New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality
Abstract
:1. Introduction
2. Results
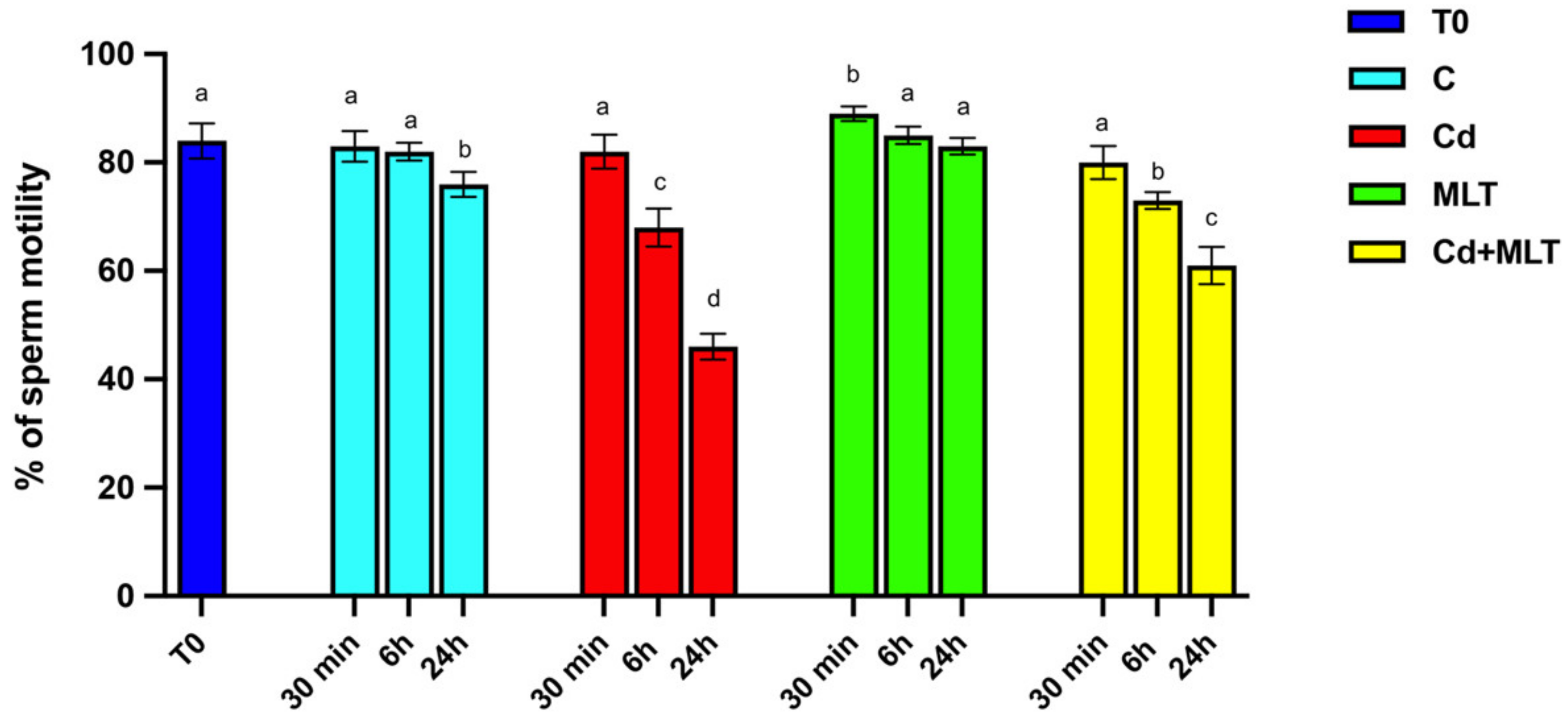
2.1. Cd and/or MLT Effects on SPZ Motility
2.2. Cd and/or MLT Effects on SPZ DNA Integrity and Apoptosis
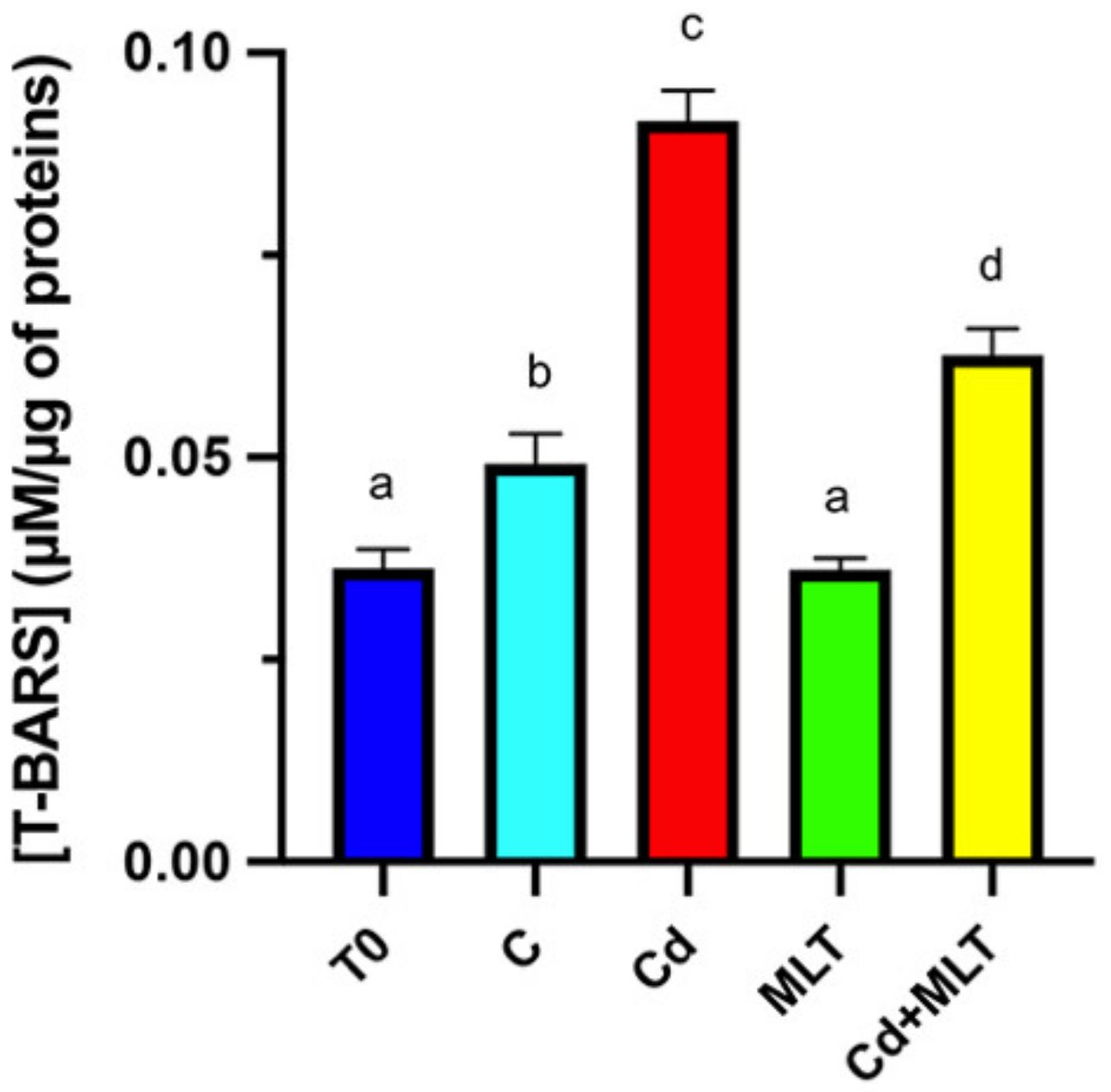
2.3. Cd and/or MLT Effects on Oxidative Stress
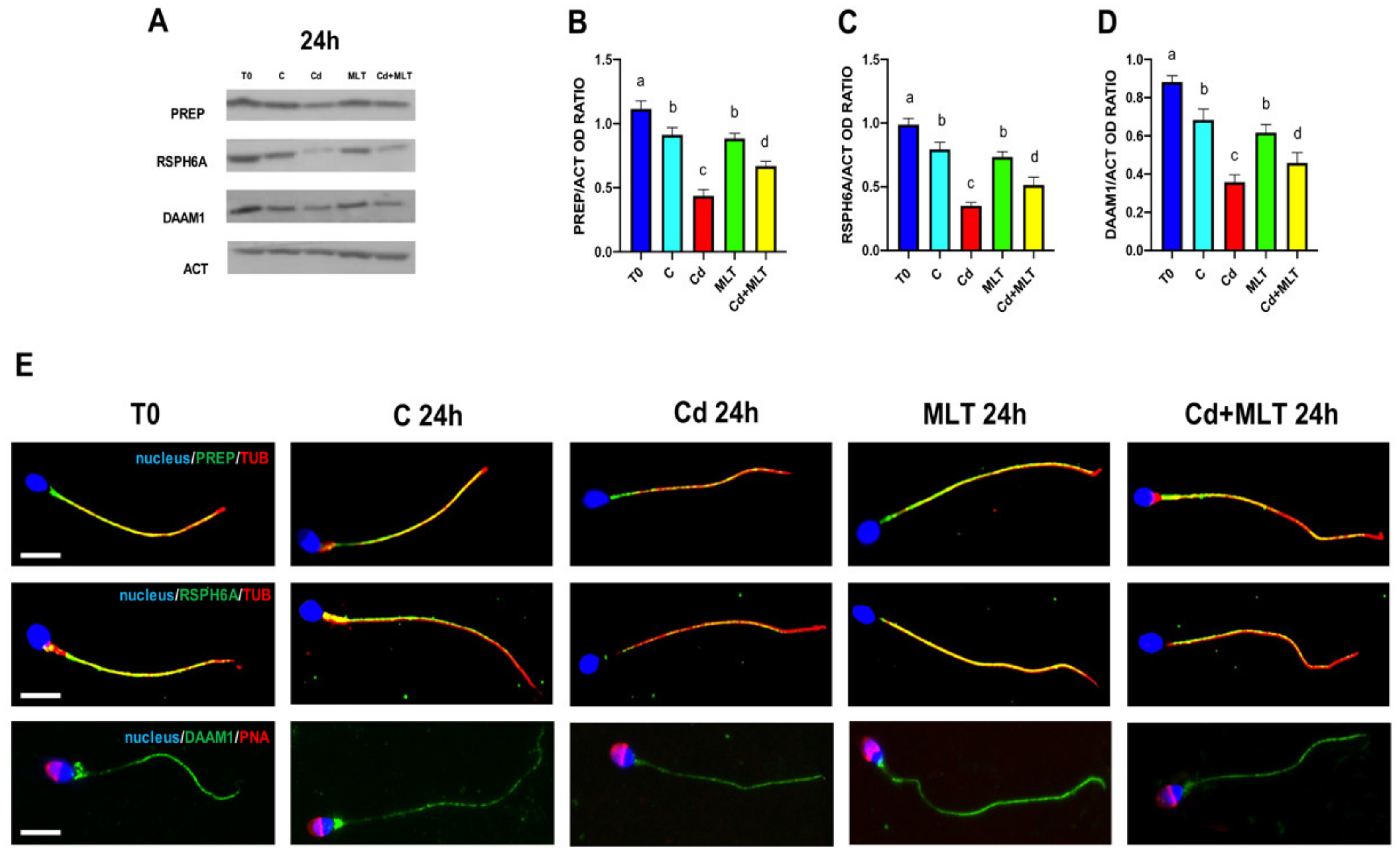
2.4. Cd and/or MLT Effects on PREP, RSPH6A and DAAM1
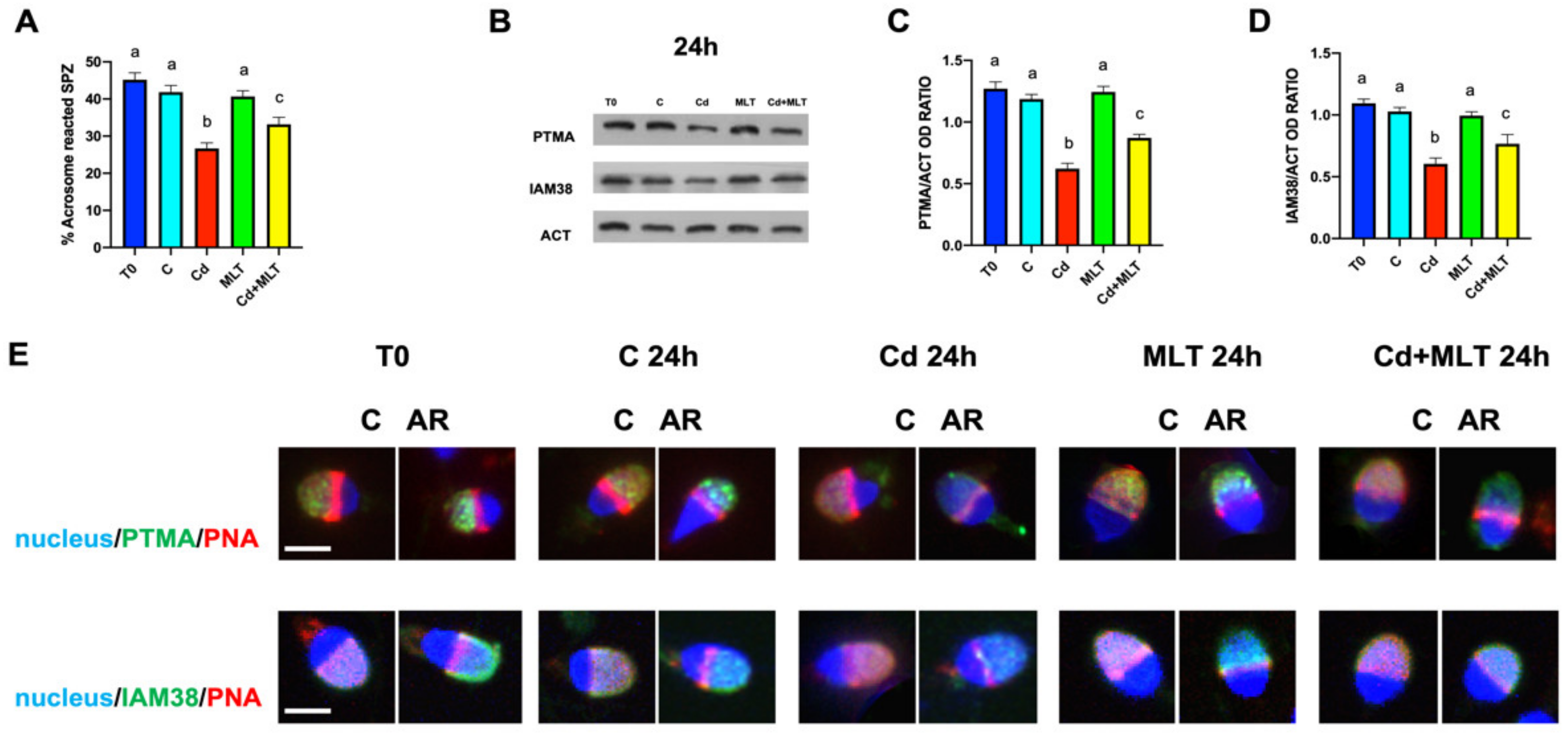
2.5. Cd and/or MLT Effects on Acrosome Reaction, PTMA and IAM38
3. Discussion
4. Materials and Methods
4.1. Human Semen Samples, Exposure Procedure, In Vitro AR and Ethical Approval
4.2. DNA Integrity and Apoptosis Assessment
4.3. TBARS Assay
4.4. Protein Extraction and WB Analysis
4.5. IF Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teves, M.E.; Roldan, E.R.S.; Krapf, D.; Strauss, J.F., III; Bhagat, V.; Sapao, P. Sperm Differentiation: The Role of Trafficking of Proteins. Int. J. Mol. Sci. 2020, 21, 3702. [Google Scholar] [CrossRef] [PubMed]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Sengupta, P.; Henkel, R.; Alguraigari, A.; Sinigaglia, M.M.; Kayal, M.; Joumah, A.; Agarwal, A. Microtubular dysfunction and male infertility. World J. Mens Health. 2020, 38, 9–23. [Google Scholar] [CrossRef]
- Concepción-Zavaleta, M.; Paz Ibarra, J.L.; Ramos-Yataco, A.; Coronado-Arroyo, J.; Concepción-Urteaga, L.; Roseboom., P.J.; Williams, C.A. Assessment of hormonal status in male infertility. An update. Diabetes Metab. Syndr. 2022, 16, 102447. [Google Scholar] [CrossRef]
- Balawender, K.; Orkisz, S. The impact of selected modifiable lifestyle factors on male fertility in the modern world. Cent. Eur. J. Urol. 2020, 73, 563–568. [Google Scholar] [CrossRef]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef]
