Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells
Abstract
1. Introduction
2. Results
2.1. Tumor Development
2.2. Body Weight, Tumor Size, and Tumor Volume
2.3. Blood Biochemical Parameters
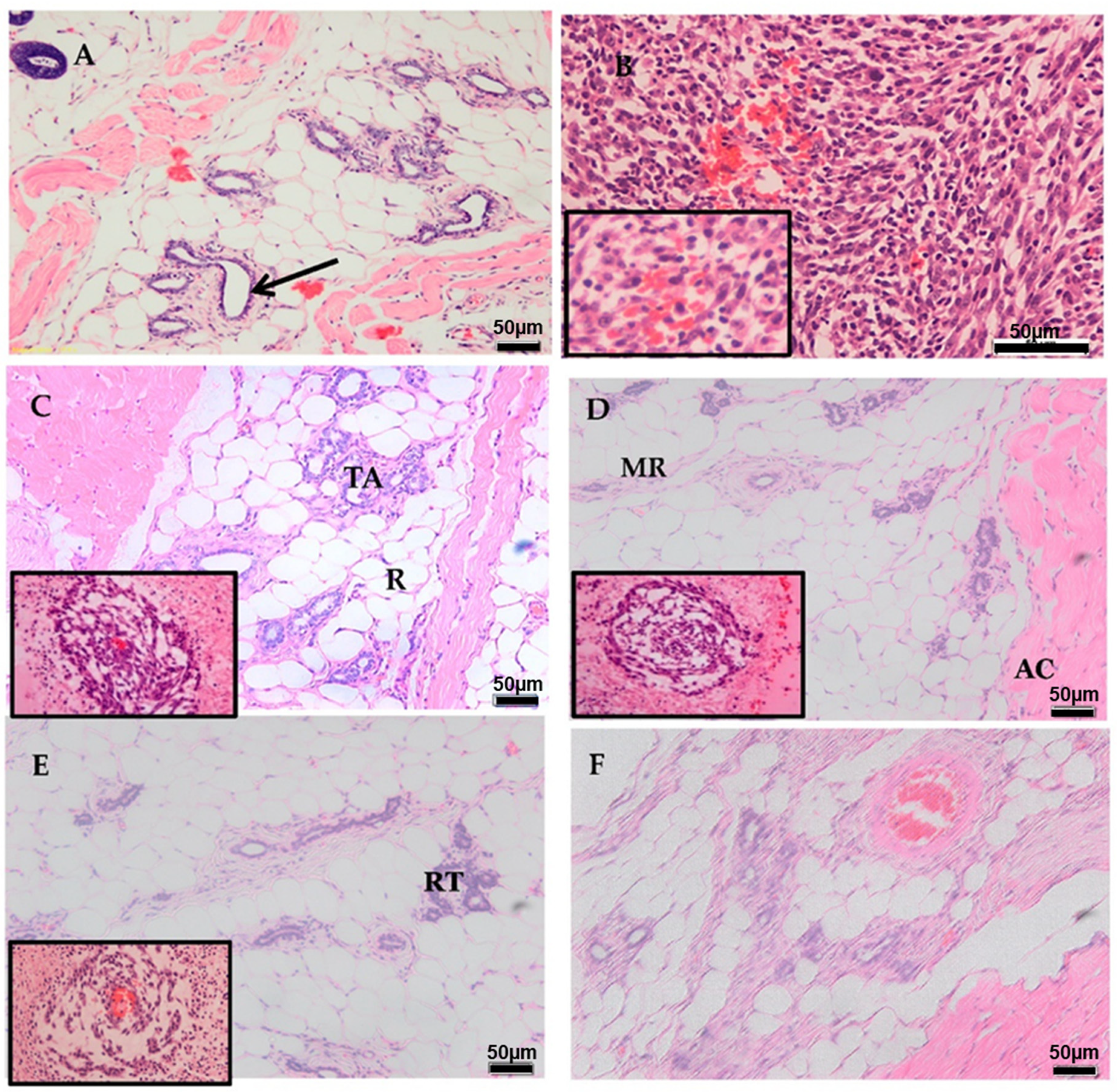
2.4. Histopathology
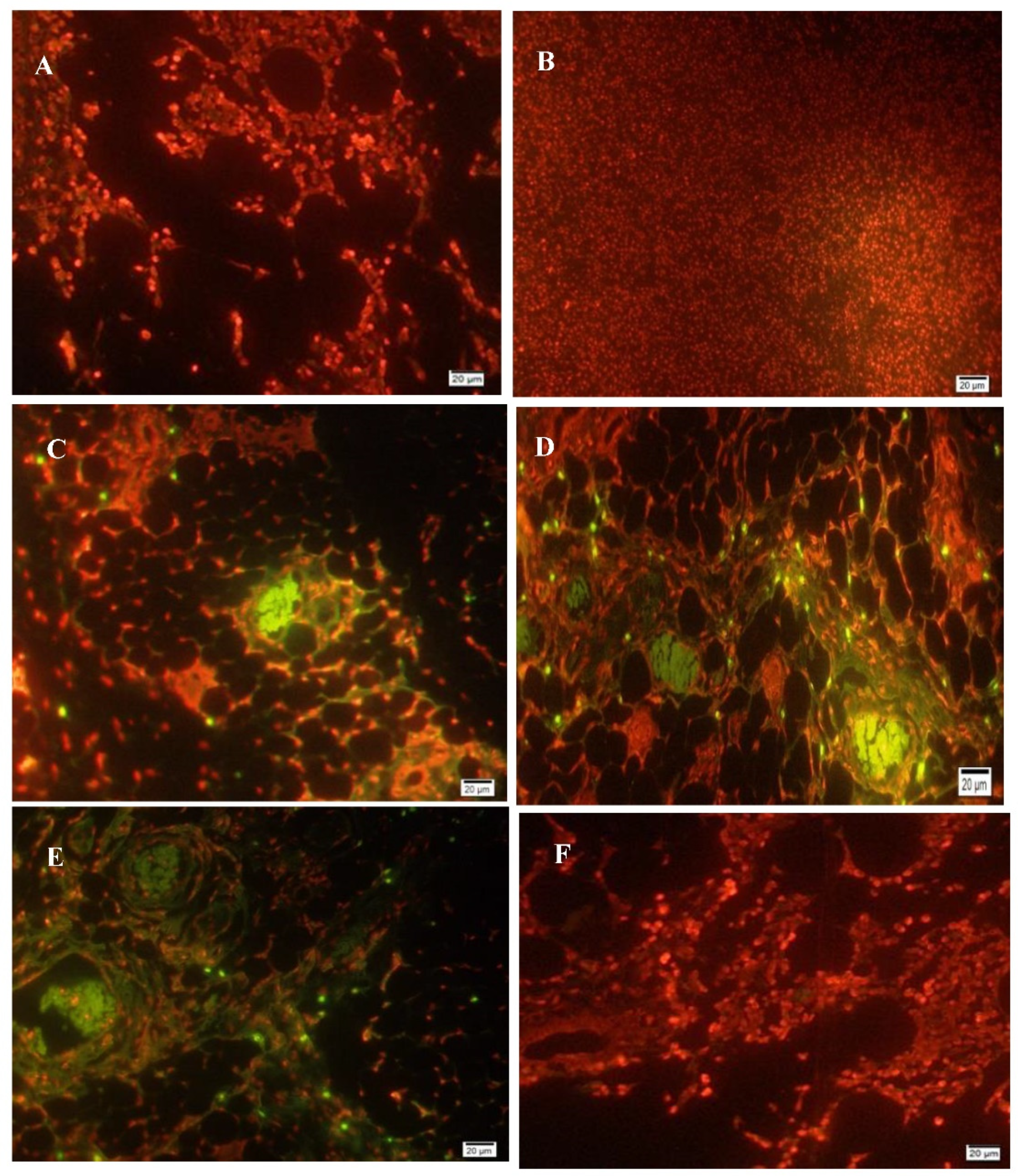
2.5. Apoptosis
2.6. Antioxidant Activity
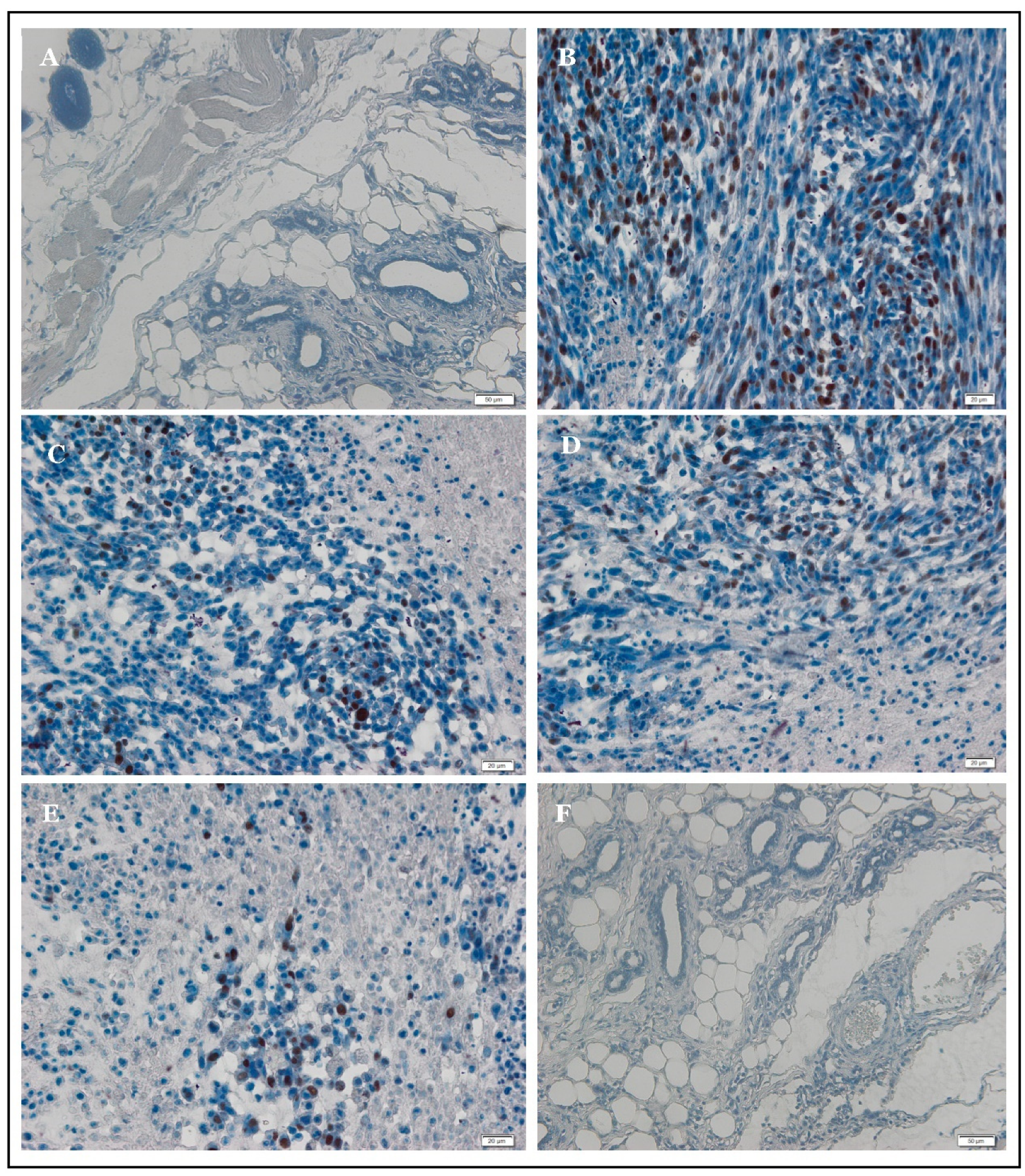
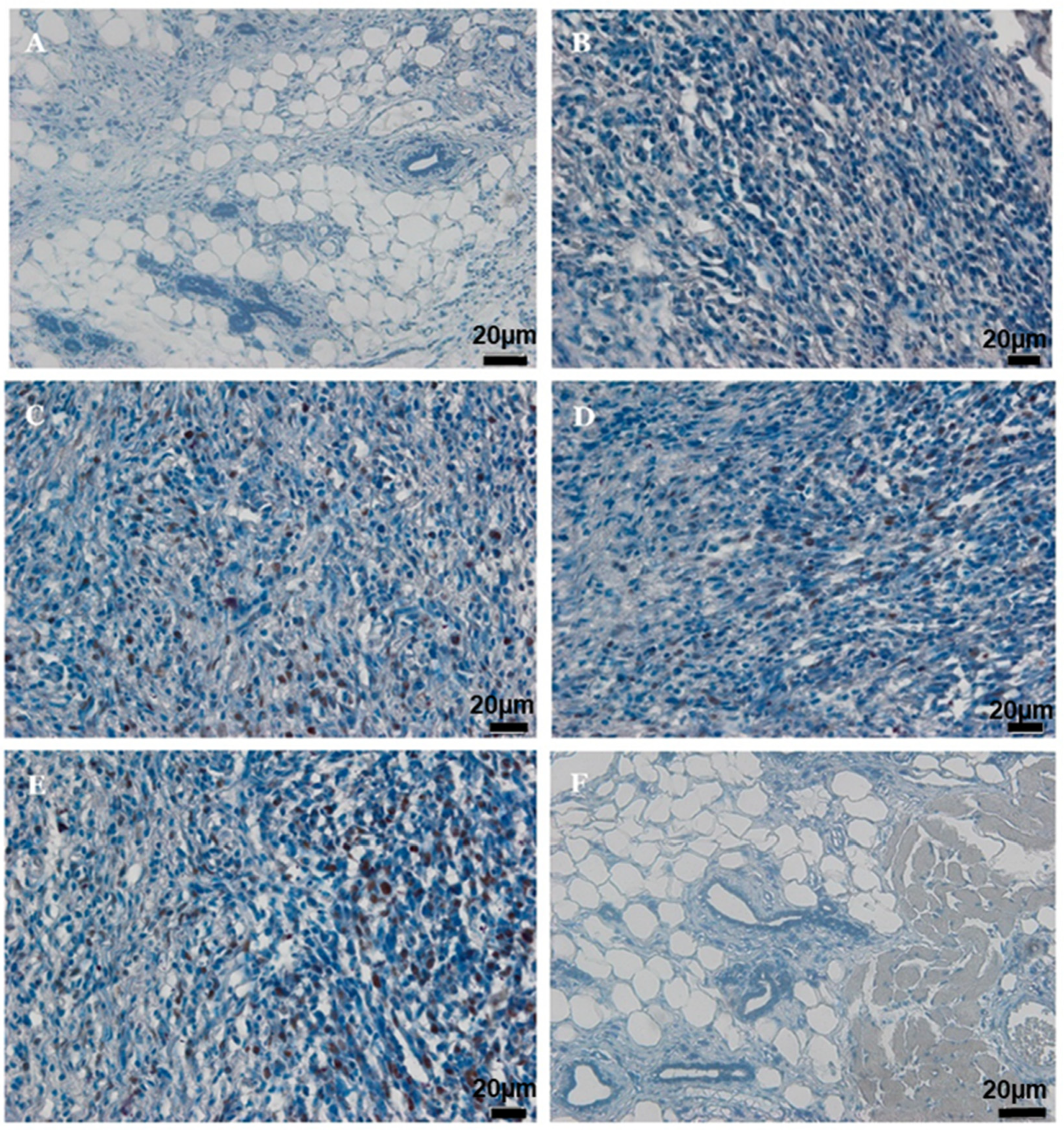
2.7. Immunohistochemistry of PCNA and p53
3. Discussion
4. Materials and Methods
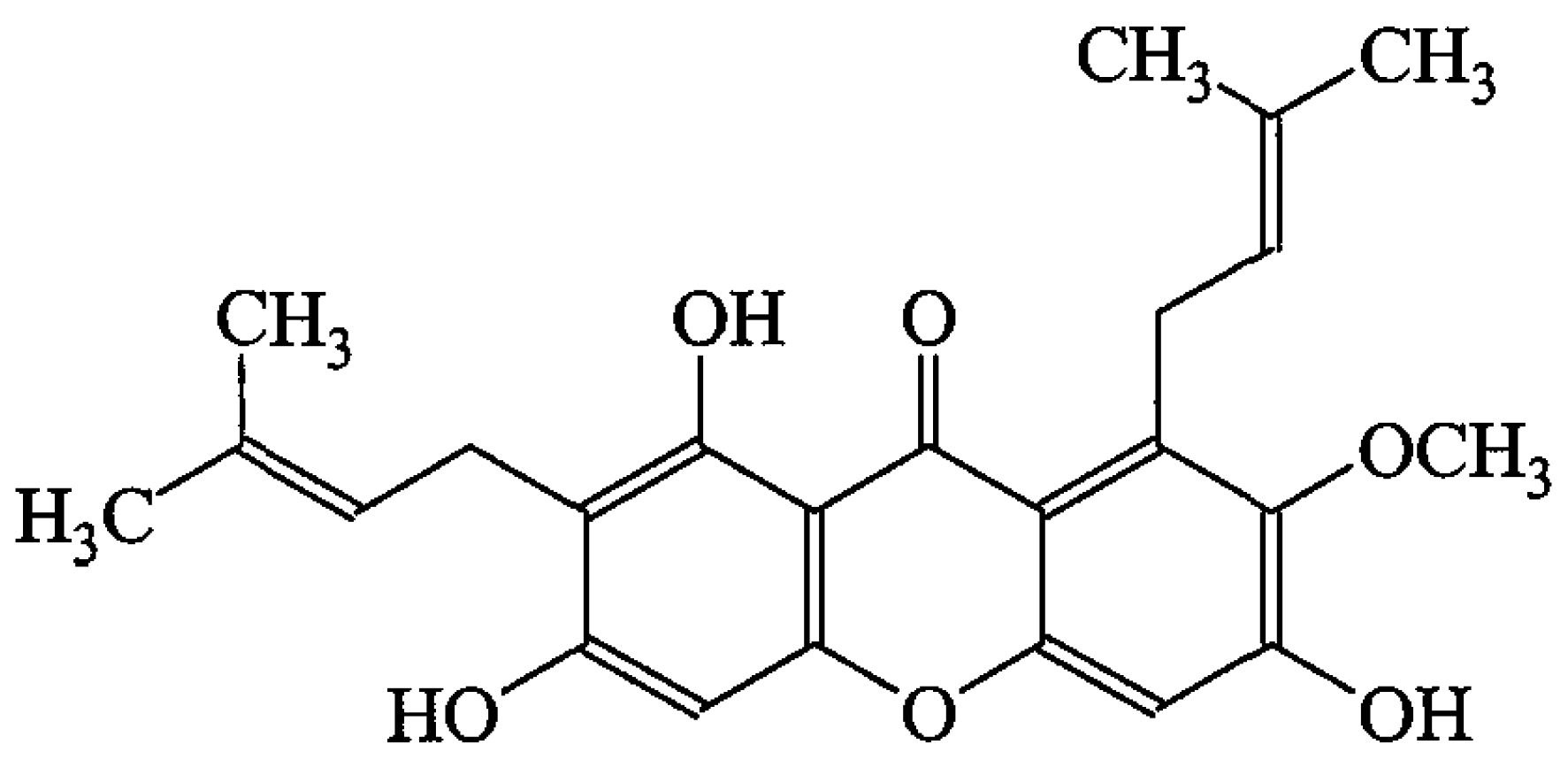
4.1. Extraction and Isolation of AM from C. arborescens
4.2. Identification of AM
4.3. Chemicals, Reagents, and Cell Culture
4.4. Ethical Issues
4.5. Cell Preparation
4.6. Induction of Mammary Gland Tumors
4.7. Experimental Design and Animal Treatment
4.8. Serum Biochemical Parameters
4.9. Biochemical Parameters of Mammary and Liver Tissues
4.9.1. Estimation of Superoxide Dismutase (SOD) Activity
4.9.2. Inhibition of LPx Formation
4.9.3. Estimation of Glutathione Peroxidase GPx Activity
4.9.4. Estimation of CAT Activity
4.10. Macroscopic and Microscopic Analysis
4.11. TUNEL Assay
4.12. Immunohistochemistry Examination
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Gao, Y.; Heller, S.L. Health Disparity and Breast Cancer Outcomes in Asian Women. RadioGraphics 2022, 42, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yan, L.; Liu, Y.; Yuan, F.; Li, H.; Ni, J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J. Hematol. Oncol. 2019, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Ki, J.-S. Antioxidant and chemotherapeutic efficacies of seaweed-derived phlorotannins in cancer treatment: A review regarding novel anticancer drugs. Phytother. Res. 2023, 37, 2067–2091. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Aggarwal, L.M.; Mishra, S.P.; Khanna, H.D.; Shahi, U.P.; Pradhan, S. Free radicals and antioxidants in normal versus cancerous cells-An overview. Indian J. Biochem. Biophys. 