Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice
Abstract
1. Introduction
2. Results
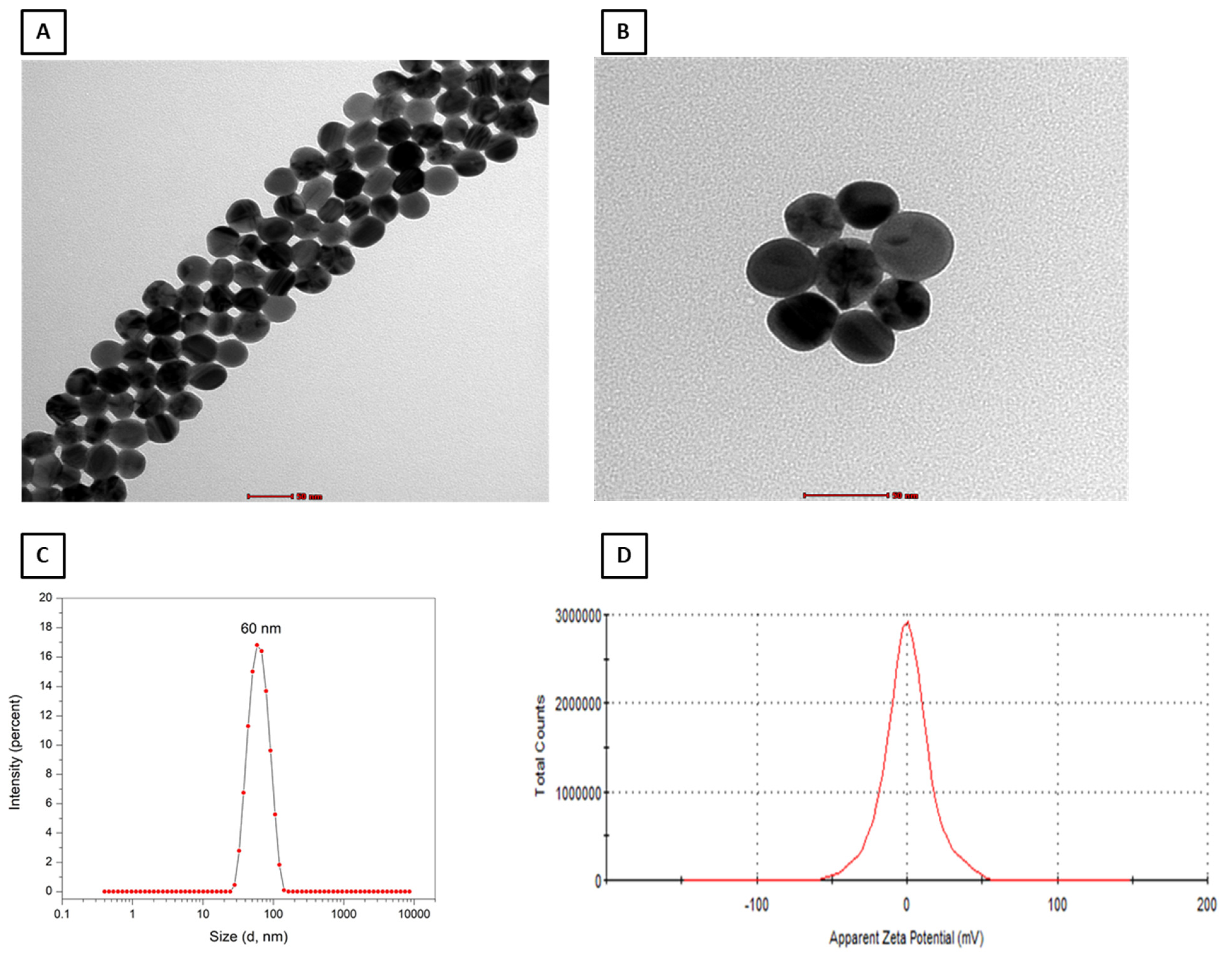
2.1. Characterization of PEG–AgNPs
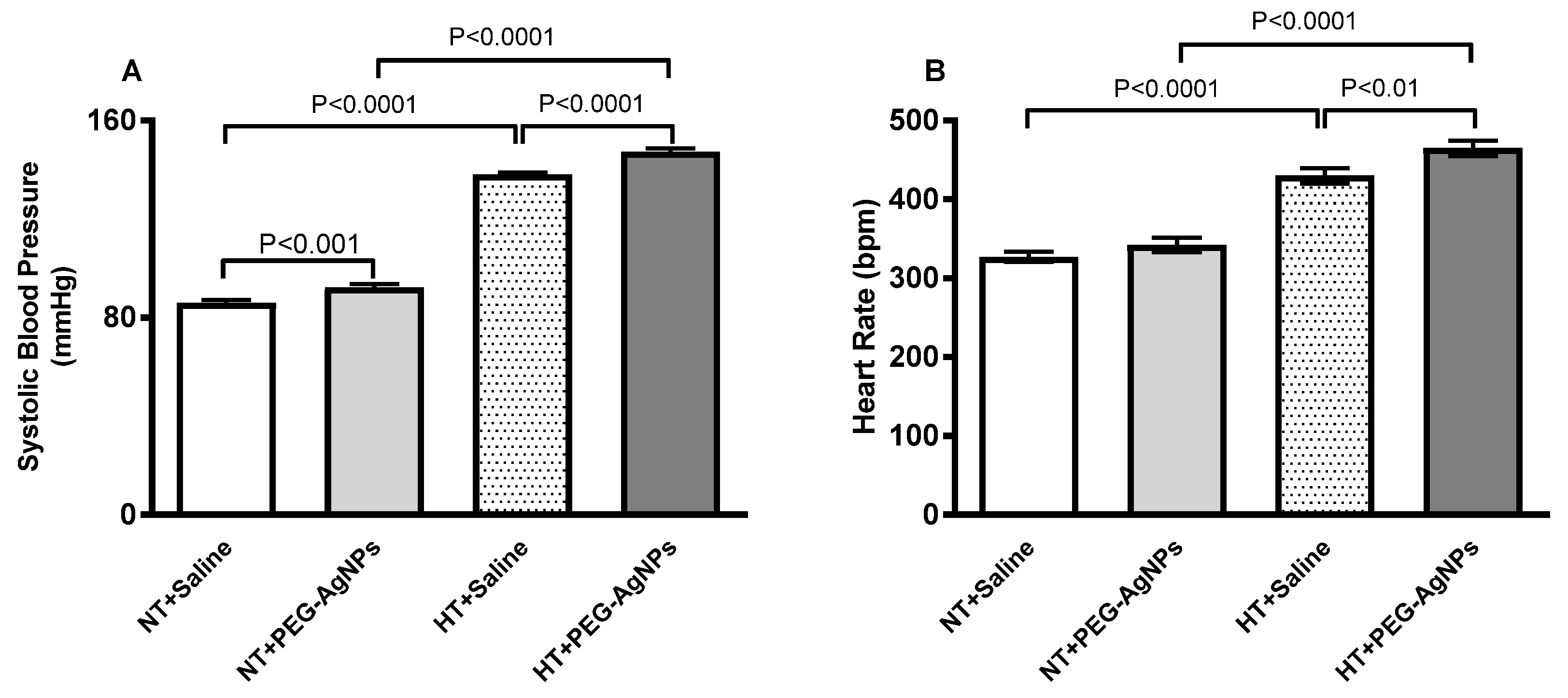
2.2. Systolic Blood Pressure (SBP) and Heart Rate (HR)
2.3. Relative Heart Weight
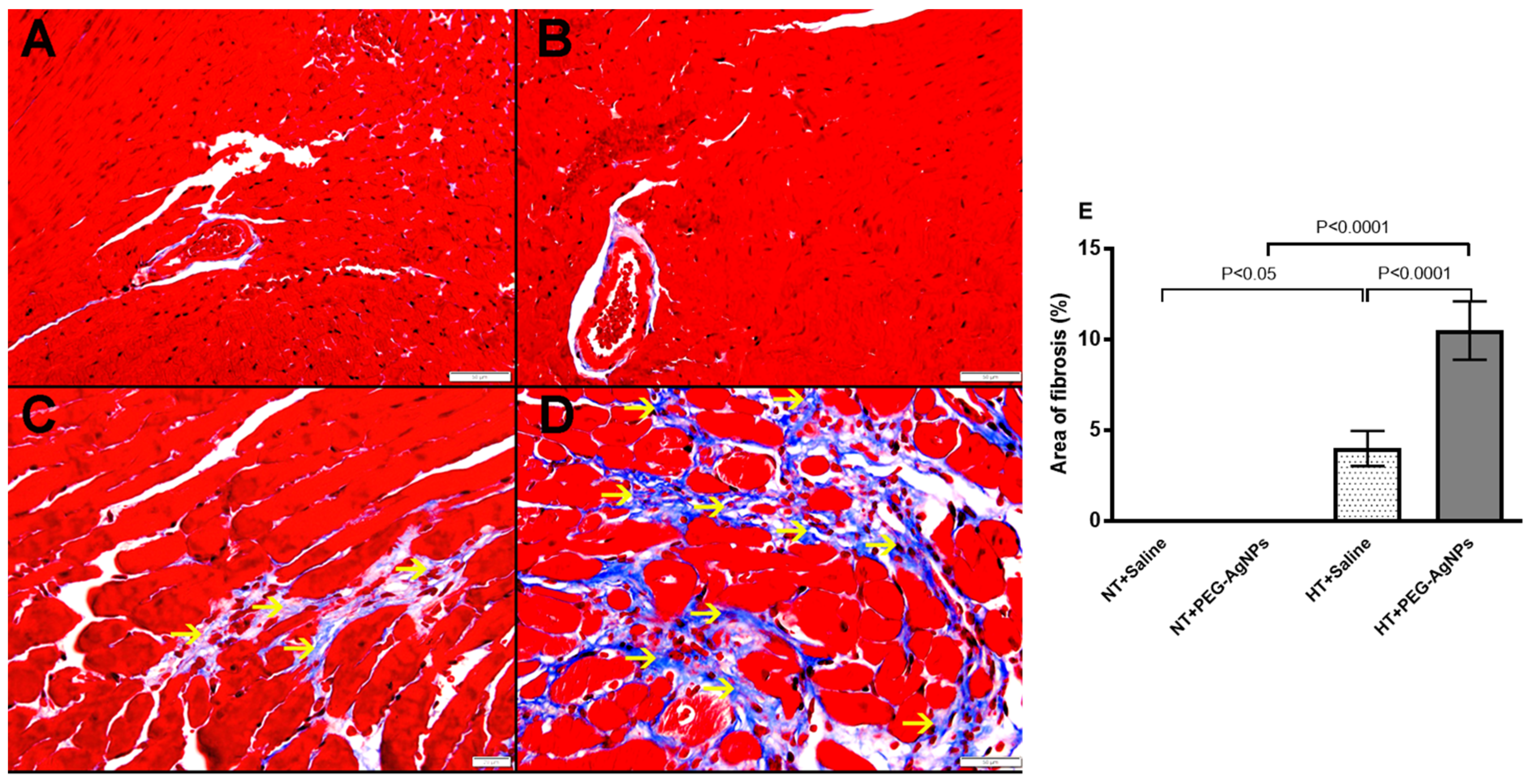
2.4. Histopathological Analysis of the Heart
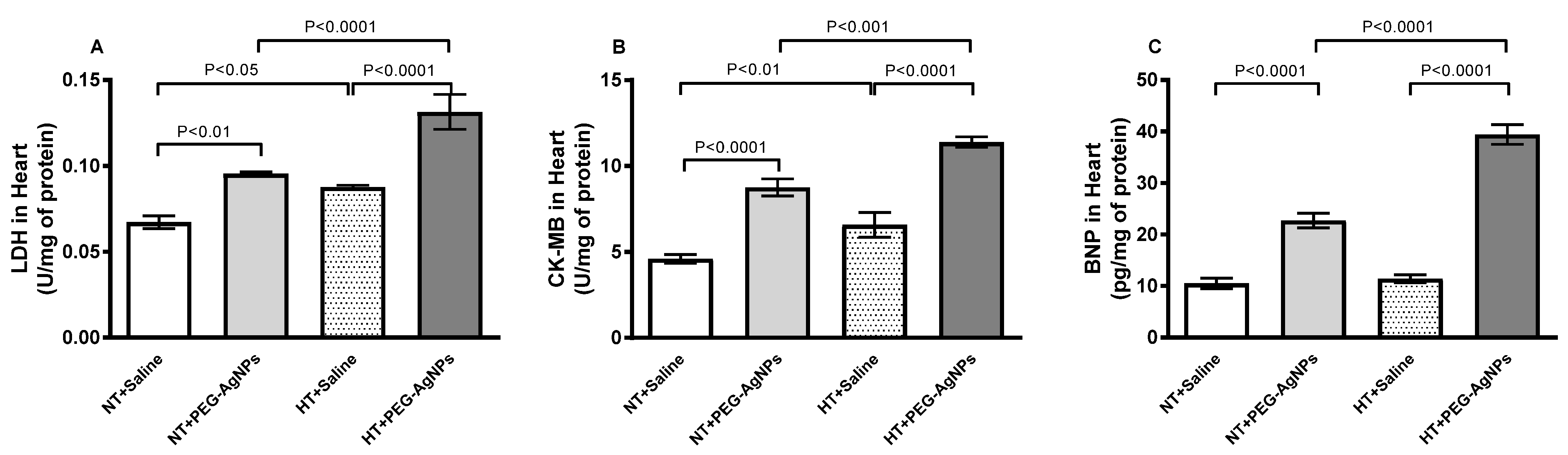
2.5. Lactate Dehydrogenase (LDH) and Creatine Kinase-MB (CK-MB) Activities, and Brain Natriuritic Peptide (BNP) Concentration in Heart
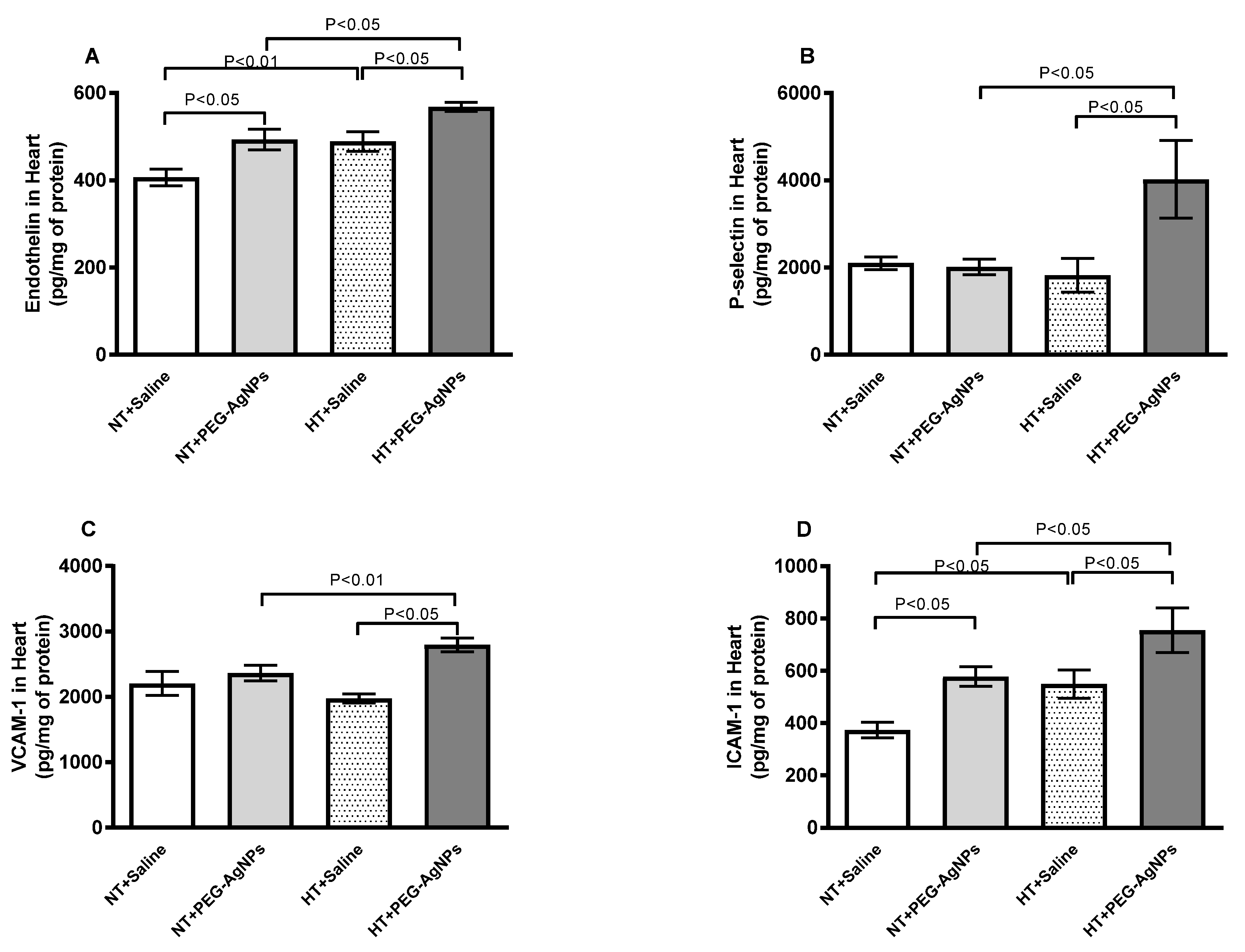
2.6. Endothelin-1, P-Selectin, Vascular Cell Adhesion Molecule-1 (VCAM-1), and Intercellular Adhesion Molecule-1 (ICAM-1) Concentrations in Heart
2.7. Tumor Necrosis Factor-α (TNFα) and Interleukin-6 (IL-6) Concentrations in Heart
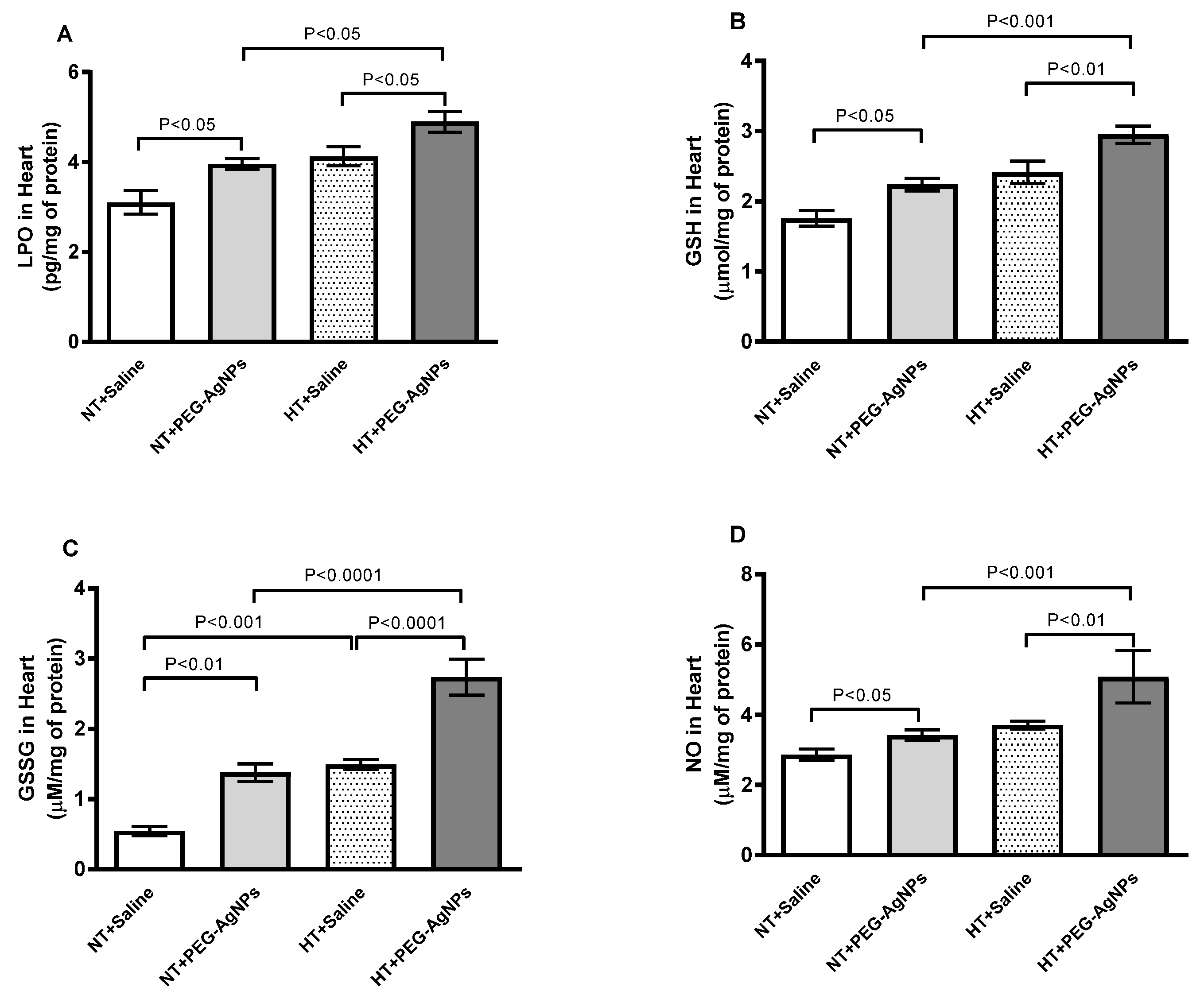
2.8. Lipid Peroxidation (LPO), Reduced Glutathione (GSH), Oxidized Glutathione (GSSG), and Total Nitric Oxide (NO) Concentrations in Heart
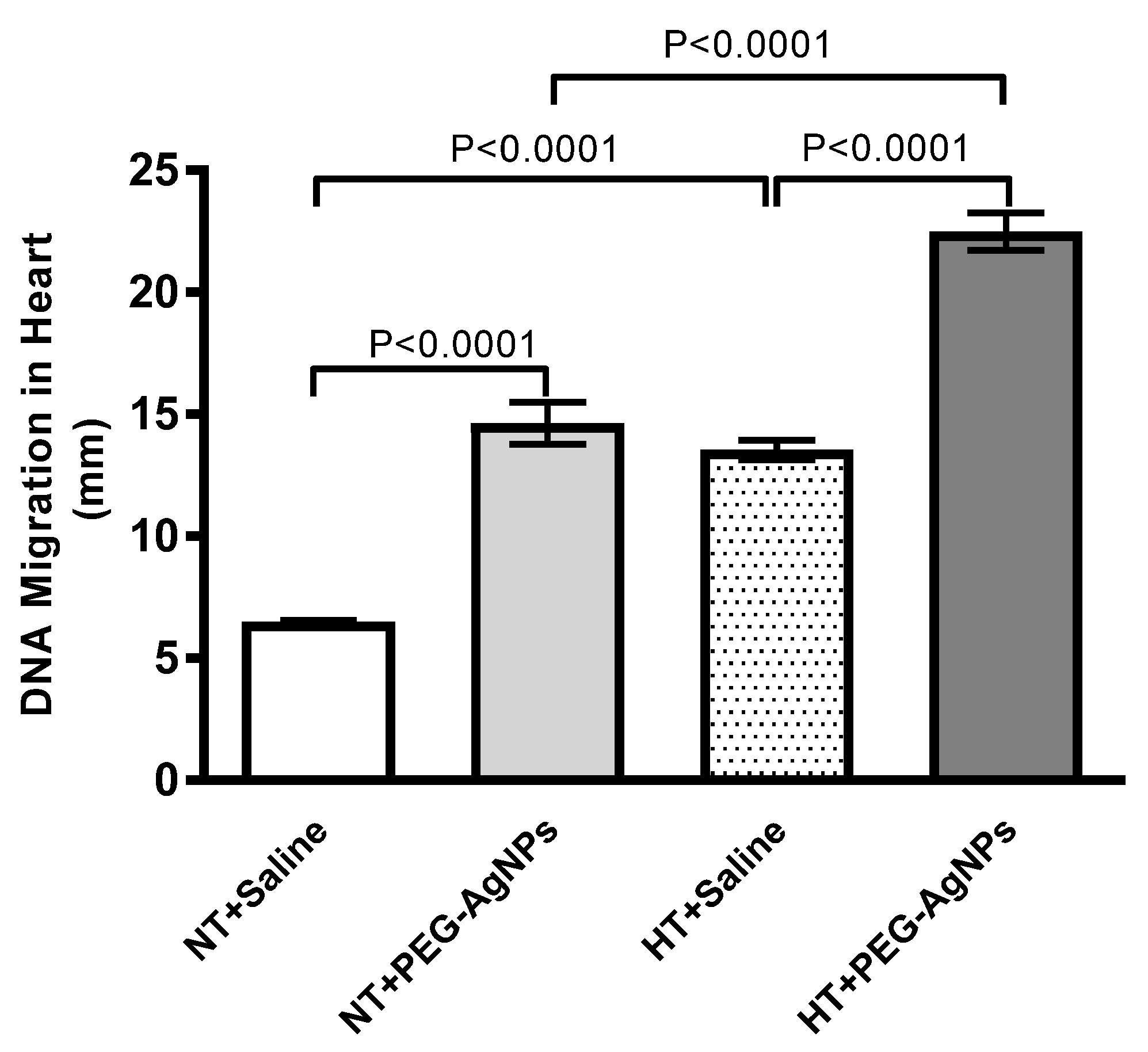
2.9. DNA Damage in Heart
3. Discussion
4. Materials and Methods
4.1. PEG–AgNPs
4.2. PEG–AgNP Characterization by Transmission Electron Microscopy and Surface Charge Analysis by Zeta Potential
4.3. Treatments
4.4. SBP and HR Measurement
4.5. Heart Histology and Weight
4.6. Quantification of the Concentrations of LDH, CK-MB, BNP, Endothelin-1, P-selectin, VCAM-1, and ICAM-1 in Heart Tissue
4.7. Measurement of TNF-α, IL-6, LPO, GSH, GSSG, and Total Nitric Oxide in Heart
4.8. Evaluation of DNA Injury in the Heart by COMET Assay