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2021, 53, e13646. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility-An Overview. Antioxidants (Basel) 2021, 10, 1473. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of environmental toxin exposure on male fertility potential. Transl. Androl. Urol. 2020, 9, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, H.; Venditti, M.; Ben Salah-Abbès, J.; Minucci, S.; Abbès, S. Potential protective effect of lactic acid bacteria against zearalenone causing reprotoxicity in male mice. Toxicon 2022, 209, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Pereira, S.C.; Seco-Rovira, V.; Oliveira, P.F.; Alves, M.G.; Pereira, M.L. Pesticides and Male Fertility: A Dangerous Crosstalk. Metabolites 2021, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Duca, R.C.; Galea, K.S.; Maric, T.; Garcia, K.; Bloom, M.S.; Andersen, H.R.; Vena, J.E. Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides. Int. J. Environ. Res. Public. Health. 2021, 18, 6576. [Google Scholar] [CrossRef]
- D’Angelo, S.; Meccariello, R. Microplastics: A Threat for Male Fertility. Int. J. Environ. Res. Public. Health. 2021, 18, 2392. [Google Scholar] [CrossRef]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. (China) 2019, 77, 210–217. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Pomeroy, K.O.; Comizzoli, P.; Rushing, J.S.; Lersten, I.L.; Nel-Themaat, L. The ART of cryopreservation and its changing landscape. Fertil. Steril. 2022, 117, 469–476. [Google Scholar] [CrossRef]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm Selection for ICSI: Do We Have a Winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants (Basel) 2022, 11, 306. [Google Scholar] [CrossRef]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants (Basel) 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Almeida, H.; Castro, J.P. (In) Fertility and Oxidative Stress: New Insights into Novel Redox Mechanisms Controlling Fundamental Reproductive Processes. Oxid. Med. Cell. Longev. 