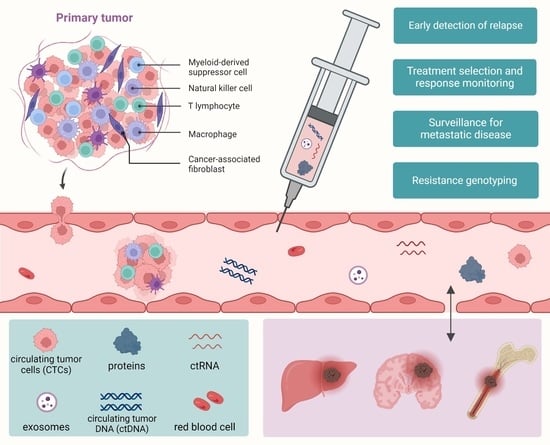
Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis
Abstract
1. Introduction
2. Current Liquid Biopsy Samples and Biomarkers for Late-Stage Lung Cancer
2.1. Sample Subtypes of Liquid Biopsies Used in Advanced-Stage Lung Cancer
2.2. Biomarker Subtypes of Liquid Biopsies Used in Advanced-Stage Lung Cancer
2.2.1. Extracellular Vesicles (EVs)
2.2.2. Circulating Tumor DNA (ctDNA)
2.2.3. Cell-Free RNAs (cfRNAs)
2.2.4. DNA Methylation Markers
3. Monitoring Minimal Residual Disease (MRD)
3.1. Clonal Evolution and Tumor Heterogeneity within MRD
3.2. Minimal Residual Disease (MRD) and the Role of Liquid Biopsy in Detecting Lung Cancer Recurrence
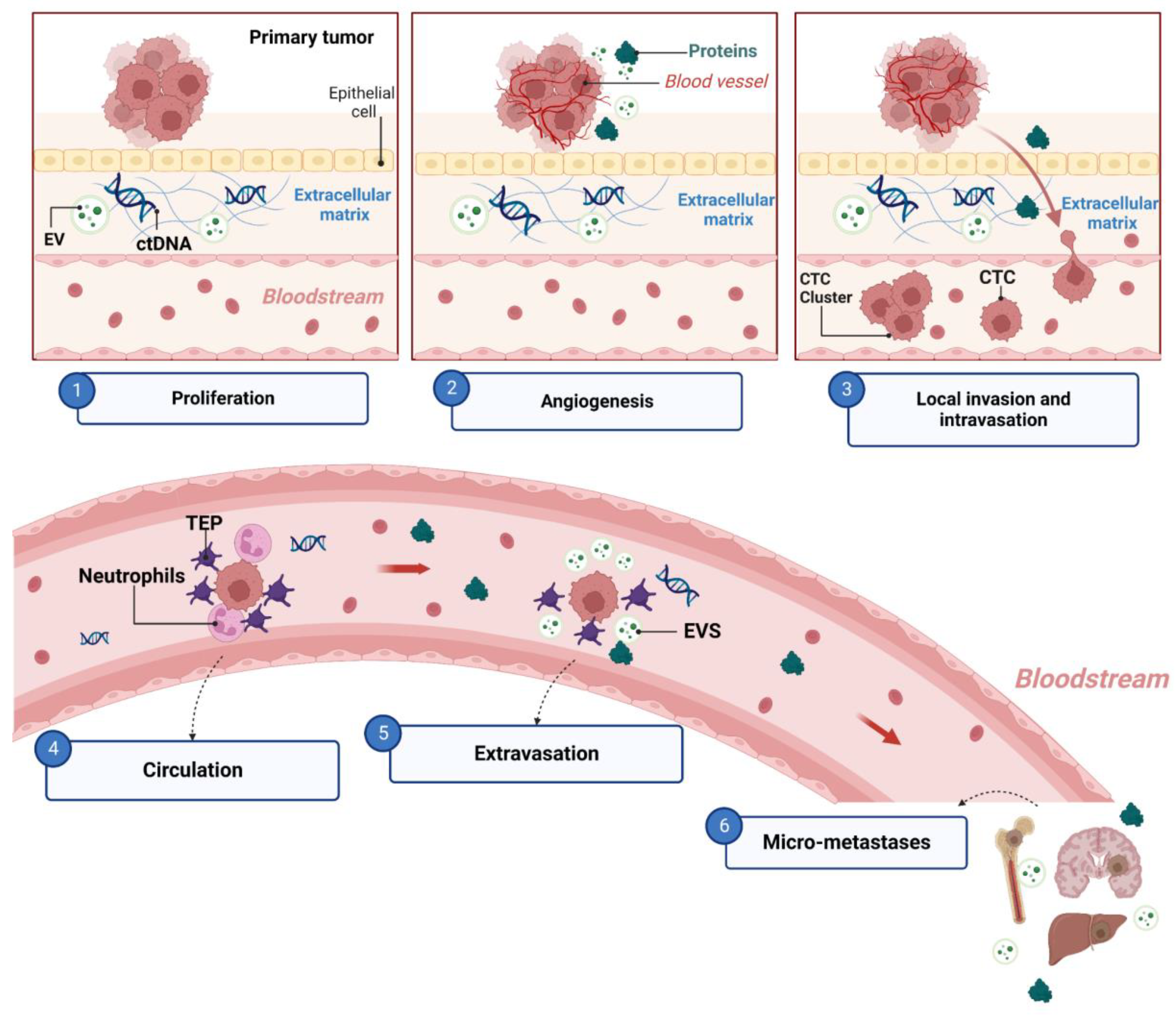
4. Metastasis
4.1. The Metastatic Cascade and Associated Biomarkers
4.2. Applications of Liquid Biopsy in Metastatic Lung Cancer
4.2.1. Liquid Biopsy for the Early Detection of Lung Cancer Metastasis
4.2.2. Liquid Biopsy to Monitor Treatment Response and Identify Actionable Mutations in the Clinic
5. Nucleic-Acid-Based Methods for Detecting Lung Cancer in Liquid Biopsies
5.1. Multiplex-Hybridization-Based and PCR-Based Methods
5.2. NGS Methods
| Detection Method | Multiplexing (Number of Markers) | Turnaround Time (TAT) | Sensitivity/Limit of Detection (LOD) |
|---|---|---|---|
| Multiplex-hybridization-based methods | nanoString nCounter: 800+ target genes | Dependent on panel [212] | Sensitivity of 95% and specificity of 82% [213] 0.02–2% MAF [214] |
| PCR-based methods | Small, predetermined gene panels | ~2–3 days depending on panel [72] | qPCR: >10% MAF [215] dPCR: ~0.01% MAF [216] |
| NGS-based methods | WES: entire exome. WGS: entire genome. Targeted panels: large number of genes (e.g., TSO500 uses 500-gene panel [217]) | ~13 days depending on panel [72,218] | <1% MAF, can be <0.1% with specialized methods [211] |
5.3. Fragmentomics and Long-Read Sequencing
5.4. Single-Cell Sequencing
6. Emerging Approaches for Liquid Biopsy of Lung Cancer
6.1. Microbiome
6.2. Tumor-Educated Platelets (TEPs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lung and Bronchus Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 30 April 2023).