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, C.; Rosas-Hernandez, H.; Ramirez-Lee, M.A.; Salazar-Garcia, S.; Ali, S.F. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch. Toxicol. 2016, 90, 493–511. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [PubMed]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.A.; Chen, K.L. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ. Sci. Technol. 2011, 45, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Priest, T.; Zhang, G.; Sun, X.; Patel, D.N.; McNally, L.R.; van Berkel, V.; Gobin, A.M.; Frieboes, H.B. Enhanced penetration into 3D cell culture using two and three layered gold nanoparticles. Int. J. Nanomed. 2013, 8, 3603–3617. [Google Scholar]
- Bastos, V.; Ferreira de Oliveira, J.M.; Brown, D.; Jonhston, H.; Malheiro, E.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. The influence of Citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line. Toxicol.Lett. 2016, 249, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Beegam, S.; Zaaba, N.E.; Elzaki, O.; Tariq, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Exacerbation of Thrombotic Responses to Silver Nanoparticles in Hypertensive Mouse Model. Oxid. Med. Cell. Longev. 2022, 2022, 2079630. [Google Scholar] [CrossRef]
- Ramirez-Lee, M.A.; Aguirre-Banuelos, P.; Martinez-Cuevas, P.P.; Espinosa-Tanguma, R.; Chi-Ahumada, E.; Martinez-Castanon, G.A.; Gonzalez, C. Evaluation of cardiovascular responses to silver nanoparticles (AgNPs) in spontaneously hypertensive rats. Nanomedicine 2018, 14, 385–395. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Volker, C.; Oetken, M.; Oehlmann, J. The biological effects and possible modes of action of nanosilver. Rev. Environ. Contam Toxicol. 