2020, 4674896. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.A.; Yániz, J.L.; Peña, F.J.; Santolaria, P.; Castelló-Ruiz, M. Role of Antioxidants in Cooled Liquid Storage of Mammal Spermatozoa. Antioxidants (Basel) 2021, 10, 1096. [Google Scholar] [CrossRef]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Galanio, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Zhao, F.; Whiting, S.; Lambourne, S.; Aitken, R.J.; Sun, Y.P. Melatonin alleviates heat stress-induced oxidative stress and apoptosis in human spermatozoa. Free Radic. Biol. Med. 2021, 164, 410–416. [Google Scholar] [CrossRef]
- Malmir, M.; Naderi Noreini, S.; Ghafarizadeh, A.; Faraji, T.; Asali, Z. Ameliorative effect of melatonin on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the idiopathic asthenoteratospermic men: In vitro. Andrologia 2021, 53, e13944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Xiong, Y.M.; Tan, Y.J.; Wang, L.; Li, R.; Zhang, Y.; Liu, X.M.; Lin, X.H.; Jin, L.; Hu, Y.T.; et al. Melatonin rescues impaired penetration ability of human spermatozoa induced by mitochondrial dysfunction. Reproduction 2019, 158, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Monllor, F.; Espino, J.; Marchena, A.M.; Ortiz, Á.; Lozano, G.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; Bejarano, I. Melatonin diminishes oxidative damage in sperm cells, improving assisted reproductive techniques. Turk. J. Biol. 2017, 41, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Karimfar, M.H.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef]
- Bejarano, I.; Monllor, F.; Marchena, A.M.; Ortiz, A.; Lozano, G.; Jiménez, M.I.; Gaspar, P.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 2014, 57, 333–339. [Google Scholar] [CrossRef]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J Pineal Res. 2011, 50, 132–139. [Google Scholar] [CrossRef]
