Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis
Abstract
1. Introduction
2. Results
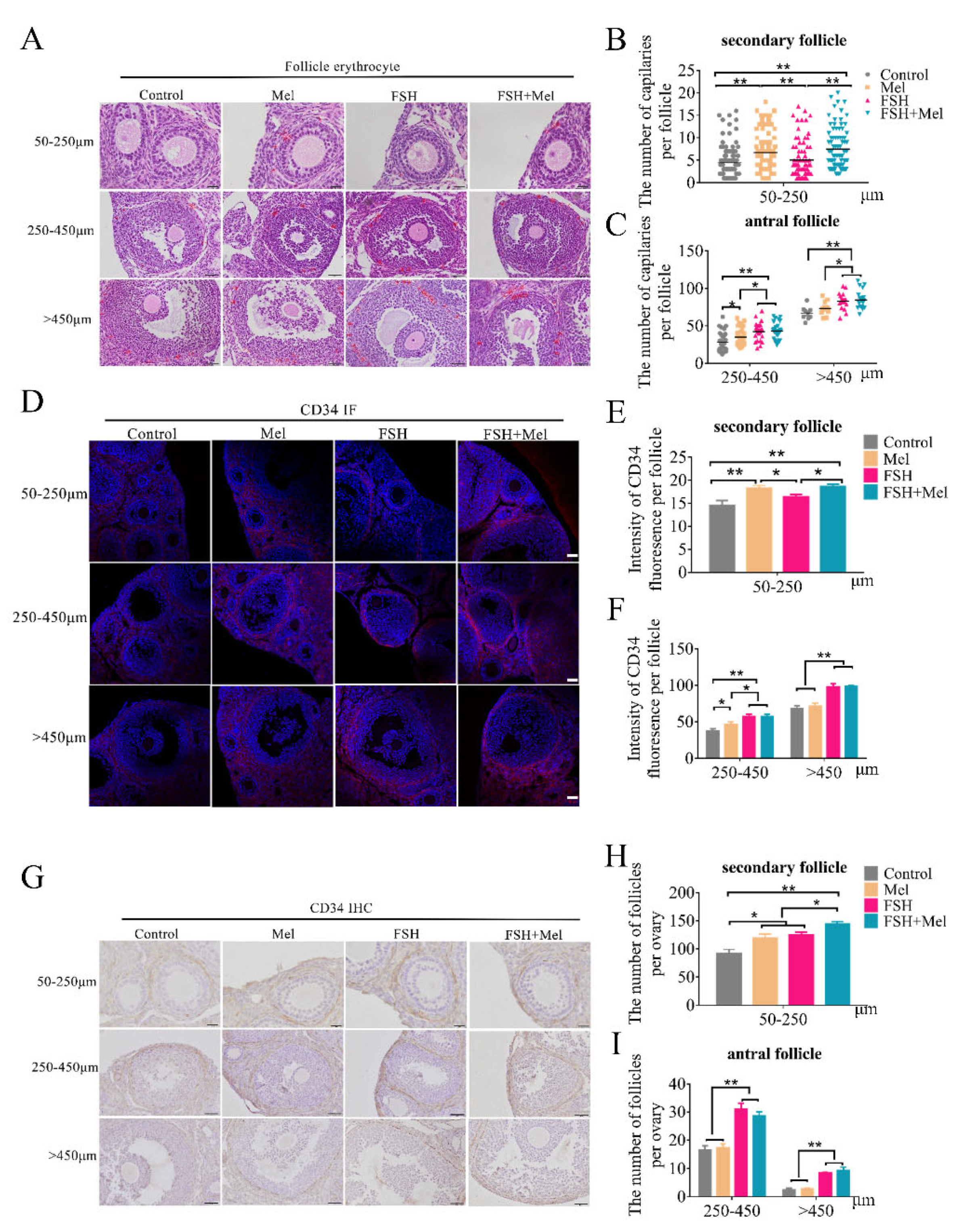
2.1. Melatonin Promoted Secondary Follicles Development and Periphery Angiogenesis, While FSH Remarkably Increased the Number of Antral Follicles and Periphery Angiogenesis
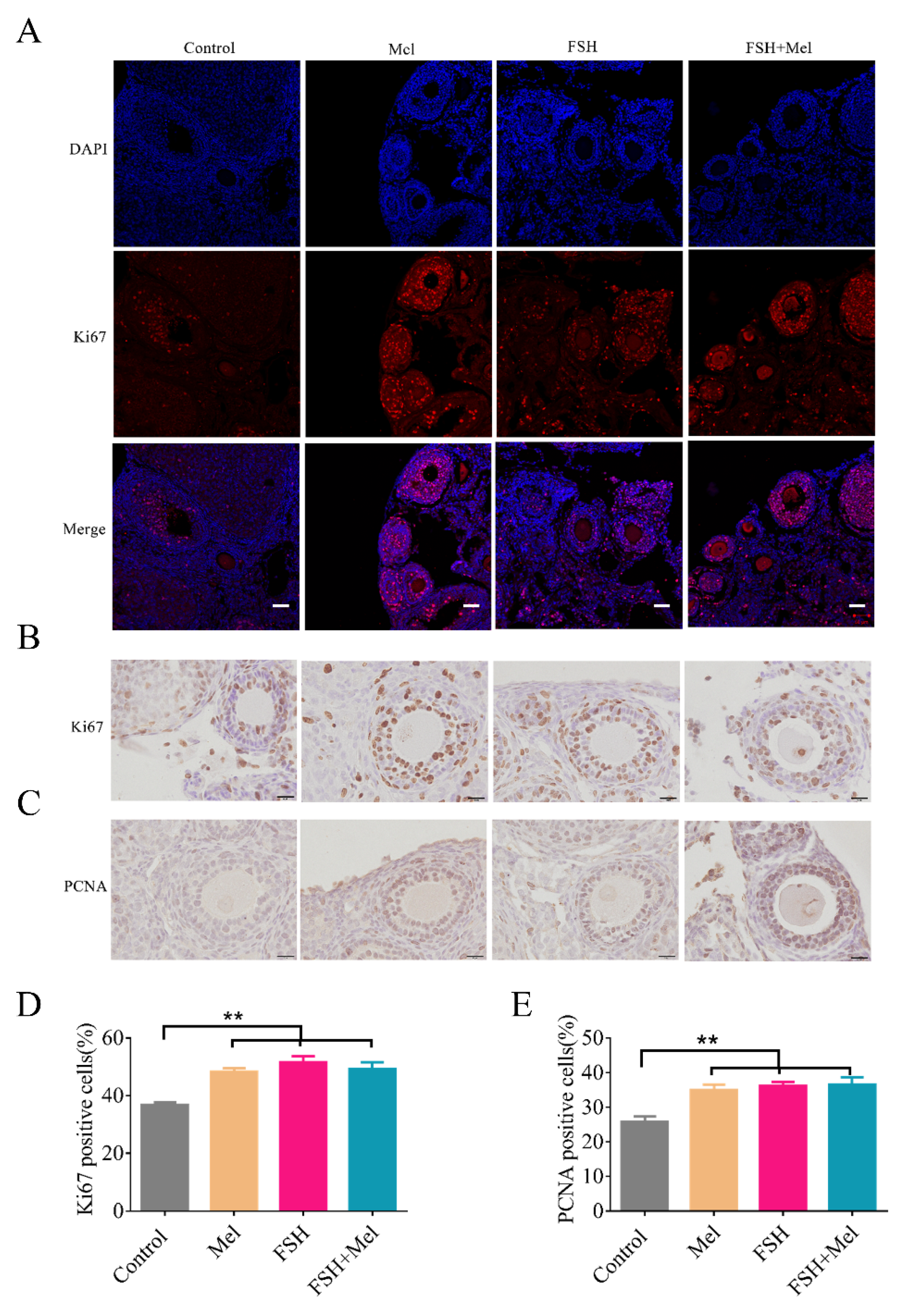
2.2. Melatonin Enhanced Cell Proliferative Capacity in Ovary
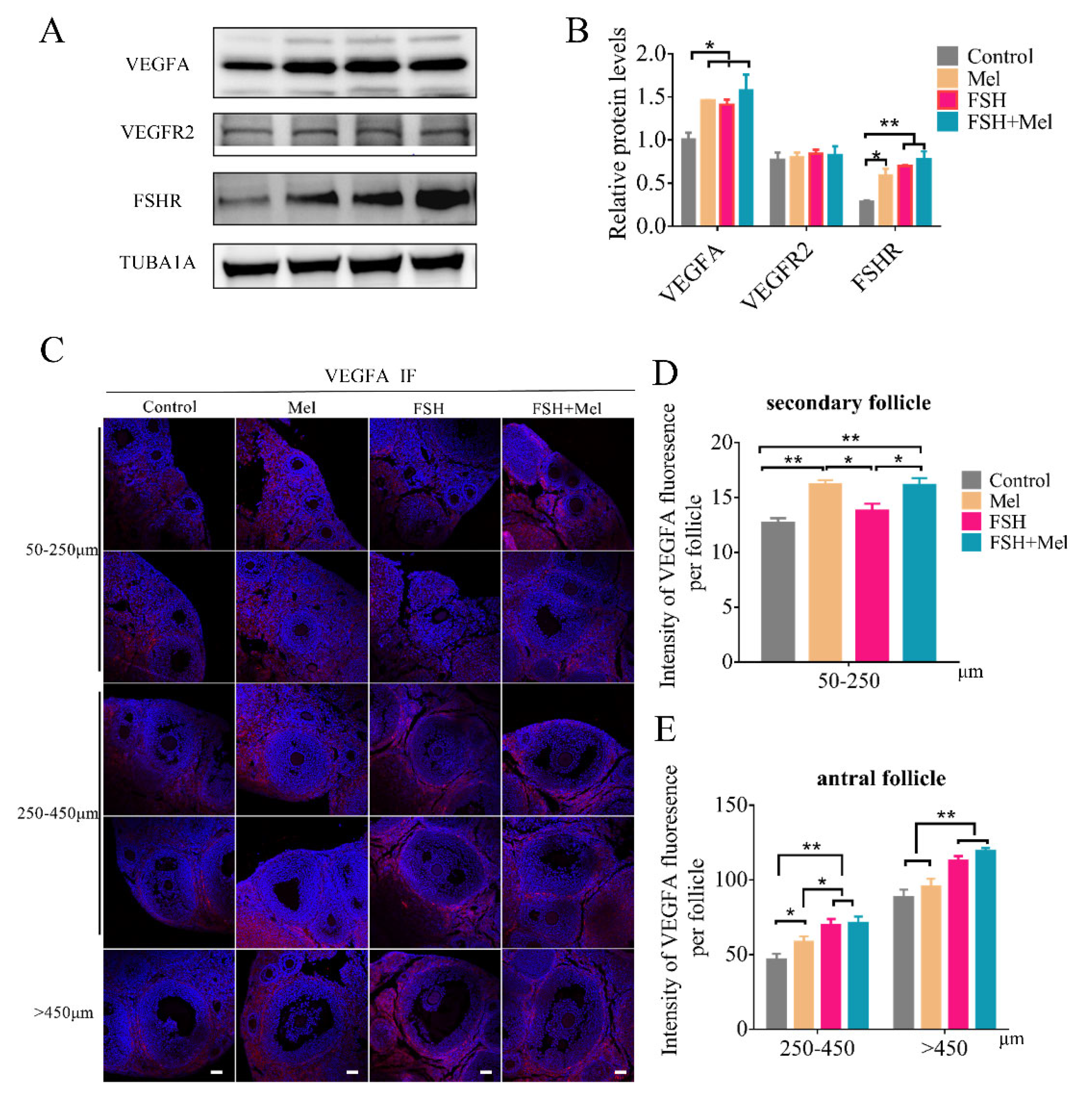
2.3. Melatonin Raised the Expression of VEGFA in Secondary Follicles, While FSH Conspicuously Enhanced the Expression of VEGFA in Antral Follicles
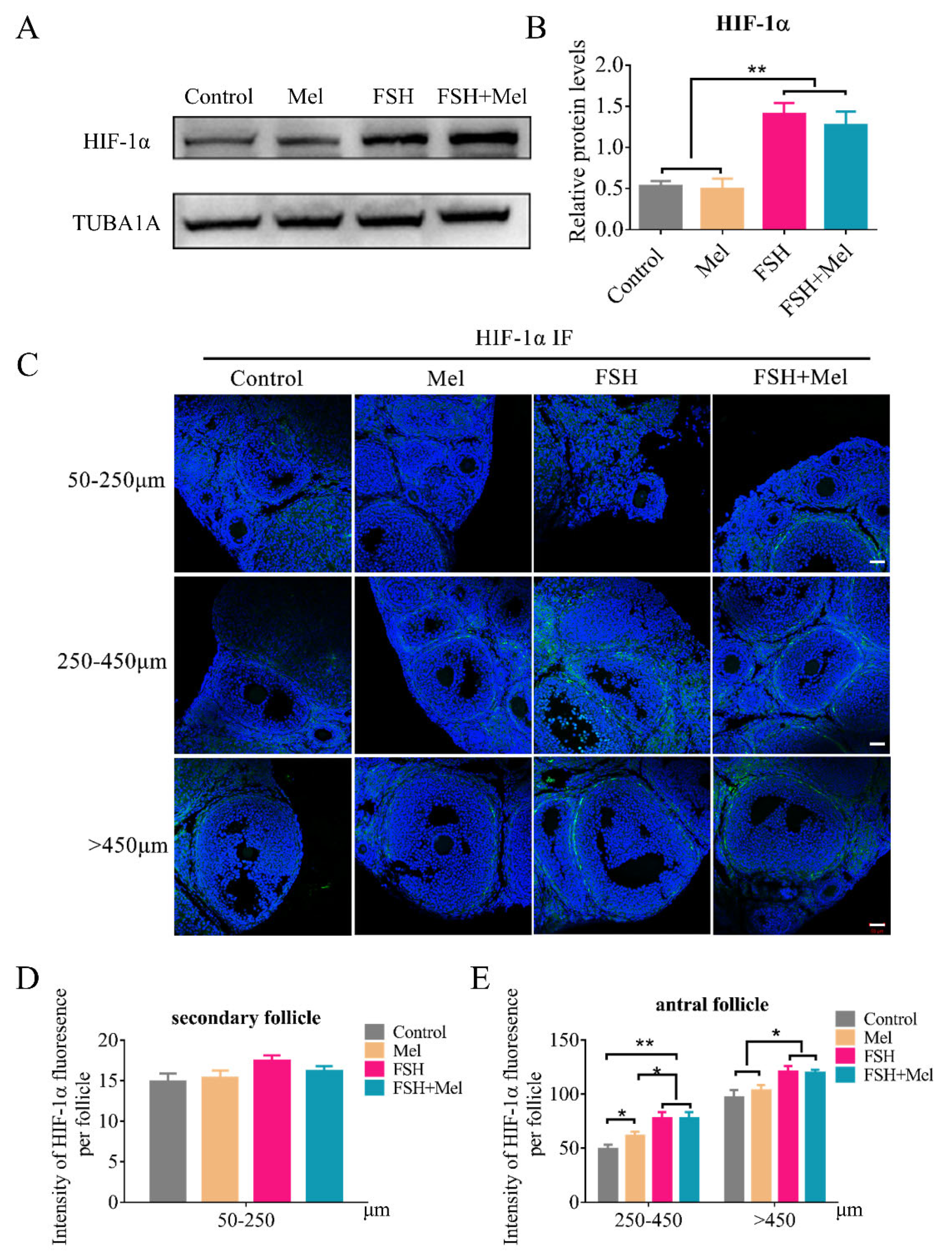
2.4. Melatonin-Regulated Angiogenesis May Not through the HIF-1α/VEGF Signaling Pathway in Secondary Follicles
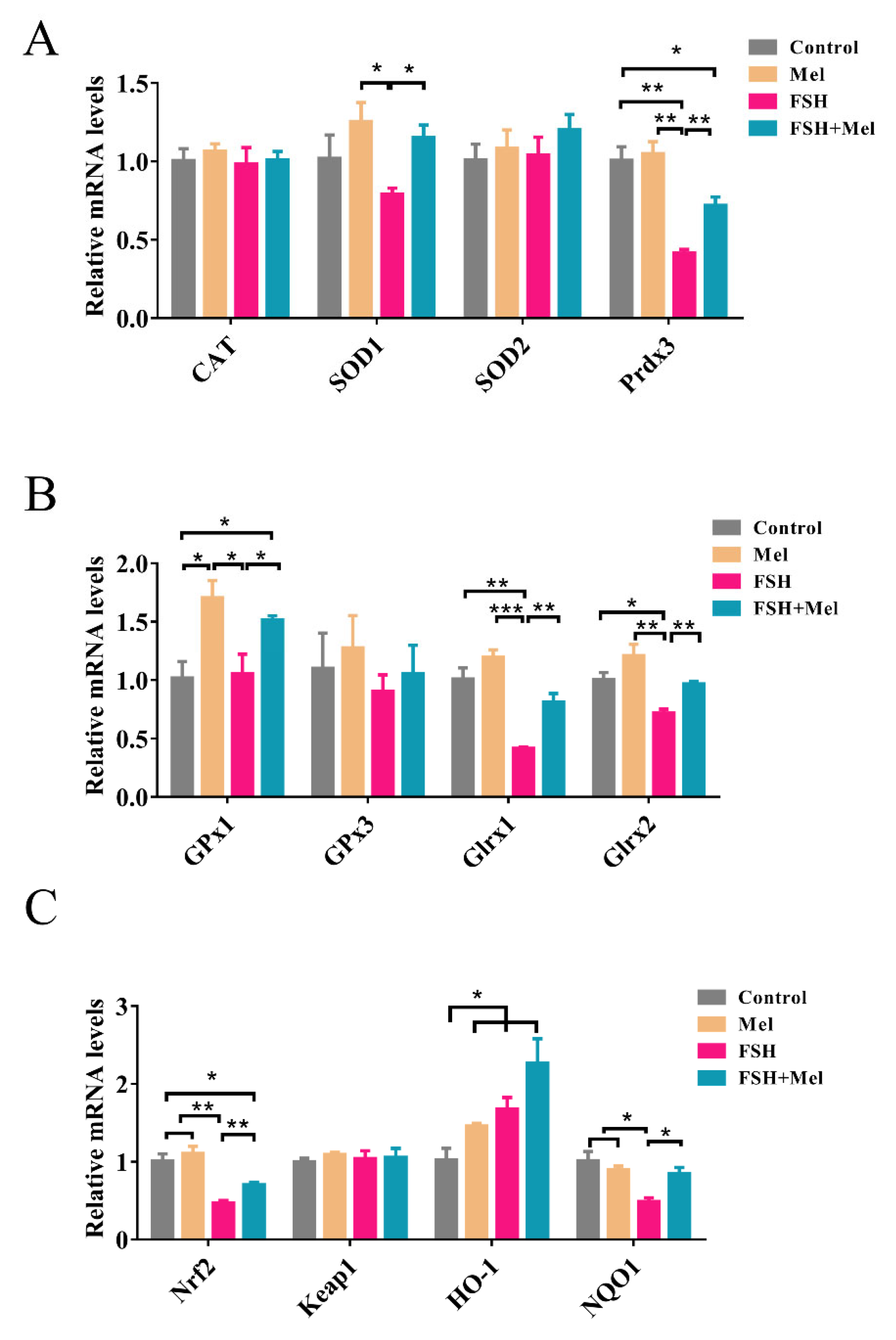
2.5. Melatonin Increased the Expression of Antioxidant Enzymes and Alleviated the Declined Expression of Antioxidant Enzymes Induced by FSH
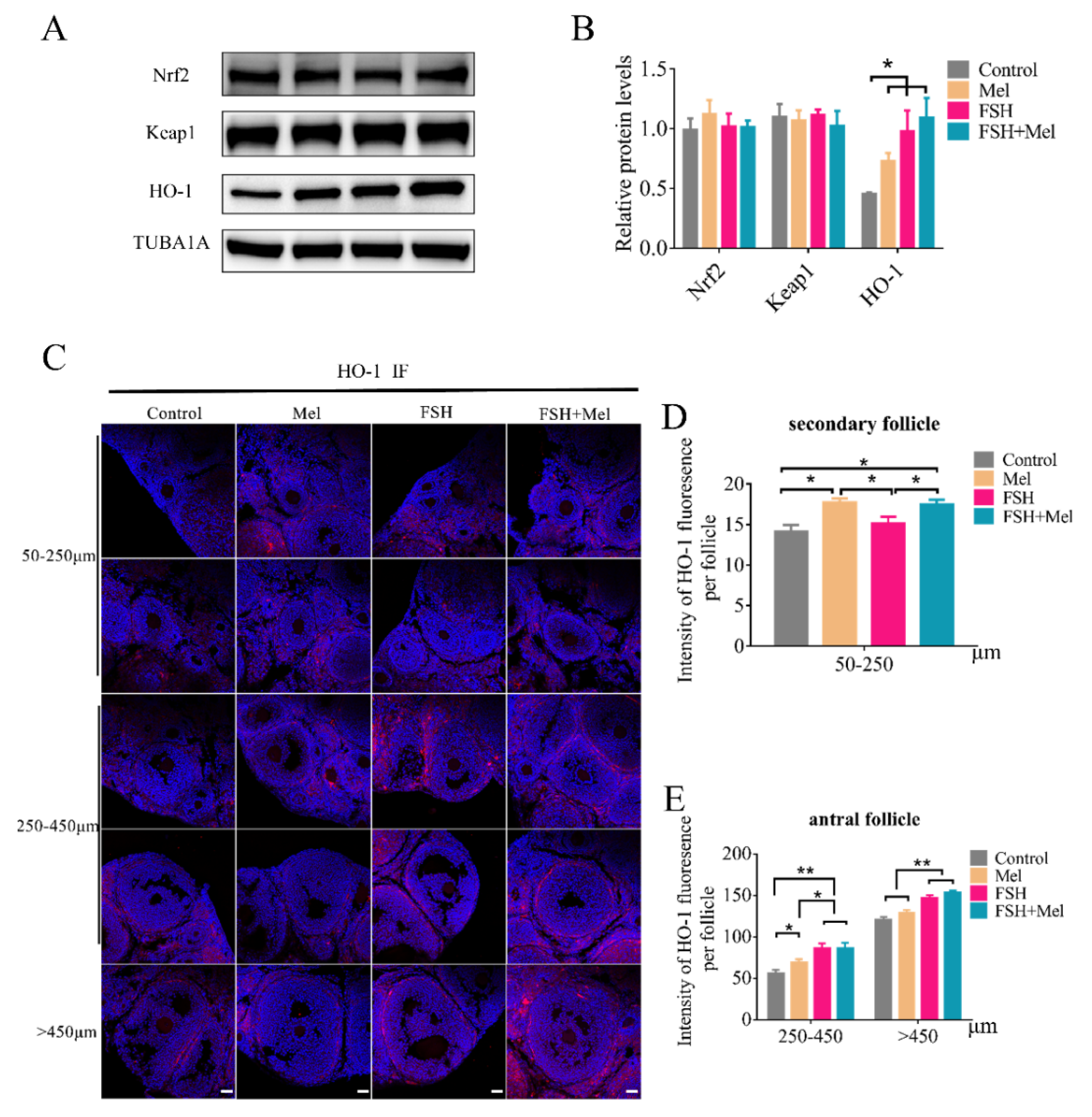
2.6. Melatonin Increased the Expression of HO-1 in Secondary Follicles
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents and Antibodies