2013, 223, 81–106. [Google Scholar] [PubMed]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepulveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdorster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Tamagawa, E.; Bai, N.; Suda, K.; Yang, H.H.; Li, Y.; Chiang, G.; Yatera, K.; Mukae, H.; Sin, D.D.; et al. Particulate Matter Induces IL-6 Translocation from the Lung to the Systemic Circulation. Am. J. Respir. Cell Mol. Biol. 2011, 44, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, I.G.; Ryan, M.P.; Tetley, T.D.; Porter, A.E. Inhalation of silver nanomaterials—Seeing the risks. Int. J. Mol. Sci. 2014, 15, 23936–23974. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mun, J.; Park, J.D.; Yu, I.J. A health surveillance case study on workers who manufacture silver nanomaterials. Nanotoxicology 2012, 6, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Ditto, A.J.; Panzner, M.J.; Medvetz, D.A.; Han, D.S.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Cannon, C.L.; et al. The antimicrobial efficacy of sustained release silver-carbene complex-loaded L-tyrosine polyphosphate nanoparticles: Characterization, in vitro and in vivo studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef]
- Jang, S.; Park, J.W.; Cha, H.R.; Jung, S.Y.; Lee, J.E.; Jung, S.S.; Kim, J.O.; Kim, S.Y.; Lee, C.S.; Park, H.S. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int. J. Nanomed. 2012, 7, 1329–1343. [Google Scholar]
- Silva, R.M.; Anderson, D.S.; Franzi, L.M.; Peake, J.L.; Edwards, P.C.; Van Winkle, L.S.; Pinkerton, K.E. Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol. Sci. 2015, 144, 151–162. [Google Scholar] [CrossRef]