2019, 56, 7–19. [Google Scholar]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An update on the anticancer activity of xanthone derivatives: A review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Abbasalipourkabir, R.; Salehzadeh, A.; Abdullah, R. Antitumor activity of tamoxifen loaded solid lipid nanoparticles on induced mammary tumor gland in Sprague-Dawley rats. Afr. J. Biotechnol. 2010, 9, 7337–7345. [Google Scholar]
- Abbasalipourkabir, R.; Dehghan, A.; Salehzadeh, A.; Shamsabadi, F.; Abdullah, R. Induction of mammary gland tumor in female Sprague-Dawley rats with LA7 cells. Afr. J. Biotechnol. 2010, 9, 4491–4498. [Google Scholar]
- Liu, Y.; Yin, T.; Feng, Y.; Cona, M.M.; Huang, G.; Liu, J.; Song, S.; Jiang, Y.; Xia, Q.; Swinnen, J.V. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant. Imaging. Med. Surg. 2015, 5, 708. [Google Scholar]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Li, R.R.; Zeng, D.Y. The effects and mechanism of α-mangostin on chemosensitivity of gastric cancer cells. Kaohsiung J. Med. Sci 2021, 37, 709–717. [Google Scholar] [CrossRef]
- Chandra Boinpelly, V.; Verma, R.K.; Srivastav, S.; Srivastava, R.K.; Shankar, S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J. Cell. Mol. Med. 2020, 24, 11343–11354. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Kamalidehghan, B.; Ghaderian, M.; Dehghan, F.; Ali, L.Z.; Arbab, I.A.; Yahayu, M. α-Mangostin from Cratoxylum arborescens demonstrates apoptogenesis in MCF-7 with regulation of NF-κB and Hsp70 protein modulation in vitro, and tumor reduction in vivo. Drug Des. Dev. Ther. 2014, 8, 1629–1647. [Google Scholar] [CrossRef]
