Thermally Stable and Reusable Silica and Nano-Fructosome Encapsulated CalB Enzyme Particles for Rapid Enzymatic Hydrolysis and Acylation
Abstract
1. Introduction
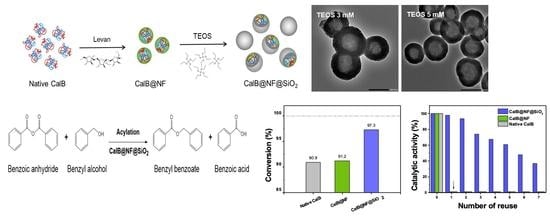
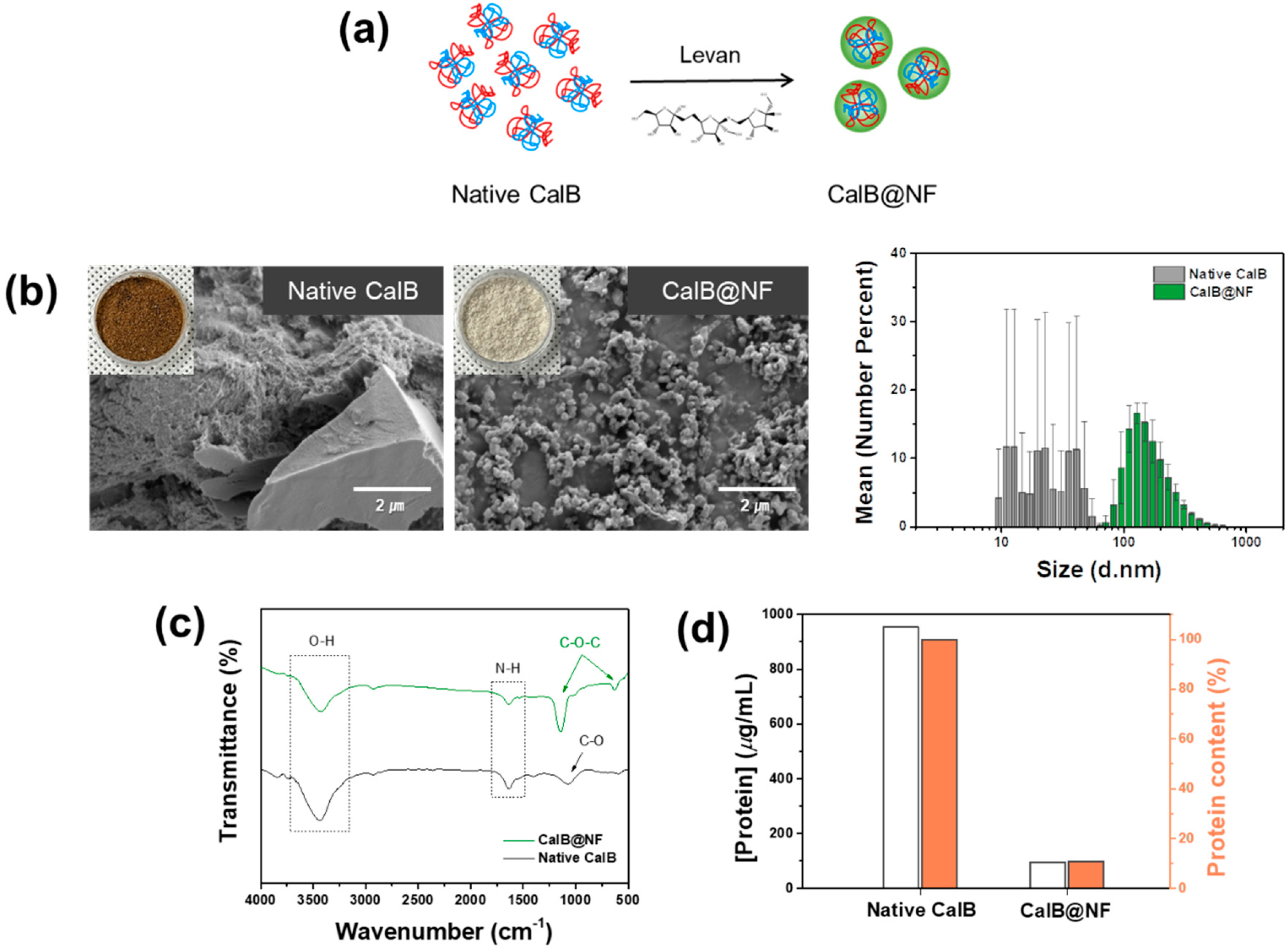
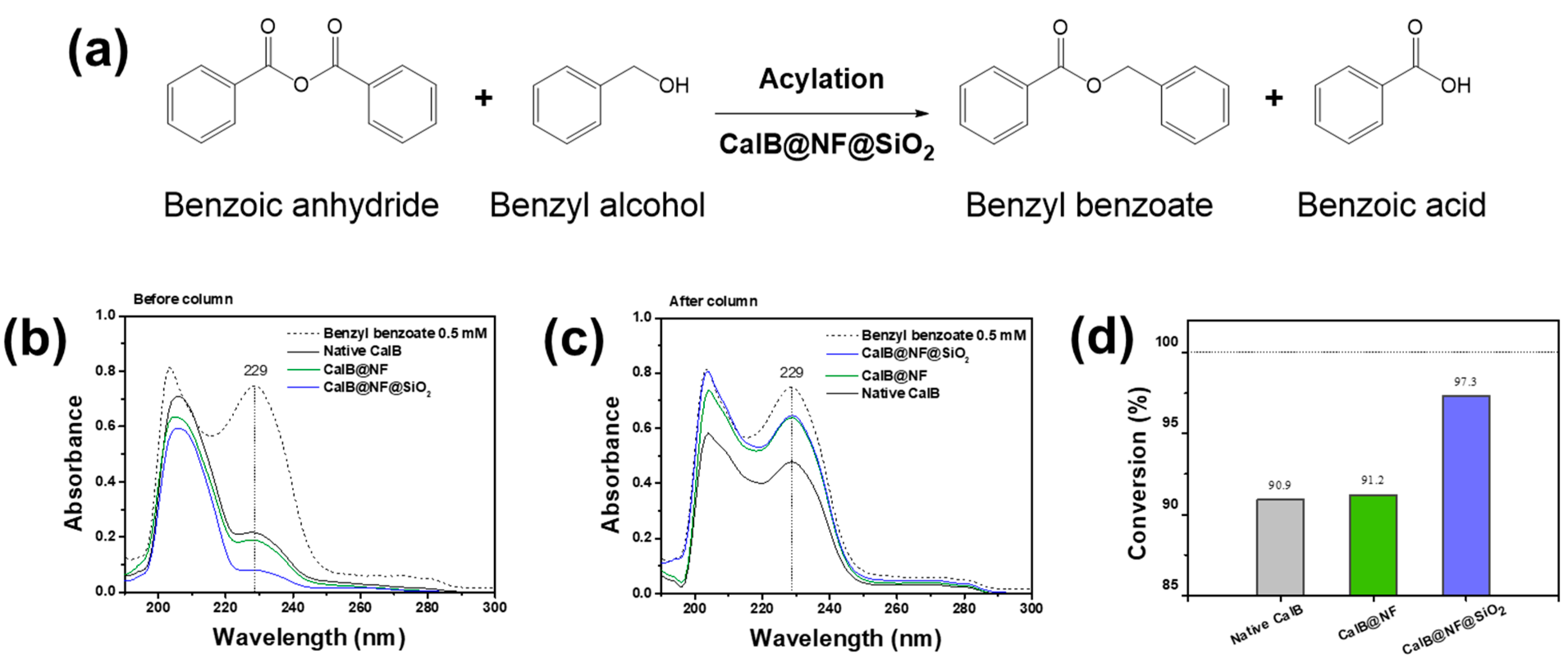
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silica Encapsulated CalB@NF (CalB@NF@SiO2)
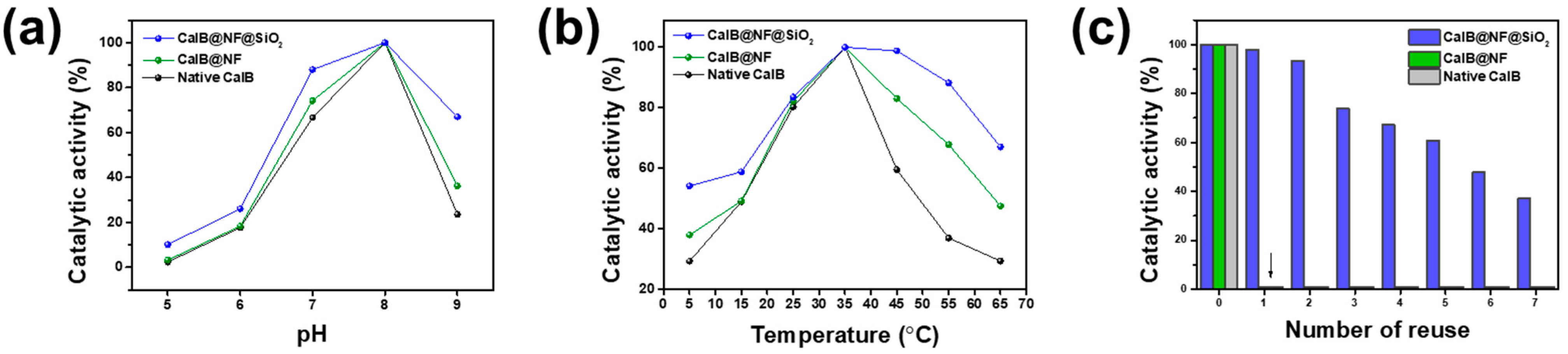
3.3. pH and Thermal Stability of Native CalB, CalB@NF, and CalB@NF@SiO2
3.4. Reusability of Native CalB, CalB@NF, and CalB@NF@SiO2
3.5. Enzymatic Hydrolysis for p-Nitrophenyl Butyrate with Native CalB, CalB@NF, and CalB@NF@SiO2
3.6. Enzymatic Synthesis for Benzyl Benzoate with Native CalB, CalB@NF, and CalB@NF@SiO2
3.7. Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sousa, S.F. Special Issue on “Enzymes as Biocatalysts: Current Research Trends and Applications”. Int. J. Mol. Sci. 2022, 23, 16209. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.N.; Howard, R.M.; Kumar, R.; Thompson, M.P.; Truppo, M.D.; Turner, N.J. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2018, 2, 409–421. [Google Scholar] [CrossRef]
- Thiele, I.; Yehia, H.; Krausch, N.; Birkholz, M.; Bournazou, M.N.C.; Sitanggang, A.B.; Kraume, M.; Neubauer, P.; Kurreck, A. Production of Modified Nucleosides in a Continuous Enzyme Membrane Reactor. Int. J. Mol. Sci. 2023, 24, 6081. [Google Scholar] [CrossRef]
- Fernandes, P. Miniaturization in Biocatalysis. Int. J. Mol. Sci. 2010, 11, 858–879. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Ge, J.; Lu, D.; Liu, Z.; Liu, Z. Recent advances in nanostructured biocatalysts. Biochem. Eng. J. 2009, 44, 53–59. [Google Scholar] [CrossRef]