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.M.; Vandermeer, R. Delays in the Diagnosis of Lung Cancer. J. Thorac. Dis. 2011, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic Sites and Survival in Lung Cancer. Lung Cancer Amst. Neth. 2014, 86, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Popper, H.H. Progression and Metastasis of Lung Cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Souza, V.G.P.; de Araújo, R.P.; Santesso, M.R.; Seneda, A.L.; Minutentag, I.W.; Felix, T.F.; Hamamoto Filho, P.T.; Pewarchuk, M.E.; Brockley, L.J.; Marchi, F.A.; et al. Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers 2023, 15, 722. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific Organ Metastases and Survival in Metastatic Non-Small-Cell Lung Cancer. Mol. Clin. Oncol. 2015, 3, 217–221. [Google Scholar] [CrossRef]
- Little, A.G.; Gay, E.G.; Gaspar, L.E.; Stewart, A.K. National Survey of Non-Small Cell Lung Cancer in the United States: Epidemiology, Pathology and Patterns of Care. Lung Cancer Amst. Neth. 2007, 57, 253–260. [Google Scholar] [CrossRef]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific Organ Metastases and Survival in Small Cell Lung Cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef]
- Lou, F.; Sima, C.S.; Rusch, V.W.; Jones, D.R.; Huang, J. Differences in Patterns of Recurrence in Early-Stage versus Locally Advanced Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 98, 1755–1760; discussion 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after Surgery in Patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Qiao, L.; Liang, N.; Zhang, J. Risk Factors for Recurrence in Patients with Resected N1 Non-Small Cell Lung Cancer—A Systematic Review and Meta-Analysis. J. BUON Off. J. Balk. Union Oncol. 2015, 20, 791–799. [Google Scholar]
- Cruz, C.; Afonso, M.; Oliveiros, B.; Pêgo, A. Recurrence and Risk Factors for Relapse in Patients with Non-Small Cell Lung Cancer Treated by Surgery with Curative Intent. Oncology 2017, 92, 347–352. [Google Scholar] [CrossRef]
- al-Kattan, K.; Sepsas, E.; Fountain, S.W.; Townsend, E.R. Disease Recurrence after Resection for Stage I Lung Cancer. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 1997, 12, 380–384. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, X.; Zhang, X.; Wen, Y.; Lin, P.; Rong, T.; Cai, L.; Zhang, L. Time-Varying Pattern of Postoperative Recurrence Risk of Early-Stage (T1a-T2bN0M0) Non-Small Cell Lung Cancer (NSCLC): Results of a Single-Center Study of 994 Chinese Patients. PLoS ONE 2014, 9, e106668. [Google Scholar] [CrossRef]
- Inoue, A.; Kobayashi, K.; Maemondo, M.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Updated Overall Survival Results from a Randomized Phase III Trial Comparing Gefitinib with Carboplatin-Paclitaxel for Chemo-Naïve Non-Small Cell Lung Cancer with Sensitive EGFR Gene Mutations (NEJ002). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 54–59. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus Cisplatin plus Docetaxel in Patients with Non-Small-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Blackhall, F.; Kim, D.-W.; Besse, B.; Nokihara, H.; Han, J.-Y.; Wilner, K.D.; Reisman, A.; Iyer, S.; Hirsh, V.; Shaw, A.T. Patient-Reported Outcomes and Quality of Life in PROFILE 1007: A Randomized Trial of Crizotinib Compared with Chemotherapy in Previously Treated Patients with ALK-Positive Advanced Non-Small-Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 1625–1633. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. PD-1/PD-L1 Inhibitors for Non-Small Cell Lung Cancer: Incorporating Care Step Pathways for Effective Side-Effect Management. J. Adv. Pract. Oncol. 2019, 10, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet Lond. Engl. 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Q.; Yao, R.; Liu, J. Current Status and Development of Anti-PD-1/PD-L1 Immunotherapy for Lung Cancer. Int. Immunopharmacol. 2020, 79, 106088. [Google Scholar] [CrossRef]
- Chouaid, C.; Dujon, C.; Do, P.; Monnet, I.; Madroszyk, A.; Le Caer, H.; Auliac, J.B.; Berard, H.; Thomas, P.; Lena, H.; et al. Feasibility and Clinical Impact of Re-Biopsy in Advanced Non Small-Cell Lung Cancer: A Prospective Multicenter Study in a Real-World Setting (GFPC Study 12-01). Lung Cancer Amst. Neth. 2014, 86, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Fintelmann, F.J.; Troschel, F.M.; Kuklinski, M.W.; McDermott, S.; Petranovic, M.; Digumarthy, S.R.; Sharma, A.; Troschel, A.S.; Price, M.C.; Hariri, L.P.; et al. Safety and Success of Repeat Lung Needle Biopsies in Patients with Epidermal Growth Factor Receptor-Mutant Lung Cancer. Oncologist 2019, 24, 1570–1576. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Ho, H.-L.; Jiang, Y.; Chiang, C.-L.; Karwowska, S.; Yerram, R.; Sharma, K.; Scudder, S.; Chiu, C.-H.; Tsai, C.-M.; Palma, J.F.; et al. Efficacy of Liquid Biopsy for Disease Monitoring and Early Prediction of Tumor Progression in EGFR Mutation-Positive Non-Small Cell Lung Cancer. PLoS ONE 2022, 17, e0267362. [Google Scholar] [CrossRef]
- Liang, H.; Huang, J.; Wang, B.; Liu, Z.; He, J.; Liang, W. The Role of Liquid Biopsy in Predicting Post-Operative Recurrence of Non-Small Cell Lung Cancer. J. Thorac. Dis. 2018, 10, S838–S845. [Google Scholar] [CrossRef]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.; Indraccolo, S. Liquid Biopsy and Non-Small Cell Lung Cancer: Are We Looking at the Tip of the Iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.G.O.; Sousa, C.; Pereira Reis, J.; Cruz-Martins, N.; Souto Moura, C.; Guimarães, S.; Justino, A.; Pina, M.J.; Magalhães, A.; Queiroga, H.; et al. Liquid Biopsy for Disease Monitoring in Non-Small Cell Lung Cancer: The Link between Biology and the Clinic. Cells 2021, 10, 1912. [Google Scholar] [CrossRef]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.-M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, H. Liquid Biopsy in Tumors: Opportunities and Challenges. Ann. Transl. Med. 2018, 6, S89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Chen, Y.; Xiao, N.; Zheng, Z.; Liu, H.; Wan, J. Liquid Biopsy on the Horizon in Immunotherapy of Non-Small Cell Lung Cancer: Current Status, Challenges, and Perspectives. Cell Death Dis. 2023, 14, 230. [Google Scholar] [CrossRef]
- Zhu, W.; Love, K.; Gray, S.W.; Raz, D.J. Liquid Biopsy Screening for Early Detection of Lung Cancer: Current State and Future Directions. Clin. Lung Cancer 2023, 24, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, B.; Skrzypski, M.; Bieńkowski, M.; Dziadziuszko, R.; Jassem, J. Current and Future Applications of Liquid Biopsy in Non-Small-Cell Lung Cancer-a Narrative Review. Transl. Lung Cancer Res. 2023, 12, 594–614. [Google Scholar] [CrossRef]
- Melichar, B. Biomarkers in the Management of Lung Cancer: Changing the Practice of Thoracic Oncology. Clin. Chem. Lab. Med. 2023, 61, 906–920. [Google Scholar] [CrossRef]
- Khan, S.R.; Scheffler, M.; Soomar, S.M.; Rashid, Y.A.; Moosajee, M.; Ahmad, A.; Raza, A.; Uddin, S. Role of Circulating-Tumor DNA in the Early-Stage Non-Small Cell Lung Carcinoma as a Predictive Biomarker. Pathol. Res. Pract. 2023, 245, 154455. [Google Scholar] [CrossRef]
- Lianidou, E.; Pantel, K. Liquid Biopsies. Genes. Chromosomes Cancer 2019, 58, 219–232. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.-B.; Hou, L.-K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.-M.; Sun, F.; Lu, H.-M.; Deng, J.; et al. Liquid Biopsy in Lung Cancer: Significance in Diagnostics, Prediction, and Treatment Monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Brockley, L.J.; Souza, V.G.P.; Forder, A.; Pewarchuk, M.E.; Erkan, M.; Telkar, N.; Benard, K.; Trejo, J.; Stewart, M.D.; Stewart, G.L.; et al. Sequence-Based Platforms for Discovering Biomarkers in Liquid Biopsy of Non-Small-Cell Lung Cancer. Cancers 2023, 15, 2275. [Google Scholar] [CrossRef]
- Fernandes, M.G.O.; Cruz-Martins, N.; Souto Moura, C.; Guimarães, S.; Pereira Reis, J.; Justino, A.; Pina, M.J.; Magalhães, A.; Queiroga, H.; Machado, J.C.; et al. Clinical Application of Next-Generation Sequencing of Plasma Cell-Free DNA for Genotyping Untreated Advanced Non-Small Cell Lung Cancer. Cancers 2021, 13, 2707. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Wu, W.; Yang, H.; Jin, Y.; Wu, M.; Liu, W.; Yang, A.; Chervova, O.; Zhang, S.; et al. Multi-Omics Integrated Circulating Cell-Free DNA Genomic Signatures Enhanced the Diagnostic Performance of Early-Stage Lung Cancer and Postoperative Minimal Residual Disease. eBioMedicine 2023, 91, 104553. [Google Scholar] [CrossRef]
