Multifunctional Oxidized Dextran as a Matrix for Stabilization of Octahedral Molybdenum and Tungsten Iodide Clusters in Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
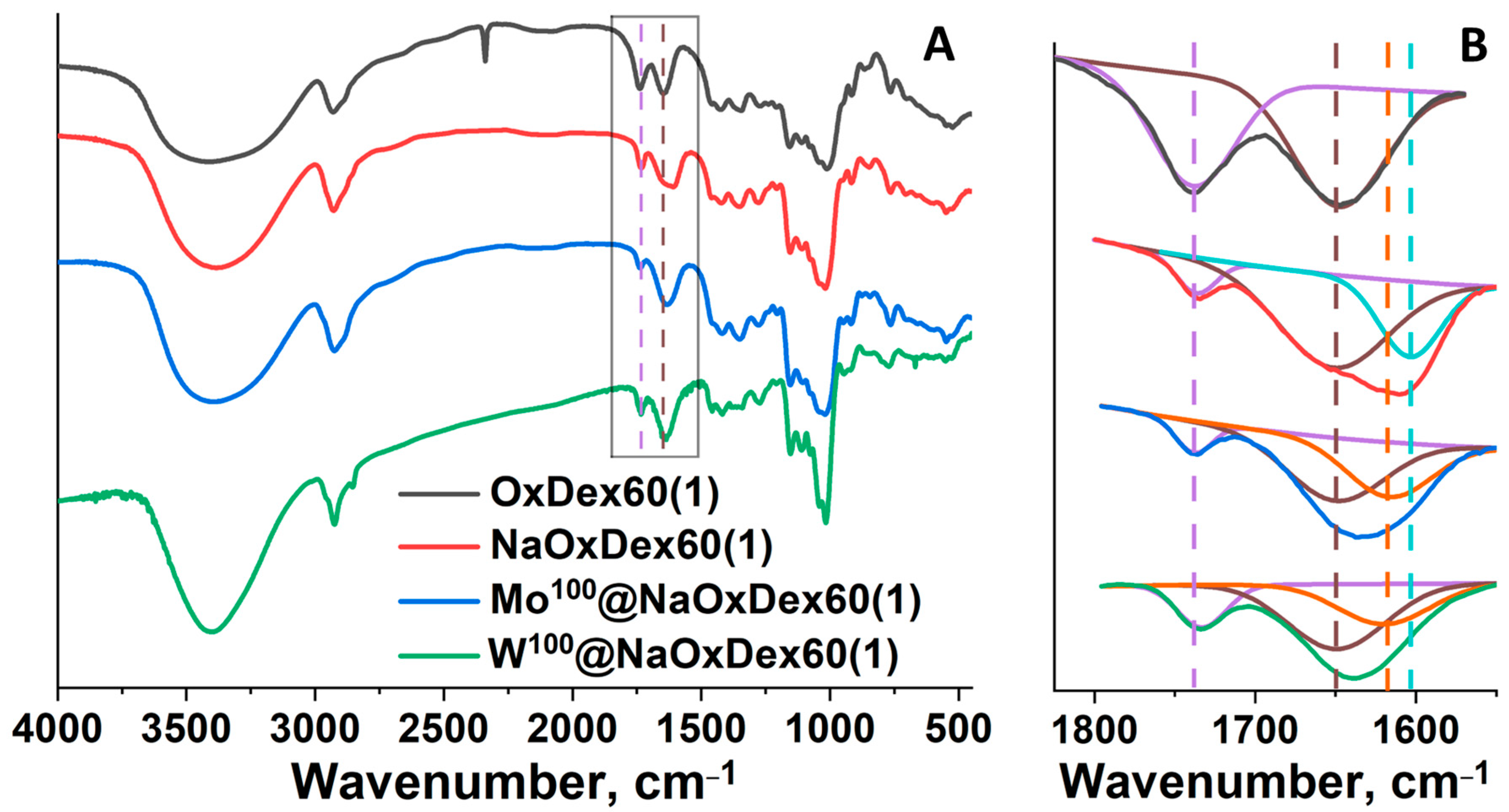
2.1. Preparation and Characterization of Mx@DexQ and Mx@NaOxDexQ(n)
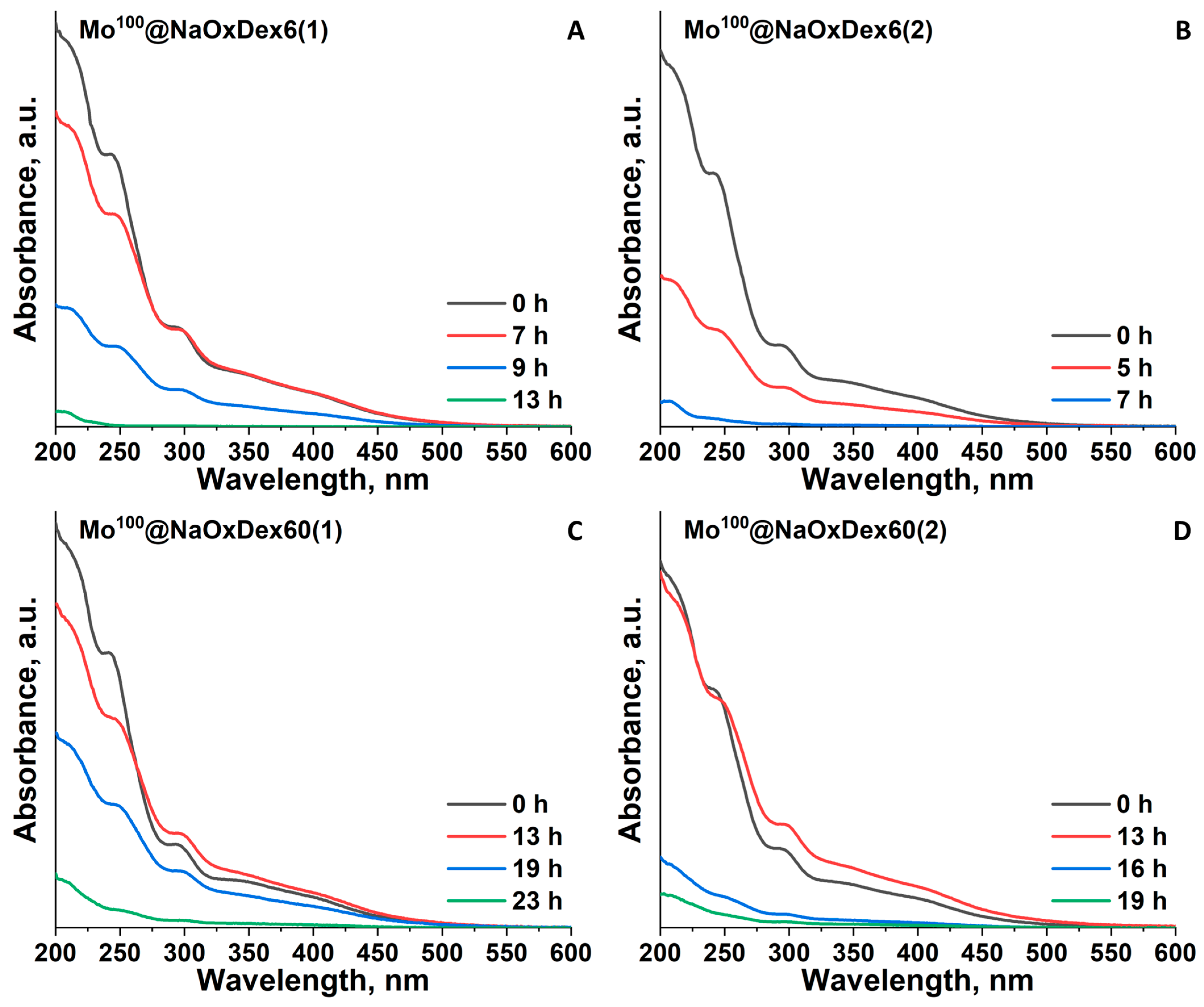
2.2. Stability of the Materials in Water and Culture Medium
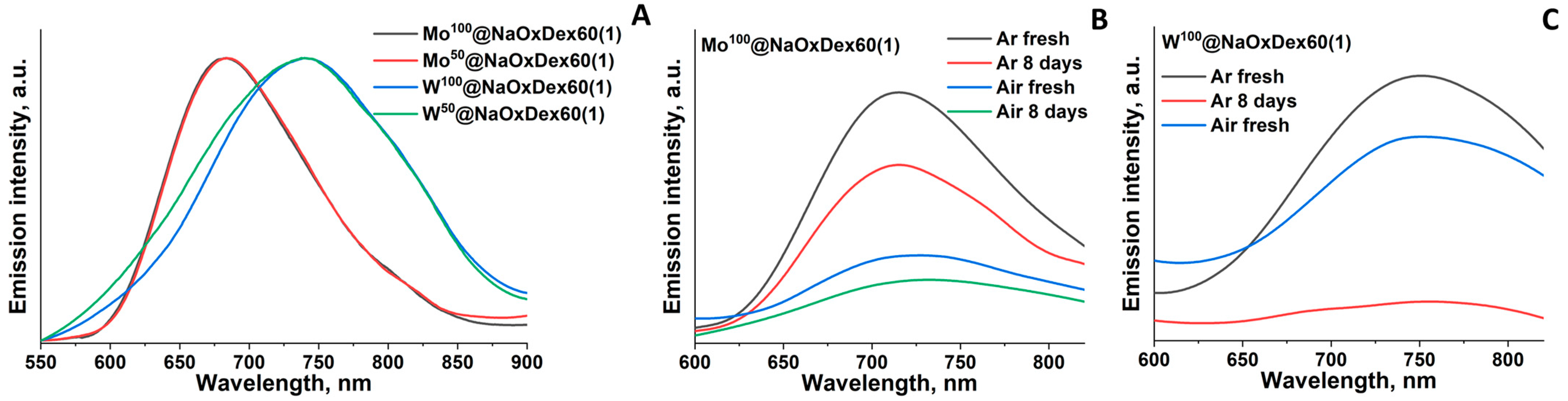
2.3. Luminescence Properties
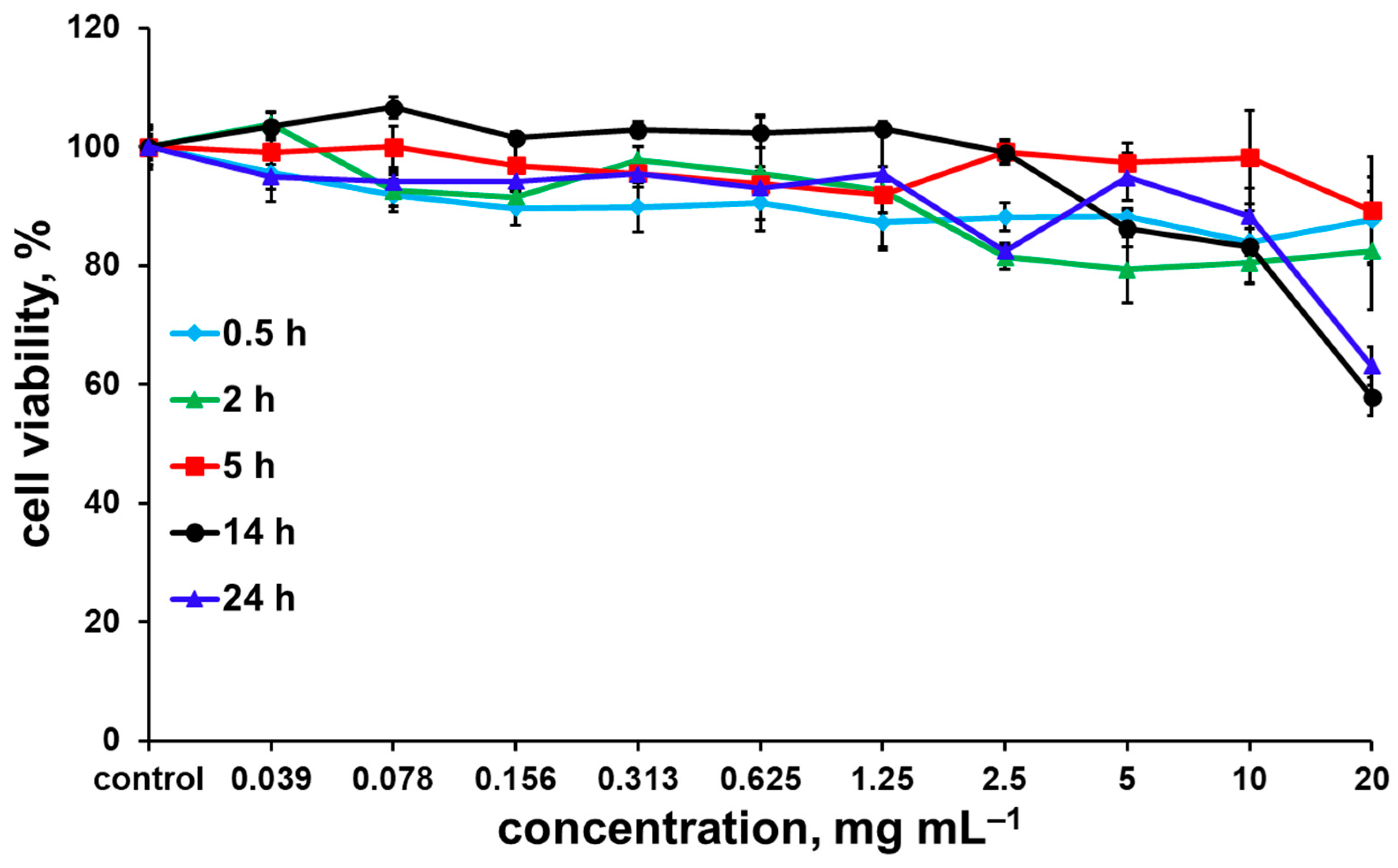
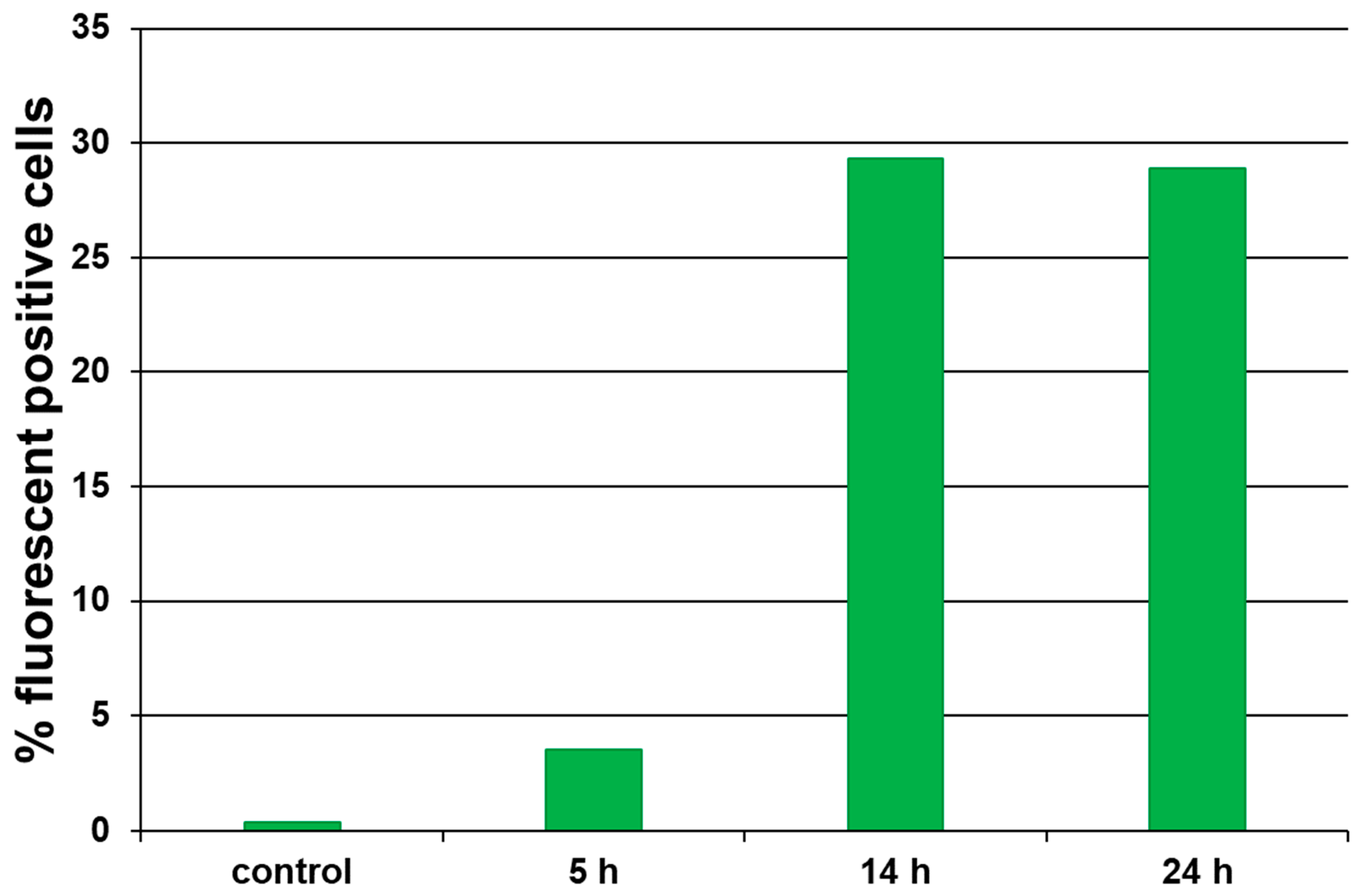
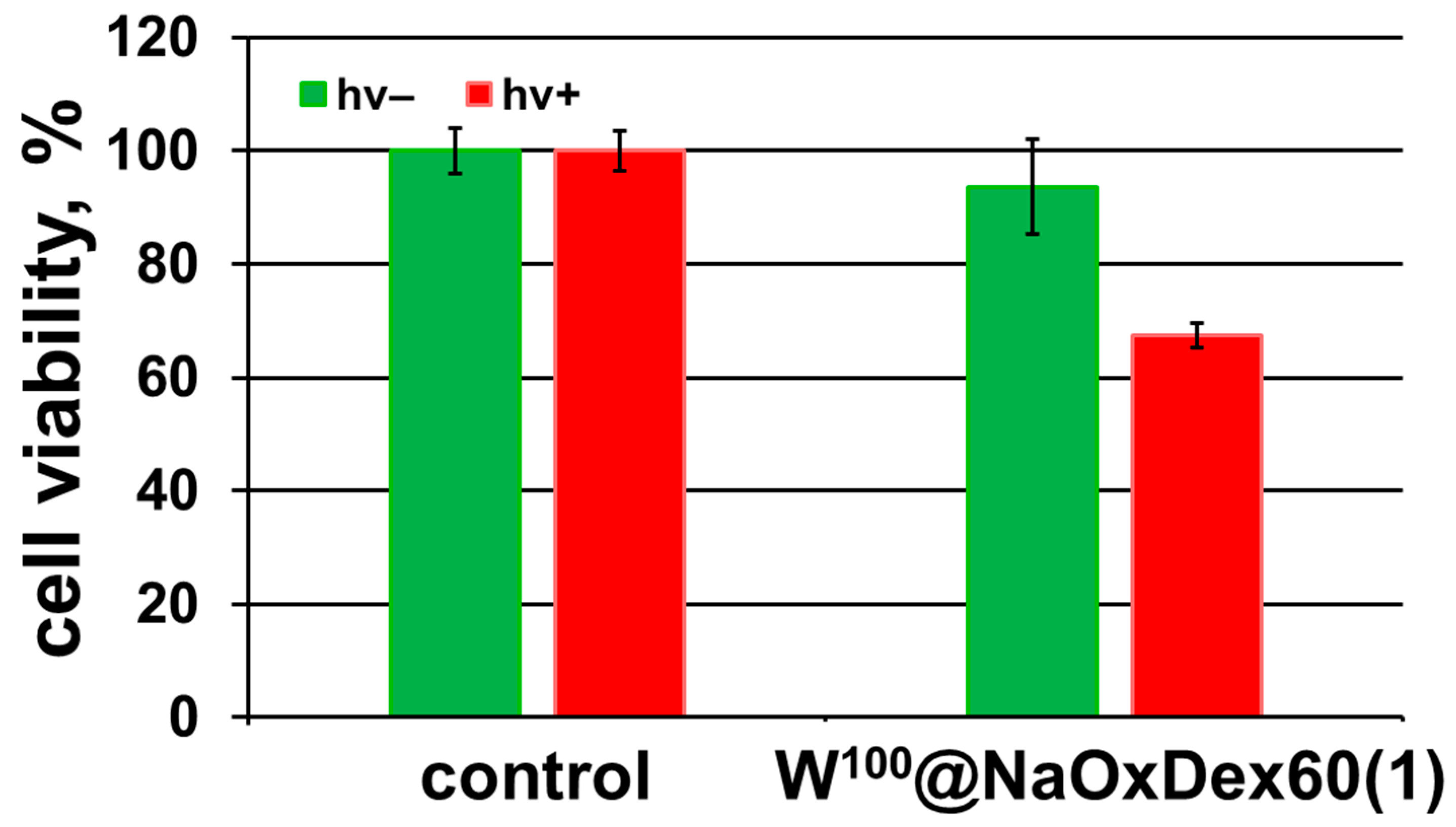
2.4. Biological Properties
3. Materials and Methods
3.1. Dextran Oxidation (OxDexQ(n))
3.2. Synthesis of Oxidized Dextran Sodium Salts (NaOxDexQ(n))
3.3. Immobilization of [{M6I8}(DMSO)6](NO3)4 (M = Mo, W) into Dextrans
3.4. Stability in Culture Medium
3.5. Reduction of OxDex60(2) (NaOxDex60(2)-Red) and Its Immobilization with Clusters (Mo100@NaOxDex60(2)-Red)
3.6. Biological Studies
3.6.1. MTT-Assay
3.6.2. Flow Cytometry (FACS) Analysis
3.6.3. Evaluation of the Photoinduced Cytotoxicity
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Polysaccharide-Based Nucleic Acid Nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hejazi, M.; Omidi, Y.; Dolatabadi, J.E.N. Bacterial-Derived Biopolymers: Advanced Natural Nanomaterials for Drug Delivery and Tissue Engineering. TrAC Trends Anal. Chem. 2016, 82, 367–384. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Design and Application of Dextran Carrier. J. Drug. Deliv. Sci. Technol. 2020, 55, 101392. [Google Scholar] [CrossRef]
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced Sunlight Photocatalytic Activity and Biosafety of Marine-Driven Synthesized Cerium Oxide Nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef] [PubMed]
