Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions
Abstract
1. Introduction
2. Results
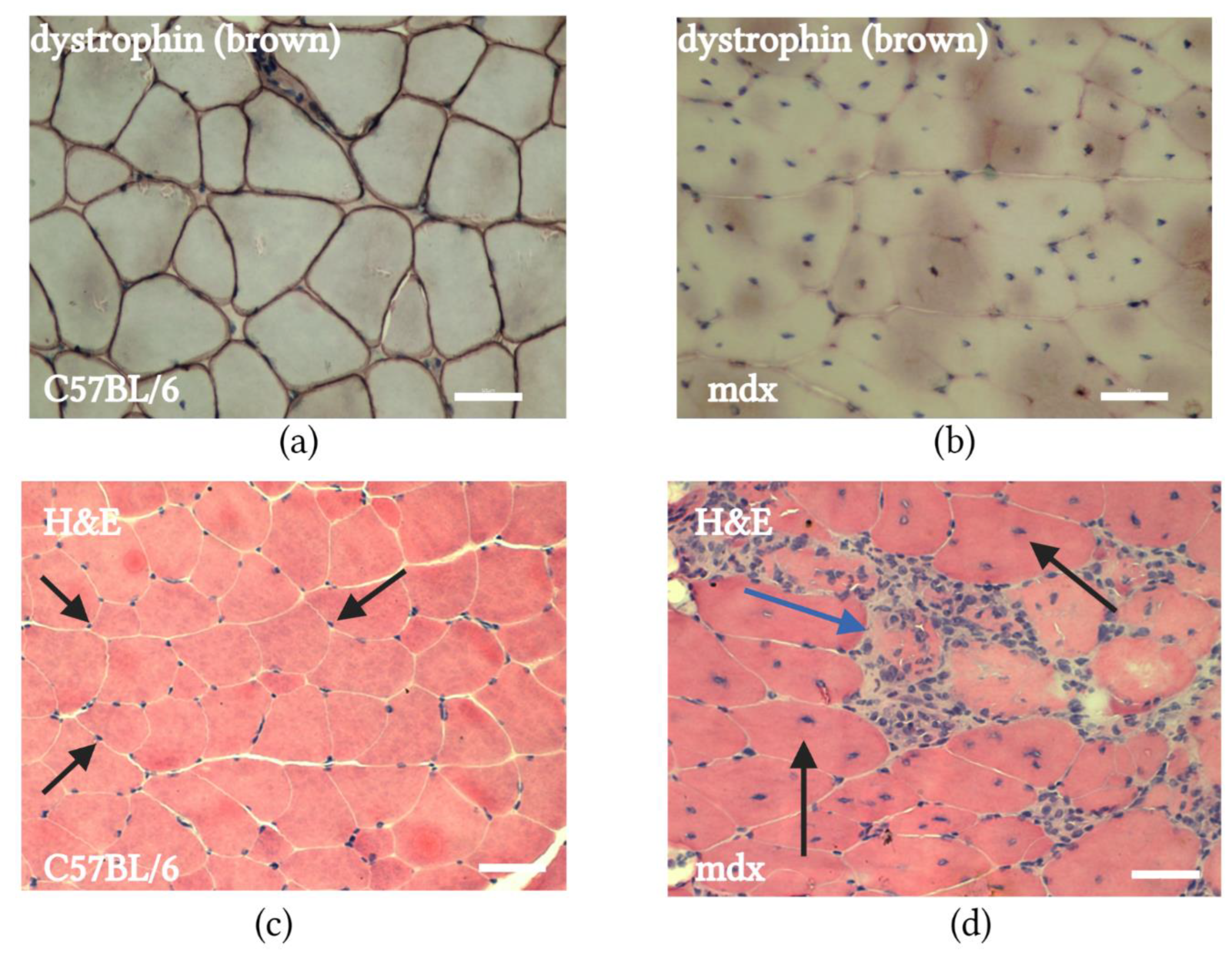
2.1. mdx Mouse Muscle Histology
2.2. mdx Mouse Muscle Contained GFP-Positive Cells and SMFs after Transplantation of BMCs from Transgenic GFP-Expressing C57BL/6 Mice
2.3. Transplantation of BMCs from C57BL/6 Mice Restored SMFs in mdx Mice
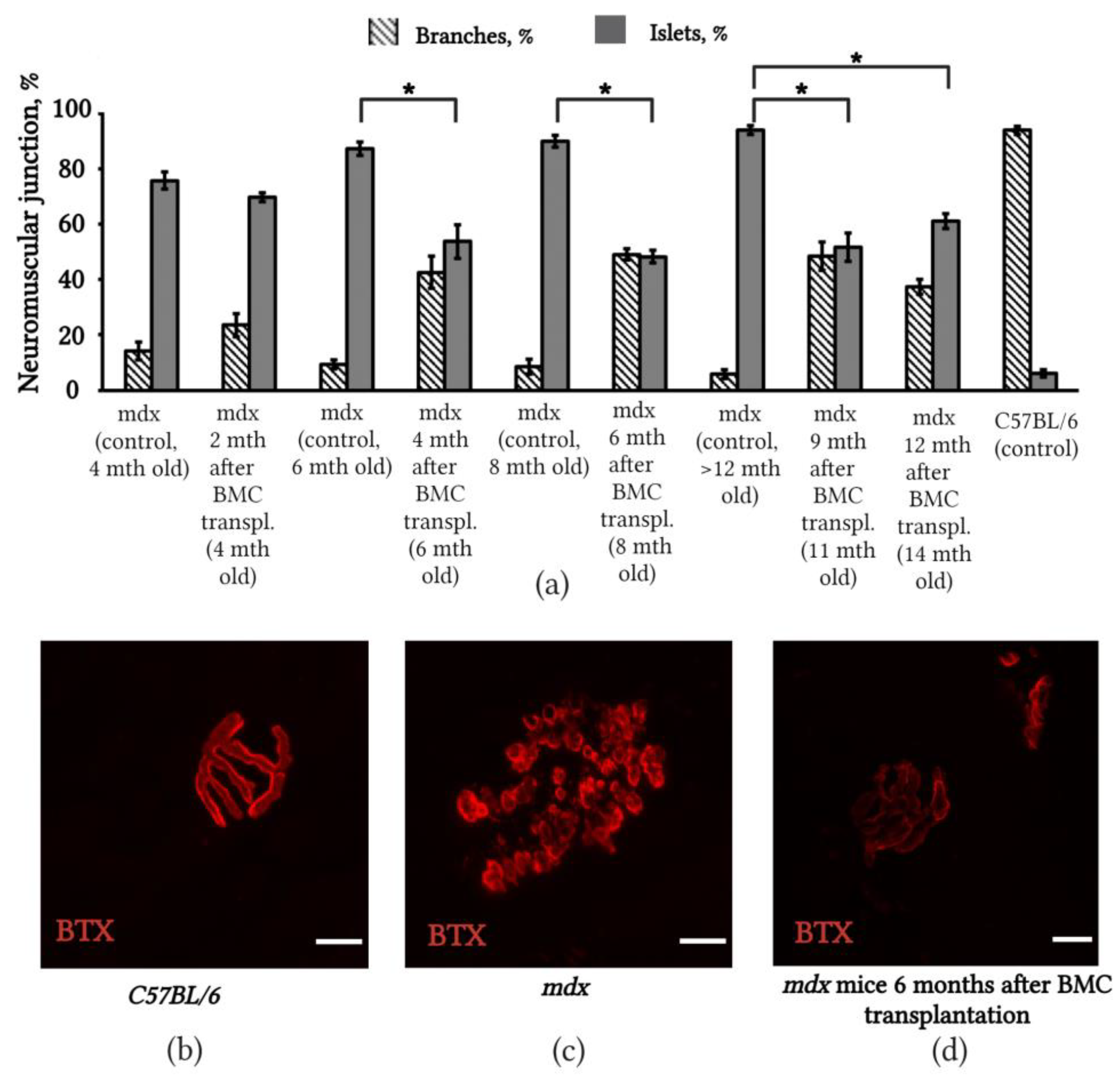
2.4. Changes in the Structure of Neuromuscular Junctions in mdx Mice after BMC Transplantation from C57BL/6 Mice
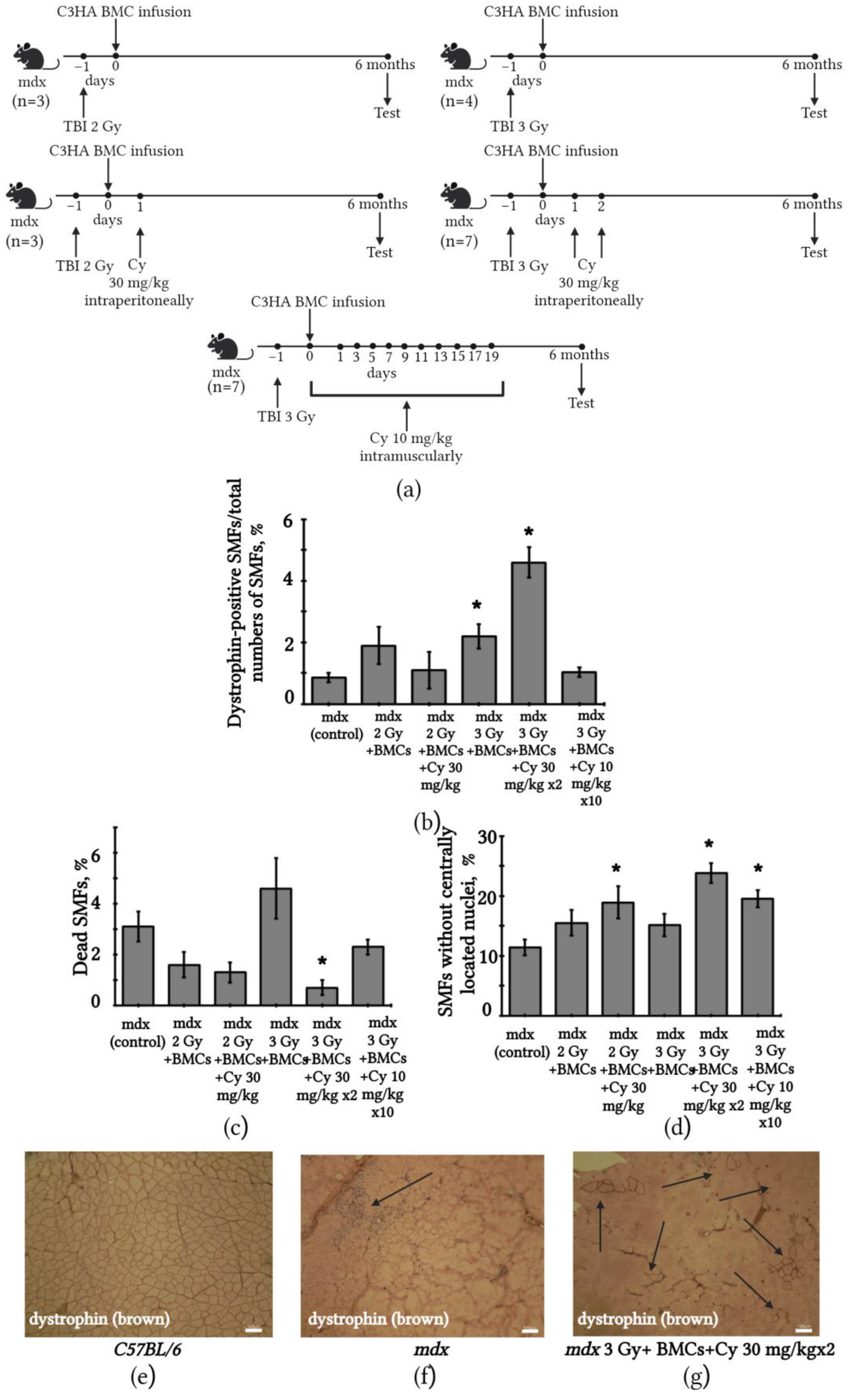
2.5. Changes in SMFs of mdx Mice after BMC Transplantation from C3HA Mice
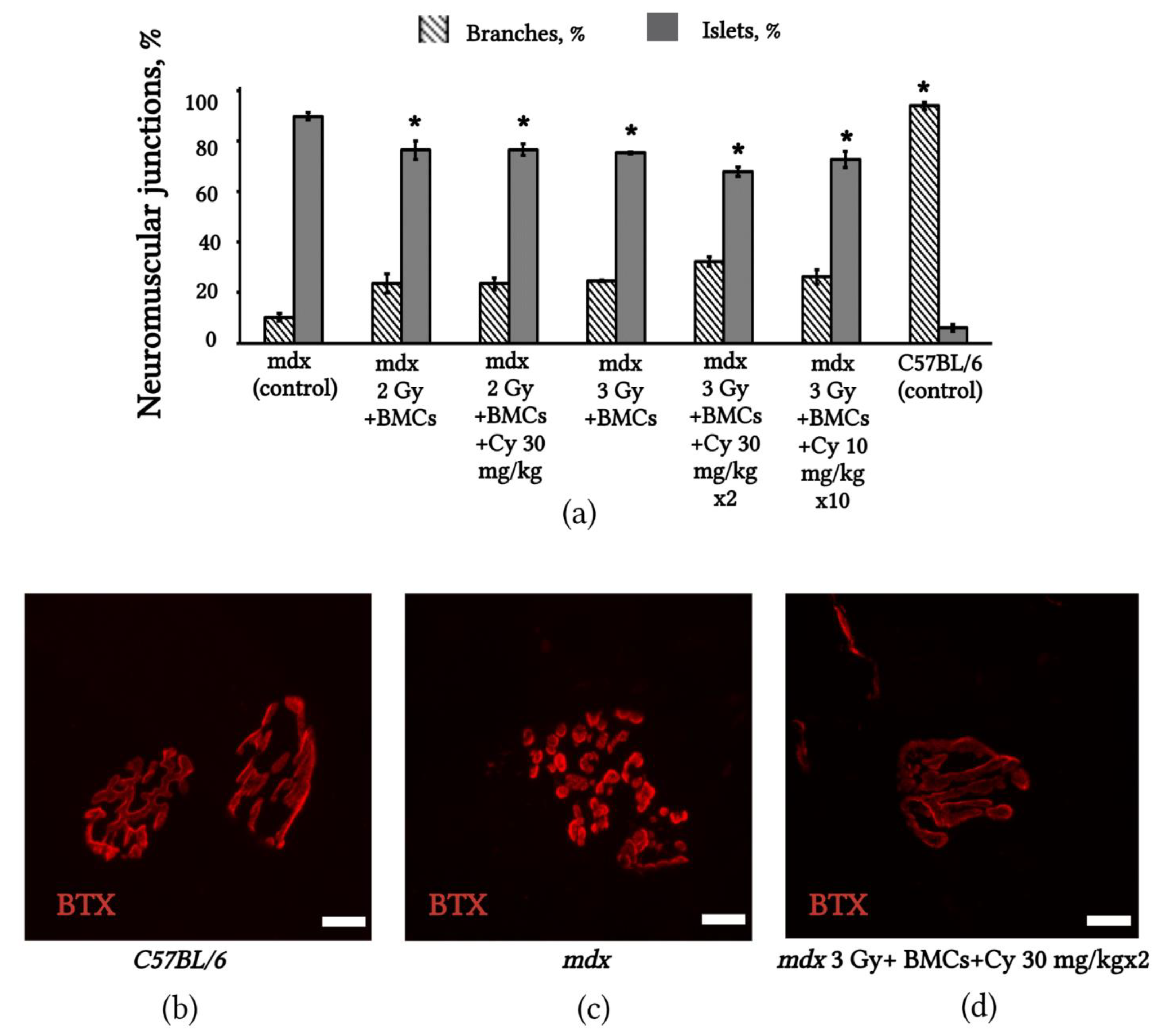
2.6. Changes in NMJs’ Structure in mdx Mice after BMC Transplantation from C3HA Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bone Marrow Cell Isolation
4.3. Bone Marrow Cell Transplantation
4.4. Muscle Collection
4.5. Assessment of Presence of GFP-Positive Cells and SMFs in Muscles after Transplantation of BMCs from Transgenic GFP-Expressing C57BL/6 Mice Donors
4.6. Immunohistochemistry
4.7. The Number of Dead SMFs Evaluation
4.8. Masson’s Trichrome Staining
4.9. Investigation of the Neuromuscular Junctions’ Structure
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, C.A.; Morgan, J.E. Duchenne’s muscular dystrophy: Animal models used to investigate pathogenesis and develop therapeutic strategies. Int. J. Exp. Pathol. 2003, 84, 165–172. [Google Scholar] [CrossRef]
- Dalkilic, I.; Kunkel, L.M. Muscular dystrophies: Genes to pathogenesis. Curr. Opin. Genet. Dev. 2003, 13, 231–238. [Google Scholar] [CrossRef]
- Otto, A.; Coolins-Hooper, H.; Patel, K. The origin, molecurlar regulation and therapeutic potencial of myogenic stem cell populations. J. Anat. 2009, 215, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Negroni, E.; Gidaro, T.; Bigot, A.; Mouly, V.; Trollet, C. Stem cells and muscle diseases: Advances in cell therapy strategies. J. Neuropathol. Appl. Neurobiol. 2015, 41, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Gaynetdinova, D.D.; Novoselova, A.A. Current diagnosis and treatment of Duchenne muscular dystrophy. Kazan Med. J. 2020, 101, 530–537. [Google Scholar] [CrossRef]