4.3. Animals
4.4. Ovarian Follicle Collection and H&E Staining
4.5. Immunohistochemistry (IHC) and Immunofluorescence (IF)
4.6. Western Blotting
4.7. Real-Time Quantitative RT-PCR Analysis
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CAT | catalase |
| FSH | follicle-stimulating hormone |
| FSHR | FSH receptor |
| GnRH | gonadotropin releasing hormone |
| Glrx1 | glutaredoxin 1 |
| Glrx2 | glutaredoxin 2 |
| Gpx1 | glutathione peroxidase-1 |
| HIF-1 | hypoxia-inducible factor-1 |
| HO-1 | heme oxygenase-1 |
| H&E | hematoxylin-eosin staining |
| IF | immunofluorescence |
| IHC | immunohistochemistry |
| LH | luteinizing hormone |
| Mel | melatonin |
| NQO1 | NAD(P)H-quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PCNA | proliferating cell nuclear antigen |
| Prdx3 | peroxiredoxin-3 |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| VEGFs | vascular endothelial growth factors |
| VEGFR2 | VEGF receptor 2 |
| WB | Western blotting |
References
- Kerr, J.B.; Myers, M.; Anderson, R.A. The dynamics of the primordial follicle reserve. Reproduction 2013, 146, R205–R215. [Google Scholar] [CrossRef]
