Abstract
Melanin is a complex natural pigment that is widely present in fungi. The mushroom Ophiocordyceps sinensis has a variety of pharmacological effects. The active substances of O. sinensis have been extensively studied, but few studies have focused on the O. sinensis melanin. In this study, the production of melanin was increased by adding light or oxidative stress, namely, reactive oxygen species (ROS) or reactive nitrogen species (RNS), during liquid fermentation. Subsequently, the structure of the purified melanin was characterized using elemental analysis, ultraviolet-visible absorption spectrum, Fourier transform infrared (FTIR), electron paramagnetic resonance (EPR), and pyrolysis gas chromatography and mass spectrometry (Py-GCMS). Studies have shown that O. sinensis melanin is composed of C (50.59), H (6.18), O (33.90), N (8.19), and S (1.20), with maximum absorbance at 237 nm and typical melanin structures such as benzene, indole, and pyrrole. Additionally, the various biological activities of O. sinensis melanin have been discovered; it can chelate heavy metals and shows a strong ultraviolet-blocking ability. Moreover, O. sinensis melanin can reduce the levels of intracellular reactive oxygen species and counteract the oxidative damage of H2O2 to cells. These results can help us to develop applications of O. sinensis melanin in radiation resistance, heavy metal pollution remediation, and antioxidant use.
1. Introduction
Melanin is a dark-colored polymer of phenols and indoles that is widely distributed among animals, plants, and microorganisms. Melanin can be categorized into eumelanin, phaeomelanin, and allomelanin based on its color and elemental composition [1]. Eumelanin is black or dark brown, contains nitrogen elements but not sulfur elements, and mainly exists in microorganisms and the eyes and fur of animals; phaeomelanin is red, brown, or yellow, contains nitrogen and sulfur elements, and mainly exists in animal hair; allomelanin is black or brown, sometimes contains a small amount of nitrogen, and generally exists only in plants and fungi [2,3,4]. The precursors of melanin include DOPA, DHN (1,8-dihydroxynaphthalene), 5,6-dihydroxyindole, catechol, and HGA (homogentisic acid), it has extremely complex and diverse chemical structures [5].
Melanin contains multiple functional groups and complex structures, which help to resist extreme temperature, ultraviolet (UV) radiation, heavy metal ions, and other environmental stresses, and is widely used in the fields of food, cosmetics, optoelectronic biomaterials, and ecological restoration [6,7]. Various studies have reported many biological activities of melanin. Melanin isolated from Lachnum YM226 lowered blood lipid levels and showed antitumor activity in a mouse model [8]. The melanin synthesized from Inonotus hispidus has a strong antioxidant capacity, which can significantly reduce reactive oxygen species (ROS) in LO2 cells and thus protect the liver from oxidative damage [9]. Melanin also has antibacterial, anti-radiation, DNA protection, and other physiological activities [10,11,12].
The requirement for melanin has increased rapidly due to its extensive biological activity [1,13]. Through microbial fermentation, melanin can be extracted quickly, cheaply, and on a large scale to meet market demands [5,14]. Fungi, especially edible and medicinal fungi (mushrooms), are excellent sources of natural melanin because they are safe to consume [15]. Ophiocordyceps sinensis, a fungus belonging to Ascomycota, Sordariomycetes, Hypocreales, Ophiocordycipitaceae, and Ophiocordyceps, is the anamorph of Chinese cordyceps and a precious Chinese traditional medicine. O. sinensis has many pharmacological effects such as antioxidative, anti-inflammatory, hypoglycemic, and immunomodulatory activities [16,17,18,19]. Previous studies have found that O. sinensis can synthesize melanin during liquid fermentation; it shows good antioxidant activity against 2,2-diphenyl-1-picryl-hydrazyl (DPPH) and Fe2+ chelating activity [20]. However, the structure of O. sinensis melanin has not been analyzed in detail, and its biological activity needs to be further explored.
In this study, we showed that the production of O. sinensis melanin was significantly increased by inducing NO stress with sodium nitroferricyanide dihydrate (SNP). The melanin structure was characterized using full wavelength scanning absorption spectroscopy, Fourier transform infrared spectroscopy (FTIR), elemental analysis, and pyrolysis gas chromatography and mass spectrometry (Py–GCMS). The data on the structure of melanin can help us to better understand its physiological activity. The structure of melanin varies among different species, resulting in different physiological functions. This study found that the structure of O. sinensis melanin is complex; in addition, we preliminarily demonstrated that O. sinensis melanin has multiple biological and physiological activities, such as blocking UV radiation, chelating metal ions, and clearing intracellular ROS to inhibit cell apoptosis.
2. Results
2.1. Induction Conditions of Melanin
O. sinensis proliferated in the form of hyphal aggregates in OS1 medium. The hyphal aggregates were white in the dark, and the color of the culture medium did not appreciably change. The color of the hyphal aggregates and the culture medium considerably darkened under light-induced circumstances (Figure 1A). Similarly, the addition of H2O2 and SNP, which induce ROS and reactive nitrogen species (RNS) oxidative stress, could also stimulate the production of melanin. After the fermentation products under different conditions were extracted for melanin, the absorbance was measured at 237 nm. The results showed that the applying of RNS stress significantly promoted melanin yield (Figure 1B).
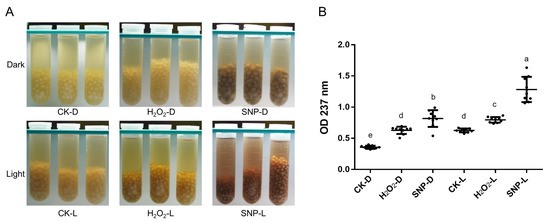
Figure 1.
