The Lysyl Oxidase G473A Polymorphism Exacerbates Oral Cancer Development in Humans and Mice
Abstract
1. Introduction
2. Results
2.1. Increased Human Oral-Cancer Incidence in rs1800449 Polymorphic-Variant Subjects
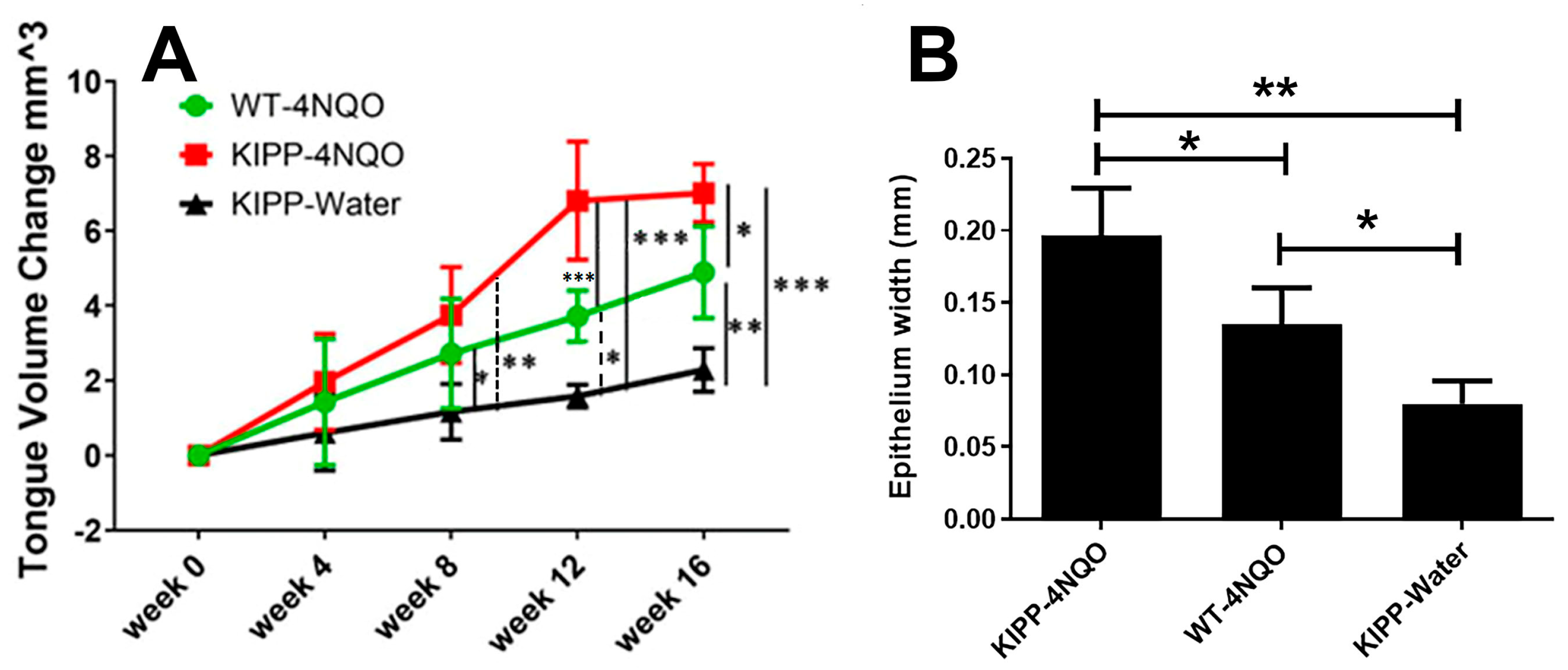
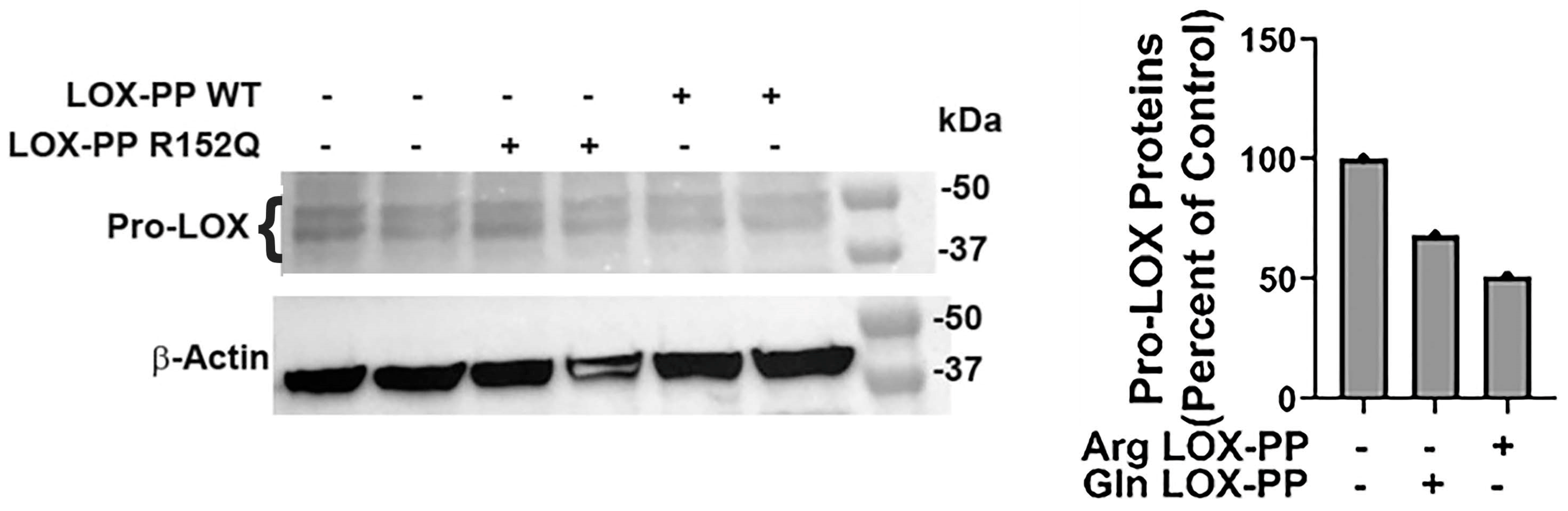
2.2. Increased Lesion Occurrence in Arg to Gln LOX-PP Knockin Mice
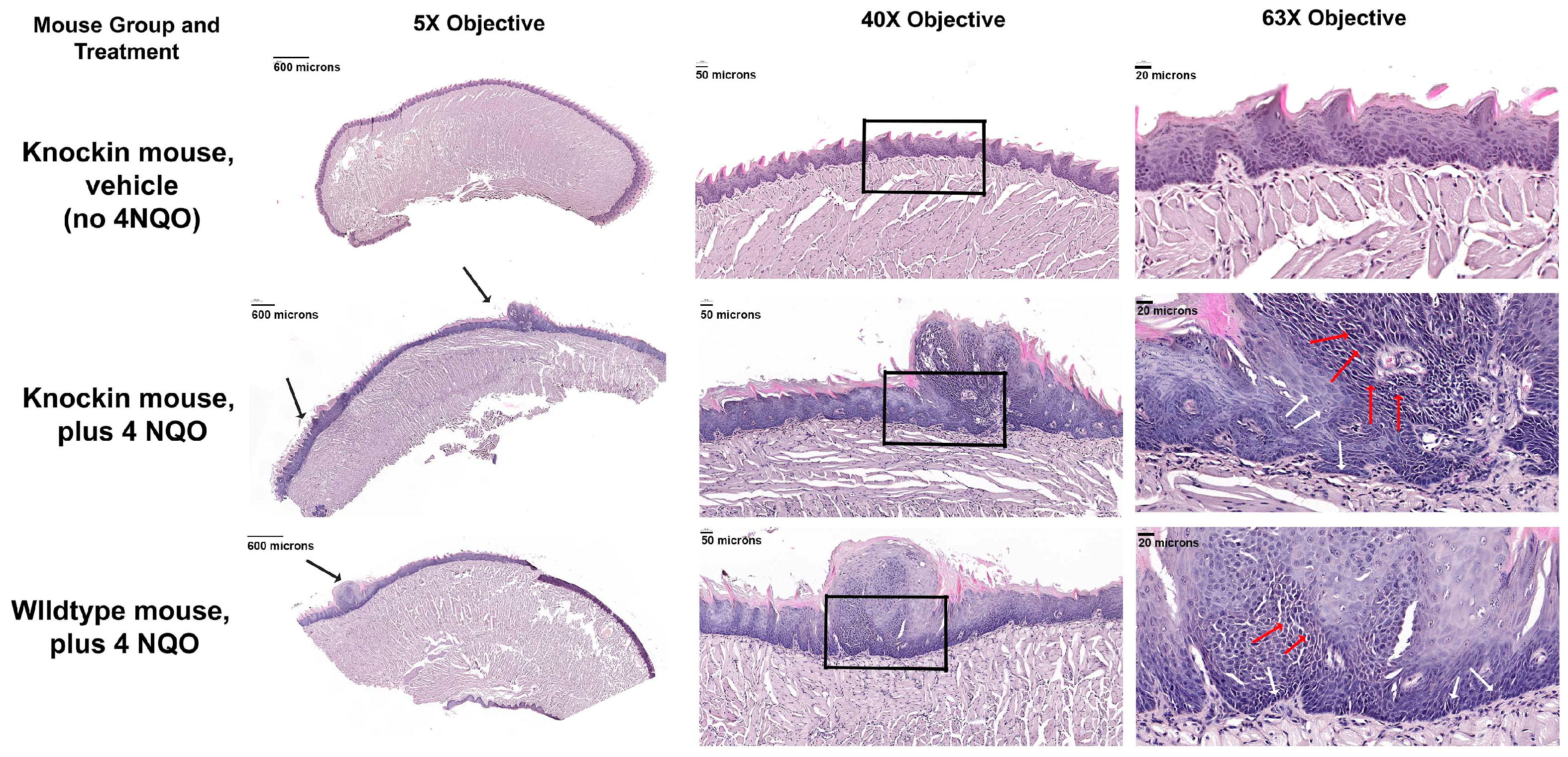
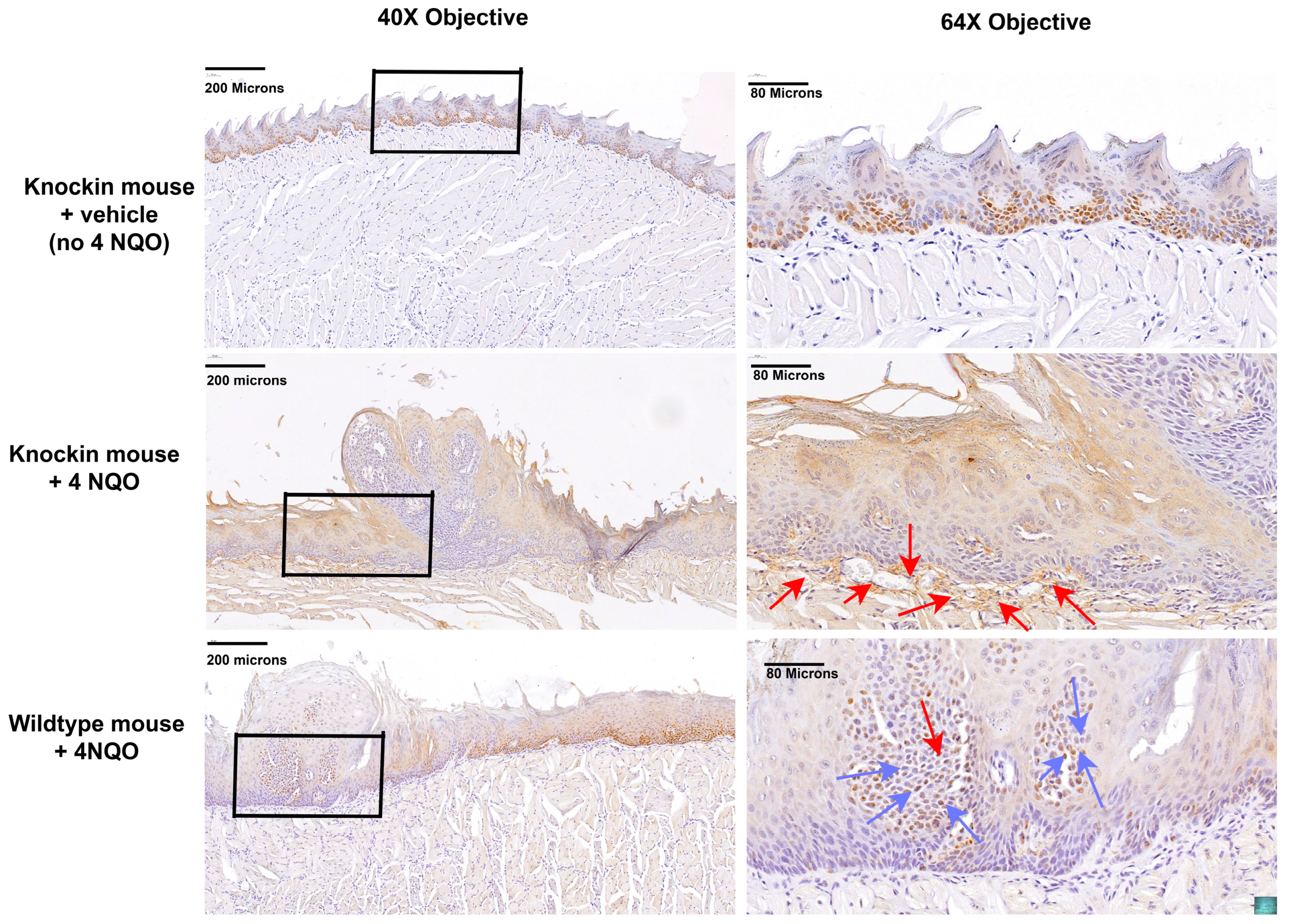
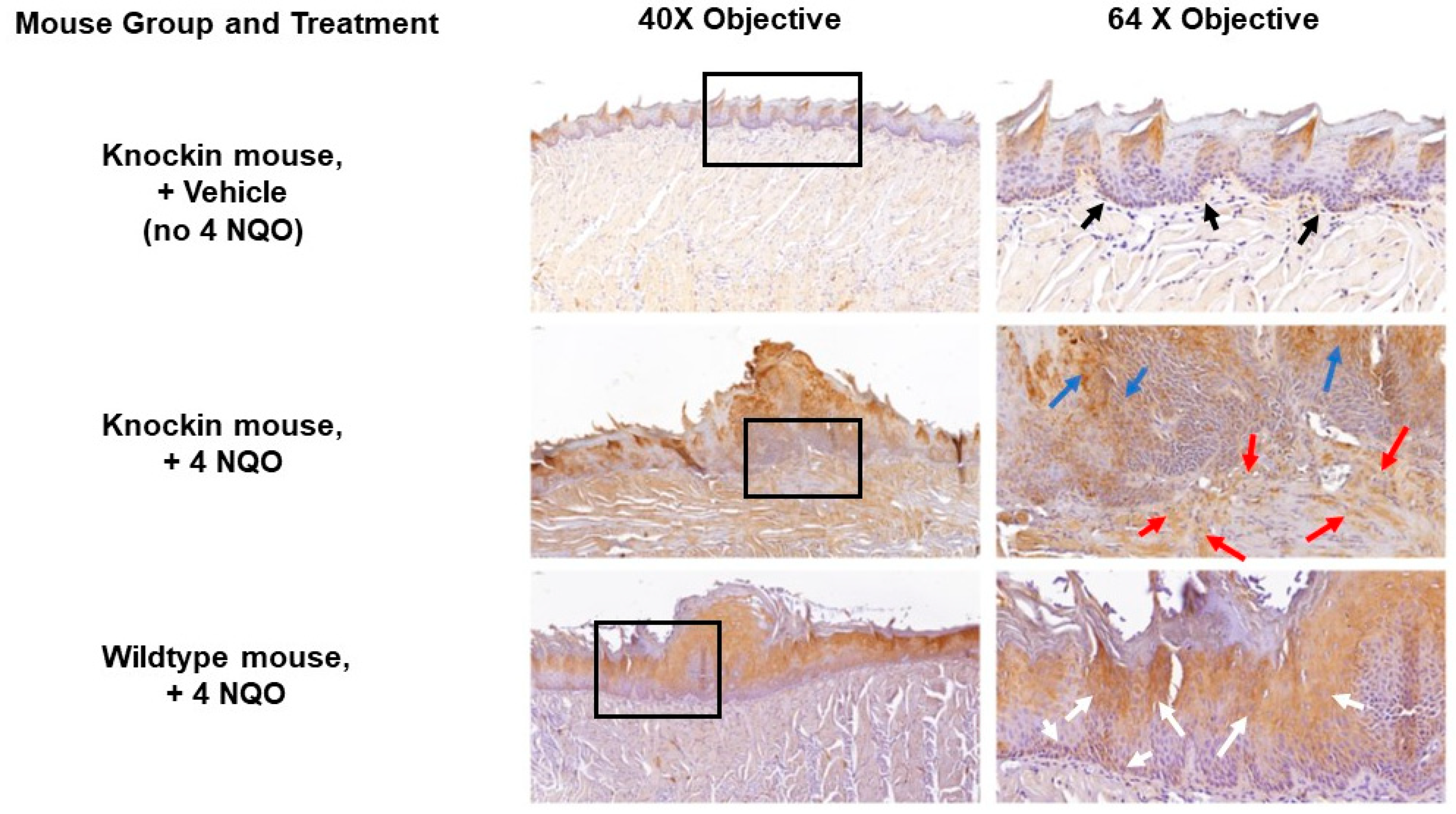
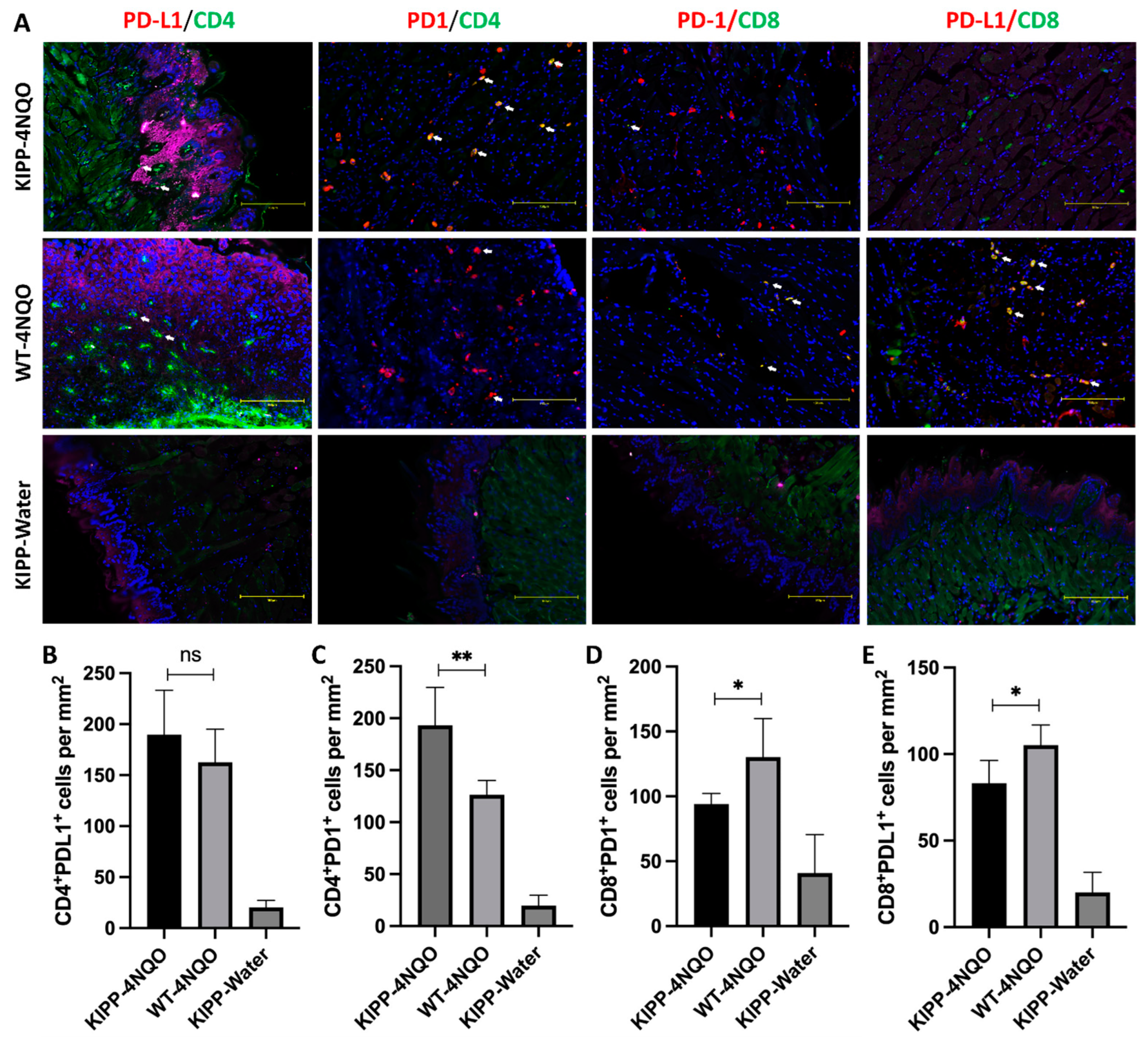
2.3. Histopathology of the Tongue Lesions
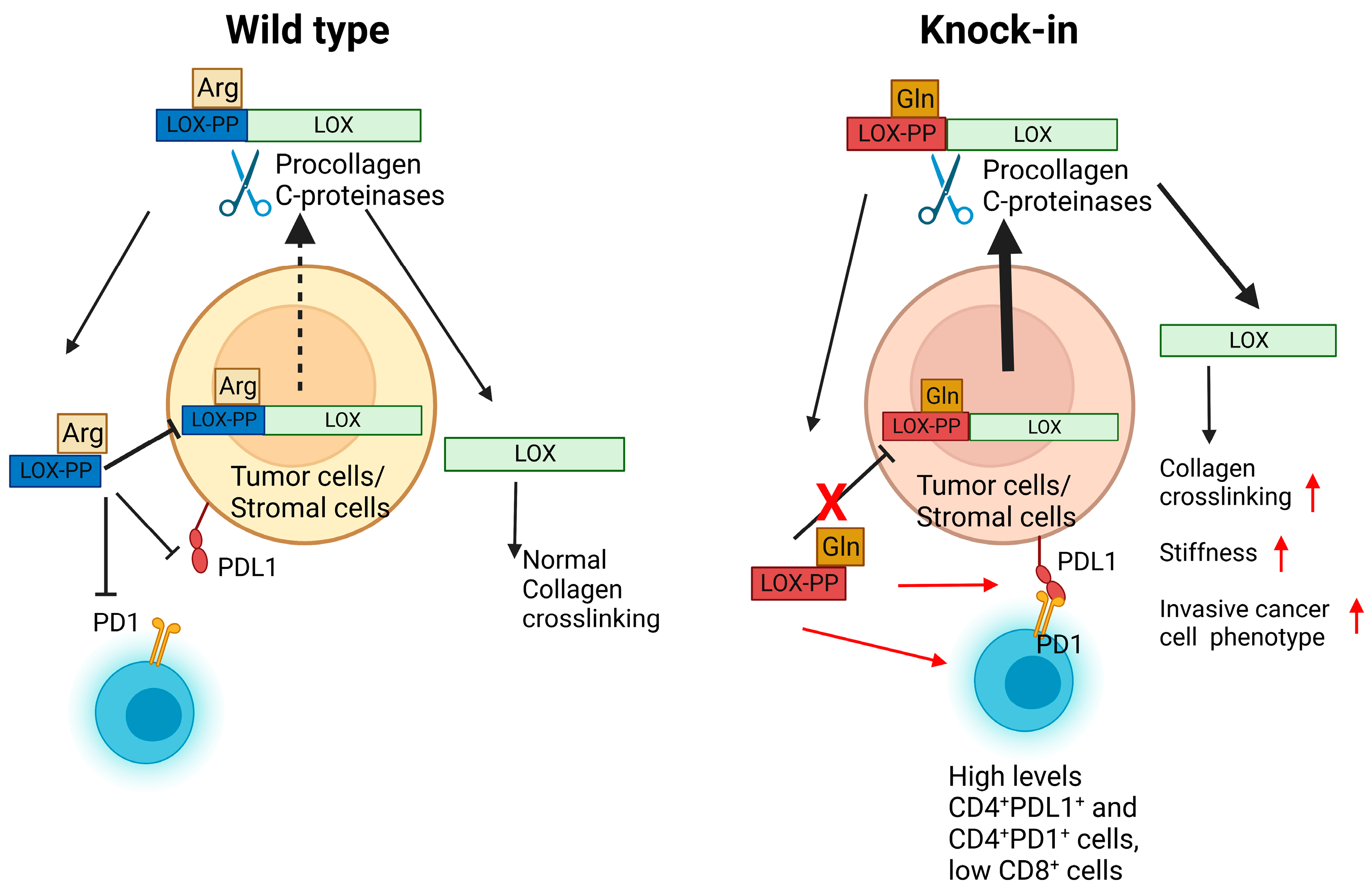
3. Discussion
4. Materials and Methods
4.1. LOX G473A Polymorphism in Oral-Cancer Patients
4.2. Animals Study and 4 NQO Treatment
4.3. Histology and Immunohistochemistry
4.4. Cell Culture and Western Blots
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kagan, H.M.; Trackman, P.C. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 1991, 5, 206–210. [Google Scholar] [CrossRef]
- Trackman, P.C.; Bedell-Hogan, D.; Tang, J.; Kagan, H.M. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J. Biol. Chem. 1992, 267, 8666–8671. [Google Scholar] [CrossRef]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef]
- Palamakumbura, A.H.; Jeay, S.; Guo, Y.; Pischon, N.; Sommer, P.; Sonenshein, G.E.; Trackman, P.C. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J. Biol. Chem. 2004, 279, 40593–40600. [Google Scholar] [CrossRef]