- Aggarwal, C.; Rolfo, C.D.; Oxnard, G.R.; Gray, J.E.; Sholl, L.M.; Gandara, D.R. Strategies for the Successful Implementation of Plasma-Based NSCLC Genotyping in Clinical Practice. Nat. Rev. Clin. Oncol. 2021, 18, 56–62. [Google Scholar] [CrossRef]
- Mack, P.C.; Miao, J.; Redman, M.W.; Moon, J.; Goldberg, S.B.; Herbst, R.S.; Melnick, M.A.; Walther, Z.; Hirsch, F.R.; Politi, K.; et al. Circulating Tumor DNA Kinetics Predict Progression-Free and Overall Survival in EGFR TKI-Treated Patients with EGFR-Mutant NSCLC (SWOG S1403). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 3752–3760. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating Exosomal MicroRNAs as Prognostic Biomarkers for Non-Small-Cell Lung Cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef] [PubMed]
- Hallermayr, A.; Benet-Pagès, A.; Steinke-Lange, V.; Mansmann, U.; Rentsch, M.; Holinski-Feder, E.; Pickl, J.M.A. Liquid Biopsy Hotspot Variant Assays: Analytical Validation for Application in Residual Disease Detection and Treatment Monitoring. Clin. Chem. 2021, 67, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Politi, K.; Pitteri, S.J.; Lockwood, W.W.; Faça, V.M.; Kelly-Spratt, K.; Wong, C.-H.; Zhang, Q.; Chin, A.; Park, K.-S.; et al. Lung Cancer Signatures in Plasma Based on Proteome Profiling of Mouse Tumor Models. Cancer Cell 2011, 20, 289–299. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Jin, Y.; Si, X.; Jiao, W.; He, W.; Mao, W.; Li, M.; Luo, G. Plasma Long Non-Coding RNA RP11-438N5.3 as a Novel Biomarker for Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 1513–1521. [Google Scholar] [CrossRef]
- Giallombardo, M.; Jorge Chacartegui, J.; Reclusa, P.; Van Meerbeeck, J.P.; Alessandro, R.; Peeters, M.; Pauwels, P.; Rolfo, C.D. Follow up Analysis by Exosomal MiRNAs in EGFR Mutated Non-Small Cell Lung Cancer (NSCLC) Patients during Osimertinib (AZD9291) Treatment: A Potential Prognostic Biomarker Tool. J. Clin. Oncol. 2016, 34, e23035. [Google Scholar] [CrossRef]
- Hori, S.; Nishiumi, S.; Kobayashi, K.; Shinohara, M.; Hatakeyama, Y.; Kotani, Y.; Hatano, N.; Maniwa, Y.; Nishio, W.; Bamba, T.; et al. A Metabolomic Approach to Lung Cancer. Lung Cancer Amst. Neth. 2011, 74, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, S.; Iannantuono, G.M.; Barchiesi, G.; Magri, V.; Gelibter, A.; Cortesi, E. Prolactin as a Potential Early Predictive Factor in Metastatic Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Oncology 2017, 93, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Duréndez-Sáez, E.; Torres-Martinez, S.; Calabuig-Fariñas, S.; Meri-Abad, M.; Ferrero-Gimeno, M.; Camps, C. Exosomal MicroRNAs in Non-Small Cell Lung Cancer. Transl. Cancer Res. 2021, 10, 3128–3139. [Google Scholar] [CrossRef]
- Wang, C.-F.; Peng, S.-J.; Liu, R.-Q.; Yu, Y.-J.; Ge, Q.-M.; Liang, R.-B.; Li, Q.-Y.; Li, B.; Shao, Y. The Combination of CA125 and NSE Is Useful for Predicting Liver Metastasis of Lung Cancer. Dis. Mark. 2020, 2020, 8850873. [Google Scholar] [CrossRef]
- Lou, E.; Johnson, M.; Sima, C.; Gonzalez-Espinoza, R.; Fleisher, M.; Kris, M.G.; Azzoli, C.G. Serum Biomarkers for Assessing Histology and Outcomes in Patients with Metastatic Lung Cancer. Cancer Biomark. Sect. Dis. Mark. 2014, 14, 207–214. [Google Scholar] [CrossRef]
- Varella-Garcia, M.; Schulte, A.P.; Wolf, H.J.; Feser, W.J.; Zeng, C.; Braudrick, S.; Yin, X.; Hirsch, F.R.; Kennedy, T.C.; Keith, R.L.; et al. The Detection of Chromosomal Aneusomy by Fluorescence in Situ Hybridization in Sputum Predicts Lung Cancer Incidence. Cancer Prev. Res. Phila. Pa 2010, 3, 447–453. [Google Scholar] [CrossRef]
- Guo, T.; Hu, C.; Qin, L.; Yang, H.; Gu, Q.; Cao, L.; Deng, P.; Han-Zhang, H.; Li, B.; Ye, J.; et al. Sputum Supernatant as an Alternative Source for Liquid Biopsy in Patients with Advanced Lung Cancer. J. Clin. Oncol. 2020, 38, e21617. [Google Scholar] [CrossRef]
- Xie, X.; Wu, J.; Guo, B.; Wang, L.; Deng, H.; Lin, X.; Liu, M.; Qin, Y.; Luo, W.; Yang, Y.; et al. Comprehensive Characterization Reveals Sputum Supernatant as a Valuable Alternative Liquid Biopsy for Genome Profiling in Advanced Non-Small Cell Lung Cancer. Respir. Res. 2022, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Guo, T.; Yang, H.; Deng, P.; Gu, Q.; Liu, C.; Wu, M.; Lizaso, A.; Li, B.; Zhang, S.; et al. The Utility of Sputum Supernatant as an Alternative Liquid Biopsy Specimen for Next-Generation Sequencing-Based Somatic Variation Profiling. Ann. Transl. Med. 2022, 10, 462. [Google Scholar] [CrossRef]
- Rennard, S.I. Bronchoalveolar Lavage in the Assessment of Primary and Metastatic Lung Cancer. Respir. Int. Rev. Thorac. Dis. 1992, 59 (Suppl. 1), 41–43. [Google Scholar] [CrossRef]
- Matthiesen, R. MS-Based Biomarker Discovery in Bronchoalveolar Lavage Fluid for Lung Cancer. Proteomics Clin. Appl. 2020, 14, e1900077. [Google Scholar] [CrossRef]
- Porcel, J.M.; Esquerda, A.; Martínez-Alonso, M.; Bielsa, S.; Salud, A. Identifying Thoracic Malignancies Through Pleural Fluid Biomarkers: A Predictive Multivariate Model. Medicine 2016, 95, e3044. [Google Scholar] [CrossRef] [PubMed]
- Jany, B.; Welte, T. Pleural Effusion in Adults-Etiology, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2019, 116, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hur, J.Y.; Kim, I.A.; Kim, H.J.; Choi, C.M.; Lee, J.C.; Kim, W.S.; Lee, K.Y. Liquid Biopsy Using the Supernatant of a Pleural Effusion for EGFR Genotyping in Pulmonary Adenocarcinoma Patients: A Comparison between Cell-Free DNA and Extracellular Vesicle-Derived DNA. BMC Cancer 2018, 18, 1236. [Google Scholar] [CrossRef]
- Oshi, M.; Murthy, V.; Takahashi, H.; Huyser, M.; Okano, M.; Tokumaru, Y.; Rashid, O.M.; Matsuyama, R.; Endo, I.; Takabe, K. Urine as a Source of Liquid Biopsy for Cancer. Cancers 2021, 13, 2652. [Google Scholar] [CrossRef]
- Huang, W.-L.; Chen, Y.-L.; Yang, S.-C.; Ho, C.-L.; Wei, F.; Wong, D.T.; Su, W.-C.; Lin, C.-C. Liquid Biopsy Genotyping in Lung Cancer: Ready for Clinical Utility? Oncotarget 2017, 8, 18590–18608. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Sandúa, A.; Alegre, E.; González, Á. Exosomes in Lung Cancer: Actors and Heralds of Tumor Development. Cancers 2021, 13, 4330. [Google Scholar] [CrossRef]
- Rizwan, M.N.; Ma, Y.; Nenkov, M.; Jin, L.; Schröder, D.C.; Westermann, M.; Gaßler, N.; Chen, Y. Tumor-Derived Exosomes: Key Players in Non-Small Cell Lung Cancer Metastasis and Their Implication for Targeted Therapy. Mol. Carcinog. 2022, 61, 269–280. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, L.-Z.; Dong, M. Progress on Pivotal Role and Application of Exosome in Lung Cancer Carcinogenesis, Diagnosis, Therapy and Prognosis. Mol. Cancer 2021, 20, 22. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, C.; Du, T.; Gabriel, A.N.A.; Wang, X.; Li, X.; Sun, L.; Wang, N.; Jiang, X.; Zhang, Y. Progress of Exosomes in the Diagnosis and Treatment of Lung Cancer. Biomed. Pharmacother. Biomedecine Pharmacother. 2021, 134, 111111. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Forder, A.; Hsing, C.-Y.; Trejo Vazquez, J.; Garnis, C. Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis. Cells 2021, 10, 3429. [Google Scholar] [CrossRef]
- Kwok, H.-H.; Ning, Z.; Chong, P.W.-C.; Wan, T.S.-K.; Ng, M.H.-L.; Ho, G.Y.F.; Ip, M.S.-M.; Lam, D.C.-L. Transfer of Extracellular Vesicle-Associated-RNAs Induces Drug Resistance in ALK-Translocated Lung Adenocarcinoma. Cancers 2019, 11, 104. [Google Scholar] [CrossRef]
- Pasini, L.; Ulivi, P. Extracellular Vesicles in Non-Small-Cell Lung Cancer: Functional Role and Involvement in Resistance to Targeted Treatment and Immunotherapy. Cancers 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y.; Lee, J.S.; Kim, I.A.; Kim, H.J.; Kim, W.S.; Lee, K.Y. Extracellular Vesicle-Based EGFR Genotyping in Bronchoalveolar Lavage Fluid from Treatment-Naive Non-Small Cell Lung Cancer Patients. Transl. Lung Cancer Res. 2019, 8, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Herrero, E.; Campos-Silva, C.; Cáceres-Martell, Y.; Robado de Lope, L.; Sanz-Moreno, S.; Serna-Blasco, R.; Rodríguez-Festa, A.; Ares Trotta, D.; Martín-Acosta, P.; Patiño, C.; et al. ALK-Fusion Transcripts Can Be Detected in Extracellular Vesicles (EVs) from Nonsmall Cell Lung Cancer Cell Lines and Patient Plasma: Toward EV-Based Noninvasive Testing. Clin. Chem. 2022, 68, 668–679. [Google Scholar] [CrossRef]
- Yuwen, D.-L.; Sheng, B.-B.; Liu, J.; Wenyu, W.; Shu, Y.-Q. MiR-146a-5p Level in Serum Exosomes Predicts Therapeutic Effect of Cisplatin in Non-Small Cell Lung Cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2650–2658. [Google Scholar]