Effects of light and oxidative stress on melanin synthesis. (A) Color phenotypes of fermented liquid and mycelial pellets under light-induced and oxidative stress (100 μmol/L H2O2 or SNP). (B) Quantitative analysis of melanin yield under different induction conditions. Each point represents one experimental datum, and each group consists of three biological replicates and three technical replicates. D and L represent dark and light conditions, respectively. Different lowercase letters indicate significant differences between groups (p < 0.05).
2.2. Elemental Analysis
Despite the structure complexity of fungal melanin, it can be generally categorized depending on the composition and content of elements. The elemental analysis of O. sinensis and other fungi melanin are shown in Table 1. The percentages of C, H, O, N, and S in O. sinensis melanin were 50.59%, 6.18%, 33.90%, 8.19%, and 1.20%, respectively.

Table 1.
Comparison of elemental analysis of O. sinensis melanin and other fungi.
Melanin extracted from O. sinensis, Ganoderma lucidum, and Boletus griseus were similar and contained a small amount of S; they are likely to be eumelanin. But O. sinensis melanin contained lower C/N, C/H, and C/O, indicating that it may contain more heterocycles or fatty groups.
2.3. Spectral Characterization
The UV-visible spectrum of O. sinensis melanin exhibited strong optical absorbance in the UV region, and the maximum absorption peak was observed at 237 nm (Figure 2A). In addition, the log of the optical density of O. sinensis melanin against its wavelengths produced a linear curve with a negative slope of −0.0031, which is consistent with the absorption characteristics of melanin (Figure 2B), indicating that the purity of the extracted melanin was high.
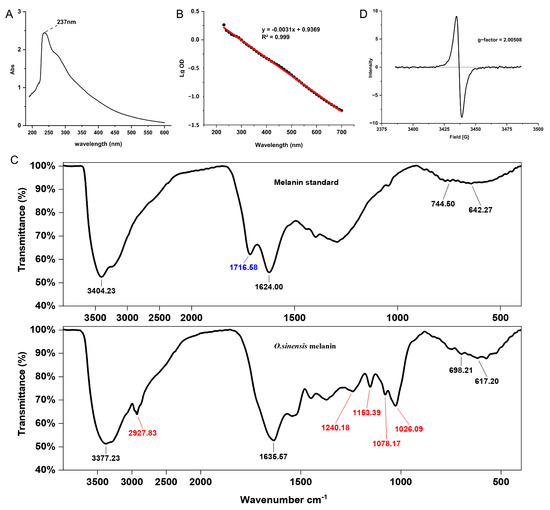
Figure 2.
The spectroscopy analysis of O. sinensis melanin. (A) UV-visible absorption spectra. (B) The lg value of absorbance at different wavelengths. The black dots represent the measured data points, and the red line represents the linear fitting result. (C) Fourier transform infrared spectra. (D) Electron paramagnetic resonance spectra.
The FTIR spectrum of O. sinensis melanin and qualitative standard displayed several typical spectral bands (Figure 2C). The strong and broad complex bands at 3400 cm−1 corresponded to the -NH group connected to the -OH group of the indole ring, which is the characteristic group of melanin. Compared with the qualitative standard, O. sinensis melanin had a unique absorption peak at 2930–2830 cm−1, combined with the weak absorptive peak at 1240 cm−1, indicating an asymmetric vibration of -CH2. The peak at 1720–1715 cm−1 corresponded to ketones, indicating that the standard substance contains C=O, while O. sinensis melanin does not contain this group. The peak at 1650–1620 cm−1 corresponded to the asymmetric deformation vibration of C=O and N-H in amide, indicating the presence of a substituted carboxyl. The weak peaks at 1150 cm−1 corresponded to the amino group C-N. The peak at 1078 cm−1 corresponded to the stretching vibration of C-O in phenol or carboxyl. Because the O. sinensis melanin contains the element S, it may also be a sulfonic acid group -SO3H. The peak at 1026 cm−1 corresponded to the skeletal vibration of C-CH3. The peaks at 800–600 cm−1 were weak, indicating that the aromatic ring was replaced to form a conjugated system with low aromatic hydrogen. The above characteristics indicate that the infrared spectrum of O. sinensis melanin obtained through extraction and purification conforms to the structural characteristics of traditional melanin.
The EPR spectrum of O. sinensis melanin showed a strong single slightly asymmetric line, indicating the presence of stable organic free radicals, and the g-factor was 2.00508 (Figure 2D).
2.4. Py-GCMS Analysis
Py-GCMS can provide monomer information about complex compounds from their pyrolysis products; therefore, it is perfectly suited for the structural characterization of melanin [25]. In this study, Py-GCMS was used to further characterize the structure of O. sinensis melanin, and a total of 24 compounds were identified (Table 2). Benzene, indole, and pyrrole were detected in O. sinensis melanin (components 4 and 10), which are the characteristic products of the thermal decomposition of eumelanin [26]. Moreover, furan was detected in O. sinensis melanin (component 19), which is similar to B. griseus melanin [25]. In addition, the high abundance of alkanes in the thermal cracking products of O. sinensis melanin indicates there are more saturated bonds, which is consistent with the lower C/N and C/H in the elemental analysis (Table 1).

Table 2.
The Py-GCMS products of O. sinensis melanin.