- Nurakhmetova, Z.A.; Azhkeyeva, A.N.; Klassen, I.A.; Tatykhanova, G.S. Tatykhanova. Synthesis and Stabilization of Gold Nanoparticles Using Water-Soluble Synthetic and Natural Polymers. Polymers 2020, 12, 2625. [Google Scholar] [CrossRef]
- Tagad, C.K.; Rajdeo, K.S.; Kulkarni, A.; More, P.; Aiyer, R.C.; Sabharwal, S. Green Synthesis of Polysaccharide Stabilized Gold Nanoparticles: Chemo Catalytic and Room Temperature Operable Vapor Sensing Application. RSC Adv. 2014, 4, 24014–24019. [Google Scholar] [CrossRef]
- Lopes, L.C.; Lima, D.; Hacke, A.C.M.; Schveigert, B.S.; Calaça, G.N.; Simas, F.F.; Pereira, R.P.; Iacomini, M.; Viana, A.G.; Pessôa, C.A. Gold Nanoparticles Capped with Polysaccharides Extracted from Pineapple Gum: Evaluation of Their Hemocompatibility and Electrochemical Sensing Properties. Talanta 2021, 223, 121634. [Google Scholar] [CrossRef]
- Ghormade, V.; Gholap, H.; Kale, S.; Kulkarni, V.; Bhat, S.; Paknikar, K. Fluorescent Cadmium Telluride Quantum Dots Embedded Chitosan Nanoparticles: A Stable, Biocompatible Preparation for Bio-Imaging. J. Biomater. Sci. Polym. Ed. 2015, 26, 42–56. [Google Scholar] [CrossRef]
- Lai, P.-Y.; Huang, C.-C.; Chou, T.-H.; Ou, K.-L.; Chang, J.-Y. Aqueous Synthesis of Ag and Mn Co-Doped In2S3/ZnS Quantum Dots with Tunable Emission for Dual-Modal Targeted Imaging. Acta Biomater. 2017, 50, 522–533. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.; Carvalho, I.C.; Costa, A.; Guedes, M.I.M.; Kroon, E.G.; Lobato, Z.I.; Mansur, H.S. Fluorescent Quantum Dots-Zika Virus Hybrid Nanoconjugates for Biolabeling, Bioimaging, and Tracking Host-Cell Interactions. Mater. Lett. 2020, 277, 128279. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Selective Biosensing of Staphylococcus Aureus Using Chitosan Quantum Dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 50–56. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Probing the Interactions of Chitosan Capped CdS Quantum Dots with Pathogenic Bacteria and Their Biosensing Application. J. Mater. Chem. B 2013, 1, 6094–6106. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, L. Studies of the Interaction of CS@ZnS:Mn with Bovine Serum Albumin under Illumination. Appl. Surf. Sci. 2015, 349, 83–88. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) Comodified Chitosan-Wrapped Upconversion Nanoparticle for Targeted Near-Infrared Photodynamic Therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual Antibacterial Activities of a Chitosan-Modified Upconversion Photodynamic Therapy System Against Drug-Resistant Bacteria in Deep Tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xia, H.; Wu, Y.; Kong, S.N.; Deivasigamani, A.; Xu, R.; Hui, K.M.; Kang, Y. Single-Layer MoS2 Nanosheet Grafted Upconversion Nanoparticles for Near-Infrared Fluorescence Imaging-Guided Deep Tissue Cancer Phototherapy. Nanoscale 2016, 8, 7861–7865. [Google Scholar] [CrossRef]
- Maia, J.; Evangelista, M.B.; Gil, H.; Ferreira, L. Dextran-Based Materials for Biomedical Applications. In Carbohydrates Applications in Medicine; Gil, M.H., Ed.; Research Signpost: Kerala, India, 2014; pp. 31–53. [Google Scholar]
- Pronina, E.A.; Vorotnikov, Y.; Pozmogova, T.N.; Solovieva, A.O.; Miroshnichenko, S.M.; Plyusnin, P.E.; Pishchur, D.P.; Eltsov, I.V.; Edeleva, M.V.; Shestopalov, M.A.; et al. No Catalyst Added Hydrogen Peroxide Oxidation of Dextran: An Environmentally Friendly Route to Multifunctional Polymers. ACS Sustain. Chem. Eng. 2020, 8, 5371–5379. [Google Scholar] [CrossRef]
- Neaime, C.; Amela-Cortes, M.; Grasset, F.; Molard, Y.; Cordier, S.; Dierre, B.; Mortier, M.; Takei, T.; Takahashi, K.; Haneda, H.; et al. Time-Gated Luminescence Bioimaging with New Luminescent Nanocolloids Based on [Mo6I8(C2F5COO)6]2− Metal Atom Clusters. Phys. Chem. Chem. Phys. 2016, 18, 30166–30173. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Vorotnikov, Y.A.; Trifonova, K.E.; Efremova, O.A.; Krasilnikova, A.A.; Brylev, K.A.; Vorontsova, E.V.; Avrorov, P.A.; Shestopalova, L.V.; Poveshchenko, A.F.; et al. Cellular Internalisation, Bioimaging and Dark and Photodynamic Cytotoxicity of Silica Nanoparticles Doped by {Mo6I8}4+ Metal Clusters. J. Mater. Chem. B 2016, 4, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Křížová, I.; Ruml, T.; Lang, K. Octahedral Molybdenum Cluster Complexes with Optimized Properties for Photodynamic Applications. Inorg. Chem. 2020, 59, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Boucaud, Y.; Amela-Cortes, M.; Molard, Y.; Cordier, S.; Brandhonneur, N. PLGA Nanoparticles Embedding Molybdenum Cluster Salts: Influence of Chemical Composition on Physico-Chemical Properties, Encapsulation Efficiencies, Colloidal Stabilitiesand in Vitro Release. Int. J. Pharm. 2020, 576, 119025. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic Octahedral Molybdenum Cluster Complexes Functionalized with Mitochondria-Targeting Ligands: Photodynamic Anticancer and Antibacterial Activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Pozmogova, T.N.; Sitnikova, N.A.; Pronina, E.V.; Miroshnichenko, S.M.; Kushnarenko, A.O.; Solovieva, A.O.; Bogachev, S.S.; Vavilov, G.D.; Efremova, O.A.; Vorotnikov, Y.A.; et al. Hybrid System {W6I8}-Cluster/dsDNA as an Agent for Targeted X-Ray Induced Photodynamic Therapy of Cancer Stem Cells. Mater. Chem. Front. 2021, 5, 7499–7507. [Google Scholar] [CrossRef]
- Kirakci, K.; Shestopalov, M.A.; Lang, K. Recent developments on luminescent octahedral transition metal cluster complexes towards biological applications. Coord. Chem. Rev. 2023, 481, 215048. [Google Scholar] [CrossRef]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A Water-Soluble Octahedral Molybdenum Cluster Complex as a Potential Agent for