- Han, B.; Marrades, R.M.; Viñolas, N.; He, Y.; Canals, J.; Díaz, T.; Molins, L.; Martinez, D.; Moisés, J.; Sánchez, D.; et al. Monitoring HOTTIP Levels on Extracellular Vesicles for Predicting Recurrence in Surgical Non-Small Cell Lung Cancer Patients. Transl. Oncol. 2021, 14, 101144. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Tian, W.; Liu, S.; Li, B. Potential Role of Exosomes in Cancer Metastasis. BioMed Res. Int. 2019, 2019, 4649705. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-Associated Exosomes Promote Lung Cancer Metastasis through Multiple Mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Kuriyama, N.; Yoshioka, Y.; Kikuchi, S.; Azuma, N.; Ochiya, T. Extracellular Vesicles Are Key Regulators of Tumor Neovasculature. Front. Cell Dev. Biol. 2020, 8, 611039. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, X.; Yu, J. Exosomes and Organ-Specific Metastasis. Mol. Ther. Methods Clin. Dev. 2021, 22, 133–147. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Jiang, L.; Li, Y.; Zheng, Q. Tumor Endothelial Cell-Derived Extracellular Vesicles Contribute to Tumor Microenvironment Remodeling. Cell Commun. Signal. CCS 2022, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Guo, S.-C.; Zhang, C.-Q. Platelet-Derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Hynes, R.O. The Initial Hours of Metastasis: The Importance of Cooperative Host-Tumor Cell Interactions during Hematogenous Dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Stroun, M.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Beljanski, M. Neoplastic Characteristics of the DNA Found in the Plasma of Cancer Patients. Oncology 1989, 46, 318–322. [Google Scholar] [CrossRef]
- Sánchez-Herrero, E.; Serna-Blasco, R.; Robado de Lope, L.; González-Rumayor, V.; Romero, A.; Provencio, M. Circulating Tumor DNA as a Cancer Biomarker: An Overview of Biological Features and Factors That May Impact on CtDNA Analysis. Front. Oncol. 2022, 12, 943253. [Google Scholar] [CrossRef]
- Wong, F.C.K.; Sun, K.; Jiang, P.; Cheng, Y.K.Y.; Chan, K.C.A.; Leung, T.Y.; Chiu, R.W.K.; Lo, Y.M.D. Cell-Free DNA in Maternal Plasma and Serum: A Comparison of Quantity, Quality and Tissue Origin Using Genomic and Epigenomic Approaches. Clin. Biochem. 2016, 49, 1379–1386. [Google Scholar] [CrossRef]
- Devonshire, A.S.; Whale, A.S.; Gutteridge, A.; Jones, G.; Cowen, S.; Foy, C.A.; Huggett, J.F. Towards Standardisation of Cell-Free DNA Measurement in Plasma: Controls for Extraction Efficiency, Fragment Size Bias and Quantification. Anal. Bioanal. Chem. 2014, 406, 6499–6512. [Google Scholar] [CrossRef]
- Parsons, H.A.; Beaver, J.A.; Park, B.H. Circulating Plasma Tumor DNA. Adv. Exp. Med. Biol. 2016, 882, 259–276. [Google Scholar] [CrossRef]
- Danesi, R.; Lo, Y.M.D.; Oellerich, M.; Beck, J.; Galbiati, S.; Re, M.D.; Lianidou, E.; Neumaier, M.; van Schaik, R.H.N. What Do We Need to Obtain High Quality Circulating Tumor DNA (CtDNA) for Routine Diagnostic Test in Oncology?—Considerations on Pre-Analytical Aspects by the IFCC Workgroup CfDNA. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 520, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Elazezy, M.; Joosse, S.A. Techniques of Using Circulating Tumor DNA as a Liquid Biopsy Component in Cancer Management. Comput. Struct. Biotechnol. J. 2018, 16, 370–378. [Google Scholar] [CrossRef]
- Heitzer, E.; van den Broek, D.; Denis, M.G.; Hofman, P.; Hubank, M.; Mouliere, F.; Paz-Ares, L.; Schuuring, E.; Sültmann, H.; Vainer, G.; et al. Recommendations for a Practical Implementation of Circulating Tumor DNA Mutation Testing in Metastatic Non-Small-Cell Lung Cancer. ESMO Open 2022, 7, 100399. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Z.J.F.; Zou, W.; Fine, A.D.; Socinski, M.A.; Young, A.; Lipson, D.; Freidin, J.F.; Kennedy, M.; Polisecki, E.; Nishio, M.; et al. A Longitudinal Circulating Tumor DNA-Based Model Associated with Survival in Metastatic Non-Small-Cell Lung Cancer. Nat. Med. 2023, 29, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yaung, S.J.; Fuhlbrück, F.; Ballinger, M.; Peters, E.; Palma, J.F.; Shames, D.S.; Gandara, D.; Jiang, Y.; Patil, N.S. CtDNA Predicts Overall Survival in Patients With NSCLC Treated With PD-L1 Blockade or With Chemotherapy. JCO Precis. Oncol. 2021, 5, 827–838. [Google Scholar] [CrossRef]
- Buder, A.; Hochmair, M.J.; Setinek, U.; Pirker, R.; Filipits, M. EGFR Mutation Tracking Predicts Survival in Advanced EGFR-Mutated Non-Small Cell Lung Cancer Patients Treated with Osimertinib. Transl. Lung Cancer Res. 2020, 9, 239–245. [Google Scholar] [CrossRef]
- Cheng, M.L.; Lau, C.J.; Milan, M.S.D.; Supplee, J.G.; Riess, J.W.; Bradbury, P.A.; Jänne, P.A.; Oxnard, G.R.; Paweletz, C.P. Plasma CtDNA Response Is an Early Marker of Treatment Effect in Advanced NSCLC. JCO Precis. Oncol. 2021, 5, 393–402. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized Circulating Tumor DNA Analysis as a Predictive Biomarker in Solid Tumor Patients Treated with Pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Alessi, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early Plasma Circulating Tumor DNA (CtDNA) Changes Predict Response to First-Line Pembrolizumab-Based Therapy in Non-Small Cell Lung Cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.-X.; Li, J.; Shao, Y.; Li, M.-T.; Li, J.-J.; Kuang, P.-P.; Liu, Z.; Sun, T.-Y.; Wu, H.-Q.; et al. Perioperative Circulating Tumor DNA as a Potential Prognostic Marker for Operable Stage I to IIIA Non-Small Cell Lung Cancer. Cancer 2022, 128, 708–718. [Google Scholar] [CrossRef]
- Gale, D.; Heider, K.; Ruiz-Valdepenas, A.; Hackinger, S.; Perry, M.; Marsico, G.; Rundell, V.; Wulff, J.; Sharma, G.; Knock, H.; et al. Residual CtDNA after Treatment Predicts Early Relapse in Patients with Early-Stage Non-Small Cell Lung Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Zhang, J.; Xing, P.; Wu, M.; Meng, F.; Jiang, F.; Wang, J.; Bao, H.; Huang, J.; et al. Circulating Tumor DNA Integrating Tissue Clonality Detects Minimal Residual Disease in Resectable Non-Small-Cell Lung Cancer. J. Hematol. Oncol. 2022, 15, 137. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Huang, Z.; Wang, F.; Lin, Y.; Wen, Y.; Liu, L.; Li, J.; Liu, X.; Xie, W.; et al. Development and Validation of a Preoperative Noninvasive Predictive Model Based on Circular Tumor DNA for Lymph Node Metastasis in Resectable Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Tzimagiorgis, G.; Michailidou, E.Z.; Kritis, A.; Markopoulos, A.K.; Kouidou, S. Recovering Circulating Extracellular or Cell-Free RNA from Bodily Fluids. Cancer Epidemiol. 2011, 35, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, T.; Yang, X.; Zhou, Q.; Zhu, S.; Zeng, J.; Chen, H.; Sun, J.; Li, L.; Xu, J.; et al. Polyadenylation Ligation-Mediated Sequencing (PALM-Seq) Characterizes Cell-Free Coding and Non-Coding RNAs in Human Biofluids. Clin. Transl. Med. 2022, 12, e987. [Google Scholar] [CrossRef]
- Cabús, L.; Lagarde, J.; Curado, J.; Lizano, E.; Pérez-Boza, J. Current Challenges and Best Practices for Cell-Free Long RNA Biomarker Discovery. Biomark. Res. 2022, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, T.; Guglas, K.; Baranowski, D.; Sobocińska, J.; Kopczyńska, M.; Teresiak, A.; Bliźniak, R.; Lamperska, K. CfRNAs as Biomarkers in Oncology—Still Experimental or Applied Tool for Personalized Medicine Already? Rep. Pract. Oncol. Radiother. J. Gt. Cancer Cent. Poznan Pol. Soc. Radiat. Oncol. 2020, 25, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Fontanini, G. Next Generation Sequencing for Gene Fusion Analysis in Lung Cancer: A Literature Review. Diagnostics 2020, 10, 521. [Google Scholar] [CrossRef]
- Mensah, M.; Borzi, C.; Verri, C.; Suatoni, P.; Conte, D.; Pastorino, U.; Orazio, F.; Sozzi, G.; Boeri, M. MicroRNA Based Liquid Biopsy: The Experience of the Plasma MiRNA Signature Classifier (MSC) for Lung Cancer Screening. J. Vis. Exp. JoVE 2017, 128, 56326. [Google Scholar] [CrossRef]
- Ishiba, T.; Hoffmann, A.-C.; Usher, J.; Elshimali, Y.; Sturdevant, T.; Dang, M.; Jaimes, Y.; Tyagi, R.; Gonzales, R.; Grino, M.; et al. Frequencies and Expression Levels of Programmed Death Ligand 1 (PD-L1) in Circulating Tumor RNA (CtRNA) in Various Cancer Types. Biochem. Biophys. Res. Commun. 2018, 500, 621–625. [Google Scholar] [CrossRef]