2.5. Ability to Chelate Metal Ions
The ability to chelate metal ions of O. sinensis melanin was explored through coincubation experiments (Figure 3A). It was found that the inhibiting effect of metal solutions on S. cerevisiae cell growth was greatly diminished following O. sinensis melanin incubation (Figure 3B). Furthermore, the metal ion concentration in the preincubation and postincubation supernatant metal solutions and precipitate melanin solutions (dissolved in DMSO) was detected; the results showed that melanin can transfer metal ions from supernatant metal solutions to precipitate melanin solutions (dissolved in DMSO) through chelation (Figure 3C).
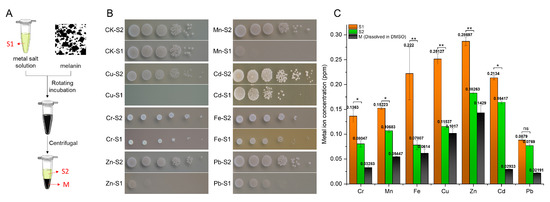
Figure 3.
The metal-ion-chelating ability of O. sinensis melanin. (A) Schematic diagram of metal ion chelation experiment. (B) Inhibition effect of the metal solution on S. cerevisiae growth. (C) Concentration of metal ions in each component during the incubation experiment. Values represent the mean ± SD of three experiments; ANOVA was used to compare the concentration of metal ions in the solution of melanin before (S1) and after (S2) incubation. (* p < 0.05; ** p < 0.01; ns, no significant difference).
2.6. UV-Blocking Activity
S. cerevisiae was mixed with melanin solution (0.1% DMSO as a control), and irradiated under UV light at different times (Figure 4A). The survival rate of yeast cells was used to evaluate the resistance to UV radiation. The result showed that, after 20 min of UV radiation, the survival rate of the control group decreased significantly, and all the yeast cells died after 3 h exposure to UV light. In contrast, the survival of yeast cells mixed with O. sinensis melanin was essentially unaffected; its survival rate still kept comparable to the unirradiated group even after 3 h of UV irradiation. The results indicated that melanin can block the damage of UV radiation and free radicals caused by UV-generated ozone (Figure 4B).
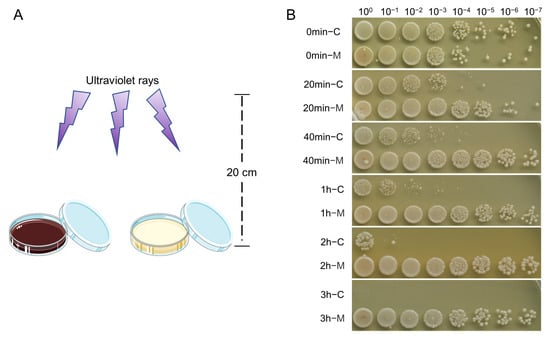
Figure 4.
The UV-blocking ability of O. sinensis melanin. (A) Schematic diagram of UV-blocking experiment. (B) Vitality of S. cerevisiae cells at different times of UV radiation.
2.7. Antioxidant Activity
The free-radical-scavenging assay of O. sinensis melanin is shown in Figure 5A. Both DPPH and ABTS free radicals could be scavenged by O. sinensis melanin, and the activity was dose-dependent. In this assay, the radical-scavenging activity of O. sinensis melanin was lower than that of Trolox.
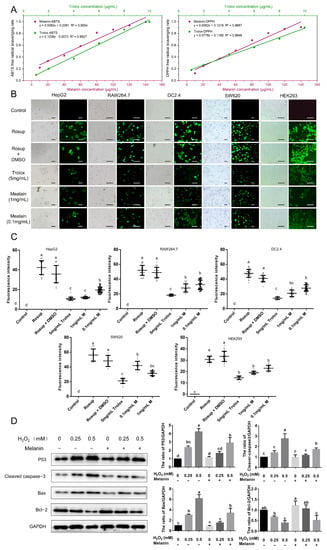
Figure 5.
Antioxidant activity of O. sinensis melanin. (A) ABTS and DPPH radical scavenging activities. (B) The scavenging effect of O. sinensis melanin on intracellular ROS; green fluorescence indicates DCFH-DA labeled ROS. Bar = 20 μm. (C) Quantification and statistical analysis of the scavenging effect of O. sinensis melanin on intracellular ROS. (D) Preincubation with O. sinensis melanin reduces the apoptosis induced by H2O2 in HEK293 cells. Different lowercase letters indicate significant differences between groups (p < 0.05).
Moreover, the activity of O. sinensis melanin in removing intracellular ROS was examined using five cell lines, and the level of intracellular ROS was shown by the DCF fluorescence intensity. The use of an active oxygen inducer (Rosup) dramatically enhanced intracellular ROS, and when the cells were preincubated with O. sinensis melanin, the levels of intracellular ROS were greatly inhibited, and this action was dose-dependent (Figure 5B). The quantitative analysis of DCF fluorescence intensity revealed that O. sinensis melanin has a stronger ability to neutralize intracellular ROS than Trolox, indicating a strong antioxidant capacity (Figure 5C).
It is well established that treatment of HEK293 cells with H2O2 causes an increase in ROS, which lead to apoptosis. To analyze the protective effect of O. sinensis melanin on H2O2-mediated cell damage, Western blotting was used to detect the expression of the apoptotic-related proteins. The results showed that the expression of the apoptosis-inhibitory protein Bcl-2 decreased after H2O2 treatment, while pretreatment with O. sinensis melanin could restore the expression level of Bcl-2 (Figure 5D). In contrast, the apoptosis-related protein P53, the apoptosis-marker-cleaved caspase-3, and the proapoptotic protein Bax were down-regulated after being pretreated with O. sinensis melanin. These results indicate that O. sinensis melanin can exert antiapoptotic effects by neutralizing ROS.
