Halogen Bond-Involving Self-Assembly of Iodonium Carboxylates: Adding a Dimension to Supramolecular Architecture
Abstract
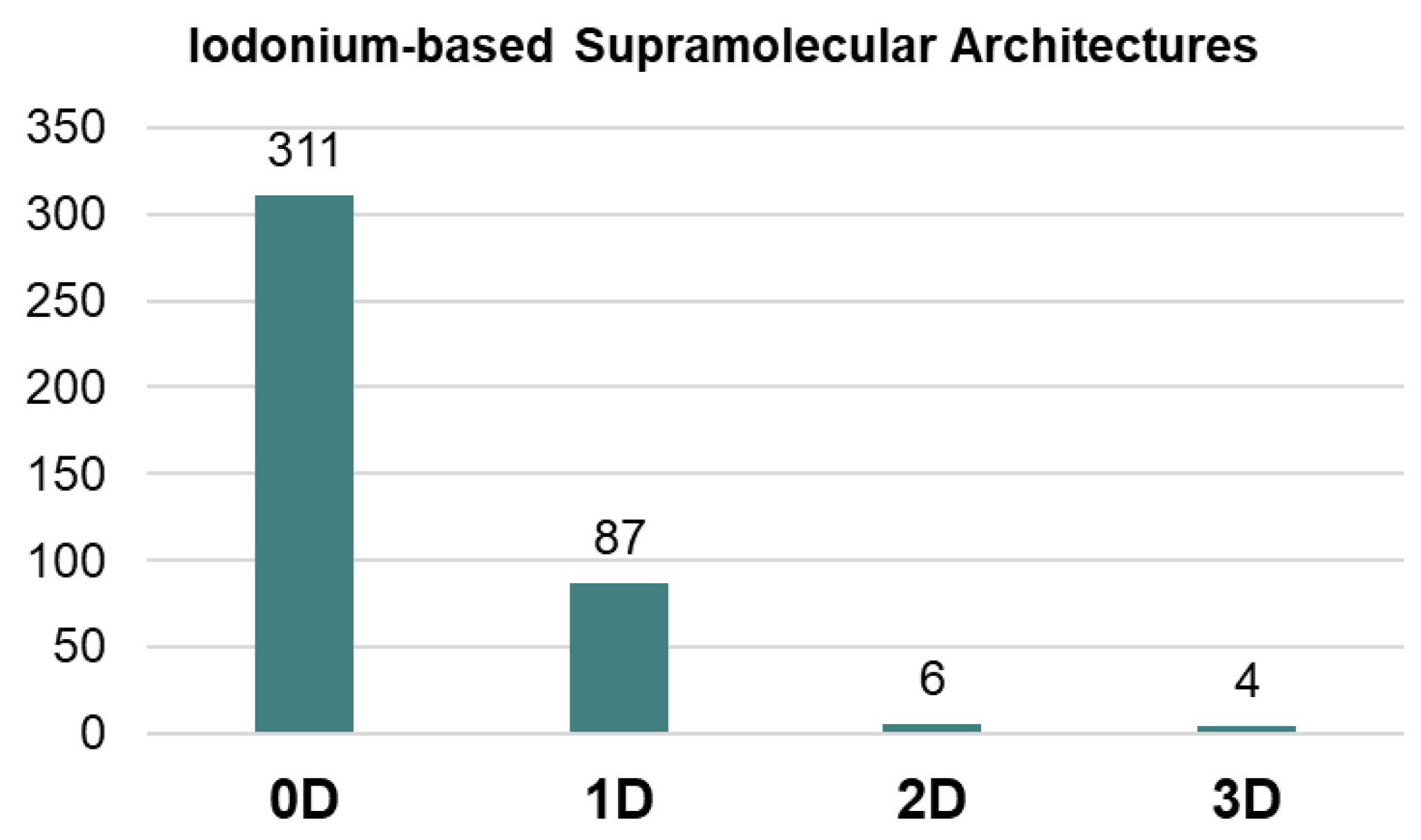
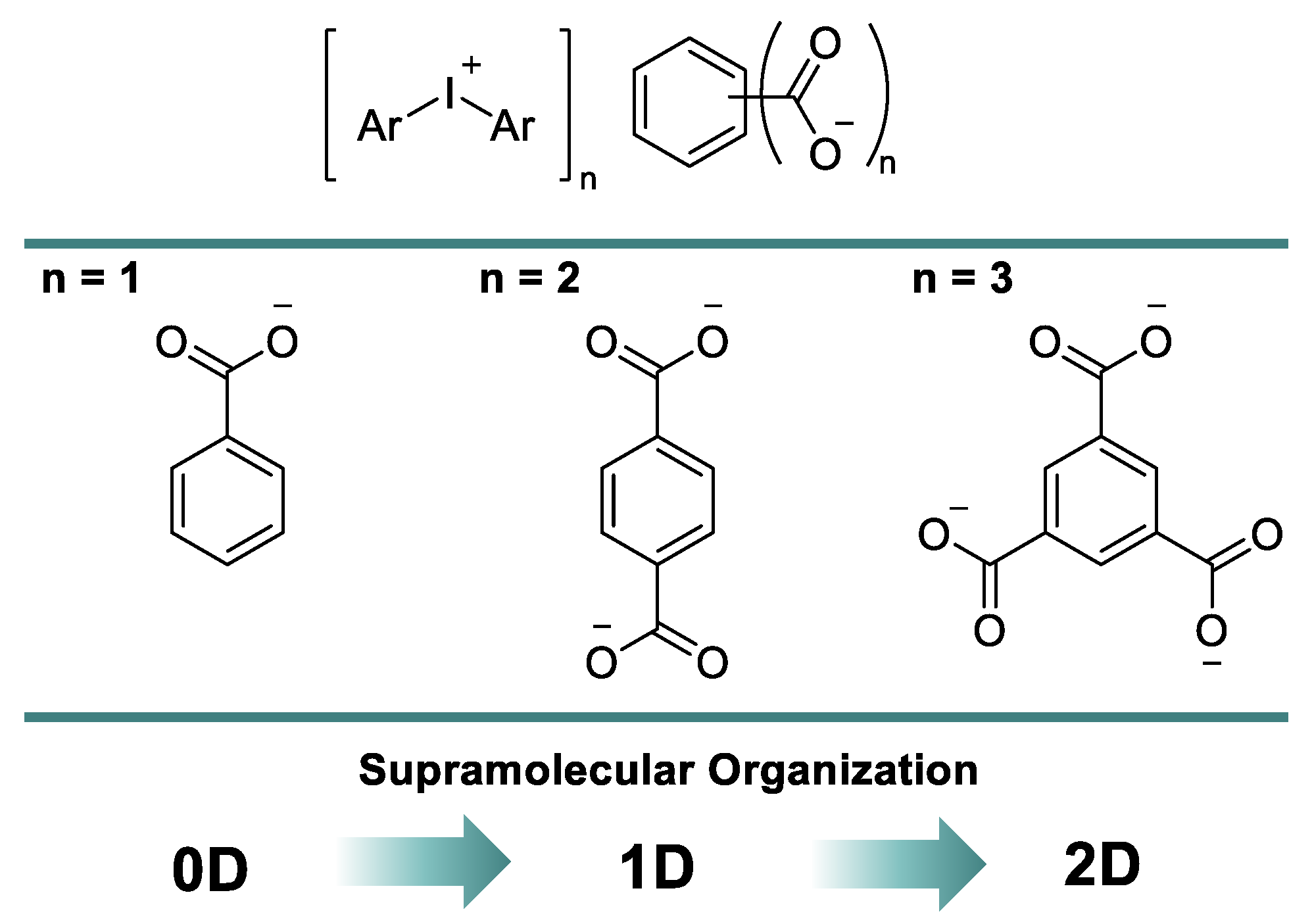
:1. Introduction
2. Results and Discussion
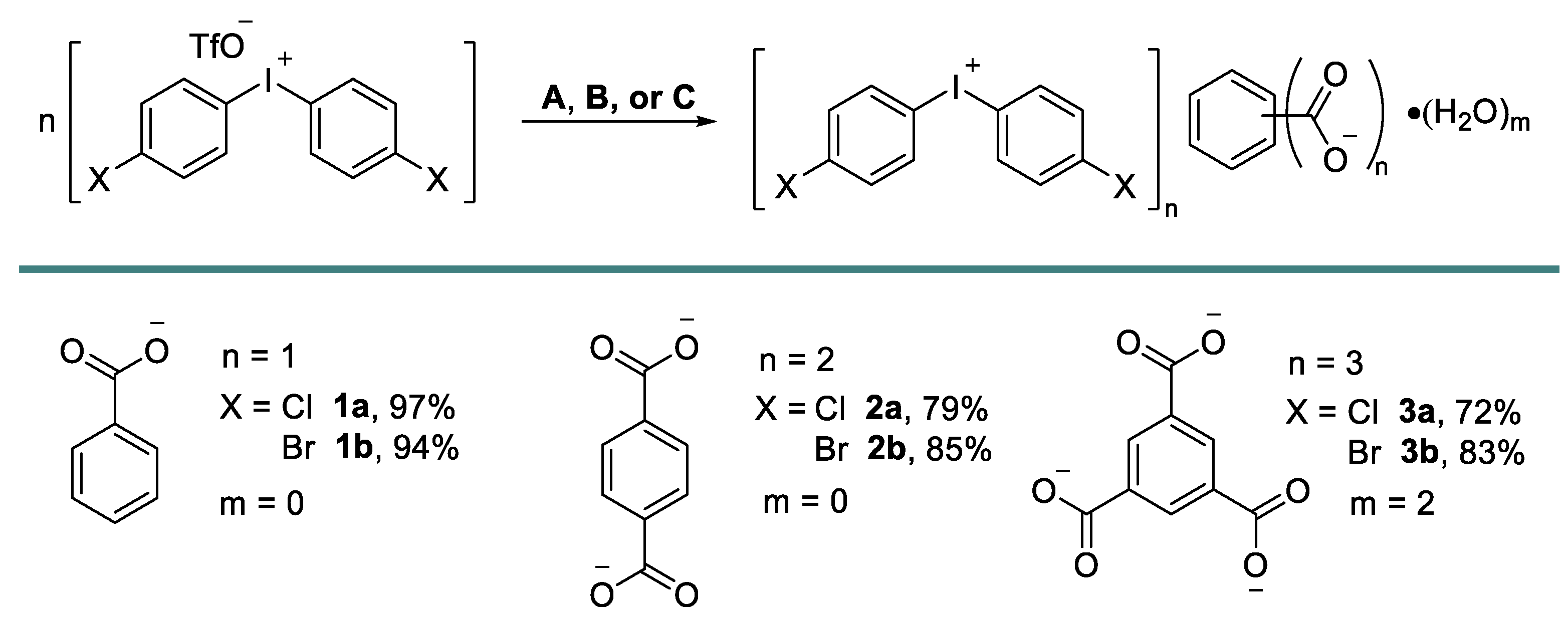
2.1. Synthesis and Crystal Growth
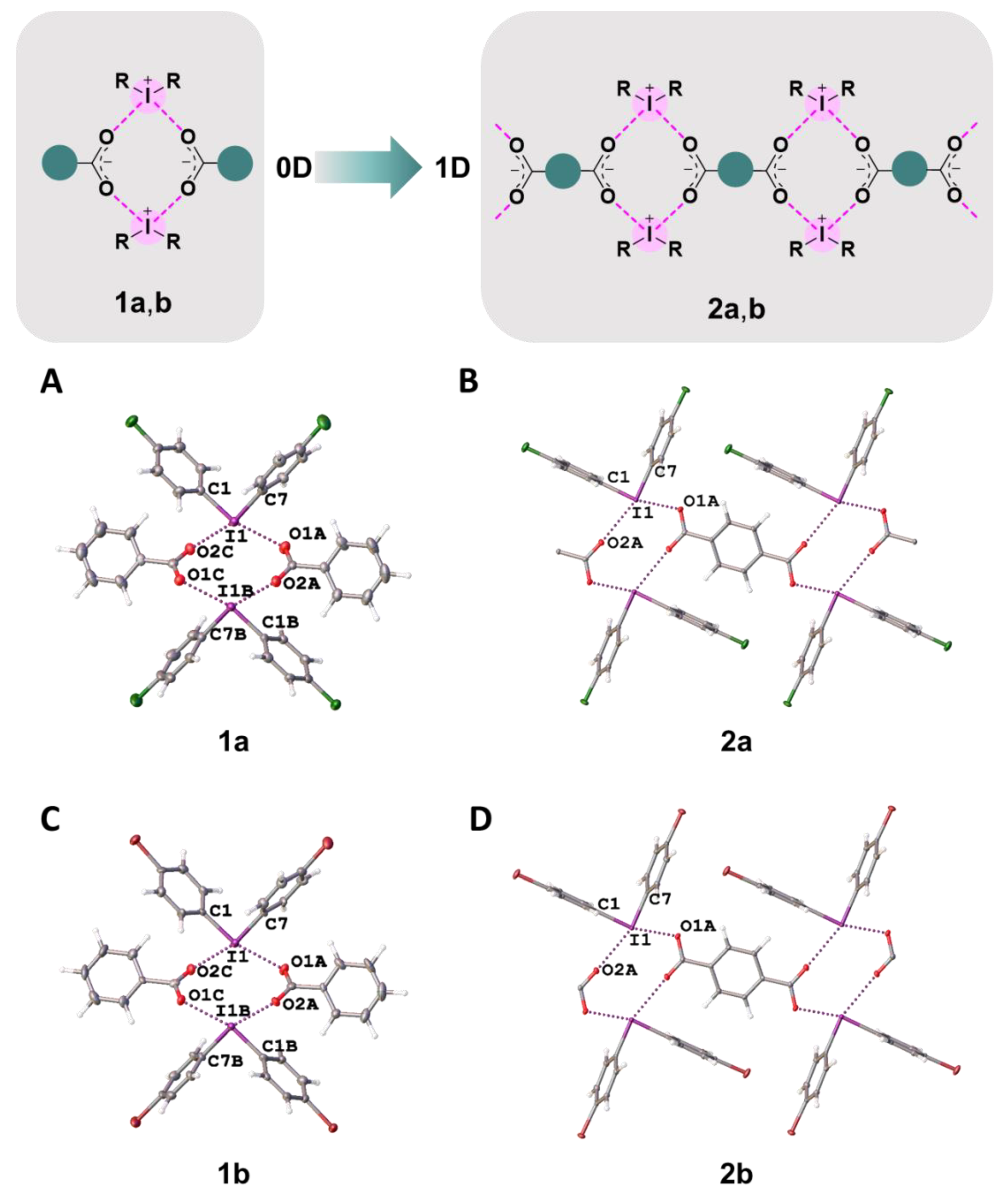
2.2. General Consideration of the XRD Structures
2.3. XRD Structures: Supramolecular Assembly
2.4. Theoretical Calculations
3. Materials and Methods
3.1. General Information
3.2. X-ray Structure Determinations
3.3. Computational Details
3.4. Synthetic Procedures
3.4.1. Preparation of Diaryliodonium Benzoates 1
3.4.2. Preparation of Diaryliodonium Terephtalates 2
3.4.3. Preparation of Diaryliodonium Trimesates 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cotelle, Y.; Sakai, N.; Matile, S. Unorthodox Interactions at Work. J. Am. Chem. Soc. 2016, 138, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.Q.; Wang, L.Y.; Mak, T.C.W. Halogen Bonding: A Powerful, Emerging Tool for Constructing High-Dimensional Metal-Containing Supramolecular Networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen Bonding (X-Bonding): A Biological Perspective. Protein Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef]
- Riel, A.M.S.; Rowe, R.K.; Ho, E.N.; Carlsson, A.-C.C.; Rappé, A.K.; Berryman, O.B.; Ho, P.S. Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry. Acc. Chem. Res. 2019, 52, 2870–2880. [Google Scholar] [CrossRef]
- Ho, P.S. Biomolecular Halogen Bonds. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 241–276. [Google Scholar]
- Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Looking Back, Looking Forward at Halogen Bonding in Drug Discovery. Molecules 2017, 22, 1397. [Google Scholar] [CrossRef]
- Pancholi, J.; Beer, P.D. Halogen Bonding Motifs for Anion Recognition. Coord. Chem. Rev. 2020, 416, 213281. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D. Halogen Bonding and Chalcogen Bonding Mediated Sensing. Chem. Sci. 2022, 13, 7098–7125. [Google Scholar] [CrossRef] [PubMed]
- Tay, H.M.; Tse, Y.C.; Docker, A.; Gateley, C.; Thompson, A.L.; Kuhn, H.; Zhang, Z.; Beer, P.D. Halogen-Bonding Heteroditopic [2]Catenanes for Recognition of Alkali Metal/Halide Ion Pairs. Angew. Chem. Int. Ed. 2023, 62, e202214785. [Google Scholar] [CrossRef] [PubMed]