- Shibata, M.-A.; Iinuma, M.; Morimoto, J.; Kurose, H.; Akamatsu, K.; Okuno, Y.; Akao, Y.; Otsuki, Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011, 9, 69. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Tian, B.; Wang, Y.; Du, L.; Sun, T.; Shi, Y.; Zhao, X.; Jing, J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin. Chim. Acta 2017, 470, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hing, J.; Mok, C.; Tan, P.; Sudhakar, S.; Seah, C.; Lee, W.; Tan, S. Clinical utility of tumour marker velocity of cancer antigen 15–3 (CA 15–3) and carcinoembryonic antigen (CEA) in breast cancer surveillance. Breast 2020, 52, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Liang, M.-M.; Ye, X.-B.; Zhao, W.-Y. Association of CA 15-3 and CEA with clinicopathological parameters in patients with metastatic breast cancer. Mol. Clin. Oncol. 2015, 3, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.S.; Sateesh, M.; Bashir, M.; Ganie, M.A.; Nabi, S. Chemopreventive and anti-breast cancer activity of compounds isolated from leaves of Abrus precatorius L. Biotech 2018, 8, 371. [Google Scholar] [CrossRef]
- Hutadilok-Towatana, N.; Reanmongkol, W.; Wattanapiromsakul, C.; Bunkrongcheap, R. Acute and subchronic toxicity evaluation of the hydroethanolic extract of mangosteen pericarp. J. Med. Plant. Res. 2010, 4, 969–974. [Google Scholar]
- Jeong, S.; Park, M.-J.; Song, W.; Kim, H.-S. Current immunoassay methods and their applications to clinically used biomarkers of breast cancer. Clin. Biochem. 2020, 78, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Kimbung, S.; Johansson, I.; Danielsson, A.; Veerla, S.; Egyhazi Brage, S.; Frostvik Stolt, M.; Skoog, L.; Carlsson, L.; Einbeigi, Z.; Lidbrink, E. Transcriptional Profiling of Breast Cancer Metastases Identifies Liver Metastasis–Selective Genes Associated with Adverse Outcome in Luminal A Primary Breast Cancer. Clin. Cancer Res. 2016, 22, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.D.; Gu, R.; Pierce, C.; Kil, J. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anticancer Drugs. 2005, 16, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-J.; Lim, Y.S.; Kim, M.S.; Lee, B.; Kim, B.-Y.; Kim, Z.; Lee, J.E.; Lee, M.H.; Kim, S.G.; Kim, Y.S. Risk of fatty liver after long-term use of tamoxifen in patients with breast cancer. PLoS ONE 2020, 15, e0236506. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Kontomanolis, E.; Giatromanolaki, A.; Sivridis, E.; Liberis, V. Serum and tissue LDH levels in patients with breast/gynaecological cancer and benign diseases. Gynecol. Obstet. Investig. 2009, 67, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Hu, C.-T.; Prijatelj, V.; Weng, C.-F. Antrodia cinnamomea is a potentially effective complementary medicine for adjuvant therapy against breast cancer with bone metastasis: A case report. Medicine 2020, 99, e20808. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Das, U.N. Introduction to Free Radicals, Antioxidants, Lipid Peroxidation, and Their Effects on Cell Proliferation. In Molecular Biochemical Aspects of Cancer; Humana: New York, NY, USA, 2020; pp. 41–65. [Google Scholar]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Kohan, R.; Collin, A.; Guizzardi, S.; Tolosa de Talamoni, N.; Picotto, G. Reactive oxygen species in cancer: A paradox between pro-and anti-tumour activities. Cancer Chemother. Pharmacol. 2020, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Camargo, L.L. Reactive oxygen species and oxidative stress. Primer Auton. Nerv. Syst. 2023, 345–352. [Google Scholar] [CrossRef]
- Durgadevi, P.K.S.; Saravanan, A.; Uma, S. Antioxidant potential and antitumour activities of Nendran banana peels in breast cancer cell line. Indian J. Pharm. Sci. 2019, 81, 464–473. [Google Scholar]