- Gu, X.; He, J.; Ji, G. Combined Use of Circulating Tumor Cells and Salivary MRNA to Detect Non-Small-Cell Lung Cancer. Medicine 2020, 99, e19097. [Google Scholar] [CrossRef] [PubMed]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Characteristics and Applications. Eur. J. Hum. Genet. EJHG 2018, 26, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ye, L.; Huang, L.; Zhou, L.; Chen, X.; Ye, M.; Wu, G.; Zhan, P.; Lv, T.; Song, Y. Serum Exosomal MiRNA Might Be a Novel Liquid Biopsy to Identify Leptomeningeal Metastasis in Non-Small Cell Lung Cancer. OncoTargets Ther. 2021, 14, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, B.; Zhao, B.; Liu, Y.; Yang, Y.; Zhang, L.; Chen, J. Circulating MicroRNA-422a Is Associated with Lymphatic Metastasis in Lung Cancer. Oncotarget 2017, 8, 42173–42188. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Sousa, C.; Machado, F.; Serino, M.; Santos, V.; Cruz-Martins, N.; Teixeira, A.; Cunha, A.; Pereira, T.; Oliveira, H.P.; et al. The Role of Liquid Biopsy in Early Diagnosis of Lung Cancer. Front. Oncol. 2021, 11, 634316. [Google Scholar] [CrossRef]
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.S.; Lam, S.; Lam, W.L.; Martinez, V.D. MicroRNAs As Biomarkers For Clinical Features Of Lung Cancer. Metab. Open Access 2012, 2, 1000108. [Google Scholar] [CrossRef]
- Shen, L.; Chen, L.; Wang, Y.; Jiang, X.; Xia, H.; Zhuang, Z. Long Noncoding RNA MALAT1 Promotes Brain Metastasis by Inducing Epithelial-Mesenchymal Transition in Lung Cancer. J. Neurooncol. 2015, 121, 101–108. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, Y.; Wang, Z.; Zheng, J.; Chen, Y.; Li, X.; Wang, Y.; Ming, H. Serum Long Non Coding RNA MALAT-1 Protected by Exosomes Is up-Regulated and Promotes Cell Proliferation and Migration in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2017, 490, 406–414. [Google Scholar] [CrossRef]
- Weber, D.G.; Johnen, G.; Casjens, S.; Bryk, O.; Pesch, B.; Jöckel, K.-H.; Kollmeier, J.; Brüning, T. Evaluation of Long Noncoding RNA MALAT1 as a Candidate Blood-Based Biomarker for the Diagnosis of Non-Small Cell Lung Cancer. BMC Res. Notes 2013, 6, 518. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, A.; Bodei, L.; Kidd, M.; Modlin, I.M. Blood MRNA Measurement (NETest) for Neuroendocrine Tumor Diagnosis of Image-Negative Liver Metastatic Disease. J. Clin. Endocrinol. Metab. 2019, 104, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.; Peeters, M.; Van Camp, G.; Op de Beeck, K. Methylation Biomarkers for Early Cancer Detection and Diagnosis: Current and Future Perspectives. Eur. J. Cancer Oxf. Engl. 1990 2023, 178, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Locke, W.J.; Guanzon, D.; Ma, C.; Liew, Y.J.; Duesing, K.R.; Fung, K.Y.C.; Ross, J.P. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Front. Genet. 2019, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Constantin, N.; Sina, A.A.I.; Korbie, D.; Trau, M. Opportunities for Early Cancer Detection: The Rise of CtDNA Methylation-Based Pan-Cancer Screening Technologies. Epigenomes 2022, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.S.M.C.C.; Seiden, M.V.; Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Smith, D.; Richards, D.; et al. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, W.; Wen, B.; Kang, Y. A Sight of the Diagnostic Value of Aberrant Cell-Free DNA Methylation in Lung Cancer. Dis. Markers 2022, 2022, 9619357. [Google Scholar] [CrossRef]
- Janke, F.; Angeles, A.K.; Riediger, A.L.; Bauer, S.; Reck, M.; Stenzinger, A.; Schneider, M.A.; Muley, T.; Thomas, M.; Christopoulos, P.; et al. Longitudinal Monitoring of Cell-Free DNA Methylation in ALK-Positive Non-Small Cell Lung Cancer Patients. Clin. Epigenetics 2022, 14, 163. [Google Scholar] [CrossRef]
- Mo, S.; Dai, W.; Wang, H.; Lan, X.; Ma, C.; Su, Z.; Xiang, W.; Han, L.; Luo, W.; Zhang, L.; et al. Early Detection and Prognosis Prediction for Colorectal Cancer by Circulating Tumour DNA Methylation Haplotypes: A Multicentre Cohort Study. eClinicalMedicine 2023, 55, 101717. [Google Scholar] [CrossRef]
- Kandimalla, R.; Xu, J.; Link, A.; Matsuyama, T.; Yamamura, K.; Parker, M.I.; Uetake, H.; Balaguer, F.; Borazanci, E.; Tsai, S.; et al. EpiPanGI Dx: A Cell-Free DNA Methylation Fingerprint for the Early Detection of Gastrointestinal Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 6135–6144. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.; Li, W.; Zhu, X.; Li, C. DNA Methylation in Lung Cancer Patients: Opening a “Window of Life” under Precision Medicine. Biomed. Pharmacother. 2021, 144, 112202. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Du, L.; Mohseni, G.; Zhang, Y.; Wang, C. Liquid Biopsies Based on DNA Methylation as Biomarkers for the Detection and Prognosis of Lung Cancer. Clin. Epigenetics 2022, 14, 118. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, X.; Zhang, L.; Zhou, J.; Deng, D.; Ji, J. A Panel of DNA Methylated Markers Predicts Metastasis of PN0M0 Gastric Carcinoma: A Prospective Cohort Study. Br. J. Cancer 2019, 121, 529–536. [Google Scholar] [CrossRef]
- Liu, L.; Toung, J.M.; Jassowicz, A.F.; Vijayaraghavan, R.; Kang, H.; Zhang, R.; Kruglyak, K.M.; Huang, H.J.; Hinoue, T.; Shen, H.; et al. Targeted Methylation Sequencing of Plasma Cell-Free DNA for Cancer Detection and Classification. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mei, W.; Ma, K.; Zeng, C. Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Front. Oncol. 2021, 11, 763790. [Google Scholar] [CrossRef] [PubMed]
- Früh, M.; Rolland, E.; Pignon, J.-P.; Seymour, L.; Ding, K.; Tribodet, H.; Winton, T.; Le Chevalier, T.; Scagliotti, G.V.; Douillard, J.Y.; et al. Pooled Analysis of the Effect of Age on Adjuvant Cisplatin-Based Chemotherapy for Completely Resected Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3573–3581. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Black, J.R.M.; McGranahan, N. Genetic and Non-Genetic Clonal Diversity in Cancer Evolution. Nat. Rev. Cancer 2021, 21, 379–392. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Roper, N.; Brown, A.-L.; Wei, J.S.; Pack, S.; Trindade, C.; Kim, C.; Restifo, O.; Gao, S.; Sindiri, S.; Mehrabadi, F.; et al. Clonal Evolution and Heterogeneity of Osimertinib Acquired Resistance Mechanisms in EGFR Mutant Lung Cancer. Cell Rep. Med. 2020, 1, 100007. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Facchinetti, F.; Braye, F.; Yurchenko, A.A.; Bigot, L.; Ponce, S.; Planchard, D.; Gazzah, A.; Nikolaev, S.; Michiels, S.; et al. Single-Cell DNA-Seq Depicts Clonal Evolution of Multiple Driver Alterations in Osimertinib-Resistant Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Pradines, A.; Favre, G.; Mazieres, J. Current and Future Applications of Liquid Biopsy in Nonsmall Cell Lung Cancer from Early to Advanced Stages. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2020, 29, 190052. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Honoré, N.; Galot, R.; van Marcke, C.; Limaye, N.; Machiels, J.-P. Liquid Biopsy to Detect Minimal Residual Disease: Methodology and Impact. Cancers 2021, 13, 5364. [Google Scholar] [CrossRef]
- Verzè, M.; Pluchino, M.; Leonetti, A.; Corianò, M.; Bonatti, F.; Armillotta, M.P.; Perrone, F.; Casali, M.; Minari, R.; Tiseo, M. Role of CtDNA for the Detection of Minimal Residual Disease in Resected Non-Small Cell Lung Cancer: A Systematic Review. Transl. Lung Cancer Res. 2022, 11, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef]
- Xia, L.; Mei, J.; Kang, R.; Deng, S.; Chen, Y.; Yang, Y.; Feng, G.; Deng, Y.; Gan, F.; Lin, Y.; et al. Perioperative CtDNA-Based Molecular Residual Disease Detection for Non–Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin. Cancer Res. 2022, 28, 3308–3317. [Google Scholar] [CrossRef]
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive Liquid Biopsy Analysis as a Tool for the Early Detection of Minimal Residual Disease in Breast Cancer. Sci. Rep. 2023, 13, 1258. [Google Scholar] [CrossRef]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating Tumor DNA Predicts Neoadjuvant Immunotherapy Efficacy and Recurrence-Free Survival in Surgical Non-Small Cell Lung Cancer Patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef]
- O’Sullivan, H.M.; Feber, A.; Popat, S. Minimal Residual Disease Monitoring in Radically Treated Non-Small Cell Lung Cancer: Challenges and Future Directions. OncoTargets Ther. 2023, 16, 249–259. [Google Scholar] [CrossRef]
- Marsico, G.; Sharma, G.; Perry, M.; Hackinger, S.; Forshew, T.; Howarth, K.; Platt, J.; Rosenfeld, N.; Osborne, R. Abstract 3097: Analytical Development of the RaDaRTM Assay, a Highly Sensitive and Specific Assay for the Monitoring of Minimal Residual Disease. Cancer Res. 2020, 80, 3097. [Google Scholar] [CrossRef]