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne muscular dystrophy: Myonecrosis, inflammation and oxidative stress. Dis. Model. Mech. 2020, 13, dmm043638. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H., Jr.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Rybakova, I.N.; Patel, J.R.; Ervasti, J.M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 2000, 150, 1209–1214. [Google Scholar] [CrossRef]
- Zaynitdinova, M.I.; Lavrov, A.V.; Smirnikhina, S.A. Animal models for researching approaches to therapy of Duchenne muscular dystrophy. Transgenic Res. 2021, 30, 709–725. [Google Scholar] [CrossRef]
- Bulfield, G.; Siller, W.G.; Wight, P.A.; Moore, K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 1984, 81, 1189–1192. [Google Scholar] [CrossRef]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science 1989, 244, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Muscle and cardiac therapeutic strategies for Duchenne muscular dystrophy: Past, present, and future. Pharmacol. Rep. 2020, 72, 1227–1263. [Google Scholar] [CrossRef]
- Fortunato, F.; Farnè, M.; Ferlini, A. The DMD gene and therapeutic approaches to restore dystrophin. Neuromuscul. Disord. 2021, 31, 1013–1020. [Google Scholar] [CrossRef]
- Mbakam, C.H.; Lamothe, G.; Tremblay, J.P. Therapeutic Strategies for Dystrophin Replacement in Duchenne Muscular Dystrophy. Front. Med. 2022, 9, 859930. [Google Scholar] [CrossRef]
- Matsuo, M. Antisense Oligonucleotide-Mediated Exon-skipping Therapies: Precision Medicine Spreading from Duchenne Muscular Dystrophy. JMA J. 2021, 4, 232–240. [Google Scholar]
- Xu, X.; Wilschut, K.J.; Kouklis, G.; Tian, H.; Hesse, R.; Garland, C.; Sbitany, H.; Hansen, S.; Seth, R.; Knott, P.D.; et al. Human Satellite Cell Transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep. 2015, 5, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, A.; Kozlowska, U.; Kurpisz, M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch. Immunol. Ther. Exp. 2018, 66, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Galvez, B.G.; Sampaolesi, M.; Brunelli, S.; Covarello, D.; Gavina, M.; Rossi, B.; Constantin, G.; Torrente, Y.; Cossu, G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J. Cell Biol. 2006, 174, 231–243. [Google Scholar] [CrossRef]