- Docker, A.; Guthrie, C.H.; Kuhn, H.; Beer, P.D. Modulating Chalcogen Bonding and Halogen Bonding Sigma-Hole Donor Atom Potency and Selectivity for Halide Anion Recognition. Angew. Chem. Int. Ed. 2021, 60, 21973–21978. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Huber, S.M. Catalysis of Organic Reactions through Halogen Bonding. ACS Catal. 2019, 9, 9622–9639. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Reinhard, D.L.; Engelage, E.; Huber, S.M. A Bidentate Iodine(III)-Based Halogen-Bond Donor as a Powerful Organocatalyst**. Angew. Chem. Int. Ed. 2021, 60, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S.M. Iodine(III) Derivatives as Halogen Bonding Organocatalysts. Angew. Chem. Int. Ed. 2018, 57, 3830–3833. [Google Scholar] [CrossRef]
- Brammer, L.; Peuronen, A.; Roseveare, T.M. Halogen Bonds, Chalcogen Bonds, Pnictogen Bonds, Tetrel Bonds and Other σ-Hole Interactions: A Snapshot of Current Progress. Acta Crystallogr. Sect. C Struct. Chem. 2023, 79, 204–216. [Google Scholar] [CrossRef]
- Robidas, R.; Reinhard, D.L.; Legault, C.Y.; Huber, S.M. Iodine(III)-Based Halogen Bond Donors: Properties and Applications. Chem. Rec. 2021, 21, 1912–1927. [Google Scholar] [CrossRef]
- Catalano, L.; Cavallo, G.; Metrangolo, P.; Resnati, G.; Terraneo, G. Halogen Bonding in Hypervalent Iodine Compounds. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 373, pp. 289–309. [Google Scholar]
- Cavallo, G.; Murray, J.S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. Halogen Bonding in Hypervalent Iodine and Bromine Derivatives: Halonium Salts. IUCrJ 2017, 4, 411–419. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a Double σ-Hole Donor: The Dichotomy of Thiocyanate Halogen Bonding Provides Divergent Solid State Arylation by Diaryliodonium Cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Semenov, A.V.; Baykov, S.V.; Soldatova, N.S.; Geyl, K.K.; Ivanov, D.M.; Frontera, A.; Boyarskiy, V.P.; Postnikov, P.S.; Kukushkin, V.Y. Noncovalent Chelation by Halogen Bonding in the Design of Metal-Containing Arrays: Assembly of Double σ-Hole Donating Halolium with Cu I-Containing O,O-Donors. Inorg. Chem. 2023, 62, 6128–6137. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Suslonov, V.V.; Ivanov, D.M.; Yusubov, M.S.; Resnati, G.; Postnikov, P.S.; Kukushkin, V.Y. Controlled Halogen-Bond-Involving Assembly of Double-σ-Hole-Donating Diaryliodonium Cations and Ditopic Arene Sulfonates. Cryst. Growth Des. 2023, 23, 413–423. [Google Scholar] [CrossRef]
- Fedorova, I.I.; Soldatova, N.S.; Ivanov, D.M.; Nikiforova, K.; Aliyarova, I.S.; Yusubov, M.S.; Tolstoy, P.M.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y.; et al. Benzothienoiodolium Cations Doubly Bonded to Anions via Halogen–Chalcogen and Halogen–Hydrogen Supramolecular Synthons. Cryst. Growth Des. 2023, 23, 2661–2674. [Google Scholar] [CrossRef]