- Ferdous, Z.; Al-Salam, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Pulmonary exposure to silver nanoparticles impairs cardiovascular homeostasis: Effects of coating, dose and time. Toxicol. Appl. Pharmacol. 2019, 367, 36–50. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxid. Med. Cell. Longev. 2017, 2017, 9639035. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Yue, P.; Xu, X.; Zhong, M.; Sun, Q.; Mikolaj, M.; Wang, A.; Brook, R.D.; Chen, L.C.; Rajagopalan, S. Air pollution and cardiac remodeling: A role for RhoA/Rho-kinase. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1540–H1550. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Xie, X.; Bai, Y.; Chen, M.; Wang, X.; Zhang, X.; Morishita, M.; Sun, Q.; Rajagopalan, S. Exposure to concentrated ambient particulate matter induces reversible increase of heart weight in spontaneously hypertensive rats. Part. Fibre Toxicol. 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bhatia, J.; Arora, S.; Golechha, M.; Kumari, S.; Arya, D.S. Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity: A biochemical and histopathological evaluation. J. Environ. Biol. 2011, 32, 731–738. [Google Scholar]
- Tankersley, C.G.; Champion, H.C.; Takimoto, E.; Gabrielson, K.; Bedja, D.; Misra, V.; El-Haddad, H.; Rabold, R.; Mitzner, W. Exposure to inhaled particulate matter impairs cardiac function in senescent mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R252–R263. [Google Scholar] [CrossRef]