- Kaja, B.S.; Lumor, S.; Besong, S.; Taylor, B.; Ozbay, G. Investigating Enzyme Activity of Immobilized Candida rugosa Lipase. J. Food Qual. 2018, 2018, 1618085. [Google Scholar]
- Chiplunkar, P.P.; Zhao, X.; Tomke, P.D.; Noro, J.; Xu, B.; Wang, Q.; Silva, C.; Pratap, A.P.; Cavaco-Paulo, A. Ultrasound-assisted lipase catalyzed hydrolysis of aspirin methyl ester. Ultrason. Sonochem. 2018, 40, 587–593. [Google Scholar] [CrossRef]
- Juhl, P.B.; Doderer, K.; Hollmann, F.; Thum, O.; Pleiss, J. Engineering of Candida antarctica lipase B for hydrolysis of bulky carboxylic acid esters. J. Biotechnol. 2010, 150, 474–480. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A. Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts 2019, 9, 1012. [Google Scholar] [CrossRef]
- Okulus, M.; Gliszczyńska, A. Enzymatic Synthesis of O-Methylated Phenophospholipids by Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Anisic and Veratric Acids. Catalysis 2020, 10, 538. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Li, S.; Huang, H.; Hu, Y. Improving Catalytic Performance of Candida rugosa Lipase by Chemical Modification with Polyethylene Glycol Functional Ionic Liquids. Ind. Eng. Chem. Res. 2015, 54, 8072–8079. [Google Scholar] [CrossRef]
- Siódmiak, T.; Haraldsson, G.G.; Dulęba, J.; Ziegler-Borowska, M.; Siódmiak, J.; Marszałł, M.P. Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts 2020, 10, 876. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Zou, D.; Wang, T.; Liu, T.; Wang, L.; Elfalleh, W.; Jiang, L. Immobilized CALB Catalyzed Transesterification of Soybean Oil and Phytosterol. Food Biophys. 2018, 13, 208–215. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, Z.; Arab, S.S. Solvent-dependent activity of Candida antarctica lipase B and its correlation with a regioselective mono aza-Michael addition—experimental and molecular dynamics simulation studies. Heliyon 2022, 8, e10336. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Liu, M.; Ma, L.; Zhang, W. Co-Immobilization of Lipases with Different Specificities for Efficient and Recyclable Biodiesel Production from Waste Oils: Optimization Using Response Surface Methodology. Int. J. Mol. Sci. 2023, 24, 4726. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization, Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecular 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how, Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes, Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilization. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V. Ananthanarayan, Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, F.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Santos, J.C.S.D.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Ami, D.; de Divitiis, M.; Brocca, S.; Catelani, T.; Natalello, A.; Lotti, M. Short-chain alcohols inactivate an immobilized industrial lipase through two different mechanisms. Biotechnol. J. 2022, 17, e2100712. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Carvalho, H.; Natalello, A.; Ferrario, V.; Pennati, M.L.; Barbiroli, A.; Lotti, M.; Pleiss, J.; Brocca, S. Diverse effects of aqueous polar co-solvents on Candida antarctica lipase B. Int. J. Biol. Macromol. 2020, 150, 930–940. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Chidambaram, J.S.C.A.; Veerapandian, B.; Sarwareddy, K.K.; Mani, K.P.; Shanmugam, S.R.; Venkatachalam, P. Studies on solvent precipitation of levan synthesized using Bacillus subtilis MTCC 441. Heliyon 2019, 5, e02414. [Google Scholar] [CrossRef]
