Volatile Composition and Classification of Paeonia lactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes
Abstract
1. Introduction
2. Results
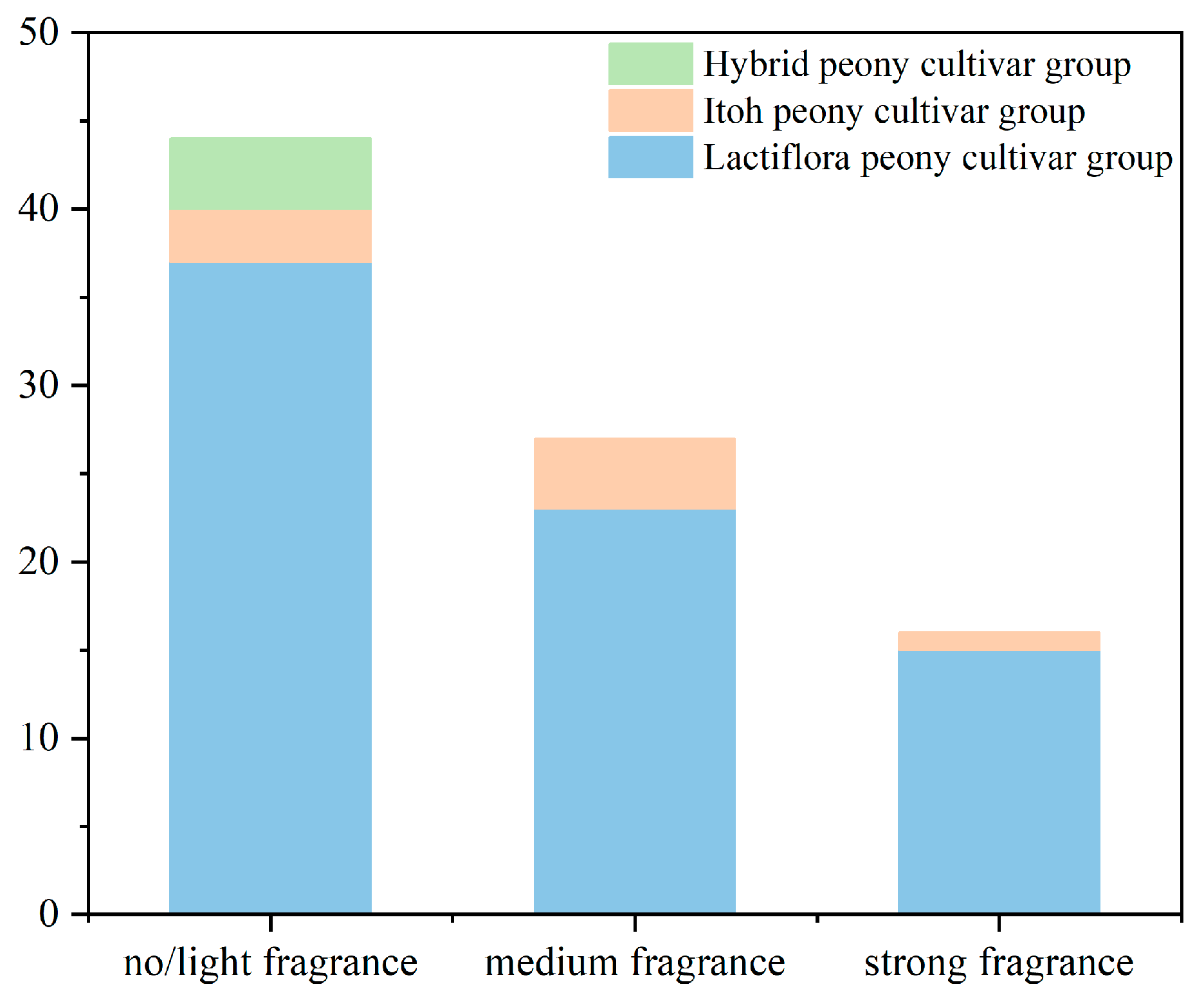
2.1. Three Levels of Sensory Evaluation
2.2. Types of Volatile Components from Herbaceous Peony Cultivars
2.3. Analysis of Major Volatile Components from Herbaceous Peony Cultivars
2.4. Odor Classification and OAVs of Volatile Compounds
2.5. Grouping of Herbaceous Peony Cultivars Based on Their Fragrance
- A rose scent
- A lily scent
- A mixed scent
2.6. Analysis of Genes Related to Monoterpene Synthesis
2.7. Analysis of Genes Related to 2-PE Synthesis
3. Discussion
3.1. Complexity and Diverse of Herbaceous Peony Floral Aroma Compounds
3.2. Terpene Biosynthesis-Related Genes Contribute to the Fragrance of Herbaceous Peony
3.3. 2-PE Biosynthesis-Related Genes Contribute to the Fragrance of Herbaceous Peony
4. Materials and Methods
4.1. Plant Materials
4.2. Sensory Evaluation of Herbaceous Peony Fragrance
4.3. Floral Scent Collection and Analysis
4.4. Classification of Scents
4.5. RNA Isolation and cDNA Synthesis
4.6. Expression Analysis of Related Genes by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Z.; Leng, P.; Hu, Z.; Wu, J.; Dou, D. Transcriptome sequencing reveals terpene biosynthesis pathway genes accounting for volatile terpene of tree peony. Planta 2021, 254, 67–80. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Yao, K.; Zhang, W.; Yang, L.; Li, C. Research progress of the device form of solid phase microextraction. Heilongjiang Anim. Sci. Vet. Med. 2017, 03, 55–58. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotech. 2008, 19, 181–189. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J.; Croteau, R. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, J.; Sangwan, N.; Narnoliya, L.; Singh, N.; Bansal, S.; Mishra, B.; Sangwan, R. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: Active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 2017, 159, 381–400. [Google Scholar] [CrossRef]
- Munoz-Bertomeu, J.; Arrillaga, I.; Ros, R.; Segura, J. Up-Regulation of 1-Deoxy-d-Xylulose-5-Phosphate Synthase Enhances Production of Essential Oils in Transgenic Spike Lavender. Plant. Physiol. 2006, 142, 890–900. [Google Scholar] [CrossRef]
- Nagegowda, D.; Gutensohn, M.; Wilkerson, C.; Dudareva, N. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. Plant. J. 2008, 55, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Pattanaik, S.; Schluttenhofer, C.; Yuan, L. A network of jasmonate-responsive bHLH factors modulate monoterpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol. 2018, 217, 1566–1581. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.; Lewinsohn, E.; Croteau, R. Floral scent production in Clarkia (Onagraceae) (I. Localization and developmental modulation of monoterpene emission and linalool synthase activity). Plant Physiol. 1994, 16, 1533–1540. [Google Scholar] [CrossRef]
- Ramya, M.; Park, P.H.; Chuang, Y.; Kwon, O.; An, H.; Park, P.M.; Baek, Y.; Kang, B.; Tsai, W.; Chen, H. RNA sequencing analysis of Cymbidium goeringii identifies floral scent biosynthesis related genes. BMC Plant Biol. 2019, 19, 337. [Google Scholar] [CrossRef]