- Sethi, H.; Salari, R.; Navarro, S.; Natarajan, P.; Srinivasan, R.; Dashner, S.; Tin, T.; Balcioglu, M.; Swenerton, R.; Zimmermann, B. Abstract 4542: Analytical Validation of the SignateraTM RUO Assay, a Highly Sensitive Patient-Specific Multiplex PCR NGS-Based Noninvasive Cancer Recurrence Detection and Therapy Monitoring Assay. Cancer Res. 2018, 78, 4542. [Google Scholar] [CrossRef]
- Kohabir, K.; Wolthuis, R.; Sistermans, E.A. Fragmentomic CfDNA Patterns in Noninvasive Prenatal Testing and Beyond. J. Biomed. Transl. Res. 2021, 7, 38–47. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. Northwood Lond. Engl. 2021, 38, 3. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Naumov, G.N.; Vantyghem, S.A.; Tuck, A.B. Molecular Biology of Breast Cancer Metastasis. Clinical Implications of Experimental Studies on Metastatic Inefficiency. Breast Cancer Res. BCR 2000, 2, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The Role of the Tumor-Microenvironment in Lung Cancer-Metastasis and Its Relationship to Potential Therapeutic Targets. Cancer Treat. Rev. 2014, 40, 558–566. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. The Metastatic Cascade as the Basis for Liquid Biopsy Development. Front. Oncol. 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from Metastatic Cancer Cells Transfer Amoeboid Phenotype to Non-Metastatic Cells and Increase Endothelial Permeability: Their Emerging Role in Tumor Heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Ward, Y.; Lake, R.; Faraji, F.; Sperger, J.; Martin, P.; Gilliard, C.; Ku, K.P.; Rodems, T.; Niles, D.; Tillman, H.; et al. Platelets Promote Metastasis via Binding Tumor CD97 Leading to Bidirectional Signaling That Coordinates Transendothelial Migration. Cell Rep. 2018, 23, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Nicolaides, T. Tumor-Educated Platelets: A Review of Current and Potential Applications in Solid Tumors. Cureus 2021, 13, e19189. [Google Scholar] [CrossRef]
- Jiang, X.; Wong, K.H.K.; Khankhel, A.H.; Zeinali, M.; Reategui, E.; Phillips, M.J.; Luo, X.; Aceto, N.; Fachin, F.; Hoang, A.N.; et al. Microfluidic Isolation of Platelet-Covered Circulating Tumor Cells. Lab Chip 2017, 17, 3498–3503. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Matrix Metalloproteinases and Metastasis. Cancer Chemother. Pharmacol. 1999, 43, S42–S51. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix Metalloproteinases and Tumor Metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix Metalloproteinases Participation in the Metastatic Process and Their Diagnostic and Therapeutic Applications in Cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, H.; Neelakantan, D.; Hughes, C.J.; Hsu, J.Y.; Srinivasan, R.R.; Lewis, M.T.; Ford, H.L. VEGF-C Mediates Tumor Growth and Metastasis through Promoting EMT-Epithelial Breast Cancer Cell Crosstalk. Oncogene 2021, 40, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Huber, V.; Iero, M.; Filipazzi, P.; Parmiani, G.; Rivoltini, L. Tumor-Released Microvesicles as Vehicles of Immunosuppression. Cancer Res. 2007, 67, 2912–2915. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The Impact of VEGF on Cancer Metastasis and Systemic Disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Hosaka, K.; Andersson, P.; Wang, J.; Tholander, F.; Cao, Z.; Morikawa, H.; Tegnér, J.; Yang, Y.; et al. VEGF-B Promotes Cancer Metastasis through a VEGF-A-Independent Mechanism and Serves as a Marker of Poor Prognosis for Cancer Patients. Proc. Natl. Acad. Sci. USA 2015, 112, E2900–E2909. [Google Scholar] [CrossRef]
- Cheng, H.; Perez-Soler, R. Leptomeningeal Metastases in Non-Small-Cell Lung Cancer. Lancet Oncol. 2018, 19, e43–e55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Jiang, B.Y.; Yang, J.J.; Zhang, X.C.; Zhang, Z.; Ye, J.Y.; Zhong, W.Z.; Tu, H.Y.; Chen, H.J.; Wang, Z.; et al. Unique Genetic Profiles from Cerebrospinal Fluid Cell-Free DNA in Leptomeningeal Metastases of EGFR-Mutant Non-Small-Cell Lung Cancer: A New Medium of Liquid Biopsy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 945–952. [Google Scholar] [CrossRef]
- Brower, J.V.; Saha, S.; Rosenberg, S.A.; Hullett, C.R.; Ian Robins, H. Management of Leptomeningeal Metastases: Prognostic Factors and Associated Outcomes. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2016, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal Fluid-Derived Circulating Tumour DNA Better Represents the Genomic Alterations of Brain Tumours than Plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wen, L.; Zhao, C.; Chen, J.; Zhou, Z.; Zhou, C.; Cai, L.; Zhou, C. Cerebrospinal Fluid-Derived Circulating Tumor DNA Is More Comprehensive than Plasma in NSCLC Patients with Leptomeningeal Metastases Regardless of Extracranial Evolution. Heliyon 2022, 8, e12374. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Liang, N.; Fan, Z.; Zhao, M.; Kang, J.; Wang, X.; Hu, Q.; Mu, Y.; Wang, K.; Yuan, M.; et al. Genomic Alterations Identification and Resistance Mechanisms Exploration of NSCLC With Central Nervous System Metastases Using Liquid Biopsy of Cerebrospinal Fluid: A Real-World Study. Front. Oncol. 2022, 12, 889591. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, R.; Cai, Q.; Gao, X.; Tong, F.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. MicroRNA-330-3p Promotes Brain Metastasis and Epithelial-Mesenchymal Transition via GRIA3 in Non-Small Cell Lung Cancer. Aging 2019, 11, 6734–6761. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal MiRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Yang, X.-R.; Pi, C.; Yu, R.; Fan, X.-J.; Peng, X.-X.; Zhang, X.-C.; Chen, Z.-H.; Wu, X.; Shao, Y.; Wu, Y.-L.; et al. Correlation of Exosomal MicroRNA Clusters with Bone Metastasis in Non-Small Cell Lung Cancer. Clin. Exp. Metastasis 2021, 38, 109–117. [Google Scholar] [CrossRef]
- Couraud, S.; Vaca-Paniagua, F.; Villar, S.; Oliver, J.; Schuster, T.; Blanché, H.; Girard, N.; Trédaniel, J.; Guilleminault, L.; Gervais, R.; et al. Noninvasive Diagnosis of Actionable Mutations by Deep Sequencing of Circulating Free DNA in Lung Cancer from Never-Smokers: A Proof-of-Concept Study from BioCAST/IFCT-1002. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 4613–4624. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, F.; Cui, F.; Xu, R.; Tang, X. The Role of Circulating Tumor Cells in Evaluation of Prognosis and Treatment Response in Advanced Non-Small-Cell Lung Cancer. Cancer Chemother. Pharmacol. 2017, 79, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Abbosh, C.; Frankell, A.M.; Harrison, T.; Kisistok, J.; Garnett, A.; Johnson, L.; Veeriah, S.; Moreau, M.; Chesh, A.; Chaunzwa, T.L.; et al. Tracking Early Lung Cancer Metastatic Dissemination in TRACERx Using CtDNA. Nature 2023, 616, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Thompson, J.C.; Black, T.A.; Katz, S.I.; Fan, R.; Yee, S.S.; Chien, A.L.; Evans, T.L.; Bauml, J.M.; Alley, E.W.; et al. Clinical Implications of Plasma-Based Genotyping With the Delivery of Personalized Therapy in Metastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 173. [Google Scholar] [CrossRef] [PubMed]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S Mutation Mediates Resistance to AZD9291 in Non-Small Cell Lung Cancer Harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Shinozaki, E.; Yamaguchi, K.; Zembutsu, H. Early Change in Circulating Tumor DNA as a Potential Predictor of Response to Chemotherapy in Patients with Metastatic Colorectal Cancer. Sci. Rep. 2019, 9, 17358. [Google Scholar] [CrossRef]
- Sinoquet, L.; Jacot, W.; Quantin, X.; Alix-Panabières, C. Liquid Biopsy and Immuno-Oncology for Advanced Nonsmall Cell Lung Cancer. Clin. Chem. 2023, 69, 23–40. [Google Scholar] [CrossRef]
- Sama, S.; Le, T.; Ullah, A.; Elhelf, I.A.; Kavuri, S.K.; Karim, N.A. The Role of Serial Liquid Biopsy in the Management of Metastatic Non-Small Cell Lung Cancer (NSCLC). Clin. Pract. 2022, 12, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Crucitta, S.; Ruglioni, M.; Novi, C.; Manganiello, M.; Arici, R.; Petrini, I.; Pardini, E.; Cucchiara, F.; Marmorino, F.; Cremolini, C.; et al. Comparison of Digital PCR Systems for the Analysis of Liquid Biopsy Samples of Patients Affected by Lung and Colorectal Cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2023, 541, 117239. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Solutions—Guardant360. Available online: https://www.guardanthealthamea.com/solutions/ (accessed on 1 March 2023).