- Suslonov, V.V.; Soldatova, N.S.; Postnikov, P.S.; Resnati, G.; Kukushkin, V.Y.; Ivanov, D.M.; Bokach, N.A. Diaryliodonium Tetracyanidometallates Self-Assemble into Halogen-Bonded Square-Like Arrays. Cryst. Growth Des. 2022, 22, 2749–2758. [Google Scholar] [CrossRef]
- Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C.Y.; Kirsch, S.F.; Huber, S.M. Activation of a Metal-Halogen Bond by Halogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 16496–16500. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Cramer, C.J.; Huber, S.M. Hypervalent Iodine(III) Compounds as Biaxial Halogen Bond Donors. J. Am. Chem. Soc. 2020, 142, 8633–8640. [Google Scholar] [CrossRef]
- Boelke, A.; Kuczmera, T.J.; Lork, E.; Nachtsheim, B.J. N-Heterocyclic Iod(Az)Olium Salts–Potent Halogen-Bond Donors in Organocatalysis. Chem. Eur. J. 2021, 27, 13128–13134. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Ivanov, D.M.; Semyonov, O.V.; Kukurina, O.S.; Guselnikova, O.; Yamauchi, Y.; Wirth, T.; Zhdankin, V.V.; Yusubov, M.S.; et al. Zwitterionic Iodonium Species Afford Halogen Bond-Based Porous Organic Frameworks. Chem. Sci. 2022, 13, 5650–5658. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Mayer, R.J.; Ofial, A.R.; Mayr, H.; Legault, C.Y. Lewis Acidity Scale of Diaryliodonium Ions toward Oxygen, Nitrogen, and Halogen Lewis Bases. J. Am. Chem. Soc. 2020, 142, 5221–5233. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η 2 (Cl–Pt). Cryst. Growth Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Efimenko, Z.M.; Eliseeva, A.A.; Ivanov, D.M.; Galmés, B.; Frontera, A.; Bokach, N.A.; Kukushkin, V.Y. Bifurcated μ 2-I···(N,O) Halogen Bonding: The Case of (Nitrosoguanidinate)Ni II Cocrystals with Iodine(I)-Based σ-Hole Donors. Cryst. Growth Des. 2021, 21, 588–596. [Google Scholar] [CrossRef]
- Aliyarova, I.S.; Ivanov, D.M.; Soldatova, N.S.; Novikov, A.S.; Postnikov, P.S.; Yusubov, M.S.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Diaryliodonium Cations as Iodine(III)-Based Double-σ-Hole Donors. Cryst. Growth Des. 2021, 21, 1136–1147. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H 2 C(X)–X···X–(X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Fotović, L.; Bedeković, N.; Stilinović, V. Isostructural Halogen Exchange and Halogen Bonds: The Case of N-(4-Halogenobenzyl)-3-Halogenopyridinium Halogenides. Cryst. Growth Des. 2022, 22, 1333–1344. [Google Scholar] [CrossRef]
