Drug Discov. 2021, 16, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone Injury and Fracture Healing Biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef]
- Gardner, H. Integrin alpha 1 beta 1. In I Domain Integrins; Gullberg, D., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 21–39. [Google Scholar]
- Lundgren-Akerlund, E.; Aszodi, A. Integrin alpha 10 beta 1: A Collagen Receptor Critical in Skeletal Development. In I Domain Integrins; Gullberg, D., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 61–71. [Google Scholar]
- Madamanchi, A.; Santoro, S.A.; Zutter, M.M. alpha 2 beta 1 Integrin. In I Domain Integrins; Gullberg, D., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 41–60. [Google Scholar]
- Varas, L.; Ohlsson, L.B.; Honeth, G.; Olsson, A.; Bengtsson, T.; Wiberg, C.; Bockermann, R.; Jarnum, S.; Richter, J.; Pennington, D.; et al. alpha 10 integrin expression is up-regulated on fibroblast growth factor-2-treated mesenchymal stem cells with improved chondrogenic differentiation potential. Stem Cells Dev. 2007, 16, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Ladage, D.; Schinkothe, T.; Klausmann, U.; Ulrichsa, C.; Klinz, F.J.; Brixius, K.; Arnhold, S.; Desai, B.; Mehlhorn, U.; et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells 2006, 24, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Wenke, A.K.; Kjellman, C.; Lundgren-Akerlund, E.; Uhlmann, C.; Haass, N.K.; Herlyn, M.; Bosserhoff, A.K. Expression of integrin alpha 10 is induced in malignant melanoma. Cell. Oncol. 2007, 29, 373–386. [Google Scholar]
- Zeltz, C.; Lu, N.; Gullberg, D. Integrin alpha 11 beta 1: A Major Collagen Receptor on Fibroblastic Cells. In I Domain Integrins; Gullberg, D., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 73–83. [Google Scholar]
- Tiger, C.F.; Fougerousse, F.; Grundstrom, G.; Velling, T.; Gullberg, D. alpha 11 beta 1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 2001, 237, 116–129. [Google Scholar] [CrossRef]
- Zhang, W.M.; Kapyla, J.; Puranen, J.S.; Knight, C.G.; Tiger, C.F.; Pentikainen, O.T.; Johnson, M.S.; Farndale, R.W.; Heino, J.; Gullberg, D. alpha(11)beta(1) integrin recognizes the GFOGER sequence in interstitial collagens. J. Biol. Chem. 2003, 278, 7270–7277. [Google Scholar] [CrossRef]
| Gene | Primer | Sequence | Tm (°C) | GenBank |
|---|---|---|---|---|
| ITGA1 | Forward | 5′-CACTGATCTGCTTCTCGTCGG-3′ | 60.80 | NM_001033228.3 |
| Reverse | 5′-CTGATTCACAGCGTACACGTA-3′ | 58.14 | ||
| ITGA2 | Forward | 5′-GGGGACCTCACAAACACCT-3′ | 59.16 | NM_001033228.3 |
| Reverse | 5′-CAGTTTTCAGCTTCGACCCAT-3′ | 58.85 | ||
| ITGA10 | Forward | 5′-GCTTCTCCATCCACCGACT-3′ | 59.10 | NM_001302471.1 |
| Reverse | 5′-ACCTTCTTCAAGCCATAGCAC-3′ | 58.28 | ||
| ITGA11 | Forward | 5′-GGCACCAACAAGAATGAGACC-3′ | 59.46 | NM_176922.5 |
| Reverse | 5′-CCCCGTTCCAGTCATAGGC-3′ | 59.85 | ||
| ITGB1 | Forward | 5′-GCACACTGTCTGGAAACTCT-3′ | 57.75 | NM_010578.2 |
| Reverse | 5′-TTGTTACTCCGTCTGGCAAT-3′ | 57.15 | ||
| GAPDH | Forward | 5′-TCAACGACCCCTTCATTGACC-3′ | 60.27 | NM_008084.3 |
| Reverse | 5′-ACTGTGCCGTTGAATTTGCC-3′ | 59.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, B.; Wei, M.; Bao, B.; Pan, Z.; Elango, J.; Wu, W. The Role of Integrin Receptor’s α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen. Int. J. Mol. Sci. 2023, 24, 9110. https://doi.org/10.3390/ijms24119110
Ge B, Wei M, Bao B, Pan Z, Elango J, Wu W. The Role of Integrin Receptor’s α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen. International Journal of Molecular Sciences. 2023; 24(11):9110. https://doi.org/10.3390/ijms24119110
Chicago/Turabian StyleGe, Baolin, Mingjun Wei, Bin Bao, Zhilin Pan, Jeevithan Elango, and Wenhui Wu. 2023. "The Role of Integrin Receptor’s α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen" International Journal of Molecular Sciences 24, no. 11: 9110. https://doi.org/10.3390/ijms24119110
APA StyleGe, B., Wei, M., Bao, B., Pan, Z., Elango, J., & Wu, W. (2023). The Role of Integrin Receptor’s α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen. International Journal of Molecular Sciences, 24(11), 9110. https://doi.org/10.3390/ijms24119110