- Palamakumbura, A.H.; Vora, S.R.; Nugent, M.A.; Kirsch, K.H.; Sonenshein, G.E.; Trackman, P.C. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene 2009, 28, 3390–3400. [Google Scholar] [CrossRef]
- Vora, S.R.; Guo, Y.; Stephens, D.N.; Salih, E.; Vu, E.D.; Kirsch, K.H.; Sonenshein, G.E.; Trackman, P.C. Characterization of recombinant lysyl oxidase propeptide. Biochemistry 2010, 49, 2962–2972. [Google Scholar] [CrossRef]
- Bais, M.V.; Ozdener, G.B.; Sonenshein, G.E.; Trackman, P.C. Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways. Oncogene 2015, 34, 1928–1937. [Google Scholar] [CrossRef]
- Min, C.; Zhao, Y.; Romagnoli, M.; Trackman, P.C.; Sonenshein, G.E.; Kirsch, K.H. Lysyl oxidase propeptide sensitizes pancreatic and breast cancer cells to doxorubicin-induced apoptosis. J. Cell. Biochem. 2010, 111, 1160–1168. [Google Scholar] [CrossRef]
- Wu, M.; Min, C.; Wang, X.; Yu, Z.; Kirsch, K.H.; Trackman, P.C.; Sonenshein, G.E. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer es. 2007, 67, 6278–6285. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Wang, H.; Yan, W.; Zhang, Q.; Chang, X. Expression of the lysyl oxidase propeptide in hepatocellular carcinoma and its clinical relevance. Oncol. Rep. 2014, 31, 1669–1676. [Google Scholar] [CrossRef]