- Yorikane, R.; Sakai, S.; Miyauchi, T.; Sakurai, T.; Sugishita, Y.; Goto, K. Increased production of endothelin-1 in the hypertrophied rat heart due to pressure overload. FEBS Lett. 1993, 332, 31–34. [Google Scholar] [CrossRef]
- Archer, C.R.; Robinson, E.L.; Drawnel, F.M.; Roderick, H.L. Endothelin-1 promotes hypertrophic remodelling of cardiac myocytes by activating sustained signalling and transcription downstream of endothelin type A receptors. Cell. Signal. 2017, 36, 240–254. [Google Scholar] [CrossRef]
- Yang, L.L.; Gros, R.; Kabir, M.G.; Sadi, A.; Gotlieb, A.I.; Husain, M.; Stewart, D.J. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation 2004, 109, 255–261. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. A review about biomarkers for the investigation of vascular function and impairment in diabetes mellitus. Vasc. Health Risk Manag. 2016, 12, 415–419. [Google Scholar] [CrossRef]
- Kacimi, R.; Karliner, J.S.; Koudssi, F.; Long, C.S. Expression and Regulation of Adhesion Molecules in Cardiac Cells by Cytokines. Circ. Res. 1998, 82, 576–586. [Google Scholar] [CrossRef]
- Kiarash, A.; Pagano, P.J.; Tayeh, M.; Rhaleb, N.-E.; Carretero, O.A. Upregulated Expression of Rat Heart Intercellular Adhesion Molecule-1 in Angiotensin II—but Not Phenylephrine—Induced Hypertension. Hypertension 2001, 37, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial cell-cardiomyocyte crosstalk in heart development and disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Haryono, A.; Ramadhiani, R.; Ryanto, G.R.; Emoto, N. Endothelin and the Cardiovascular System: The Long Journey and Where We Are Going. Biology 2022, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.A.W.; Buckley, B.; Farraj, A.K.; Thompson, L.C. The heart as an extravascular target of endothelin-1 in particulate matter-induced cardiac dysfunction. Pharmacol. Ther. 2016, 165, 63–78. [Google Scholar] [CrossRef]