X-Ray Induced Photodynamic Therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-Soluble Octahedral Molybdenum Cluster Compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, Luminescence, and in Vitro Studies. Inorganica Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Vorotnikov, Y.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.; Ivanov, A.; Evtushok, D.V.; Miroshnichenko, S.; Yanshole, V.; Eling, C.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem. Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef]
- Pronina, E.V.; Pozmogova, T.N.; Vorotnikov, Y.A.; Ivanov, A.A.; Shestopalov, M.A. The Role of Hydrolysis in Biological Effects of Molybdenum Cluster with DMSO Ligands. J. Biol. Inorg. Chem. 2022, 27, 111–119. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Solovieva, A.O.; Vorotnikov, Y.A.; Kurskaya, O.G.; Brylev, K.A.; Tsygankova, A.R.; Edeleva, M.V.; Gyrylova, S.N.; Kitamura, N.; Efremova, O.A.; et al. Water-Soluble Hybrid Materials Based on {Mo6X8}4+ (X = Cl, Br, I) Cluster Complexes and Sodium Polystyrene Sulfonate. New J. Chem. 2017, 41, 1670–1676. [Google Scholar] [CrossRef]
- Elistratova, J.; Mikhailov, M.; Burilov, V.; Babaev, V.; Rizvanov, I.; Mustafina, A.; Abramov, P.; Sokolov, M.; Konovalov, A.; Fedin, V. Supramolecular Assemblies of Triblock Copolymers with Hexanuclear Molybdenum Clusters for Sensing Antibiotics in Aqueous Solutions via Energy Transfer. RSC Adv. 2014, 4, 27922–27930. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Boucaud, Y.; Verger, A.; Dumait, N.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum Cluster Loaded PLGA Nanoparticles as Efficient Tools Against Epithelial Ovarian Cancer. Int. J. Pharm. 2021, 592, 120079. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Novikova, E.D.; Solovieva, A.O.; Shanshin, D.V.; Tsygankova, A.R.; Shcherbakov, D.N.; Efremova, O.A.; Shestopalov, M.A. Single-Domain Antibody C7b for Address Delivery of Nanoparticles to HER2-Positive Cancers. Nanoscale 2020, 12, 21885–21894. [Google Scholar] [CrossRef]