- Küçükaşik, F.; Kazak, H.; Güney, D.; Finore, I.; Poli, A.; Yenigün, O.; Nicolaus, B.; Öner, E.T.J.A.M. Biotechnology, Molasses as fermentation substrate for levan production by Halomonas sp. Biotechnol. Bioprocess Eng. 2010, 89, 1729–1740. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jampala, P.; Preethi, M.; Ramanujam, S.; Harish, B.; Uppuluri, K.B.; Anbazhagan, V. Immobilization of levan-xylanase nanohybrid on an alginate bead improves xylanase stability at wide pH and temperature. Int. J. Biol. Macromol. 2017, 95, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Terassi, G.; Cristiani, B.; Colabone, C.M.A.P. Immobilization of Levansucrase: Strategies and Biotechnological Applications. J. Chil. Chem. Soc. 2019, 64, 4377–4381. [Google Scholar]
- Vīna, I.; Karsakevich, A.; Bekers, M. Stabilization of anti-leukemic enzyme l-asparaginase by immobilization on polysaccharide levan. J. Mol. Catal. B: Enzym. 2001, 11, 551–558. [Google Scholar] [CrossRef]
- Sonh, J.H.; Bae, J.H. Levan-Protein Nanocomposite and Uses Thereof. KR Patent 10-2021-0012979, 7 July 2021. [Google Scholar]
- Ma, H.-Z.; Yu, X.-W.; Song, C.; Xue, Q.-L.; Jiang, B. Immobilization of Candida Antarctica lipase B on epoxy modified silica by sol-gel process. J. Mol. Catal. B Enzym. 2016, 127, 76–81. [Google Scholar] [CrossRef]
- Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Cazaban, D.; Illanes, A.; Wilson, L.; Betancor, L. Bio-inspired silica lipase nanobiocatalysts for the synthesis of fatty acid methyl esters. Process. Biochem. 2018, 74, 86–93. [Google Scholar] [CrossRef]