- FoundationOne CDx|Foundation Medicine. Available online: https://www.foundationmedicine.com/test/foundationone-cdx (accessed on 1 March 2023).
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- NCounter® Pro Analysis System|Gene Expression|NanoString. Available online: https://nanostring.com/products/ncounter-analysis-system/ncounter-pro/?utm_source=google&utm_medium=cpc&utm_campaign=ncounterpro&utm_id=Instruments_CombinedTier_Search&gad=1&gclid=CjwKCAjwge2iBhBBEiwAfXDBRx2KQC1n2oqYorQMQD9kIQvcKUQ99ybmkm5693ugCR2WYENahNuUkBoCx9UQAvD_BwE (accessed on 10 May 2023).
- Marin, E.; Reyes, R.; Arcocha, A.; Viñolas, N.; Mezquita, L.; Gonzalvo, E.; Saez de Gordoa, K.; Jares, P.; Reguart, N.; Teixido, C. Prospective Evaluation of Single Nucleotide Variants by Two Different Technologies in Paraffin Samples of Advanced Non-Small Cell Lung Cancer Patients. Diagnostics 2020, 10, 902. [Google Scholar] [CrossRef]
- Giménez-Capitán, A.; Bracht, J.; García, J.J.; Jordana-Ariza, N.; García, B.; Garzón, M.; Mayo-de-Las-Casas, C.; Viteri-Ramirez, S.; Martinez-Bueno, A.; Aguilar, A.; et al. Multiplex Detection of Clinically Relevant Mutations in Liquid Biopsies of Cancer Patients Using a Hybridization-Based Platform. Clin. Chem. 2021, 67, 554–563. [Google Scholar] [CrossRef]
- Wilkening, S.; Hemminki, K.; Thirumaran, R.K.; Bermejo, J.L.; Bonn, S.; Försti, A.; Kumar, R. Determination of Allele Frequency in Pooled DNA: Comparison of Three PCR-Based Methods. BioTechniques 2005, 39, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Iwama, E.; Takayama, K.; Harada, T.; Okamoto, I.; Ookubo, F.; Kishimoto, J.; Baba, E.; Oda, Y.; Nakanishi, Y. Highly Sensitive and Quantitative Evaluation of the EGFR T790M Mutation by Nanofluidic Digital PCR. Oncotarget 2015, 6, 20466–20473. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Kang, J.; Kibukawa, M.; Arreaza, G.; Maguire, M.; Chen, L.; Qiu, P.; Lang, L.; Aurora-Garg, D.; Cristescu, R.; et al. Evaluation of the TruSight Oncology 500 Assay for Routine Clinical Testing of Tumor Mutational Burden and Clinical Utility for Predicting Response to Pembrolizumab. J. Mol. Diagn. JMD 2022, 24, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Clark, E.W.; Milan, M.S.D.; Champagne, C.; Michael, K.S.; Awad, M.M.; Barbie, D.A.; Cheng, M.L.; Kehl, K.L.; Marcoux, J.P.; et al. Turnaround Time of Plasma Next-Generation Sequencing in Thoracic Oncology Patients: A Quality Improvement Analysis. JCO Precis. Oncol. 2020, 4, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Baranova, A.; Butler, T.; Spellman, P.; Mileyko, V. Non-Random Fragmentation Patterns in Circulating Cell-Free DNA Reflect Epigenetic Regulation. BMC Genom. 2015, 16 (Suppl. 13), S1. [Google Scholar] [CrossRef]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, Fragmentomics, and Topology of Cell-Free DNA in Liquid Biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef]
- Ding, S.C.; Lo, Y.M.D. Cell-Free DNA Fragmentomics in Liquid Biopsy. Diagnostics 2022, 12, 978. [Google Scholar] [CrossRef]
- Zheng, G.X.Y.; Lau, B.T.; Schnall-Levin, M.; Jarosz, M.; Bell, J.M.; Hindson, C.M.; Kyriazopoulou-Panagiotopoulou, S.; Masquelier, D.A.; Merrill, L.; Terry, J.M.; et al. Haplotyping Germline and Cancer Genomes with High-Throughput Linked-Read Sequencing. Nat. Biotechnol. 2016, 34, 303–311. [Google Scholar] [CrossRef]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Jensen, S.Ø.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-Wide Cell-Free DNA Fragmentation in Patients with Cancer. Nature 2019, 570, 385–389. [Google Scholar] [CrossRef]
- Foda, Z.H.; Annapragada, A.V.; Boyapati, K.; Bruhm, D.C.; Vulpescu, N.A.; Medina, J.E.; Mathios, D.; Cristiano, S.; Niknafs, N.; Luu, H.T.; et al. Detecting Liver Cancer Using Cell-Free DNA Fragmentomes. Cancer Discov. 2023, 13, 616–631. [Google Scholar] [CrossRef]
- Vessies, D.C.L.; Schuurbiers, M.M.F.; van der Noort, V.; Schouten, I.; Linders, T.C.; Lanfermeijer, M.; Ramkisoensing, K.L.; Hartemink, K.J.; Monkhorst, K.; van den Heuvel, M.M.; et al. Combining Variant Detection and Fragment Length Analysis Improves Detection of Minimal Residual Disease in Postsurgery Circulating Tumour DNA of Stage II-IIIA NSCLC Patients. Mol. Oncol. 2022, 16, 2719–2732. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of Nanopore Sequencing to the Genomics Community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-Time DNA Sequencing from Single Polymerase Molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore Sequencing Technology, Bioinformatics and Applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Martignano, F.; Munagala, U.; Crucitta, S.; Mingrino, A.; Semeraro, R.; Del Re, M.; Petrini, I.; Magi, A.; Conticello, S.G. Nanopore Sequencing from Liquid Biopsy: Analysis of Copy Number Variations from Cell-Free DNA of Lung Cancer Patients. Mol. Cancer 2021, 20, 32. [Google Scholar] [CrossRef]
- Choy, L.Y.L.; Peng, W.; Jiang, P.; Cheng, S.H.; Yu, S.C.Y.; Shang, H.; Olivia Tse, O.Y.; Wong, J.; Wong, V.W.S.; Wong, G.L.H.; et al. Single-Molecule Sequencing Enables Long Cell-Free DNA Detection and Direct Methylation Analysis for Cancer Patients. Clin. Chem. 2022, 68, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Di Lee, W.; Vasudevan, J.; Lim, W.-T.; Lim, C.T. Liquid Biopsy: One Cell at a Time. NPJ Precis. Oncol. 2019, 3, 23. [Google Scholar] [CrossRef]
- Lu, S.; Chang, C.-J.; Guan, Y.; Szafer-Glusman, E.; Punnoose, E.; Do, A.; Suttmann, B.; Gagnon, R.; Rodriguez, A.; Landers, M.; et al. Genomic Analysis of Circulating Tumor Cells at the Single-Cell Level. J. Mol. Diagn. JMD 2020, 22, 770–781. [Google Scholar] [CrossRef]
- Ni, X.; Zhuo, M.; Su, Z.; Duan, J.; Gao, Y.; Wang, Z.; Zong, C.; Bai, H.; Chapman, A.R.; Zhao, J.; et al. Reproducible Copy Number Variation Patterns among Single Circulating Tumor Cells of Lung Cancer Patients. Proc. Natl. Acad. Sci. USA 2013, 110, 21083–21088. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H.; et al. Inferring the Evolution and Progression of Small-Cell Lung Cancer by Single-Cell Sequencing of Circulating Tumor Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, Y.; Liu, Z.; Li, J.; Li, X.; Yang, F.; Qiu, M. Circulating Microbiome DNA: An Emerging Paradigm for Cancer Liquid Biopsy. Cancer Lett. 2021, 521, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association and Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, A.T.; Okuma, Y. Onco-Biome in Pharmacotherapy for Lung Cancer: A Narrative Review. Transl. Lung Cancer Res. 2022, 11, 2332–2345. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota Triggers STING-Type I IFN-Dependent Monocyte Reprogramming of the Tumor Microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef]
- Hilmi, M.; Kamal, M.; Vacher, S.; Dupain, C.; Ibadioune, S.; Halladjian, M.; Sablin, M.P.; Marret, G.; Ajgal, Z.C.; Nijnikoff, M.; et al. Intratumoral Microbiome Is Driven by Metastatic Site and Associated with Immune Histopathological Parameters: An Ancillary Study of the SHIVA Clinical Trial. Eur. J. Cancer Oxf. Engl. 1990 2023, 183, 152–161. [Google Scholar] [CrossRef]
- Liu, B.; Chau, J.; Dai, Q.; Zhong, C.; Zhang, J. Exploring Gut Microbiome in Predicting the Efficacy of Immunotherapy in Non-Small Cell Lung Cancer. Cancers 2022, 14, 5401. [Google Scholar] [CrossRef]
- Verschueren, M.V.; van der Welle, C.C.; Tonn, M.; Schramel, F.M.N.H.; Peters, B.J.M.; van de Garde, E.M.W. The Association between Gut Microbiome Affecting Concomitant Medication and the Effectiveness of Immunotherapy in Patients with Stage IV NSCLC. Sci. Rep. 2021, 11, 23331. [Google Scholar] [CrossRef]