- Sampaolesi, M.; Blot, S.; D’Antona, G.; Granger, N.; Tonlorenzi, R.; Innocenzi, A.; Mognol, P.; Thibaud, J.L.; Galvez, B.G.; Barthélémy, I.; et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006, 444, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Seale, P.; Girgis-Gabardo, A.; Rudnicki, M.A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002, 159, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, E.; Soneoka, Y.; Strickland, C.D.; Buzney, E.A.; Khan, M.K.; Flint, A.F.; Kunkel, L.M.; Mulligan, F.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 401, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.A.; Mi, T.; Goodell, M.A. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA 1999, 96, 14482–14486. [Google Scholar] [CrossRef]
- Muskiewicz, K.R.; Frank, N.Y.; Flint, A.F.; Gussoni, E. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. J. Histochem. Cytochem. 2005, 53, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Torrente, Y.; Belicchi, M.; Sampaolesi, M.; Pisati, F.; Meregalli, M.; D’Antona, G.; Tonlorenzi, R.; Porretti, L.; Gavina, M.; Mamchaoui, K.; et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Investig. 2004, 114, 182–195. [Google Scholar] [CrossRef]
- Benchaouir, R.; Meregalli, M.; Farini, A.; D’Antona, G.; Belicchi, M.; Goyenvalle, A.; Battistelli, M.; Bresolin, N.; Bottinelli, R.; Garcia, L.; et al. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell 2007, 1, 646–657. [Google Scholar] [CrossRef]
- Miura, Y.; Sato, M.; Kuwahara, T.; Ebata, T.; Tabata, Y.; Sakurai, H. Transplantation of human iPSC-derived muscle stem cells in the diaphragm of Duchenne muscular dystrophy model mice. PLoS ONE 2022, 17, e0266391. [Google Scholar] [CrossRef]
- Siemionow, M.; Langa, P.; Harasymczuk, M.; Cwykiel, J.; Sielewicz, M.; Smieszek, J.; Heydemann, A. Human Dystrophin Expressing Chimeric (DEC) Cell Therapy Ameliorates Cardiac, Respiratory, and Skeletal Muscle’s Function in Duchenne Muscular Dystrophy. Stem Cells Transl. Med. 2021, 10, 1406–1418. [Google Scholar] [CrossRef]