3. Discussion
O. sinensis is an important edible medicinal fungus; its active substances such as polysaccharides and peptides have been extensively studied [17,19], but few studies have focused on the medicinal value of O. sinensis melanin. Melanin is a crucial part of the fungal cell structure and has a variety of physiological and biochemical functions [14]. Numerous fungi, including I. hispidus, B. griseus, and Auricularia auricula, have been used to produce melanin, and their structures and functions have been studied [9,25,27]. Reports of preliminary studies on the structure and function of O. sinensis melanin indicate that it shows antioxidant activities [20]. In this study, we initially explored the induction conditions of O. sinensis melanin, and the structural characteristics of O. sinensis melanin were detected using elemental analysis, UV-visible absorption spectroscopy, FTIR spectroscopy, and Py-GCMS. In addition, we studied the biological activities of O. sinensis melanin such as chelating metal ions, UV blocking, and antioxidant properties.
The average yield of melanin varies greatly among different fungal species; for example, it is 0.54 mg/g in Phoma sp. RDSE17 and 0.938 g/L in Hortaea werneckii AS1 [22,28]. Generally, the production of fungi melanin is not very high; therefore, the culture medium and conditions need to be optimized to boost melanin production [29]. Aspergillus niger intensely accumulated melanin under light, suggesting that light can be used as an inducer to increase melanin production [30]. It is widely believed that fungi can perceive and respond to light signals. On the one hand, fungi can respond to light signals through photoreceptor proteins, thereby initiating the transcription of downstream genes. On the other hand, light stress can also directly induce intracellular ROS, leading to changes in the physiological balance of cells and thereby triggering a series of antagonistic reactions [31]. Similarly, in this study, the yield of O. sinensis melanin could be increased under light or oxidative stress (ROS and RNS).
Analysis of the chemical structure of melanin helps to understand its biological activity. It has been reported that fungi can synthesize allomelanin through the DHN pathway and synthesize pheomelanin (contains 9–12% S) and eumelanin (contains 0–1% S) through the DOPA pathway [32]. O. sinensis melanin contains 1.2% S, and the C/N ratio is 7.21, which conforms more to eumelanin. In the UV-visible absorption spectrum, O. sinensis melanin has maximum absorption values at 237 nm, and a plot of the log of absorption against wavelength is a straight line. The FTIR spectroscopy showed the characteristic absorption peaks of O. sinensis melanin. Combined with the Py-GCMS results, we demonstrated that O. sinensis melanin contains benzene, indole, pyrrole, and furan, which are the most significant thermal degradation products of eumelanin [25]. Compared with dopa melanin standards, O. sinensis melanin contains more saturated bonds, which is consistent with the lower C/N and C/H ratios in the elemental analysis. Through elemental analysis, the results showed that O. sinensis melanin has a high N element content and a very low C/N ratio when compared with other fungal melanin. In conjunction with Py-GCMS, we found that there are a lot of -NH2 and -NH in O. sinensis melanin. As -NH2 is an active and easily oxidized group, these results further indicated the potent antioxidant properties of O. sinensis melanin.
Melanin contains numerous functional groups, such as -OH, -COOH, and -NH, which make it an ideal choice for heavy metal remediation [33]. Therefore, melanin can fix metal ions through chelation to reduce the harm caused by heavy metals to organisms. Azotobacter chrooccum can synthesize dark brown melanin, which can chelate Cd2+, Cr2+, and Ni2+. Melanin not only makes A. chrooccum highly metal tolerant but also protects plants in heavy-metal-polluted environments [34]. Owing to the diversity of its structure, different melanin has different characteristics for adsorbing metal ions. Pseudomonas stutzeri melanin has a higher adsorption capacity for Cu2+ and Pb2+ than Hg2+, while Ommastrephes bartrami melanin has a higher adsorption capacity for Pb2+ and Cd2+ [35,36]. The adsorption ability of O. sinensis melanin for seven heavy metals was investigated in this study, and the results indicated that O. sinensis melanin has the best adsorption capacity for Pb2+, Cd2+, and Mn2+, but a poor adsorption capacity for Fe2+ and Cu2+.
In general, melanin exists in the form of insoluble granular particles and is localized on the fungal cell wall. Numerous studies of melanin using microscopy demonstrated that melanin is cross-linked with polysaccharide components to form the components of spore walls. This is also the reason why high-temperature and strong alkaline treatments are necessarily used to separate melanin from the stable spore wall structure [37,38]. Studies have shown that melanin is stored as granules within the reticular scaffold of chitin aggregation; therefore, the mutation of chitin synthase will lead to the disruption of the structure of the fungal cell wall and the release of melanin into the culture medium [39,40]. When O. sinensis melanin is induced, the color of the culture medium will change from faint yellow to black; this indicates that some melanin is secreted into the culture medium. The secretory melanin is likely to have other functions due to their water solubility; we mainly focused on the melanin which functions as the structural components of the O. sinensis in this study.