- Buldakov, A.V.; Kinzhalov, M.A.; Kryukova, M.A.; Ivanov, D.M.; Novikov, A.S.; Smirnov, A.S.; Starova, G.L.; Bokach, N.A.; Kukushkin, V.Y. Isomorphous Series of Pd II-Containing Halogen-Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostructural Exchange. Cryst. Growth Des. 2020, 20, 1975–1984. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals 2020, 10, 289. [Google Scholar] [CrossRef]
- Aliyarova, I.S.; Tupikina, E.Y.; Soldatova, N.S.; Ivanov, D.M.; Postnikov, P.S.; Yusubov, M.; Kukushkin, V.Y. Halogen Bonding Involving Gold Nucleophiles in Different Oxidation States. Inorg. Chem. 2022, 61, 15398–15407. [Google Scholar] [CrossRef] [PubMed]
- United States Patent Application: 0050054626. Available online: https://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220050054626%22.PGNR.&OS=DN/20050054626&RS=DN/20050054626 (accessed on 26 September 2022).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef] [PubMed]
- LIPPERT, B.G.; PARRINELLO, J.H. and M. A Hybrid Gaussian and Plane Wave Density Functional Scheme. Mol. Phys. 1997, 92, 477–488. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: London, UK, 1990; ISBN 9780198558651. [Google Scholar]
- Bader, R.F.W.; Nguyen-Dang, T.T. Quantum Theory of Atoms in Molecules–Dalton Revisited. Adv. Quantum Chem. 1981, 14, 63–124. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From Weak to Strong Interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving X-H⋯F-Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Maiti, B.; Sarkar, U. Philicity: A Unified Treatment of Chemical Reactivity and Selectivity. J. Phys. Chem. A 2003, 107, 4973–4975. [Google Scholar] [CrossRef]
- Parsaee, F.; Senarathna, M.C.; Kannangara, P.B.; Alexander, S.N.; Arche, P.D.E.; Welin, E.R. Radical Philicity and Its Role in Selective Organic Transformations. Nat. Rev. Chem. 2021, 5, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, R.H.; Schmauck, J.; Perryman, M.S.; Yue, H.; Riegger, J.; Schweitzer-Chaput, B.; Breugst, M.; Klussmann, M. Philicity of Acetonyl and Benzoyl Radicals: A Comparative Experimental and Computational Study. Chem. Eur. J. 2019, 25, 9088–9097. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G. The Exact One-Electron Model of Molecular Structure. Int. J. Quantum Chem. 1986, 29, 197–204. [Google Scholar] [CrossRef]
- Chan, W.-T.; Hamilton, I.P. Valence Shell Structures in the Distributions of the Laplacian of the Electron Density and the One-Electron Potential for Diatomic Molecules. J. Chem. Phys. 1998, 108, 2473–2485. [Google Scholar] [CrossRef]
- Tsirelson, V.; Stash, A. On Functions and Quantities Derived from the Experimental Electron Density. Acta Crystallogr. Sect. A Found. Crystallogr. 2004, 60, 418–426. [Google Scholar] [CrossRef]