- Iqbal, T.; Cao, M.; Zhao, Z.; Zhao, Y.; Chen, L.; Chen, T.; Li, C.; Zhou, X. Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium. Int. J. Environ. Res. Public. Health 2021, 18, 6038. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Cadmium as a testicular toxicant: A Review. J. Appl. Toxicol. 2021, 41, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials (Basel) 2020, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Tamburrino, L.; Farnetani, G.; Muratori, M.; Vignozzi, L.; Baldi, E. Acute effects on human sperm exposed in vitro to cadmium chloride and diisobutyl phthalate. Reproduction 2019, 158, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis. Genes (Basel) 2021, 12, 1016. [Google Scholar] [CrossRef]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol. Environ. Saf. 2021, 226, 112878. [Google Scholar] [CrossRef]
- Venditti, M.; Fasano, C.; Minucci, S.; Serino, I.; Sinisi, A.A.; Dale, B.; Di Matteo, L. DAAM1 and PREP are involved in human spermatogenesis. Reprod. Fertil. Dev. 2020, 32, 484–494. [Google Scholar] [CrossRef]
- Ergoli, M.; Venditti, M.; Picillo, E.; Minucci, S.; Politano, L. Study of expression of genes potentially responsible for reduced fitness in patients with myotonic dystrophy type 1 and identification of new biomarkers of testicular function. Mol. Reprod. Dev. 2020, 87, 45–52. [Google Scholar] [CrossRef]
- Ferrara, D.; Pariante, P.; Di Matteo, L.; Serino, I.; Oko, R.; Minucci, S. First evidence of prothymosin α localization in the acrosome of mammalian male gametes. J. Cell. Physiol. 2013, 228, 1629–1637. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants (Basel) 2021, 10, 1025. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M. Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants. Biology (Basel) 2020, 9, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Chemek, M.; Minucci, S.; Messaoudi, I. Cadmium-induced toxicity increases prolyl endopeptidase (PREP) expression in the rat testis. Mol. Reprod. Dev. 2020, 87, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chemek, M.; Venditti, M.; Boughamoura, S.; Mimouna, S.B.; Messaoudi, I.; Minucci, S. Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J. Cell. Physiol. 2018, 233, 630–640. [Google Scholar] [CrossRef]
- Zhao, L.L.; Ru, Y.F.; Liu, M.; Tang, J.N.; Zheng, J.F.; Wu, B.; Gu, Y.H.; Shi, H.J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One 2017, 12, e0186727. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.L.; Stein, P.; Jefferson, W.N.; Padilla-Banks, E.; Williams, C.J. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc. Natl. Acad. Sci. U S A 2012, 109, 4169–4174. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.F.; Chang, M.; Peng, T.T.; Yang, Y.; Li, N.; Luo, T.; Cheng, Y.M.; Zhou, M.Z.; Zeng, X.H.; Zheng, L.P. Exposure to Cadmium Impairs Sperm Functions by Reducing CatSper in Mice. Cell. Physiol. Biochem. 2017, 42, 44–54. [Google Scholar] [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Espino, J.; Bejarano, I.; Ortiz, A.; Lozano, G.M.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil. Steril. 2010, 94, 1915–1917. [Google Scholar] [CrossRef]
- Dotolo, R.; Kim, J.D.; Pariante, P.; Minucci, S.; Diano, S. Prolyl Endopeptidase (PREP) is Associated With Male Reproductive Functions and Gamete Physiology in Mice. J. Cell. Physiol. 2016, 231, 551–557. [Google Scholar] [CrossRef]