- Siemionow, M.; Langa, P.; Brodowska, S.; Kozlowska, K.; Zalants, K.; Budzynska, K.; Heydemann, A. Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy. Stem Cell Rev. Rep. 2022, 18, 2872–2892. [Google Scholar] [CrossRef]
- Bittner, R.E.; Schöfer, C.; Weipoltshammer, K.; Ivanova, S.; Streubel, B.; Hauser, E.; Freilinger, M.; Höger, H.; Elbe-Bürger, A.; Wachtler, F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat. Embryol. 1999, 199, 391–396. [Google Scholar] [CrossRef]
- Ferrari, G.; Cusella-De Angelis, G.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, M.A.; Blau, H.M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 2002, 111, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chretien, F.; Dreyfus, P.A.; Christov, C.; Caramelle, P.; Lagrange, J.L.; Chazaud, B.; Gherardi, R.K. In vivo fusion of circulating fluorescent cells with dystrophin-deficient myofibers results in extensive sarcoplasmic fluorescence expression but limited dystrophin sarcolemmal expression. Am. J. Pathol. 2005, 166, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Wernig, G.; Janzen, V.; Schäfer, R.; Zweyer, M.; Knauf, U.; Hoegemeier, O.; Mundegar, R.R.; Garbe, S.; Stier, S.; Franz, T.; et al. The vast majority of bone-marrow-derived cells integrated into mdx muscle fibers are silent despite long-term engraftment. Proc. Natl. Acad. Sci. USA 2005, 102, 11852–11857. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ohsawa, Y.; Asakura, S.; Murakami, T.; Teshima, T.; Sunada, Y. Bone marrow transplantation improves outcome in a mouse model of congenital muscular dystrophy. FEBS Lett. 2006, 580, 4463–4468. [Google Scholar] [CrossRef]
- Cossu, G. Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes. J Clin. Invest. 2004, 114, 1540–1543. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, L.; Wu, S.; Zuo, L. Effects of Ionizing Irradiation on Mouse Diaphragmatic Skeletal Muscle. Front. Physiol. 2017, 8, 506. [Google Scholar] [CrossRef]