- Bertolotti, F.; Shishkina, A.V.; Forni, A.; Gervasio, G.; Stash, A.I.; Tsirelson, V.G. Intermolecular Bonding Features in Solid Iodine. Cryst. Growth Des. 2014, 14, 3587–3595. [Google Scholar] [CrossRef]
- Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V. Testing the Tools for Revealing and Characterizing the Iodine–Iodine Halogen Bond in Crystals. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 217–226. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of Chemical Bonds Based on Topological Analysis of Electron Localization Functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Zou, W.; Cai, Z.; Wang, J.; Xin, K. An Open Library of Relativistic Core Electron Density Function for the QTAIM Analysis with Pseudopotentials. J. Comput. Chem. 2018, 39, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond Lengths in Organic and Metal-Organic Compounds Revisited: X —H Bond Lengths from Neutron Diffraction Data. Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 380–386. [Google Scholar] [CrossRef]
- Frigo, M.; Johnson, S.G. The Design and Implementation of FFTW3. Proc. IEEE 2005, 93, 216–231. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and Accurate Density Functional Calculations Using a Mixed Gaussian and Plane Waves Approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. <scp>cp2k:</Scp> Atomistic Simulations of Condensed Matter Systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef]
- Borštnik, U.; VandeVondele, J.; Weber, V.; Hutter, J. Sparse Matrix Multiplication: The Distributed Block-Compressed Sparse Row Library. Parallel Comput. 2014, 40, 47–58. [Google Scholar] [CrossRef]
- Schütt, O.; Messmer, P.; Hutter, J.; VandeVondele, J. GPU-Accelerated Sparse Matrix-Matrix Multiplication for Linear Scaling Density Functional Theory. In Electronic Structure Calculations on Graphics Processing Units; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 173–190. [Google Scholar]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A Look at the Density Functional Theory Zoo with the Advanced GMTKN55 Database for General Main Group Thermochemistry, Kinetics and Noncovalent Interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package-Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Golze, D.; Iannuzzi, M.; Hutter, J. Local Fitting of the Kohn–Sham Density in a Gaussian and Plane Waves Scheme for Large-Scale Density Functional Theory Simulations. J. Chem. Theory Comput. 2017, 13, 2202–2214. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.S.; Wahiduzzaman, M.; Park, J.; Muschi, M.; Martineau-Corcos, C.; Tissot, A.; Cho, K.H.; Marrot, J.; Shepard, W.; et al. A Robust Large-Pore Zirconium Carboxylate Metal–Organic Framework for Energy-Efficient Water-Sorption-Driven Refrigeration. Nat. Energy 2018, 3, 985–993. [Google Scholar] [CrossRef]