- Song, M.; Chang, J.-H. Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. Int. J. Mol. Sci. 2022, 23, 2459. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamanishi, T.; Kamegawa, T.; Mori, K.; Che, M.; Yamashita, H. Lipase-embedded silica nanoparticles with oil-filled core–shell structure: Stable and recyclable platforms for biocatalysts. Chem. Commun. 2012, 48, 2882–2884. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Fuentes, M.; Podrasky, O.; Kuncova, G.; Guisán, J.M.; Fernández-Lafuente, R. Advantages of the Pre-Immobilization of Enzymes on Porous Supports for Their Entrapment in Sol−Gels. Biomacromolecules 2005, 6, 1027–1030. [Google Scholar] [CrossRef]
- Escobar, S.; Bernal, C.; Bolivar, J.M.; Nidetzky, B.; López-Gallego, F.; Mesa, M. Understanding the silica-based sol-gel encapsulation mechanism of Thermomyces lanuginosus lipase: The role of polyethylenimine. Mol. Catal. 2018, 449, 106–113. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, I.-W. Encapsulation of protein molecules in transparent porous silica matrices via an aqueous colloidal sol–gel process. Acta Mater. 1999, 47, 4535–4544. [Google Scholar] [CrossRef]
- Kang, K.; Choi, J.; Nam, J.H.; Lee, S.C.; Kim, K.J.; Lee, S.-W.; Chang, J.H. Preparation and Characterization of Chemically Functionalized Silica-Coated Magnetic Nanoparticles as a DNA Separator. J. Phys. Chem. B 2009, 113, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, V.; Obuobi, S.A.O.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- De Meneses, A.C.; Balen, M.; de Andrade Jasper, E.; Korte, I.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Enzymatic synthesis of benzyl benzoate using different acyl donors: Comparison of solvent-free reaction techniques. Process Biochem. 2020, 92, 261–268. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef]