- Huang, M.; Fan, R.; Ye, X.; Lin, R.; Luo, Y.; Fang, N.; Zhong, H.; Chen, S. The transcriptome of flower development provides insight into floral scent formation in Freesia hybrida. Plant. Growth Regul. 2018, 86, 93–104. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.Y.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, T.; Fan, J.; Liu, Z.; Zong, J.; Fan, W.; Han, Y.; Grierson, D. Volatile composition and classification of Lilium flower aroma types and identification, polymorphisms, and alternative splicing of their monoterpene synthase genes. Hortic. Res. 2019, 6, 110. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yue, Y.; Ding, W.; Xu, C.; Shi, T.; Chen, G.; Wang, L. Transcriptomic analysis of the candidate genes related to aroma formation in Osmanthus fragrans. Molecules 2018, 23, 1604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, W.; Zhou, Y.; Zhang, J.; Li, Y.; Ahmad, S.; Zhang, Q. Comparative transcriptome reveals benzenoid biosynthesis regulation as inducer of floral scent in the woody plant Prunus mume. Front. Plant Sci. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Wang, S.; Yuan, M.; Niu, L.; Shi, Q. Transcriptome and volatile compounds profiling analyses provide insights into the molecular mechanism underlying the floral fragrance of tree peony. Ind. Crop. Prod. 2021, 162, 113286. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Mei, X.; Zhou, Y.; Du, B.; Watanabe, N.; Yang, Z. Regulation of biosynthesis and emission of volatile phenylpropanoids/benzenoids in Petunia x hybrida flowers by multi-factors of circadian clock, light, and temperature. Plant Physiol. Bioch. 2016, 17, 1–8. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, K.; Chen, L. Molecular cloning and expression of Chimonanthus praecox farnesyl pyrophosphate synthase gene and its possible involvement in the biosynthesis of floral volatile sesquiterpenoids. Plant Physiol. Biochem. 2010, 48, 845–850. [Google Scholar] [CrossRef]
- Tieman, D.; Loucas, H.; Kim, J.; Clark, D.; Klee, H. Tomato phenylacetaldehyde reductases catalyze the last step in the synthesis of the aroma volatile 2-phenylethanol. Phytochemistry 2007, 68, 2660–2669. [Google Scholar] [CrossRef]
- Negre, F.; Kish, C.; Boatright, J.; Underwood, B.; Shibuya, K.; Wagner, C.; Clark, D.; Dudareva, N. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 2003, 15, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, J.; Ric de Vos, C.; Verhoeven, H.; Haring, M.; Tunen, A.; Schuurink, R. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 2003, 62, 997–1008. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.; Hanson, A.; Klee, H. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef]
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-phenylethanol in roses as the dominant floral scent compound from L-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci. Biotechnol. Biochem. 2007, 71, 2408–2419. [Google Scholar] [CrossRef]
- Watanabe, S.; Hayashi, K.; Yagi, K.; Asai, T.; Mactavish, H.; Picone, J.; Turnbull, C.; Watanabe, N. Biogenesis of 2-phenylethanol in rose flowers: Incorporation of [2H8] L-phenylalanine into 2-phenylethanol and its β-D-glucopyranoside during the flower opening of Rosa ‘Hoh-Jun’ and Rosa damascene Mill. Biosci. Biotechnol. Biochem. 2002, 66, 943–947. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, B.; Wu, Q.; Zhang, J.; Leng, P.; Zhang, K. Transcriptome sequencing analysis reveals a difference in monoterpene biosynthesis between scented Lilium ‘Siberia’ and unscented Lilium ‘Novano’. Front. Plant Sci. 2017, 8, 1351. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Du, D.; Wang, T.; Yang, W.; Wang, J.; Zhang, Q. A comparative analysis of characteristic floral scent compounds in Prunus mume and related species. Biosci. Biotechnol. Biochem. 2014, 78, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Clery, R.; Owen, N.; Chambers, S.; Thornton-Wood, S. An investigation into the scent of carnations. J. Essent. Oil Res. 1999, 11, 355–359. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Haketa, T.; Furuichi, H.; Iioka, S. Characteristics of floral scent compounds in a new fragrant Petunia cultivar TX-794 ‘Evening Scentsation’. Hortic. J. 2018, 87, 258–263. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Tsuji, T. Analysis of floral scent compounds and classification by scent quality in tulip cultivars. J. Jpn. Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef]
- Datta, S.K. Breeding of new ornamental varieties: Rose. Curr. Sci. 2018, 114, 1194–1205. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Silva, J.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, Y.; Sheng, L.; Li, T.; Zhao, D.; Tao, J. Comparative Analysis of Headspace Volatiles of Different Herbaceous Peony (Paeonia lactiflora Pall.) Cultivars. J. Essent. Oil-Bear. Plants 2016, 19, 167–175. [Google Scholar] [CrossRef]
- Myung, S.; Pue, H.; Young, N.; Manjulatha, M.; Suk, W.; Eun, Y.; Jae, A.; Jong, T.; Oh, K. Discrimination of floral scents and metabolites in cut flowers of peony (Paeonia lactiflora Pall.) cultivars. Korean J. Plant. Res. 2018, 31, 641–651. [Google Scholar] [CrossRef]
- Song, C.; Yu, X. Analysis of the aroma composition of different flower organs of two peony varieties. J. Beijing For. Univ. 2017, 39, 92–99. [Google Scholar] [CrossRef]
- Song, C.; Wang, Q.; Silva, J.; Yu, X. Identification of floral fragrances and analysis of fragrance patterns in herbaceous peony cultivars. J. Am. Soc. Hort. Sci. 2018, 143, 248–258. [Google Scholar] [CrossRef]
- Hong, P.; Chen, F.; Yang, Y.; Chen, Y.; Cai, H.; Ni, H. Sensory characteristics and volatile components of three pummelo (Citrus Maxima) essential oils. Mod. Food Sci. Technol. 2014, 30, 274–281. [Google Scholar] [CrossRef]
- Yao, L.; Mo, Y.; Chen, D.; Feng, T.; Song, S.; Wang, H.; Sun, M. Characterization of key aroma compounds in Xinjiang dried figs (Ficus carica L.) by GC–MS, GC–olfactometry, odor activity values, and sensory analyses. LWT-Food Sci. Technol. 2021, 150, 111982. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Xu, Y.; Wang, L.; Wang, L.S. Identification of floral fragrances in tree peony cultivars by gas chromatography–mass spectrometry. Sci. Hortic. 2012, 142, 158–165. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z. Genetic and Biochemical Aspects of Floral Scents in Roses. Int. J. Mol. Sci. 2022, 23, 8014. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary–secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kroymann, J. Natural diversity and adaptation in plant secondary metabolism. Curr. Opin. Plant Biol. 2011, 14, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Poiroux-Gonord, F.; Bidel, L.; Fanciullino, A.L.; Gautier, H.; Lauri–Lopez, F.; Urban, L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects to Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Vandermoten, S.; Haubruge, E.; Cusson, M. New insights into short-chain prenyltransferases: Structural features, evolutionary history and potential for selective inhibition. Cell. Mol. Life Sci. 2009, 66, 3685–3695. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Li, J. Research Progress on 2-phenylethanol Biosynthesis in Plants. Acta. Hortic. Sin. 2010, 37, 1690–1694. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Zhu, J.; Shi, G.; Niu, Z.; Liu, Z.; Li, K.; Guo, X. Associations between the 1-deoxy-d-xylulose-5-phosphate synthase gene and aroma in different grapevine varieties. Genes Genom. 2017, 39, 1059–1067. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, X.; Lu, Y.; Wang, H.; Chen, D.; Luo, C.; Liu, H.; Gao, S.; Lei, T.; Huang, C.; et al. Gas chromatography-mass spectrometry analysis of floral fragrance-related compounds in scented rose (Rosa hybrida) varieties and a subsequent evaluation on the basis of the analytical hierarchy process. Plant Physiol. Biochem. 2022, 185, 368–377. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Xu, N.; Cao, C.; Liu, J.; Wu, C.; Zhang, L. Analysis of Volatile Components in Cerasus Humilis (Bge.) Sok by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Sci. Agric. Sin. 2019, 52, 3448–3459. [Google Scholar] [CrossRef]
- Xiao, W.; Li, Z.; Chen, H.; Lu, F. Analysis of volatile components in flowers of four different Phalaenopsis germplasm resources by headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. J. China Agric. Univ. 2021, 26, 38–52. [Google Scholar] [CrossRef]
- Du, W.; Duan, Q.; Jia, W.; Ma, L.; Wang, X.; Wang, J. Determination of volatile compounds and analysis of characteristic aroma components of four kind of Begonia species flowers. J. Yunnan Agric. Univ. Nat. Sci. 2022, 44, 1043–1053. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Zheng, C.; Wang, C.; Yu, Y. Studies of aroma compounds in Chrysanthemum in different florescence and inflorescence and aroma releasing. Acta Bot. Boreali-Occident. Sin. 2012, 32, 722–730. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, X.; Wang, Q.; Wang, W.; Wang, J.; Xie, Y.; Wu, Y. Analysis of volatile components of Rhododendron fortunei at different flowering stages by HS-SPME-GC-MS and PCA. Guihaia 2020, 40, 1033–1045. [Google Scholar] [CrossRef]
- Kishimoto, K.; Nakayama, M.; Yagi, M.; Onozaki, T.; Oyama-Okubo, N. Evaluation of wild dianthus species as genetic resources for fragrant carnation breeding based on their floral scent composition. J. Jpn. Soc. Hortic. Sci. 2011, 80, 175–181. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 470. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Tong, Y.; Su, P.; Zhao, Y.; Zhang, M.; Wang, X.; Liu, Y.; Zhang, X.; Gao, W.; Huang, L. Molecular Cloning and Characterization of DXS and DXR Genes in the terpenoid biosynthetic pathway of Tripterygium wilfordii. Int. J. Mol. Sci. 2015, 16, 25516. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Li, K.; Shen, Y.; Li, H.; Chen, X. Cloning and expression analysis of HDS gene from Narcissus tazetta var. Chin. Tradit. Herb. Drugs 2015, 6, 1825–1830. [Google Scholar]
- Zhang, Z.; Liao, Z.; Peng, M. Cloning and functional analysis of HDS gene from Artemisia annua. Chin. Tradit. Herb. Drugs 2012, 43, 148–154. [Google Scholar]