- Russo, J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015, 43, 145–170. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting proliferating cell nuclear antigen (PCNA) as an effective strategy to inhibit tumor cell proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Sandberg, E.N.; Goel, N.; Bishayee, A. Oncogenic and tumor suppressive components of the cell cycle in breast cancer progression and prognosis. Pharmaceutics 2021, 13, 569. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Thomas-Ahner, J.M.; Li, J.; Riedl, K.M.; Nontakham, J.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol. Nutr. Food Res. 2013, 57, 203–211. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Huang, M.-H.; Cheng, C.-W.; Hung, J.-H.; Yang, S.-F.; Hsieh, Y.-H. α-Mangostin induces mitochondrial dependent apoptosis in human hepatoma SK-Hep-1 cells through inhibition of p38 MAPK pathway. Apoptosis 2013, 18, 1548–1560. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A. Role of oncogenes and tumor-suppressor genes in carcinogenesis: A review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.; Ramos, H.; Inga, A.; Sousa, E.; Saraiva, L. Structural and drug targeting insights on mutant p53. Cancers 2021, 13, 3344. [Google Scholar] [CrossRef] [PubMed]
- D’arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V. Cellular stress and general pathological processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.H.; Ibraheem, S.A.; Jabir, M.S.; Ali, K.A.; Taqi, Z.J.; Dan, F.M. Zinc oxide nanoparticles induces apoptosis in human breast cancer cells via caspase-8 and P53 pathway. Nano Biomed. Eng. 2019, 11, 35–43. [Google Scholar]
- Mobarez, A.M.; Soleimani, N.; Esmaeili, S.-A.; Farhangi, B. Nanoparticle-based immunotherapy of breast cancer using recombinant Helicobacter pylori proteins. Eur. J. Pharm. Biopharm. 2020, 155, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A.; Yahayu, M.; Ali, L.Z.; Ishag, O.E. α-Mangostin from Cratoxylum arborescens: An in vitro and in vivo toxicological evaluation. Arab. J. Chem. 2015, 8, 129–137. [Google Scholar] [CrossRef]