- Bredin, P.; Naidoo, J. The Gut Microbiome, Immune Check Point Inhibition and Immune-Related Adverse Events in Non-Small Cell Lung Cancer. Cancer Metastasis Rev. 2022, 41, 347–366. [Google Scholar] [CrossRef]
- Dora, D.; Bokhari, S.M.Z.; Aloss, K.; Takacs, P.; Desnoix, J.Z.; Szklenárik, G.; Hurley, P.D.; Lohinai, Z. Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 2769. [Google Scholar] [CrossRef]
- Marshall, E.A.; Filho, F.S.L.; Sin, D.D.; Lam, S.; Leung, J.M.; Lam, W.L. Distinct Bronchial Microbiome Precedes Clinical Diagnosis of Lung Cancer. Mol. Cancer 2022, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Pass, H.I.; Burk, R.D.; Xue, X.; Goparaju, C.; Sollecito, C.C.; Grassi, E.; Segal, L.N.; Tsay, J.-C.J.; Hayes, R.B.; et al. The Lung Microbiome, Peripheral Gene Expression, and Recurrence-Free Survival after Resection of Stage II Non-Small Cell Lung Cancer. Genome Med. 2022, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Z.; Li, C.; Lv, K.; Tian, G.; Tang, M.; Ji, L.; Yang, J. Bacterial Biomarkers Capable of Identifying Recurrence or Metastasis Carry Disease Severity Information for Lung Cancer. Front. Microbiol. 2022, 13, 1007831. [Google Scholar] [CrossRef]
- Chen, S.; Jin, Y.; Wang, S.; Xing, S.; Wu, Y.; Tao, Y.; Ma, Y.; Zuo, S.; Liu, X.; Hu, Y.; et al. Cancer Type Classification Using Plasma Cell-Free RNAs Derived from Human and Microbes. eLife 2022, 11, e75181. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, X.; Li, Z.; Guo, W.; Cheng, H.; Cao, Q. A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing. Biomedicines 2023, 11, 631. [Google Scholar] [CrossRef]
- Kwok, B.; Wu, B.G.; Kocak, I.F.; Sulaiman, I.; Schluger, R.; Li, Y.; Anwer, R.; Goparaju, C.; Ryan, D.J.; Sagatelian, M.; et al. Pleural Fluid Microbiota as a Biomarker for Malignancy and Prognosis. Sci. Rep. 2023, 13, 2229. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Chen, H.; Zhang, T.; Yang, X.; Zhong, J.; Wang, Y.; An, T.; Wu, M.; Wang, Z.; Huang, J.; et al. The Potential Predictive Value of Circulating Immune Cell Ratio and Tumor Marker in Atezolizumab Treated Advanced Non-Small Cell Lung Cancer Patients. Cancer Biomark. 2018, 22, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Keup, C.; Kimmig, R.; Kasimir-Bauer, S. Liquid Biopsies to Evaluate Immunogenicity of Gynecological/Breast Tumors: On the Way to Blood-Based Biomarkers for Immunotherapies. Breast Care 2020, 15, 470–480. [Google Scholar] [CrossRef]
- Roweth, H.G.; Battinelli, E.M. Lessons to Learn from Tumor-Educated Platelets. Blood 2021, 137, 3174–3180. [Google Scholar] [CrossRef]
- Liu, L.; Lin, F.; Ma, X.; Chen, Z.; Yu, J. Tumor-Educated Platelet as Liquid Biopsy in Lung Cancer Patients. Crit. Rev. Oncol. Hematol. 2020, 146, 102863. [Google Scholar] [CrossRef]
- Calverley, D.C.; Phang, T.L.; Choudhury, Q.G.; Gao, B.; Oton, A.B.; Weyant, M.J.; Geraci, M.W. Significant Downregulation of Platelet Gene Expression in Metastatic Lung Cancer. Clin. Transl. Sci. 2010, 3, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Dong, Z.; Xie, Y. Identification of Tumor-Educated Platelet Biomarkers of Non-Small-Cell Lung Cancer. OncoTargets Ther. 2018, 11, 8143–8151. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef]
- Best, M.G.; In’t Veld, S.G.J.G.; Sol, N.; Wurdinger, T. RNA Sequencing and Swarm Intelligence-Enhanced Classification Algorithm Development for Blood-Based Disease Diagnostics Using Spliced Blood Platelet RNA. Nat. Protoc. 2019, 14, 1206–1234. [Google Scholar] [CrossRef] [PubMed]
| Body Fluid | Biomarkers Detected | Examples of Applications |
|---|---|---|
| Plasma | ctDNA to determine resistance mechanisms in patients with advanced NSCLC [50] Prognostic biomarkers: miR-10b-5p, miR-23b-3p, and miR-21-5p [51] Gene mutations including ALK, EGFR, KRAS with droplet digital PCR [52] | Plasma protein signatures reflect tumor biology [53] Increased RP11-438N5.3 lncRNA levels for patients’ prognosis [54] EGFR-status-related miRNA panel derived from exosomes isolated from metastatic disease [55] |
| Serum | Levels of metabolites in disease progression: lactic acid, benzoic acid, and fumaric acid [56] Prolactin, an early preventive factor in metastatic NSCLC for poor clinical outcomes [57] High exosomal microRNAs in advanced disease and poor survival: miR-378 and miR-214 [58] | CA125 levels are an indicator of metastasis to the liver [59] CYFRA 21-1 can be correlated with worsened PFS and OS in metastatic patients [60] |
| Sputum | Chromosomal aneusomy by FISH predicts lung cancer incidence [61] Sputum supernatant has comparable mutation profiling to plasma samples of NSCLC, with advantage of convenience in collection [62] | Use of nomograms to demonstrate the utility of sputum samples for genome profiling [63] Sputum as an alternative source for somatic variation profiling with NGS [64] |
| BAL | Cytologic examination of BAL has a comparable diagnostic yield to other endoscopic techniques for metastasis [65] | BAL better reflect the cancer proteome than serum samples [66] |
| Pleural effusion | MMP-9, cathepsin-B, C-reactive protein, chondroitin sulfate marker panel, and CA19-9, CA15-3, kallikrein-12 panel, are highly discriminative for malignant vs. tuberculous effusion, or lung adenocarcinoma vs. mesothelioma [67,68] | Detection of extracellular vesicles and cfDNA in pleural effusions enhances EGFR genotyping of adenocarcinoma patients [69] |
| Urine | EGFR mutations [70] | ctDNA EGFR mutation testing detects T790M mutations overlooked in tissue biopsies due to sample quality or tumor heterogeneity [71] ddPCR and Illumina MiSeq to monitor EGFR alterations during treatment [72] |
| CSF | EGFR, ROS1, ALK, BRAF, and/or EGFR T790M mutations [35] | Profiling for actionable mutation rate (EGFR, ROS1, ALK, BRAF) and resistance mutation rates (EGFR T790M mutation) [35] Higher detection sensitivity for leptomeningeal metastasis by CTCs than with MRI [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.G.P.; Forder, A.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; de Araújo, R.P.; Trejo, J.; Benard, K.; Seneda, A.L.; Minutentag, I.W.; et al. Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. Int. J. Mol. Sci. 2023, 24, 8894. https://doi.org/10.3390/ijms24108894
Souza VGP, Forder A, Brockley LJ, Pewarchuk ME, Telkar N, de Araújo RP, Trejo J, Benard K, Seneda AL, Minutentag IW, et al. Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. International Journal of Molecular Sciences. 2023; 24(10):8894. https://doi.org/10.3390/ijms24108894
Chicago/Turabian StyleSouza, Vanessa G. P., Aisling Forder, Liam J. Brockley, Michelle E. Pewarchuk, Nikita Telkar, Rachel Paes de Araújo, Jessica Trejo, Katya Benard, Ana Laura Seneda, Iael W. Minutentag, and et al. 2023. "Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis" International Journal of Molecular Sciences 24, no. 10: 8894. https://doi.org/10.3390/ijms24108894
APA StyleSouza, V. G. P., Forder, A., Brockley, L. J., Pewarchuk, M. E., Telkar, N., de Araújo, R. P., Trejo, J., Benard, K., Seneda, A. L., Minutentag, I. W., Erkan, M., Stewart, G. L., Hasimoto, E. N., Garnis, C., Lam, W. L., Martinez, V. D., & Reis, P. P. (2023). Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. International Journal of Molecular Sciences, 24(10), 8894. https://doi.org/10.3390/ijms24108894