- Bais, M.V.; Nugent, M.A.; Stephens, D.N.; Sume, S.S.; Kirsch, K.H.; Sonenshein, G.E.; Trackman, P.C. Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts. PLoS ONE 2012, 7, e31188. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Yu, Z.; Kirsch, K.H.; Zhao, Y.; Vora, S.R.; Trackman, P.C.; Spicer, D.B.; Rosenberg, L.; Palmer, J.R.; Sonenshein, G.E. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009, 69, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Friesenhengst, A.; Pribitzer-Winner, T.; Schreiber, M. Association of the G473A polymorphism and expression of lysyl oxidase with breast cancer risk and survival in European women: A hospital-based case-control study. PLoS ONE 2014, 9, e105579. [Google Scholar] [CrossRef]
- Ren, J.; Wu, X.; He, W.; Shao, J.; Cheng, B.; Huang, T. Lysyl oxidase 473 G>A polymorphism and breast cancer susceptibility in Chinese Han population. DNA Cell Biol. 2011, 30, 111–116. [Google Scholar] [CrossRef]
- Wang, X.; Cong, J.L.; Qu, L.Y.; Jiang, L.; Wang, Y. Association between lysyl oxidase G473A polymorphism and ovarian cancer in the Han Chinese population. J. Int. Med. Res. 2012, 40, 917–923. [Google Scholar] [CrossRef]
- Wang, G.; Shen, Y.; Cheng, G.; Bo, H.; Lin, J.; Zheng, M.; Li, J.; Zhao, Y.; Li, W. Lysyl Oxidase Gene G473A Polymorphism and Cigarette Smoking in Association with a High Risk of Lung and Colorectal Cancers in a North Chinese Population. Int. J. Environ. Res. Public Health 2016, 13, 635. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Chen, K.M.; Zhang, S.M.; Sun, Y.W.; Amin, S.; Stoner, G.; Guttenplan, J.B. Carcinogenesis of the Oral Cavity: Environmental Causes and Potential Prevention by Black Raspberry. Chem. Res. Toxicol. 2017, 30, 126–144. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishigamori, R. Understanding carcinogenesis for fighting oral cancer. J. Oncol. 2011, 2011, 603740. [Google Scholar] [CrossRef]
- Groome, P.A.; Rohland, S.L.; Hall, S.F.; Irish, J.; Mackillop, W.J.; O’Sullivan, B. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011, 47, 642–647. [Google Scholar] [CrossRef]
- De la Cueva, A.; Emmerling, M.; Lim, S.L.; Yang, S.; Trackman, P.C.; Sonenshein, G.E.; Kirsch, K.H. A Polymorphism in the Lysyl Oxidase Propeptide Domain Accelerates Carcinogen-induced Cancer. Carcinogenesis 2018, 39, 921–930. [Google Scholar] [CrossRef]
- Kanojia, D.; Vaidya, M.M. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Thomson, P.J. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2018, 47, 803–807. [Google Scholar] [CrossRef]
- Cooper, J.S.; Porter, K.; Mallin, K.; Hoffman, H.T.; Weber, R.S.; Ang, K.K.; Gay, E.G.; Langer, C.J. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck 2009, 31, 748–758. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.; Mohammad, S. Oral cancer: Risk factors and molecular pathogenesis. J. Maxillofac. Oral Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef]