- Beltrán, A.; Mikhailov, M.; Sokolov, M.N.; Pérez-Laguna, V.; Rezusta, A.; Revillo, M.J.; Galindo, F. A Photobleaching Resistant Polymer Supported Hexanuclear Molybdenum Iodide Cluster for Photocatalytic Oxygenations and Photodynamic Inactivation of Staphylococcus Aureus. J. Mater. Chem. B 2016, 4, 5975–5979. [Google Scholar] [CrossRef]
- Mikhailov, M.A.; Brylev, K.A.; Abramov, P.A.; Sakuda, E.; Akagi, S.; Ito, A.; Kitamura, N.; Sokolov, M.N. Synthetic Tuning of Redox, Spectroscopic, and Photophysical Properties of {Mo6I8}4+ Core Cluster Complexes by Terminal Carboxylate Ligands. Inorg. Chem. 2016, 55, 8437–8445. [Google Scholar] [CrossRef] [PubMed]
- Efremova, O.A.; Shestopalov, M.A.; Chirtsova, N.A.; Smolentsev, A.I.; Mironov, Y.V.; Kitamura, N.; Brylev, K.A.; Sutherland, A.J. A Highly Emissive Inorganic Hexamolybdenum Cluster Complex as a Handy Precursor for the Preparation of New Luminescent Materials. Dalton Trans. 2014, 43, 6021–6025. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikova, N.A.; Efremova, O.A.; Tsygankova, A.R.; Brylev, K.A.; Edeleva, M.V.; Kurskaya, O.G.; Sutherland, A.J.; Shestopalov, A.M.; Mironov, Y.V.; Shestopalov, M.A. Characterization and Cytotoxicity Studies of Thiol-Modified Polystyrene Microbeads Doped with [{Mo6X8}(NO3)6]2– (X = Cl, Br, I). Polym. Adv. Technol. 2016, 27, 922–928. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Fejfarová, K.; Martinčík, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef]
- Kirakci, K.; Demel, J.; Hynek, J.; Zelenka, J.; Rumlová, M.; Ruml, T.; Lang, K. Phosphinate Apical Ligands: A Route to a Water-Stable Octahedral Molybdenum Cluster Complex. Inorg. Chem. 2019, 58, 16546–16552. [Google Scholar] [CrossRef]