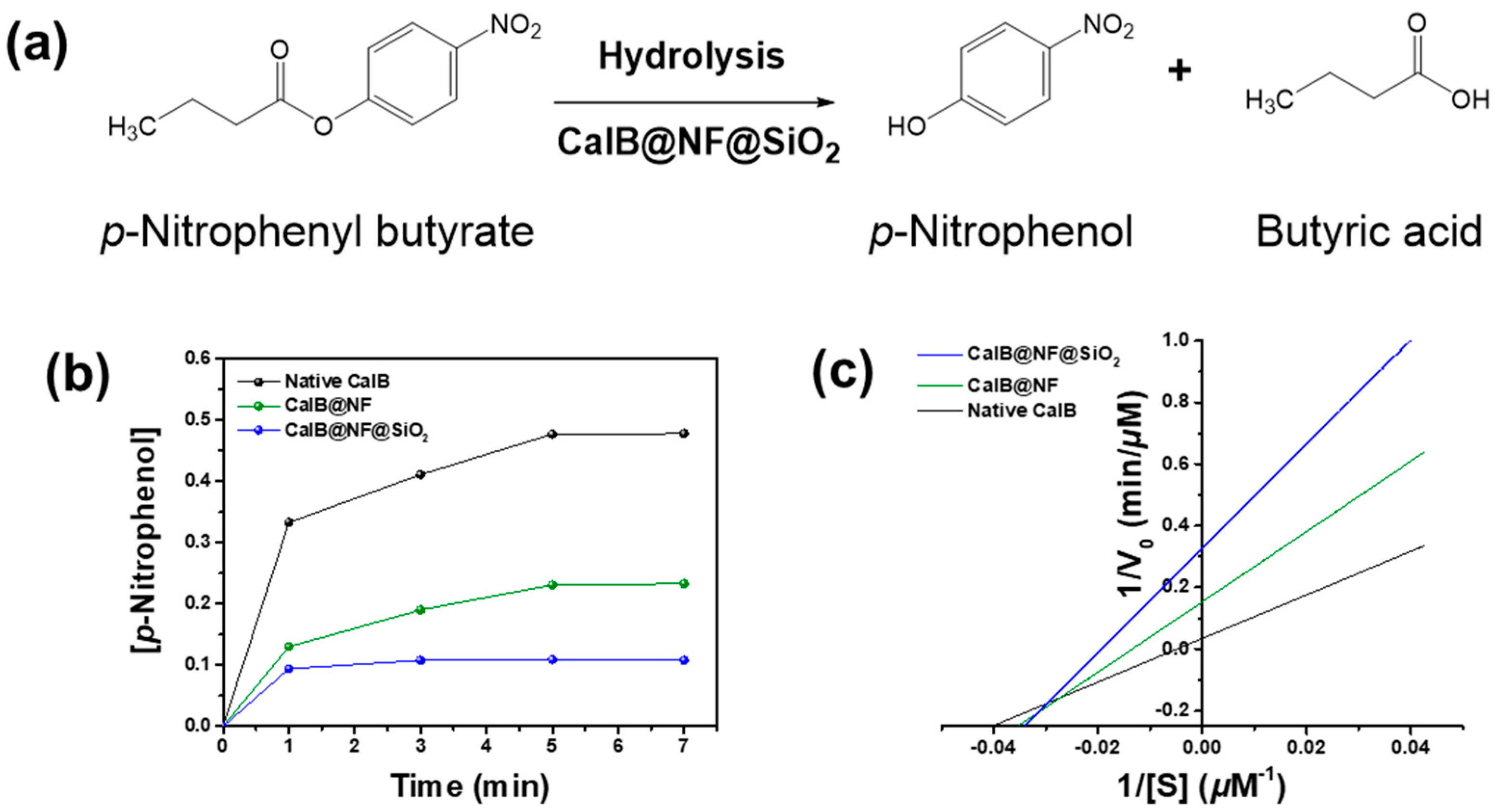
| Km (mM) | Vmax (μM∙min−1) | Kcat (min−1) | Kcat/Km (μM−1∙min−1) | |
|---|---|---|---|---|
| Native CalB | 0.204 | 28.74 | 9484 | 46,709 |
| CalB@NF | 0.074 | 11.41 | 21,473 | 289,300 |
| CalB@NF@SiO2 | 0.051 | 3.05 | 10,065 | 195,575 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.Y.; Sohn, J.H.; Chang, J.H. Thermally Stable and Reusable Silica and Nano-Fructosome Encapsulated CalB Enzyme Particles for Rapid Enzymatic Hydrolysis and Acylation. Int. J. Mol. Sci. 2023, 24, 9838. https://doi.org/10.3390/ijms24129838
Jang WY, Sohn JH, Chang JH. Thermally Stable and Reusable Silica and Nano-Fructosome Encapsulated CalB Enzyme Particles for Rapid Enzymatic Hydrolysis and Acylation. International Journal of Molecular Sciences. 2023; 24(12):9838. https://doi.org/10.3390/ijms24129838
Chicago/Turabian StyleJang, Woo Young, Jung Hoon Sohn, and Jeong Ho Chang. 2023. "Thermally Stable and Reusable Silica and Nano-Fructosome Encapsulated CalB Enzyme Particles for Rapid Enzymatic Hydrolysis and Acylation" International Journal of Molecular Sciences 24, no. 12: 9838. https://doi.org/10.3390/ijms24129838
APA StyleJang, W. Y., Sohn, J. H., & Chang, J. H. (2023). Thermally Stable and Reusable Silica and Nano-Fructosome Encapsulated CalB Enzyme Particles for Rapid Enzymatic Hydrolysis and Acylation. International Journal of Molecular Sciences, 24(12), 9838. https://doi.org/10.3390/ijms24129838