- Zolk, O.; Quattek, J.; Sitzler, G.; Schrader, T.; Nickenig, G.; Schnabel, P.; Shimada, K.; Takahashi, M.; Böhm, M. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation 1999, 99, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef]
- Lin, C.X.; Yang, S.Y.; Gu, J.L.; Meng, J.; Xu, H.Y.; Cao, J.M. The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, INa and IK1 channels and heart rhythm in mice. Nanotoxicology 2017, 11, 827–837. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Lin, Y.; Zou, X.; Li, Z.; Liao, Y.; Du, M.; Zhang, H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-kappaB pathways. Biomaterials 2014, 35, 6657–6666. [Google Scholar] [CrossRef]
- Holland, N.A.; Thompson, L.C.; Vidanapathirana, A.K.; Urankar, R.N.; Lust, R.M.; Fennell, T.R.; Wingard, C.J. Impact of pulmonary exposure to gold core silver nanoparticles of different size and capping agents on cardiovascular injury. Part. Fibre Toxicol. 2016, 13, 48. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid. Redox Signal. 2014, 20, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [PubMed]
- Recordati, C.; De, M.M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2016, 13, 12. [Google Scholar] [CrossRef]
- Ferdous, Z.; Beegam, S.; Tariq, S.; Ali, B.H.; Nemmar, A. The in Vitro Effect of Polyvinylpyrrolidone and Citrate Coated Silver Nanoparticles on Erythrocytic Oxidative Damage and Eryptosis. Cell. Physiol. Biochem. 2018, 49, 1577–1588. [Google Scholar] [CrossRef]