- Trackman, P.C.; Peymanfar, Y.; Roy, S. Functions and Mechanisms of Pro-Lysyl Oxidase Processing in Cancers and Eye Pathologies with a Focus on Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 5088. [Google Scholar] [CrossRef]
- Baker, A.-M.; Cox, T.R.; Bird, D.; Lang, G.; Murray, G.I.; Sun, X.-F.; Southall, S.M.; Wilson, J.R.; Erler, J.T. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J. Natl. Cancer Inst. 2011, 103, 407–424. [Google Scholar] [CrossRef]
- Erler, J.T.; Giaccia, A.J. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006, 66, 10238–10241. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Q.; Ma, H.; Li, L.; Liu, J.; Feng, Y.; Fang, Z.; Wu, J.; Han, X.; Zhang, J. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 18892–18897. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Spratt, D.E.; Chan, T.; Waldron, L.; Speers, C.; Feng, F.Y.; Ogunwobi, O.O.; Osborne, J.R. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2016, 2, 1070–1074. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Czerninski, R.; Amornphimoltham, P.; Patel, V.; Molinolo, A.A.; Gutkind, J.S. Chemical carcinogenesis models for evaluating molecular-targeted prevention and treatment of oral cancer. Cancer Prev. Res. 2009, 2, 419–422. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Saito, T.; Uzawa, K.; Terajima, M.; Shiiba, M.; Amelio, A.L.; Tanzawa, H.; Yamauchi, M. Aberrant Collagen Cross-linking in Human Oral Squamous Cell Carcinoma. J. Dent. Res. 2019, 98, 517–525. [Google Scholar] [CrossRef]
- Albinger-Hegyi, A.; Stoeckli, S.J.; Schmid, S.; Storz, M.; Iotzova, G.; Probst-Hensch, N.M.; Rehrauer, H.; Tinguely, M.; Moch, H.; Hegyi, I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC). Int. J. Cancer J. Int. Cancer 2010, 126, 2653–2662. [Google Scholar] [CrossRef]
- Min, C.; Kirsch, K.H.; Zhao, Y.; Jeay, S.; Palamakumbura, A.H.; Trackman, P.C.; Sonenshein, G.E. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007, 67, 1105–1112. [Google Scholar] [CrossRef]
- Nareshkumar, R.N.; Sulochana, K.N.; Coral, K. Inhibition of angiogenesis in endothelial cells by Human Lysyl oxidase propeptide. Sci. Rep. 2018, 8, 10426. [Google Scholar] [CrossRef]
- Pichu, S.; Sathiyamoorthy, J.; Vimalraj, S.; Viswanathan, V.; Chatterjee, S. Impact of lysyl oxidase (G473A) polymorphism on diabetic foot ulcers. Int. J. Biol. Macromol. 2017, 103, 242–247. [Google Scholar] [CrossRef]
- Griner, J.D.; Rogers, C.J.; Zhu, M.-J.; Du, M. Lysyl oxidase propeptide promotes adipogenesis through inhibition of FGF-2 signaling. Adipocyte 2017, 6, 12–19. [Google Scholar] [CrossRef]