- Torres, L.F.; Duchen, L.W. The mutant mdx: Inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain 1987, 110 Pt 2, 269–299. [Google Scholar] [CrossRef]
- Massopust, R.T.; Lee, Y.I.; Pritchard, A.L.; Nguyen, V.M.; McCreedy, D.A.; Thompson, W.J. Lifetime analysis of mdx skeletal muscle reveals a progressive pathology that leads to myofiber loss. Sci. Rep. 2020, 10, 17248. [Google Scholar] [CrossRef]
- Jurdana, M.; Cemazar, M.; Pegan, K.; Mars, T. Effect of ionizing radiation on human skeletal muscle precursor cells. Radiol. Oncol. 2013, 47, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, N.; Bevans, M. Non-myeloablative allogeneic hematopoietic stem cell transplantation. Semin. Oncol. Nurs. 2009, 25, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Ge, S.; Symbatyan, G.; Rosol, M.S.; Olch, A.J.; Crooks, G.M. Effects of sublethal irradiation on patterns of engraftment after murine bone marrow transplantation. Biol. Blood Marrow Transplant. 2011, 17, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Farini, A.; Sitzia, C.; Erratico, S.; Meregalli, M.; Torrente, Y. Influence of immune responses in gene/stem cell therapies for muscular dystrophies. BioMed Res. Int. 2014, 2014, 818107. [Google Scholar] [CrossRef]
- Li, N.; Parkes, J.E.; Spathis, R.; Morales, M.; Mcdonald, J.; Kendra, R.M.; Ott, E.M.; Brown, K.J.; Lawlor, M.W.; Nagaraju, K. The Effect of Immunomodulatory Treatments on Anti-Dystrophin Immune Response After AAV Gene Therapy in Dystrophin Deficient mdx Mice. J. Neuromuscul. Dis. 2021, 8, S325–S340. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, N.; Versele, R.; Planchon, C.; Selvais, C.M.; Noel, L.; Abou-Samra, M.; Davis-López de Carrizosa, M.A. Histological Methods to Assess Skeletal Muscle Degeneration and Regeneration in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2022, 23, 16080. [Google Scholar] [CrossRef]
- Kong, J.; Anderson, J.E. Dystrophin is required for organizing large acetylcholine receptor aggregates. Brain Res. 1999, 839, 298–304. [Google Scholar] [CrossRef]
- Minatel, E.; Neto, H.S.; Marques, M.J. Acetylcholine receptors and neuronal nitric oxide synthase distribution at the neuromuscular junction of regenerated muscle fibers. Muscle Nerve 2001, 24, 410–416. [Google Scholar] [CrossRef]