- Efremova, O.A.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Novozhilov, I.N.; Kuratieva, N.V.; Edeleva, M.V.; Benoit, D.M.; Kitamura, N.; Mironov, Y.V.; et al. Octahedral Molybdenum Cluster Complexes with Aromatic Sulfonate Ligands. Dalton Trans. 2016, 45, 15427–15435. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Haouas, M.; Evtushok, D.V.; Pozmogova, T.N.; Golubeva, T.S.; Molard, Y.; Cordier, S.; Falaise, C.; Cadot, E.; Shestopalov, M.A. Stabilization of Octahedral Metal Halide Clusters by Host–Guest Complexation with γ-Cyclodextrin: Toward Nontoxic Luminescent Compounds. Inorg. Chem. 2022, 61, 14462–14469. [Google Scholar] [CrossRef] [PubMed]


| Sample | Complete Disappearance of Clusters Absorption Band *, h | ||
|---|---|---|---|
| x = 10 | x = 50 | x = 100 | |
| Mox@Dex6 | - | - | unstable # |
| Mox@NaOxDex6(1) | n.d. $ | n.d. $ | ~13 |
| Mox@NaOxDex6(2) | n.d. $ | n.d. $ | ~7 |
| Mox@Dex60 | - | - | unstable # |
| Mox@NaOxDex60(1) | ~23 | ~23 | ~23 |
| Mox@NaOxDex60(2) | ~13 | ~13 | ~19 |
| Wx@Dex6 | - | - | unstable # |
| Wx@NaOxDex6(1) | n.d. $ | n.d. $ | ~54 |
| Wx@NaOxDex6(2) | n.d. $ | n.d. $ | ~54 |
| Wx@Dex60 | - | - | unstable # |
| Wx@NaOxDex60(1) | ~96 (~4 d) | ~96 (~4 d) | ~144 (~6 d) |
| Wx@NaOxDex60(2) | ~96 (~4 d) | ~96 (~4 d) | ~144 (~6 d) |
| Sample | x | Solid | In PBS, Fresh | In PBS, 8 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| λmax, nm | Φem | λmax, nm | Φem | λmax, nm | Φem | ||||
| Air | Ar | Air | Ar | ||||||
| [{Mo6I8}(DMSO)6](NO3)4 | - | 681 | 0.19 | – # | – | – | – | – | – |
| Mox@NaOxDex60(1) | 50 | 684 | 0.07 | 710 | <0.01 | 0.04 | 715 | <0.01 | 0.03 |
| 100 | 684 | 0.06 | 715 | <0.01 | 0.02 | 716 | <0.01 | 0.02 | |
| [{W6I8}(DMSO)6](NO3)4 | - | 631 | 0.18 | 668 * | 0.02 * | – | – | – | – |
| Wx@NaOxDex60(1) | 50 | 740 | 0.03 | 744 | <0.01 | 0.03 | ~750 $ | <0.01 | <0.01 |