- Wang, X.-D.; Huang, Y.-H.; Liao, J.-F.; Jiang, Y.; Zhou, L.; Zhang, X.-Y.; Chen, H.-Y.; Kuang, D.-B. In Situ Construction of a Cs 2 SnI 6 Perovskite Nanocrystal/SnS 2 Nanosheet Heterojunction with Boosted Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 13434–13441. [Google Scholar] [CrossRef]
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.; Camp, J.S.; et al. Advances, Updates, and Analytics for the Computation-Ready, Experimental Metal–Organic Framework Database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, W.; Mei, D. Theoretical Characterization of Zeolite Encapsulated Platinum Clusters in the Presence of Water Molecules. Phys. Chem. Chem. Phys. 2021, 23, 23360–23371. [Google Scholar] [CrossRef]
- Bennion, J.C.; Vogt, L.; Tuckerman, M.E.; Matzger, A.J. Isostructural Cocrystals of 1,3,5-Trinitrobenzene Assembled by Halogen Bonding. Cryst. Growth Des. 2016, 16, 4688–4693. [Google Scholar] [CrossRef]
- Oropeza, F.E.; Barawi, M.; Alfonso-González, E.; de la Peña O’Shea, V.A.; Trigo, J.F.; Guillén, C.; Saiz, F.; Villar-Garcia, I.J. Understanding Ultrafast Charge Transfer Processes in SnS and SnS 2: Using the Core Hole Clock Method to Measure Attosecond Orbital-Dependent Electron Delocalisation in Semiconducting Layered Materials. J. Mater. Chem. C 2021, 9, 11859–11872. [Google Scholar] [CrossRef]
- Chadwick, F.M.; Rees, N.H.; Weller, A.S.; Krämer, T.; Iannuzzi, M.; Macgregor, S.A. A Rhodium-Pentane Sigma-Alkane Complex: Characterization in the Solid State by Experimental and Computational Techniques. Angew. Chem. Int. Ed. 2016, 55, 3677–3681. [Google Scholar] [CrossRef]
- Pambudi, F.I.; Prasetyo, N. Insight into the Structure of the Heulandite-Type Zeolite Containing Aromatic Compounds Using Periodic Density Functional Theory. Mater. Today Commun. 2021, 26, 102028. [Google Scholar] [CrossRef]
- Hazra, A.; Bonakala, S.; Adalikwu, S.A.; Balasubramanian, S.; Maji, T.K. Fluorocarbon-Functionalized Superhydrophobic Metal–Organic Framework: Enhanced CO 2 Uptake via Photoinduced Postsynthetic Modification. Inorg. Chem. 2021, 60, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Ivanov, D.M.; Melekhova, A.A.; Bokach, N.A.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. Chameleonic Metal-Bound Isocyanides: A π-Donating CuI-Center Imparts Nucleophilicity to the Isocyanide Carbon toward Halogen Bonding. Inorg. Chem. Front. 2022, 9, 1655–1665. [Google Scholar] [CrossRef]