- Flohe, L. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef]
- Erber, R.; Hartmann, A. Histology of luminal breast cancer. Breast Care 2020, 15, 327–336. [Google Scholar] [CrossRef]
- Jayash, S.N.; Hashim, N.M.; Misran, M.; Baharuddin, N. Formulation and in vitro and in vivo evaluation of a new osteoprotegerin–chitosan gel for bone tissue regeneration. J. Biomed. Mater. Res. 2017, 105, 398–407. [Google Scholar] [CrossRef] [PubMed]
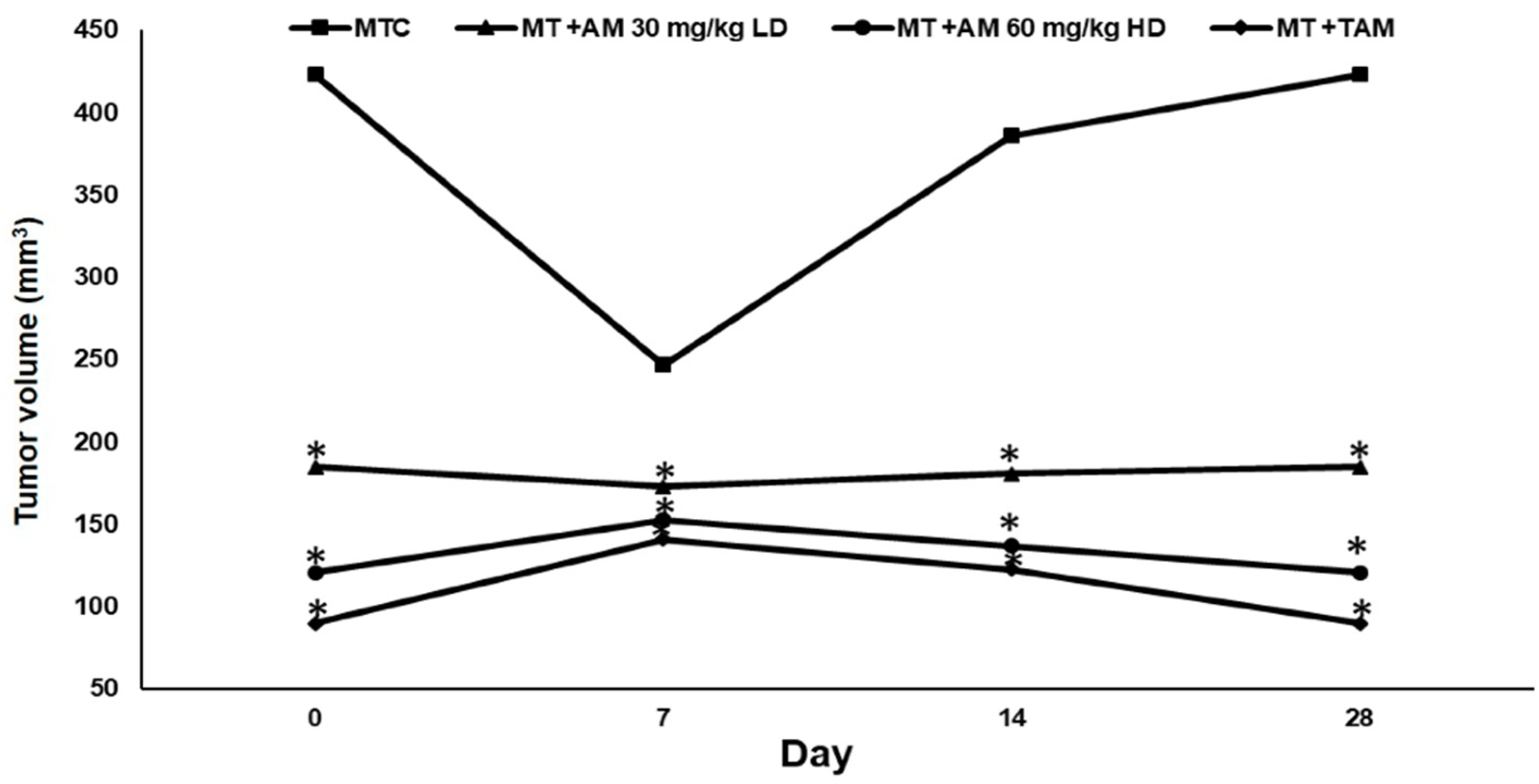
| Group | Treatment Groups | Body Weight (g) | Tumor Volume (mm3) | Reduction of Tumor Percentage (%) |
|---|---|---|---|---|
| I. | NC | 220.7 ± 2.4 * | 0 | 0 |
| II. | MTC | 183.25 ± 3.31 | 423 ± 71.2 | 0 |
| III. | MT + AM 30 mg/kg LD | 199.13 ± 2.8 * | 185 ± 12.8 * | 56.3% * |
| IV. | MT + AM 60 mg/kg HD | 205.25 ± 2 * | 121 ± 19.3 * | 71.4% * |
| V. | MT + TAM | 209.88 ± 1.7 * | 90 ± 27 * | 78.7% * |
| VI. | AM 60 mg/kg | 220.1 ± 0.5 * | 0 | 0 |
| Group | Treatment Group | Serum Biomarkers | ||||
|---|---|---|---|---|---|---|
| CEA (ng/mL) | CA15-3 (ng/mL) | ALT (U/L) | ALP (U/L) | LDH (U/L) | ||
| I | NC | 3.1 ± 0.9 * | 12.2 ± 4.0 * | 45.0 ± 3.4 * | 68.0 ± 7.3 * | 612 ± 201.34 * |
| II | MTC | 7.3 ± 2.3 | 48.0 ± 12.5 | 95.4 ± 16.1 | 453 ± 24 | 4562.0 ± 850 |
| III | MT + AM 30 mg/kg LD | 4.6 ± 1.2 * | 32.6 ± 9.4 * | 56.0 ± 14.8 * | 224.0 ± 5.5 * | 2163.8 ± 73.1 * |
| IV | MT + AM 60 mg/kg HD | 3.5 ± 0.8 * | 27.3 ± 4.6 * | 55.5 ± 14.9 * | 211.3 ± 4.1 * | 2189.7 ± 114 * |
| V | MT + TAM | 3.2 ± 1.04 * | 26.2 ± 6.9 * | 66.5 ± 16.5 * | 199.2 ± 5.2 * | 2200 ± 70 * |
| VI | AM 60 mg/kg | 3.1 ± 1.02 * | 12.3 ± 4.0 * | 45.0 ± 4.2 * | 68.0 ± 16.2 * | 614 ± 17.15 * |