- Mahjour, F.; Dambal, V.; Shrestha, N.; Singh, V.; Noonan, V.; Kantarci, A.; Trackman, P.C. Mechanism for oral tumor cell lysyl oxidase like-2 in cancer development: Synergy with PDGF-AB. Oncogenesis 2019, 8, 34. [Google Scholar] [CrossRef]
- Xiao, Q.; Ge, G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron. 2012, 5, 261–273. [Google Scholar] [CrossRef]
- NIH SNP rs1800449. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1800449 (accessed on 21 April 2023).
- Hurtado, P.A.; Vora, S.; Sume, S.S.; Yang, D.; St Hilaire, C.; Guo, Y.; Palamakumbura, A.H.; Schreiber, B.M.; Ravid, K.; Trackman, P.C. Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation. Biochem. Biophys. Res. Commun. 2008, 366, 156–161. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Wu, J.; Song, A.; Annapragada, A.; Zacharias, W. Hypoxia-induced autophagic response is associated with aggressive phenotype and elevated incidence of metastasis in orthotopic immunocompetent murine models of head and neck squamous cell carcinomas (HNSCC). Exp. Mol. Pathol. 2011, 90, 215–225. [Google Scholar] [CrossRef]



| LOX-PP Knock-in Group Treated with 4 NQO (8 Mice) | Wild Type Group Treated with 4 NQO (7 Mice) | LOX-PP Knock-in Group Treated with Water (5 Mice) | |
|---|---|---|---|
| 12th Week | 1 | 0 | 0 |
| 16th Week | 2 | 0 | 0 |
| 17th Week | 3 | 1 | 0 |