- Nieland, E.; Komisarek, D.; Hohloch, S.; Wurst, K.; Vasylyeva, V.; Weingart, O.; Schmidt, B.M. Supramolecular Networks by Imine Halogen Bonding. Chem. Commun. 2022, 58, 5233–5236. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Cheranyova, A.M.; Ivanov, D.M.; Kukushkin, V.Y.; Bokach, N.A. Polymorph-Dependent Phosphorescence of Cyclometalated Platinum(II) Complexes and Its Relation to Non-Covalent Interactions. ACS Omega 2022, 7, 34454–34462. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Bielawski, M.; Zhu, M.; Olofsson, B. Efficient and General One-Pot Synthesis of Diaryliodonium Triflates: Optimization, Scope and Limitations. Adv. Synth. Catal. 2007, 349, 2610–2618. [Google Scholar] [CrossRef]

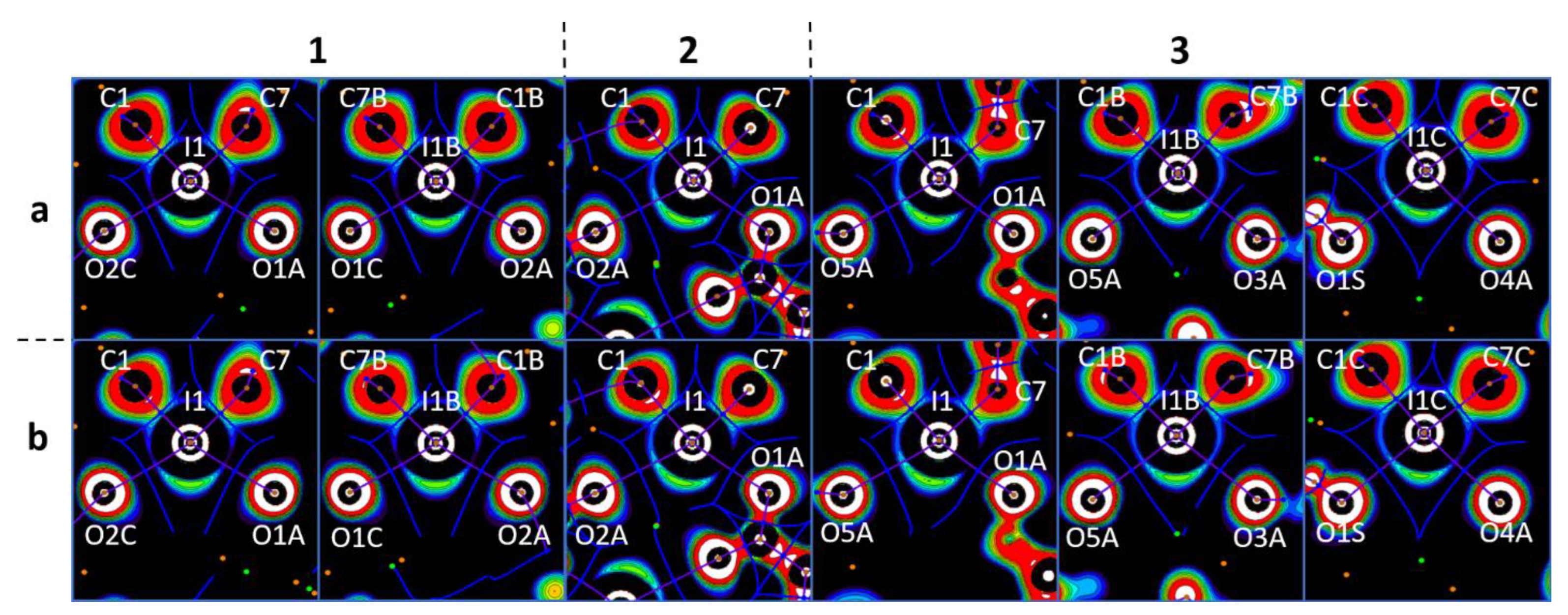
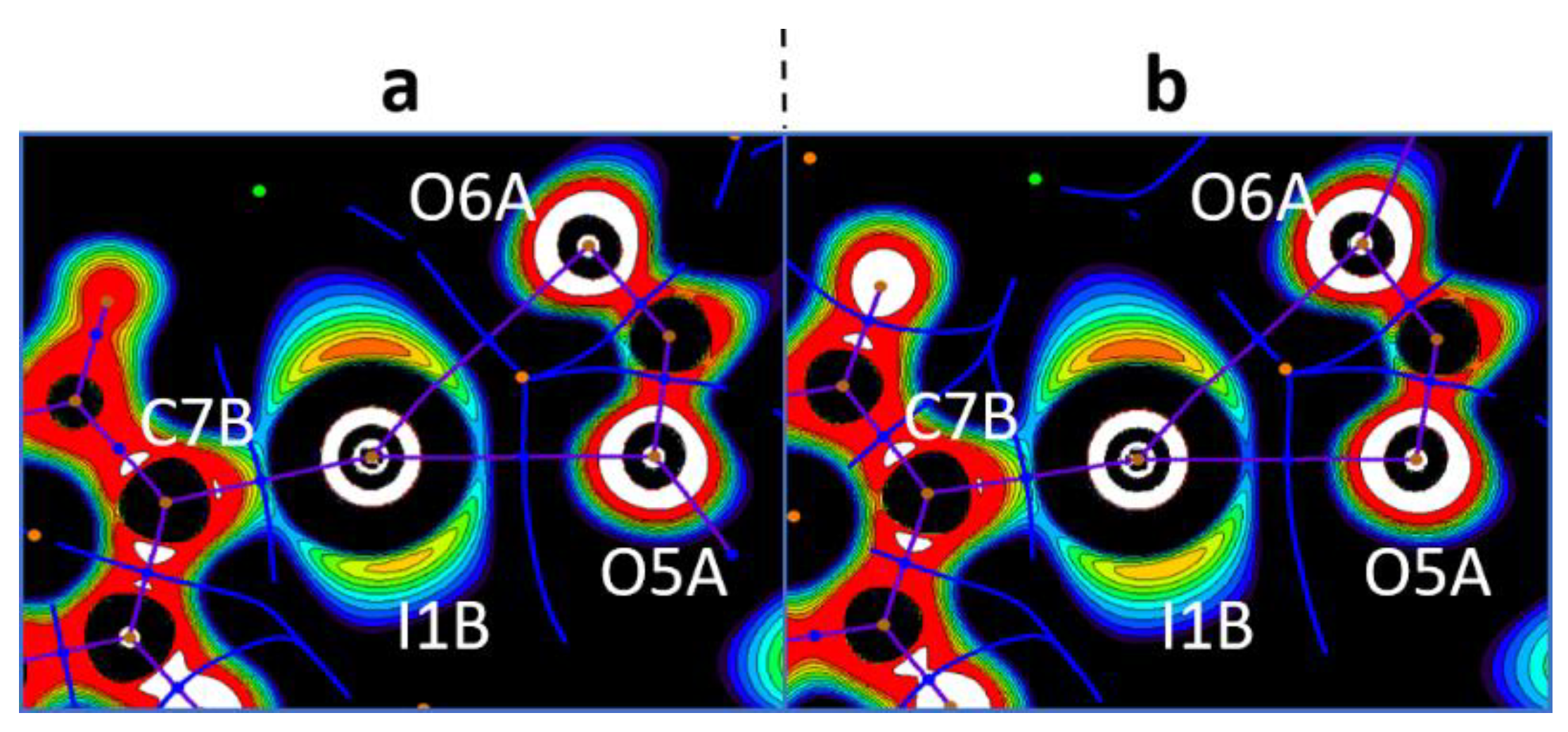
| Cation Type a | XB | d(C–I∙∙∙X) | ∠(C–I∙∙∙X) | Nc b | d(C–I∙∙∙X) | ∠(C–I∙∙∙X) | Nc b |
|---|---|---|---|---|---|---|---|
| 1a | 1b | ||||||
| A | C1–I1∙∙∙O1A | 2.622(3) | 164.04(12) | 0.75 | 2.628(4) | 164.19(19) | 0.75 |
| C7–I1∙∙∙O2C | 2.661(3) | 164.55(13) | 0.76 | 2.664(4) | 164.39(19) | 0.76 | |
| B | C1B–I1B∙∙∙O1C | 2.663(3) | 163.96(12) | 0.76 | 2.669(4) | 163.39(19) | 0.76 |
| C7B–I1B∙∙∙O2A | 2.650(3) | 164.87(12) | 0.76 | 2.647(4) | 164.17(19) | 0.76 | |
| 2a | 2b | ||||||
| C1–I1∙∙∙O1A | 2.479(2) | 165.00(9) | 0.71 | 2.480(2) | 163.95(9) | 0.71 | |
| C7–I1∙∙∙O2A | 2.9964(18) | 164.99(9) | 0.86 | 2.9612(18) | 163.98(9) | 0.85 | |
| 3a | 3b | ||||||
| A | C1–I1∙∙∙O1A | 2.479(6) | 168.4(3) | 0.71 | 2.474(4) | 167.7(2) | 0.71 |
| C7–I1∙∙∙O5A | 2.957(6) | 164.2(2) | 0.84 | 2.956(5) | 164.00(17) | 0.84 | |
| B | C1B–I1B∙∙∙O3A | 2.692(6) | 174.5(3) | 0.77 | 2.697(5) | 170.3(2) | 0.77 |
| C7B–I1B∙∙∙O5A | 2.833(6) | 166.8(2) | 0.81 | 2.766(5) | 169.5(2) | 0.79 | |
| C7B–I1B∙∙∙O6A | 3.037(6) | 148.1(2) | 0.87 | 3.097(4) | 144.8(2) | 0.88 | |
| C | C1C–I1C∙∙∙O4A | 2.630(6) | 171.2(3) | 0.75 | 2.640(5) | 171.03(19) | 0.75 |
| C7C–I1C∙∙∙O1S | 2.828(8) | 172.5(3) | 0.81 | 2.771(5) | 173.3(2) | 0.79 | |
| Structure | Contact | l | Sign(λ2)ρ(r) | ∇2ρ(r) | V(r) | G(r) | Hb |
|---|---|---|---|---|---|---|---|
| 1a | I1···O1A I1···O2C I1B···O2A I1B···O1C | 2.622 2.661 2.650 2.663 | −0.0349 −0.0321 −0.0330 −0.0325 | 0.1008 0.0948 0.0960 0.0936 | −0.0260 −0.0234 −0.0241 −0.0234 | 0.0247 0.0229 0.0233 0.0227 | −0.0013 −0.0006 −0.0008 −0.0008 |
| 1b | I1···O1A I1···O2C I1B···O2A I1B···O1C | 2.628 2.664 2.647 2.669 | −0.0345 −0.0318 −0.0330 −0.0321 | 0.0994 0.0943 0.0967 0.0926 | −0.0255 −0.0232 −0.0243 −0.0230 | 0.0243 0.0227 0.0234 0.0224 | −0.0012 −0.0005 −0.0008 −0.0006 |