- Marques, M.J.; Taniguti, A.P.; Minatel, E.; Neto, H.S. Nerve terminal contributes to acetylcholine receptor organization at the dystrophic neuromuscular junction of mdx mice. Anat. Rec. 2007, 290, 181–187. [Google Scholar] [CrossRef]
- Sokolova, A.V.; Zenin, V.V.; Mikhailov, V.M. Structure of neuromuscular junctions and differentiation of striated muscle fibers in mdx mice after bone-marrow stem cell therapy. Cell Tissue Biol. 2010, 4, 258–266. [Google Scholar] [CrossRef]
- Pratt, S.J.P.; Shah, S.B.; Ward, C.W.; Kerr, J.P.; Stains, J.P.; Lovering, R.M. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell. Mol. Life Sci. 2015, 72, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lovering, R.M.; Iyer, S.R.; Edwards, B.; Davies, K.E. Alterations of neuromuscular junctions in Duchenne muscular dystrophy. Neurosci. Lett. 2020, 737, 135304. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Ljubicic, V. Recent insights into neuromuscular junction biology in Duchenne muscular dystrophy: Impacts, challenges, and opportunities. EbioMedicine 2020, 61, 103032. [Google Scholar] [CrossRef]
- Marques, M.J.; Pertille, A.; Carvalho, C.L.; Neto, H.S. Acetylcholine receptor organization at the dystrophic extraocular muscle neuromuscular junction. Anat. Rec. 2007, 290, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Haddix, S.G.; Lee, Y.I.; Kornegay, J.N.; Thompson, W.J. Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy. PLoS ONE 2018, 13, e0205926. [Google Scholar] [CrossRef]
- Rafael, J.A.; Townsend, E.R.; Squire, S.E.; Potter, A.C.; Chamberlain, J.S.; Davies, K.E. Dystrophin and utrophin influence fiber type composition and post-synaptic membrane structure. Hum. Mol. Genet. 2000, 9, 1357–1367. [Google Scholar] [CrossRef]
- Banks, G.B.; Chamberlain, J.S.; Froehner, S.C. Truncated dystrophins can influence neuromuscular synapse structure. Mol. Cell. Neurosci. 2009, 40, 433–441. [Google Scholar] [CrossRef]