O. sinensis is naturally distributed on the high-altitude Qinghai–Tibet Plateau, where the UV level is very high because of the high altitude. Melanin pigments can protect fungi cells against UV radiation because of their ability to absorb a broad spectrum of electromagnetic waves [5]. In this study, the yeast experiments demonstrated that O. sinensis melanin has a strong UV-blocking ability, which gives it the potential to be applied in sunscreens. In addition to causing DNA damage, UV radiation can also cause harm to organisms by inducing carcinogenic ROS. The water-insoluble melanin derived from I. hispidus can clear intracellular ROS to enhance the cell viability of LO2 liver cells under H2O2 treatment, and shows strong antioxidant activity [9]. O. sinensis melanin also exhibits similar activity, which can neutralize ROS in various cells and inhibit cell apoptosis induced by H2O2 in HEK293 cells. It is generally accepted that the antioxidant activity of O. sinensis is attributed to peptides and polysaccharides. In this study, the cell experiments showed that O. sinensis melanin also has a strong ability to counteract intracellular ROS and its harmful activities, indicating that O. sinensis melanin is also one of the active components of O. sinensis that cannot be ignored.
4. Materials and Methods
4.1. Strain Cultivation and Melanin Extraction
O. sinensis was isolated from the wild Chinese cordyceps (Kangding, Sichuan, China), and preserved at the State Key Laboratory of Resource Insects (Southwest University, Beibei, Chongqing, China). O. sinensis was activated and cultivated in PDB medium (20% potato infusion and 2% glucose), shaken at 16 °C and 100 rpm for 40 days. The blastospore suspension was collected as seed solution with lens wiping paper and inoculated in OS1 liquid medium (20% potato infusion, 2% glucose, 1% yeast extract, 0.5% peptone, 0.1% KH2PO4, and 0.025% MgSO4), and cultured at 16 °C and 120 rpm. Light stress, ROS stress (induced using 100 μmol/L H2O2), and RNS stress (induced using 100 μmol/L sodium nitroprusside, SNP) were applied to observe the formation of melanin.
After fermentation, NaOH was used to bring the pH to 14 and mixed overnight at 100 °C using a magnetic stirrer. Afterward, the supernatant solution was collected using centrifugation at 10,000× g for 30 min, and then HCl solution (37%) was used to adjust the pH to 2 for 12 h. The crude melanin was collected using centrifugation at 10,000× g for 30 min and washed with ddH2O to pH = 7. This step was repeated three times to remove the proteins, carbohydrates, and lipids associated with melanin. Similarly, dichloromethane, ethyl acetate, methanol, ethanol, and water were used to remove impurities. Pure melanin was obtained using freeze-drying.
4.2. Elemental Analysis
The percentage contents of C, H, N, S, and O of 2 mg melanin were determined using an elemental analyzer (Thermo Scientific FlashSmartTM, Waltham, MA, USA).
4.3. Spectroscopy Analysis
4.3.1. UV-Visible Absorption Spectrum
The O. sinensis melanin was dissolved in 0.1 M NaOH and the UV-visible absorption spectrum was scanned in the wavelength range of 190–600 nm by SpectraMax® plus384 (Molecular Devices, Shanghai, China).
4.3.2. Fourier Transform Infrared (FTIR)
Melanin extracted from O. sinensis and qualitative standard (M832392-25mg, Macklin, Shanghai, China) was mixed with KBr (1:200) and pressed into tablets. Then, samples were scanned with FTIR (PE Spectrum Two, Waltham, MA, USA) in the scanning range of 4000–400 cm−1.
4.3.3. Electron Paramagnetic Resonance (EPR)
The O. sinensis melanin was placed in a quartz tube and the EPR analysis was recorded using EMX Nano10602 (Bruker, Germany) at a temperature of 25 °C and a frequency of 9.645 GHz.
4.4. Pyrolysis Gas Chromatography and Mass Spectrometry (Py-GCMS)
The O. sinensis melanin was lysed using the Frontier EGA/PY3030D thermal cracking apparatus (Frontier, Fukushima, Japan), and the products were analyzed using GCMS-QP2020 (SHIMADZU, Kyoto, Japan). The NIST database was used for qualitative searching.
The analysis conditions were as follows: the pyrolyzer was set at 550 °C. The GC/MS analytical column was TG-5silMS 30 m × 0.25 mm × 0.25 µm. The GC oven temperature was operated from 60 °C (isothermal for 2 min) to 320 °C at a rate of 20 °C/min, and then kept isothermal for 13 min. The GC injector was maintained at 230 °C. The ionization method was electron ionization, and the ionization source temperature was 230 °C. The acquisition method was “scan” and the mass range was m/z 29–600 amu.
4.5. Biological Activity
4.5.1. Ability to Chelate Metal Ions
Metal salt solutions (S1) of 20 mM CrCl3‧6H2O, MnCl2‧4H2O, FeSO4‧7H2O, CuSO4, ZnCl2, CdCl2‧5/2H2O, and Pb(Ac)2‧3H2O were each combined with 1 mg of melanin powder and incubated at 24°C overnight. Next, each mixture was centrifuged at 10,000× g for 10 min, the supernatant (S2) and precipitate (M) were collected, and the precipitate was dissolved in dimethyl sulfoxide (DMSO). Solution S1 or S2 was mixed in a YPD agar plate at a volume ratio of 1:15, and Saccharomyces cerevisiae was inoculated to observe the stress effect of the metal ions.
The metal ion concentrations in the preincubation (S1) and postincubation (S2) metal salt solutions and the postincubation melanin precipitation (DMSO dissolution) were determined using an atomic absorption spectrophotometer (ZA3300 FLAME AAS, Hitachi, Japan).
4.5.2. UV-Blocking
S. cerevisiae was cultured in a YPD medium to an OD600 = 1.0, and the cells were harvested via centrifugation at 700× g for 5 min, washed twice with sterile water, and resuspended in sterile water to an OD600 = 1.5. The yeast suspension was placed in a sterile culture dish, melanin (dissolved in DMSO) was added to the final concentration of 0.1 mg/mL, and an equal volume of DMSO was added as a control. The two groups of yeast were placed under UV light (F8T5GL-Z287 15w, Forbens, Guangzhou, China), irradiated for different times, subjected to 10 times gradient dilution, and then dripped onto a YPD plate medium. The plates were placed at 28 °C.