| 100 | 740 | 0.06 | 751 | <0.01 | 0.03 | ~750 $ | <0.01 | <0.01 | |
| Sample | Incubation Time, h | ||||
|---|---|---|---|---|---|
| 0.5 | 2 | 5 | 14 | 24 | |
| W100@NaOxDex60(1) | >2.50 | >2.50 | >2.50 | >2.50 | >2.50 |
| [{W6I8}(DMSO)6](NO3)4 (fresh solution) | – $ | – $ | >1.06 | – $ | >1.06 |
| [{W6I8}(DMSO)6](NO3)4 (4-day-old solution) | – $ | – $ | 0.94 ± 0.03 # | – $ | 0.89 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pronina, E.V.; Vorotnikov, Y.A.; Pozmogova, T.N.; Tsygankova, A.R.; Kirakci, K.; Lang, K.; Shestopalov, M.A. Multifunctional Oxidized Dextran as a Matrix for Stabilization of Octahedral Molybdenum and Tungsten Iodide Clusters in Aqueous Media. Int. J. Mol. Sci. 2023, 24, 10010. https://doi.org/10.3390/ijms241210010
Pronina EV, Vorotnikov YA, Pozmogova TN, Tsygankova AR, Kirakci K, Lang K, Shestopalov MA. Multifunctional Oxidized Dextran as a Matrix for Stabilization of Octahedral Molybdenum and Tungsten Iodide Clusters in Aqueous Media. International Journal of Molecular Sciences. 2023; 24(12):10010. https://doi.org/10.3390/ijms241210010
Chicago/Turabian StylePronina, Ekaterina V., Yuri A. Vorotnikov, Tatiana N. Pozmogova, Alphiya R. Tsygankova, Kaplan Kirakci, Kamil Lang, and Michael A. Shestopalov. 2023. "Multifunctional Oxidized Dextran as a Matrix for Stabilization of Octahedral Molybdenum and Tungsten Iodide Clusters in Aqueous Media" International Journal of Molecular Sciences 24, no. 12: 10010. https://doi.org/10.3390/ijms241210010
APA StylePronina, E. V., Vorotnikov, Y. A., Pozmogova, T. N., Tsygankova, A. R., Kirakci, K., Lang, K., & Shestopalov, M. A. (2023). Multifunctional Oxidized Dextran as a Matrix for Stabilization of Octahedral Molybdenum and Tungsten Iodide Clusters in Aqueous Media. International Journal of Molecular Sciences, 24(12), 10010. https://doi.org/10.3390/ijms241210010