- Weiss, D.; Kools, J.J.; Taylor, W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 2001, 103, 448–454. [Google Scholar] [CrossRef]
- Nemmar, A.; Zia, S.; Subramaniyan, D.; Fahim, M.A.; Ali, B.H. Exacerbation of thrombotic events by diesel exhaust particle in mouse model of hypertension. Toxicology 2011, 285, 39–45. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Matsuura, H.; Oshima, T.; Chayama, K. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 2002, 346, 1954–1962. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Ali, B.H. Chronic exposure to water-pipe smoke induces cardiovascular dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H329–H339. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Al, H.I.; Beegam, S.; Al-Ruqaishi, B.; Nemmar, A. Effect of Acacia gum on blood pressure in rats with adenine-induced chronic renal failure. Phytomedicine 2011, 18, 1176–1180. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. Thrombosis, systemic and cardiac oxidative stress and DNA damage induced by pulmonary exposure to diesel exhaust particles, and the effect of nootkatone thereon. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H917–H927. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Hamadi, N.K.; Ali, B.H. Prolonged Pulmonary Exposure to Diesel Exhaust Particles Exacerbates Renal Oxidative Stress, Inflammation and DNA Damage in Mice with Adenine-Induced Chronic Renal Failure. Cell. Physiol. Biochem. 2016, 38, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.E.; Al-Salam, S.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, A. Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease. Nutrients 2023, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Adham, S.A.; Ali, H.; Manoj, P.; Ashique, M.; Nemmar, A. The Effect of Metformin in Diabetic and Non-Diabetic Rats with Experimentally-Induced Chronic Kidney Disease. Biomolecules 2021, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Lung Oxidative Stress, DNA Damage, Apoptosis, and Fibrosis in Adenine-Induced Chronic Kidney Disease in Mice. Front. Physiol. 2017, 8, 896. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemmar, A.; Al-Salam, S.; Greish, Y.E.; Beegam, S.; Zaaba, N.E.; Ali, B.H. Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice. Int. J. Mol. Sci. 2023, 24, 8890. https://doi.org/10.3390/ijms24108890
Nemmar A, Al-Salam S, Greish YE, Beegam S, Zaaba NE, Ali BH. Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice. International Journal of Molecular Sciences. 2023; 24(10):8890. https://doi.org/10.3390/ijms24108890
Chicago/Turabian StyleNemmar, Abderrahim, Suhail Al-Salam, Yaser E. Greish, Sumaya Beegam, Nur E. Zaaba, and Badreldin H. Ali. 2023. "Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice" International Journal of Molecular Sciences 24, no. 10: 8890. https://doi.org/10.3390/ijms24108890
APA StyleNemmar, A., Al-Salam, S., Greish, Y. E., Beegam, S., Zaaba, N. E., & Ali, B. H. (2023). Impact of Intratracheal Administration of Polyethylene Glycol-Coated Silver Nanoparticles on the Heart of Normotensive and Hypertensive Mice. International Journal of Molecular Sciences, 24(10), 8890. https://doi.org/10.3390/ijms24108890