4.5.3. Free-Radical-Scavenging Ability
The ability of O. sinensis melanin to scavenge free radicals was measured using the DPPH method, and the procedure was adapted from Surendirakumar et al. [23]. Briefly, different concentrations of O. sinensis melanin were prepared with DMSO, and 0.05 mg/mL DPPH (ethanol solution) was mixed at a volume ratio of 1:1. After incubation in the dark at 24 °C for 30 min, a spectrophotometer (SpectraMax i3x, Molecular Devices, Shanghai, China) was used to read the absorbance at 517 nm. Trolox was used as a control. The antioxidant activity of O. sinensis melanin was also examined using the ABTS method as described by Khemakhem et al. [41]. Approximately 7.4 mM ABTS storage solution and 2.6 mM K2S2O8 storage solution were prepared, mixed with equal volumes, and reacted at 4 °C for 12–15 h (protected from light), and then diluted with ethanol to OD734 = 0.7 to obtain the ABTS working solution. The samples and ABTS working solution were mixed at a volume ratio of 1:4, kept in the dark at 24 °C for 30 min to react, and a spectrophotometer (SpectraMax i3x, Molecular Devices, Shanghai, China) was used to read the absorbance at 714 nm.
The ability to scavenge the DPPH (or ABTS) radical was calculated using the following equation:
where A0: DPPH (or ABTS)+ DMSO; A1: DPPH (or ABTS)+ melanin; and A2: melanin + ethanol.
Scavenging rate (%) = [1 − (A1 − A2)/A0] × 100
4.5.4. Antioxidant Activity on Cells
Human hepatocellular carcinoma (HepG2) cell line (HB-8065, ATCC), mouse leukemia cells of monocyte-macrophage (RAW264.7) cell line (TIB-71, ATCC), dendritic (DC2.4) cell line (HTX2245, ATCC), human colon cancer (SW620) cell line (CCL-227, ATCC), and human embryonic kidney 293 (HEK293) cell line (CRL-1573, ATCC) were purchased from the American Type Culture Collection and grown in complete growth medium supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C with 5% CO2.
The intracellular ROS levels were measured using a ROS assay kit (Beyotime Biotechnology, Nantong, China). Briefly, the cells were inoculated into 24-well plates at 105 cells per well, and melanin, DMSO (solvent control), and Trolox (positive control) were added. After 6 h, 50 μg/mL Rosup was used to induce ROS. Following the treatment, the cells were incubated with DCFH-DA for 20 min at 37 °C and then observed using fluorescence microscopy (ZESS Axio Observer A1, Oberkochen, Germany). The relative fluorescence intensity was then calculated and analyzed using GraphPad Prism (version 6.01) for the t-test.
The HEK293 cells were inoculated into six-well plates at 2 × 105 cells per well, and melanin and DMSO (solvent control) were added. Following the method of Bian et al. [42], after 12 h, 0.5 mM and 1 mM H2O2 were used to induce oxidative damage to the cells. Western blotting was used to detect P53 (anti-P53, sc-126, Santa Cruz, Dallas, TX, USA), caspase-3 (anti-caspase-3, AF1213, Beyotime, China), Bax (anti-P53, sc-20067, Santa Cruz Dallas, TX, USA), and Bcl-2 (anti-Bcl-2, sc-7382, Santa Cruz Dallas, TX, USA) to characterize the apoptosis of the cells. Specifically, the cells were collected using centrifugation at 600× g for 5 min and lysed in RIPA buffer with a protease inhibitor cocktail (P1050, Beyotime, China). The cell lysates were centrifuged for 15 min at 12,000× g and 4 °C to sediment cell debris after 10 min incubation on ice. The total proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and blocked with 5% skimmed milk for 1 h. The membranes were then probed with primary antibodies overnight at 4 °C, followed by the secondary antibody for 2 h at room temperature. The electrochemical luminescence reagent (Thermo Fisher Scientific, USA) was used for immune detection and visualization using the Azure Biosystems C300 imaging system (Azure Biosystems, Dublin, CA, USA). GAPDH (anti-GAPDH, AF1186, Beyotime) was used as a control. The developed Western blot bands were measured using ImageJ (1.52V), and the values were analyzed using GraphPad Prism (version 6.01) for the t-test.
4.6. Statistical Analysis
The experimental data were analyzed using OriginPro (2022b SR1 9.9.5.171) statistical software. The measured data (x ± SD) are presented as the mean ± standard deviation. The significance of the data was analyzed using a one-way analysis of variance; p < 0.05 represents a significant difference.
5. Conclusions
In this study, O. sinensis melanin was obtained through liquid fermentation, and its structure and biological activity were studied. The yield of melanin could be increased by applying light or oxidative stress (ROS or RNS) during liquid fermentation. O. sinensis melanin has a maximum absorption value of 237 nm and contains a small amount of the sulfur element. The elemental and Py-GCMS analysis results demonstrated that it contains the benzene, indole, and pyrrole groups, which conforms to the typical characteristics of true melanin. It was found that O. sinensis melanin has strong UV-blocking activity and broad-spectrum heavy-metal-chelating activity. In addition, our study demonstrated that O. sinensis melanin has strong in vitro antioxidant activity, which may eliminate intracellular ROS and lessen oxidative damage brought on by H2O2. This study enriched the characterization of O. sinensis melanin structure and explored its biological activity, providing a theoretical basis for its application such as radiation resistance, heavy metal pollution remediation, and antioxidant use.