| Group | Treatment Group | Tubule | Mitotic Figure | Nuclear Pleomorphism | Grade | Total Score |
|---|---|---|---|---|---|---|
| I | NC | 100% tubular structure | No mitotic figure | Uniform nuclear morphology | Grade I | 3 ± 0.0 * |
| II | MTC | 5% tubular formation | High mitotic figure more than 10 in high power per 10 magnification field | Moderate nuclear pleomorphism | Grade III | 8 ± 0.5 |
| III | MT + AM 30 mg/kg LD | 20% tubular formation | Moderate mitotic figures | Several variation in nuclear sizes | Grade II | 6 ± 0.2 * |
| IV | MT + AM 60 mg/kg HD | 50% tubular formation | Moderate mitotic figure | Moderate nuclear pleomorphism | Grade I | 5 ± 0.3 * |
| V | MT + TAM | 30% tubular formation | Low mitotic figure | Few nuclear pleomorphisms | Grade I | 4 ± 0.1 * |
| VI | 60 mg/kg AM | 95% tubular structure | No mitotic figure | Uniform nuclear morphology | Grade I | 3 ± 0.1 * |
| Group | Treatment Groups | SOD (U/mg Protein) | CAT (µmol/mg Ptotein) | GPX (µg/mg Protein) | LPx (nmol/mg Protein) |
|---|---|---|---|---|---|
| I | NC | 8.42 ± 1.12 * | 62.32 ± 2.15 * | 7.24 ± 1.56 * | 1.21 ± 0.27 * |
| II | MTC | 5.00 ± 1.7 | 39.61 ± 4.21 | 4.00 ± 0.23 | 3.13 ± 1.25 |
| III | MT + AM 30 mg/kg LD | 6.20 ± 0.47 * | 56.30 ± 3.43 * | 5.02 ± 0.93 * | 2.12 ± 0.64 * |
| IV | MT + AM 60 mg/kg HD | 7.24 ± 2.1 * | 59.40 ± 3.23 * | 5.93 ± 1.2 * | 1.12 ± 0.32 * |
| V | MT + TAM | 7.29 ± 1.23 * | 61.13 ± 3.28 * | 6.56 ± 0.31 * | 1.18 ± 0.61 * |
| VI | 60 mg/kg AM | 8.43 ± 2.11 * | 62.10 ± 2.27 * | 7.22 ± 0.21 * | 1.21 ± 0.11 * |
| Group | Treatment Groups | SOD (U/mg Protein) | CAT (µmol/mg Protein) | GPX (µg/mg Protein) | LPX (nmol/mg Protein) |
|---|---|---|---|---|---|
| I | NC | 6.32 ± 1.91 * | 57.55 ± 1.33 * | 5.13 ± 1.22 * | 1.81 ± 0.36 * |
| II | MTC | 2.56 ± 0.95 | 23.17 ± 16.1 | 2.27 ± 0.06 | 4.19 ± 0.13 |