- Pariante, P.; Dotolo, R.; Venditti, M.; Ferrara, D.; Donizetti, A.; Aniello, F.; Minucci, S. First Evidence of DAAM1 Localization During the Post-Natal Development of Rat Testis and in Mammalian Sperm. J. Cell. Physiol. 2016, 231, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Aniello, F.; Santillo, A.; Minucci, S. Study on PREP localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. Zygote 2019, 27, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Fasano, C.; Santillo, A.; Aniello, F.; Minucci, S. First evidence of DAAM1 localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. C. R. Biol. 2018, 341, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Barfield, J.P.; Cooper, T.G. Physiological volume regulation by spermatozoa. Mol. Cell. Endocrinol. 2006, 250, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fetic, S.; Yeung, C.H.; Sonntag, B.; Nieschlag, E.; Cooper, T.G. Relationship of cytoplasmic droplets to motility, migration in mucus, and volume regulation of human spermatozoa. J. Androl. 2006, 27, 294–301. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Minucci, S. Prothymosin alpha expression in the vertebrate testis: A comparative review. Zygote 2017, 25, 760–770. [Google Scholar] [CrossRef]
- Pariante, P.; Dotolo, R.; Venditti, M.; Ferrara, D.; Donizetti, A.; Aniello, F.; Minucci, S. Prothymosin alpha expression and localization during the spermatogenesis of Danio rerio. Zygote 2016, 24, 583–593. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Yi, Y.J.; Sutovsky, P.; Oko, R. The extracellular protein coat of the inner acrosomal membrane is involved in zona pellucida binding and penetration during fertilization: Characterization of its most prominent polypeptide (IAM38). Dev. Biol. 2006, 290, 32–43. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030787.
- Du Plessis, S.S.; Hagenaar, K.; Lampiao, F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar] [CrossRef]
- Tejada, R.I.; Mitchell, J.C.; Norman, A.; Marik, J.J.; Friedman, S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril. 1984, 42, 87–91. [Google Scholar] [CrossRef]