Author Contributions
Conceptualization, C.T. and Z.Z.; methodology, C.X.; software, C.T.; data curation, J.L.; writing—original draft preparation, C.T. and C.L.; writing—review and editing, J.W.; visualization, C.T. and J.L.; project administration, C.L. and Z.Z.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Chongqing (cstc2021jcyj-cxtt0005) and the Fundamental Research Funds for the Central Universities to Southwest University (XDJK2018AA001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are provided in the manuscript.
Acknowledgments
We thank Yun Wang (Department of Cell Biology, College of Basic Medical Sciences, Army Medical University, China) for their critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, K.Y. Bioprocess of Microbial Melanin Production and Isolation. Front. Bioeng. Biotechnol. 2021, 9, 765110. [Google Scholar] [CrossRef] [PubMed]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Chang, M.; Cheng, Y.; Geng, X.; Meng, J.; Zhu, M. Comparison of Physicochemical and Biochemical Properties of Natural and Arginine-Modified Melanin from Medicinal Mushroom Ganoderma lucidum. J. Basic Microbiol. 2020, 60, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and Identification of Pigments from Oyster Mushrooms with Black, Yellow and Pink Caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial Melanin: Recent Advances in Biosynthesis, Extraction, Characterization, and Applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef]
- Leite, M.O.G.; Alves, D.A.; Lecocq, A.; Malaquias, J.B.; Delalibera, I.; Jensen, A.B. Laboratory Risk Assessment of Three Entomopathogenic Fungi Used for Pest Control toward Social Bee Pollinators. Microorganisms 2022, 10, 1800. [Google Scholar] [CrossRef]
- María Del Rosario, G.-A.; Julio, M.-A.; Sylvia Páz, D.-C.; Manuel Adrián, P.-S.; Gabriela, L.-A.; Edgar Alonso, R.-S.; Lorenzo Ulises, O.-M.; Francisco, D.-V. Soluble Melanins of the Randia Echinocarpa Fruit—Structural Characteristics and Toxicity. J. Food Biochem. 2019, 43, e13077. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Yang, L.; Hou, G.; Ye, M. Hypolipidemic Effect and Protection Ability of Liver-Kidney Functions of Melanin from Lachnum YM226 in High-Fat Diet Fed Mice. Food Funct. 2018, 9, 880–889. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.; Zhang, F.; Hu, X.; Yuan, Y.; Wu, X.; Fu, J. Differences between Water-Soluble and Water-Insoluble Melanin Derived from Inonotus hispidus Mushroom. Food Chem. X 2022, 16, 100498. [Google Scholar] [CrossRef]
- Chai, W.; Wei, Q.; Deng, W.; Zheng, Y.; Chen, X.; Huang, Q.; OuYang, C.; Peng, Y. Anti-Melanogenesis Properties of Condensed Tannins from Vigna angularis Seeds with Potent Antioxidant and DNA Damage Protection Activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef]
- Ghadge, V.; Kumar, P.; Singh, S.; Mathew, D.E.; Bhattacharya, S.; Nimse, S.B.; Shinde, P.B. Natural Melanin Produced by the Endophytic Bacillus subtilis 4NP-BL Associated with the Halophyte Salicornia brachiata. J. Agric. Food Chem. 2020, 68, 6854–6863. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-Melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. New Insight into Melanin for Food Packaging and Biotechnology Applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 4629–4655. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial Production of Melanin and Its Various Applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Martinez, A.; Gosset, G. Production of Melanins with Recombinant Microorganisms. Front. Bioeng. Biotechnol. 2019, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, A.; Yin, J.; Siu, K.; Wong, W.; Wu, J. Anti-Inflammation Activity of Exopolysaccharides Produced by a Medicinal Fungus Cordyceps sinensis Cs-HK1 in Cell and Animal Models. Int. J. Biol. Macromol. 2020, 149, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Li, L.; Zhou, X. Immunostimulatory Effects of the Intracellular Polysaccharides Isolated from Liquid Culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 Cells via the MAPK and PI3K/Akt Signaling Pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef]
- Lu, Q.; Li, C.; Chen, W.; Shi, Z.; Zhan, R.; He, R. Clinical Efficacy of Jinshuibao Capsules Combined with Angiotensin Receptor Blockers in Patients with Early Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. ECAM 2018, 2018, 6806943. [Google Scholar] [CrossRef]
- Tong, X.; Guo, J. High Throughput Identification of the Potential Antioxidant Peptides in Ophiocordyceps Sinensis. Molecules 2022, 27, 438. [Google Scholar] [CrossRef]
- Dong, C.; Yao, Y. Isolation, Characterization of Melanin Derived from Ophiocordyceps sinensis, an Entomogenous Fungus Endemic to the Tibetan Plateau. J. Biosci. Bioeng. 2012, 113, 474–479. [Google Scholar] [CrossRef]
- Ito, S.; Fujita, K. Microanalysis of Eumelanin and Pheomelanin in Hair and Melanomas by Chemical Degradation and Liquid Chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Surendirakumar, K.; Pandey, R.R.; Muthukumar, T.; Sathiyaseelan, A.; Loushambam, S.; Seth, A. Characterization and Biological Activities of Melanin Pigment from Root Endophytic Fungus, Phoma sp. RDSE17. Arch. Microbiol. 2022, 204, 171. [Google Scholar] [CrossRef]