| III | MT + AM 30 mg/kg LD | 4.29 ± 0.08 * | 48.73 ± 0.15 * | 3.81 ± 0.09 * | 3.02 ± 0.02 * |
| IV | MT + AM 60 mg/kg HD | 5.24 ± 2.1 * | 59.40 ± 3.23 * | 4.41 ± 1.4 * | 2.21 ± 0.41 * |
| V | MT + TAM | 5.29 ± 0.08 * | 56.27 ± 0.12 * | 4.83 ± 0.26 * | 2.02 ± 0.17 * |
| VI | 60 mg/kg AM | 6.31 ± 0.01 * | 57.58 ± 1.27 * | 5.13 ± 1.15 * | 1.81 ± 0.25 * |
| Group | PCNA-Immunopositive Cells (%) | p53-Immunopositive Cells (%) |
|---|---|---|
| NC | 0.00 ± 0.00 | 5.10 ± 0.96 |
| MTC | 73.76 ± 22.90 a | 1.50 ± 0.42 a |
| MT + AM-LD | 41.35 ± 12.67 a,b | 3.7 ± 1.13 a,b |
| MT + AM-HD | 32.56 ± 9.85 a,b | 4.20 ± 0.91 a,b |
| MT + TAM | 30.89 ± 10.12 a,b | 4.50 ± 1.22 a,b |
| AM-HD | 0.00 ± 0.00 Ns,b | 5.0 ± 1.28 Ns,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.Y.; Hashim, N.M.; Omer, F.A.A.; Abubakar, M.S.; Mohammed, H.A.; Salama, S.M.; Jayash, S.N. Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells. Int. J. Mol. Sci. 2023, 24, 10283. https://doi.org/10.3390/ijms241210283
Ibrahim MY, Hashim NM, Omer FAA, Abubakar MS, Mohammed HA, Salama SM, Jayash SN. Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells. International Journal of Molecular Sciences. 2023; 24(12):10283. https://doi.org/10.3390/ijms241210283
Chicago/Turabian StyleIbrahim, Mohamed Yousif, Najihah Mohd Hashim, Fatima Abdelmutaal Ahmed Omer, Muhammad Salisu Abubakar, Hoyam Adam Mohammed, Suzy Munir Salama, and Soher Nagi Jayash. 2023. "Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells" International Journal of Molecular Sciences 24, no. 12: 10283. https://doi.org/10.3390/ijms241210283
APA StyleIbrahim, M. Y., Hashim, N. M., Omer, F. A. A., Abubakar, M. S., Mohammed, H. A., Salama, S. M., & Jayash, S. N. (2023). Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells. International Journal of Molecular Sciences, 24(12), 10283. https://doi.org/10.3390/ijms241210283