- Venditti, M.; Romano, M.Z.; Aniello, F.; Minucci, S. Preliminary investigation on the ameliorative role exerted by D-aspartic acid in counteracting ethane dimethane sulfonate (EDS) toxicity in the rat testis. Animals 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Venditti, M.; Minucci, S.; Chieffi Baccari, G.; Falvo, S.; Rosati, L.; Di Fiore, M.M. D-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 2019, 158, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Knani, L.; Venditti, M.; Kechiche, S.; Banni, M.; Messaoudi, I.; Minucci, S. Melatonin protects bone against cadmium-induced toxicity via activation of Wnt/β-catenin signaling pathway. Toxicol. Mech. Methods. 2020, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Santillo, A.; Falvo, S.; Di Fiore, M.M.; Chieffi Baccari, G.; Minucci, S. D-Aspartate Upregulates DAAM1 Protein Levels in the Rat Testis and Induces Its Localization in Spermatogonia Nucleus. Biomolecules 2020, 10, 677. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minucci, S.; Venditti, M. New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality. Int. J. Mol. Sci. 2022, 23, 5128. https://doi.org/10.3390/ijms23095128
Minucci S, Venditti M. New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality. International Journal of Molecular Sciences. 2022; 23(9):5128. https://doi.org/10.3390/ijms23095128
Chicago/Turabian StyleMinucci, Sergio, and Massimo Venditti. 2022. "New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality" International Journal of Molecular Sciences 23, no. 9: 5128. https://doi.org/10.3390/ijms23095128
APA StyleMinucci, S., & Venditti, M. (2022). New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality. International Journal of Molecular Sciences, 23(9), 5128. https://doi.org/10.3390/ijms23095128