- Yan, W.; Zhang, Q.; Liu, S.; Leng, P.; Hu, Z. Cloning and expression analysis of LiHDS gene from Lilium ‘Siberia’. Plant Physiol. J. 2023, 59, 362–372. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Lafond, T.P.; Gantt, E. Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J. Bacteriol. 2020, 182, 5841–5848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Lin, J.; Lu, J.; Sun, X.; Tang, K. Transformation of Ginkgo biloba with 1-hydroxy2-methyl-2-(E)-butenyl-4-diphosphate reductase gene. J. Fudan Univ. 2008, 47, 598–603. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Yang, L.; Choi, E.; Kim, S. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzyme Microb. Tech. 2015, 68, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, D.; Wang, G. Heteromeric geranyl (geranyl) diphosphate synthase is involved in monoterpene biosynthesis in Arabidopsis flowers. Mol. Plant 2015, 8, 1434–1437. [Google Scholar] [CrossRef]
- Tholl, D.; Kish, C.; Orlova, I.; Sherman, D.; Gershenzon, J.; Pichersky, E.; Dudareva, N. Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. Plant Cell. 2004, 16, 977–992. [Google Scholar] [CrossRef]
- Wang, G.; Dixon, R.A. Heterodimeric geranyl (geranyl) diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 9914–9919. [Google Scholar] [CrossRef]
- Hsiao, Y.; Jeng, M.; Tsai, W.; Chuang, Y.; Li, C.; Wu, T.; Kuoh, C.; Chen, W.; Chen, H. A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD (X) 2–4D motif. Plant J. 2008, 55, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Yu, R.; Fan, Y. Functional characterization and expression analysis of two terpene synthases involved in floral scent formation in Lilium ‘Siberia’. Planta 2019, 249, 71–93. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, H.; Ding, R.; Fang, L.; Guo, C.; Mao, J.; Yang, Q.; Chen, Z. Analysis of SPME-GC-MS on Volatile Aromatic Compounds of Jinchuanxue Pear and Qinsu. Food Res. Int. 2020, 41, 160–166. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Du, H.; Li, H.; Fang, L.; Guo, C.; Ding, R.; Mao, J.; Chen, Z. Analysis of Volatile Aroma Component in Taipo Pear by Solid Phase Micro–extraction Coupled with Gas Chromatography–Mass Spectrometry. Shandong Agric. Sci. 2018, 50, 53–58. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Van Gemert, L.J. Flavour Thresholds Compilations of Flavour Threshold Values in Water and Other Media, 2nd ed.; China Science Press: Beijing, China, 2015. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Gu, L.; Li, Y.; Zhi, H.; Luo, J.; Zhang, Y. Volatile Composition and Classification of Paeonia lactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes. Int. J. Mol. Sci. 2023, 24, 9410. https://doi.org/10.3390/ijms24119410
Zhao Q, Gu L, Li Y, Zhi H, Luo J, Zhang Y. Volatile Composition and Classification of Paeonia lactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes. International Journal of Molecular Sciences. 2023; 24(11):9410. https://doi.org/10.3390/ijms24119410
Chicago/Turabian StyleZhao, Qian, Lina Gu, Yuqing Li, Hui Zhi, Jianrang Luo, and Yanlong Zhang. 2023. "Volatile Composition and Classification of Paeonia lactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes" International Journal of Molecular Sciences 24, no. 11: 9410. https://doi.org/10.3390/ijms24119410
APA StyleZhao, Q., Gu, L., Li, Y., Zhi, H., Luo, J., & Zhang, Y. (2023). Volatile Composition and Classification of Paeonia lactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes. International Journal of Molecular Sciences, 24(11), 9410. https://doi.org/10.3390/ijms24119410