- Prados-Rosales, R.; Toriola, S.; Nakouzi, A.; Chatterjee, S.; Stark, R.; Gerfen, G.; Tumpowsky, P.; Dadachova, E.; Casadevall, A. Structural Characterization of Melanin Pigments from Commercial Preparations of the Edible Mushroom Auricularia auricula. J. Agric. Food Chem. 2015, 63, 7326–7332. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Kamat, N.M.; Nadkarni, V.S. Purification and Characterisation of a Sulphur Rich Melanin from Edible Mushroom Termitomyces albuminosus Heim. Mycology 2018, 9, 296–306. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, J.; Liu, B.; Zhuang, Y.; Sun, L. Study on the Preparation and Chemical Structure Characterization of Melanin from Boletus griseus. Int. J. Mol. Sci. 2018, 19, 3736. [Google Scholar] [CrossRef]
- Dzierzega-Lecznar, A.; Kurkiewicz, S.; Stepien, K.; Chodurek, E.; Riederer, P.; Gerlach, M. Structural Investigations of Neuromelanin by Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Neural Transm. 2006, 113, 729–734. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Wang, D.; Mai, M.; Wu, X.; Zheng, M.; Fu, J. Comprehensive Utilization of Edible Mushroom Auricularia auricula Waste Residue-Extraction, Physicochemical Properties of Melanin and Its Antioxidant Activity. Food Sci. Nutr. 2019, 7, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Elsayis, A.; Hassan, S.W.M.; Ghanem, K.M.; Khairy, H. Optimization of Melanin Pigment Production from the Halotolerant Black Yeast Hortaea werneckii AS1 Isolated from Solar Salter in Alexandria. BMC Microbiol. 2022, 22, 92. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of Melanin and Optimal Conditions for Pigment Production by an Endophytic Fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef]
- Sun, W.; Yu, Y.; Chen, J.; Yu, B.; Chen, T.; Ying, H.; Zhou, S.; Ouyang, P.; Liu, D.; Chen, Y. Light Signaling Regulates Aspergillus niger Biofilm Formation by Affecting Melanin and Extracellular Polysaccharide Biosynthesis. mBio 2021, 12, e03434-20. [Google Scholar] [CrossRef]
- Zhao, Y.; Lim, J.; Xu, J.; Yu, J.-H.; Zheng, W. Nitric Oxide as a Developmental and Metabolic Signal in Filamentous Fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, M.; Huang, H.; Wen, Y.; Zhang, X.; Wei, Y. Recent Advances and Progress on Melanin-like Materials and Their Biomedical Applications. Biomacromolecules 2018, 19, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Thaira, H.; Raval, K.; Manirethan, V.; Balakrishnan, R.M. Melanin Nano-Pigments for Heavy Metal Remediation from Water. Sep. Sci. Technol. 2019, 54, 265–274. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Bioreduction of Toxicity Influenced by Bioactive Molecules Secreted under Metal Stress by Azotobacter chroococcum. Ecotoxicology 2019, 28, 302–322. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Wang, J.; Feng, H.; Wang, Y.; Ma, Q.; Wang, D. Adsorption of Pb(II) and Cd(II) by Squid Ommastrephes bartrami Melanin. Bioinorg. Chem. Appl. 2009, 2009, 901563. [Google Scholar] [CrossRef]
- Manirethan, V.; Raval, K.; Rajan, R.; Thaira, H.; Balakrishnan, R.M. Kinetic and Thermodynamic Studies on the Adsorption of Heavy Metals from Aqueous Solution by Melanin Nanopigment Obtained from Marine Source: Pseudomonas stutzeri. J. Environ. Manag. 2018, 214, 315–324. [Google Scholar] [CrossRef]
- Chrissian, C.; Camacho, E.; Fu, M.S.; Prados-Rosales, R.; Chatterjee, S.; Cordero, R.J.B.; Lodge, J.K.; Casadevall, A.; Stark, R.E. Melanin Deposition in Two Cryptococcus Species Depends on Cell-Wall Composition and Flexibility. J. Biol. Chem. 2020, 295, 1815–1828. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Nosanchuk, J.D.; Webber, J.B.W.; Emerson, R.J.; Camesano, T.A.; Casadevall, A. Microstructure of Cell Wall-Associated Melanin in the Human Pathogenic Fungus Cryptococcus neoformans. Biochemistry 2005, 44, 3683–3693. [Google Scholar] [CrossRef]
- Perez-Dulzaides, R.; Camacho, E.; Cordero, R.J.B.; Casadevall, A. Cell-Wall Dyes Interfere with Cryptococcus neoformans Melanin Deposition. Microbiol. Read. Engl. 2018, 164, 1012–1022. [Google Scholar] [CrossRef]
- Walton, F.J.; Idnurm, A.; Heitman, J. Novel Gene Functions Required for Melanization of the Human Pathogen Cryptococcus neoformans. Mol. Microbiol. 2005, 57, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, M.; Papadimitriou, V.; Sotiroudis, G.; Zoumpoulakis, P.; Arbez-Gindre, C.; Bouzouita, N.; Sotiroudis, T.G. Melanin and Humic Acid-like Polymer Complex from Olive Mill Waste Waters. Part I. Isolation and Characterization. Food Chem. 2016, 203, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Guo, J.; Majeed, H.; Zhu, K.; Guo, X.; Peng, W.; Zhou, H. Ferulic Acid Renders Protection to HEK293 Cells against Oxidative Damage and Apoptosis Induced by Hydrogen Peroxide. Vitro Cell. Dev. Biol. Anim. 2015, 51, 722–729. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).