- Kong, J.; Yang, L.; Li, Q.; Cao, J.; Yang, J.; Chen, F.; Wang, Y.; Zhang, C. The absence of dystrophin rather than muscle degeneration causes acetylcholine receptor cluster defects in dystrophic muscle. Neuroreport 2012, 23, 82–87. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Mikhailov, V.M.; Sokolova, A.V.; Mikhailova, E.V.; Timonina, N.A.; Nikol’skii, E.E.; Krivoi, I.I. Recovery of electrogenesis in skeletal muscles after cell therapy of myodystrophy in mdx mice. Rep. Acad. Sci. 2011, 441, 272–274. [Google Scholar] [CrossRef]
- Timonina, N.; Kravtsova, V.; Mikhailova, E.; Sokolova, A.; Mikhailov, V.; Krivoi, I. Electrogenesis of end-plates of mdx mice diaphragm: Effect of cell therapy. Biol. Commun. 2015, 3, 66–74. [Google Scholar] [CrossRef]
- van Putten, M.; Hulsker, M.; Young, C.; Nadarajah, V.D.; Heemskerk, H.; van der Weerd, L.; AC’t Hoen, P.; van Ommen, G.-J.B. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J. 2013, 27, 2484–2495. [Google Scholar] [CrossRef]
- van der Pijl, E.M.; van Putten, M.; Niks, E.H.; Verschuuren, J.J.G.M.; Aartsma-Rus, A.; Plomp, J.J. Low dystrophin levels are insufficient to normalize the neuromuscular synaptic abnormalities of mdx mice. Neuromuscul. Disord. 2018, 28, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.J. What is the level of dystrophin expression required for effective therapy of Duchenne muscular dystrophy? J. Muscle Res. Cell Motil. 2019, 40, 141–150.64. [Google Scholar] [CrossRef] [PubMed]
- Recenti, M.; Ricciardi, C.; Edmunds, K.; Jacob, D.; Gambacorta, M.; Gargiulo, P. Testing soft tissue radiodensity parameters interplay with age and self-reported physical activity. Eur. J. Transl. Myol. 2021, 31, 9929. [Google Scholar] [CrossRef] [PubMed]
- Recenti, M.; Ricciardi, C.; Edmunds, K.; Gislason, M.K.; Gargiulo, P. Machine learning predictive system based upon radiodensitometric distributions from mid-thigh CT images. Eur. J. Transl. Myol. 2020, 30, 8892. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.J.; Aguiar, M.T.M.; Ribeiro, A.A.F.; Pacheco, A.G.F. Haploidentical Transplantation with Post-Transplant Cyclophosphamide versus Unrelated Donor Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2019, 25, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, L.P.; Patterson, M.T.; Eckhaus, M.A.; Venzon, D.J.; Gress, R.E.; Kanakry, C.G. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J. Clin. Investig. 2019, 129, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, H. A Review of Cyclophosphamide-Induced Transplantation Tolerance in Mice and Its Relationship With the HLA-Haploidentical Bone Marrow Transplantation/Post-Transplantation Cyclophosphamide Platform. Front. Immunol. 2021, 12, 744430. [Google Scholar] [CrossRef]
- Nakamae, H. Systematic overview of HLA-matched allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide. Int. J. Hematol. 2022, 116, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.N.; De Oliveira, S.; Gray, A.; Bowles, L.; Moore, T.B. Successful engraftment of haploidentical bone marrow with post-transplantation cyclophosphamide in patients with aplastic anemia. Pediatr. Transplant. 2020, 24, e13652. [Google Scholar] [CrossRef]
- Leick, M.; Hunter, B.; DeFilipp, Z.; Dey, B.R.; El-Jawahri, A.; Frigault, M.; McAfee, S.; Spitzer, N.R.; O’Donnell, P.; Chen, Y.-B. Posttransplant cyclophosphamide in allogeneic bone marrow transplantation for the treatment of nonmalignant hematological diseases. Bone Marrow Transplant. 2020, 55, 758–762. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, A.V.; Domnina, A.P.; Mikhailov, V.M. Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions. Int. J. Mol. Sci. 2023, 24, 8892. https://doi.org/10.3390/ijms24108892
Sokolova AV, Domnina AP, Mikhailov VM. Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions. International Journal of Molecular Sciences. 2023; 24(10):8892. https://doi.org/10.3390/ijms24108892
Chicago/Turabian StyleSokolova, Anastasiia V., Alisa P. Domnina, and Viacheslav M. Mikhailov. 2023. "Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions" International Journal of Molecular Sciences 24, no. 10: 8892. https://doi.org/10.3390/ijms24108892
APA StyleSokolova, A. V., Domnina, A. P., & Mikhailov, V. M. (2023). Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions. International Journal of Molecular Sciences, 24(10), 8892. https://doi.org/10.3390/ijms24108892