| Total | 6 (75%) * | 1 (14%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peymanfar, Y.; Mahjour, F.; Shrestha, N.; de la Cueva, A.; Chen, Y.; Huang, S.; Kirsch, K.H.; Han, X.; Trackman, P.C. The Lysyl Oxidase G473A Polymorphism Exacerbates Oral Cancer Development in Humans and Mice. Int. J. Mol. Sci. 2023, 24, 9407. https://doi.org/10.3390/ijms24119407
Peymanfar Y, Mahjour F, Shrestha N, de la Cueva A, Chen Y, Huang S, Kirsch KH, Han X, Trackman PC. The Lysyl Oxidase G473A Polymorphism Exacerbates Oral Cancer Development in Humans and Mice. International Journal of Molecular Sciences. 2023; 24(11):9407. https://doi.org/10.3390/ijms24119407
Chicago/Turabian StylePeymanfar, Yaser, Faranak Mahjour, Neha Shrestha, Ana de la Cueva, Ying Chen, Shengyuan Huang, Kathrin H. Kirsch, Xiaozhe Han, and Philip C. Trackman. 2023. "The Lysyl Oxidase G473A Polymorphism Exacerbates Oral Cancer Development in Humans and Mice" International Journal of Molecular Sciences 24, no. 11: 9407. https://doi.org/10.3390/ijms24119407
APA StylePeymanfar, Y., Mahjour, F., Shrestha, N., de la Cueva, A., Chen, Y., Huang, S., Kirsch, K. H., Han, X., & Trackman, P. C. (2023). The Lysyl Oxidase G473A Polymorphism Exacerbates Oral Cancer Development in Humans and Mice. International Journal of Molecular Sciences, 24(11), 9407. https://doi.org/10.3390/ijms24119407