| 2a | I1···O1A I1···O2A | 2.479 2.996 | −0.0483 −0.0141 | 0.1163 0.0557 | −0.0379 −0.0092 | 0.0314 0.0114 | −0.0064 0.0023 |
| 2b | I1···O1A I1···O2A | 2.480 2.961 | −0.0483 −0.0151 | 0.1151 0.0597 | −0.0377 −0.0101 | 0.0312 0.0124 | −0.0065 0.0023 |
| 3a | I1···O1A I1···O5A I1B···O3A I1B···O5A I1B···O6A I1C···O4A I1C···O1S | 2.479 2.957 2.692 2.833 3.037 2.630 2.828 | −0.0477 −0.0171 −0.0285 −0.0240 −0.0171 −0.0358 −0.0250 | 0.1186 0.0590 0.0939 0.0702 0.0554 0.0973 0.0736 | −0.0378 −0.0107 −0.0211 −0.0155 −0.0109 −0.0261 −0.0165 | 0.0318 0.0126 0.0217 0.0162 0.0123 0.0243 0.0171 | −0.0061 0.0019 0.0006 0.0007 0.0014 −0.0018 0.0007 |
| 3b | I1···O1A I1···O5A I1B···O3A I1B···O5A I1B···O6A I1C···O4A I1C···O1S | 2.474 2.956 2.697 2.766 3.097 2.640 2.771 | −0.0482 −0.0170 −0.0283 −0.0274 −0.0154 −0.0353 −0.0272 | 0.1195 0.0592 0.0935 0.0784 0.0512 0.0957 0.0810 | −0.0383 −0.0108 −0.0210 −0.0183 −0.0097 −0.0254 −0.0185 | 0.0321 0.0126 0.0216 0.0185 0.0112 0.0238 0.0189 | −0.0062 0.0019 0.0007 0.0002 0.0015 −0.0016 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzhabov, A.D.; Ledneva, A.I.; Soldatova, N.S.; Fedorova, I.I.; Ivanov, D.M.; Ivanov, A.A.; Yusubov, M.S.; Kukushkin, V.Y.; Postnikov, P.S. Halogen Bond-Involving Self-Assembly of Iodonium Carboxylates: Adding a Dimension to Supramolecular Architecture. Int. J. Mol. Sci. 2023, 24, 14642. https://doi.org/10.3390/ijms241914642
Radzhabov AD, Ledneva AI, Soldatova NS, Fedorova II, Ivanov DM, Ivanov AA, Yusubov MS, Kukushkin VY, Postnikov PS. Halogen Bond-Involving Self-Assembly of Iodonium Carboxylates: Adding a Dimension to Supramolecular Architecture. International Journal of Molecular Sciences. 2023; 24(19):14642. https://doi.org/10.3390/ijms241914642
Chicago/Turabian StyleRadzhabov, Amirbek D., Alyona I. Ledneva, Natalia S. Soldatova, Irina I. Fedorova, Daniil M. Ivanov, Alexey A. Ivanov, Mekhman S. Yusubov, Vadim Yu. Kukushkin, and Pavel S. Postnikov. 2023. "Halogen Bond-Involving Self-Assembly of Iodonium Carboxylates: Adding a Dimension to Supramolecular Architecture" International Journal of Molecular Sciences 24, no. 19: 14642. https://doi.org/10.3390/ijms241914642
APA StyleRadzhabov, A. D., Ledneva, A. I., Soldatova, N. S., Fedorova, I. I., Ivanov, D. M., Ivanov, A. A., Yusubov, M. S., Kukushkin, V. Y., & Postnikov, P. S. (2023). Halogen Bond-Involving Self-Assembly of Iodonium Carboxylates: Adding a Dimension to Supramolecular Architecture. International Journal of Molecular Sciences, 24(19), 14642. https://doi.org/10.3390/ijms241914642