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell. Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian Folliculogenesis. Results Probl. Cell. Differ. 2016, 58, 167–190. [Google Scholar] [PubMed]
- Brown, H.M.; Russell, D.L. Blood and lymphatic vasculature in the ovary: Development, function and disease. Hum. Reprod. Update 2014, 20, 29–39. [Google Scholar] [CrossRef]
- Suzuki, T.; Sasano, H.; Takaya, R.; Fukaya, T.; Yajima, A.; Nagura, H. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum. Reprod. 1998, 13, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, A.J.; Schuler, H.M.; Reichert, L.J. Gonadotropin-binding sites in the rhesus monkey ovary: Role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology 1981, 109, 356–362. [Google Scholar] [CrossRef]
- Acosta, T.J. Studies of follicular vascularity associated with follicle selection and ovulation in cattle. J. Reprod. Dev. 2007, 53, 39–44. [Google Scholar] [CrossRef]
- Acosta, T.J.; Miyamoto, A. Vascular control of ovarian function: Ovulation, corpus luteum formation and regression. Anim. Reprod. Sci. 2004, 82-83, 127–140. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Yamamoto, S.; Konishi, I.; Tsuruta, Y.; Nanbu, K.; Mandai, M.; Kuroda, H.; Matsushita, K.; Hamid, A.A.; Yura, Y.; Mori, T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol. Endocrinol. 1997, 11, 371–381. [Google Scholar] [CrossRef]
- Gordon, J.D.; Mesiano, S.; Zaloudek, C.J.; Jaffe, R.B. Vascular endothelial growth factor localization in human ovary and fallopian tubes: Possible role in reproductive function and ovarian cyst formation. J. Clin. Endocrinol. Metab. 1996, 81, 353–359. [Google Scholar] [PubMed]
- Otani, N.; Minami, S.; Yamoto, M.; Shikone, T.; Otani, H.; Nishiyama, R.; Otani, T.; Nakano, R. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J. Clin. Endocrinol. Metab. 1999, 84, 3845–3851. [Google Scholar] [CrossRef] [PubMed]
- Kamat, B.R.; Brown, L.F.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am. J. Pathol. 1995, 146, 157–165. [Google Scholar]
- Fraser, H.M. Regulation of the ovarian follicular vasculature. Reprod. Biol. Endocrinol. 2006, 4, 18. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Hartman, T.; Kavic, S.; Pauli, S.A.; Bohlen, P.; Sauer, M.V.; Kitajewski, J. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. J. Clin. Investig. 2003, 112, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sasson, R.; Dantes, A.; Tajima, K.; Amsterdam, A. Novel genes modulated by FSH in normal and immortalized FSH-responsive cells: New insights into the mechanism of FSH action. FASEB J. 2003, 17, 1256–1266. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F.; Tirelli, M.; Bussolati, S.; Tamanini, C. The effects of reduced oxygen tension on swine granulosa cell. Regul. Pept. 2004, 120, 69–75. [Google Scholar] [CrossRef]
- Wang, X.J.; Si, L.B. Advances on hypoxia inducible factor-1. Chin. Med. J. 2013, 126, 3567–3571. [Google Scholar]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Marx, J. Cell biology. How cells endure low oxygen. Science 2004, 303, 1454–1456. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993, 268, 21513–21518. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Rico, C.; Dodelet-Devillers, A.; Paquet, M.; Tsoi, M.; Lapointe, E.; Carmeliet, P.; Boerboom, D. HIF1 activity in granulosa cells is required for FSH-regulated Vegfa expression and follicle survival in mice. Biol. Reprod. 2014, 90, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythm. 2001, 16, 336–347. [Google Scholar] [CrossRef]
- Reiter, R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980, 1, 109–131. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, M.; Jiang, Y.; Sun, S.C.; Liu, H. Melatonin reduces oxidative damage in mouse granulosa cells via restraining JNK-dependent autophagy. Reproduction 2018, 155, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Mas, A. Melatonin and Other Tryptophan Metabolites Produced by Yeasts: Implications in Cardiovascular and Neurodegenerative Diseases. Front. Microbiol. 2015, 6, 1565. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jang, W.J.; Yi, E.Y.; Jang, J.Y.; Jung, Y.; Jeong, J.W.; Kim, Y.J. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J. Pineal Res. 2010, 48, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. J. Pineal Res. 2017, 63, e12430. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Sharma, A.V.; Reiter, R.J.; Swarnakar, S. Melatonin promotes angiogenesis during protection and healing of indomethacin-induced gastric ulcer: Role of matrix metaloproteinase-2. J. Pineal Res. 2010, 49, 130–140. [Google Scholar] [CrossRef]
- Ma, Q.; Reiter, R.J.; Chen, Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020, 23, 91–104. [Google Scholar] [CrossRef]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef]
- Deramaudt, B.M.; Braunstein, S.; Remy, P.; Abraham, N.G. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J. Cell. Biochem. 1998, 68, 121–127. [Google Scholar] [CrossRef]
- Stocker, R.; Perrella, M.A. Heme oxygenase-1: A novel drug target for atherosclerotic diseases? Circulation 2006, 114, 2178–2189. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Diao, C.; Chen, Z.; Qiu, T.; Liu, H.; Yang, Y.; Liu, X.; Wu, J.; Wang, L. Inhibition of PRMT5 Attenuates Oxidative Stress-Induced Pyroptosis via Activation of the Nrf2/HO-1 Signal Pathway in a Mouse Model of Renal Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2019, 2019, 2345658. [Google Scholar] [CrossRef]
- Dulak, J.; Jozkowicz, A.; Foresti, R.; Kasza, A.; Frick, M.; Huk, I.; Green, C.J.; Pachinger, O.; Weidinger, F.; Motterlini, R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox Signal. 2002, 4, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.C.; Midgley, A.J.; Richards, J.S. Hormonal regulation of ovarian cellular proliferation. Cell 1978, 14, 71–78. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Unek, G.; Ozmen, A.; Kipmen-Korgun, D.; Korgun, E.T. Immunolocalization of PCNA, Ki67, p27 and p57 in normal and dexamethasone-induced intrauterine growth restriction placental development in rat. Acta Histochem. 2012, 114, 31–40. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Jang, H.; Lee, O.H.; Lee, Y.; Yoon, H.; Chang, E.M.; Park, M.; Lee, J.W.; Hong, K.; Kim, J.O.; Kim, N.K.; et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J. Pineal Res. 2016, 60, 336–347. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Q.; Chen, Y.; Wang, X.; Ran, Z.; Fang, F.; Xiong, J.; Liu, G.; Li, X.; Yang, L.; et al. Melatonin delays ovarian aging in mice by slowing down the exhaustion of ovarian reserve. Commun. Biol. 2021, 4, 534. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Ciccimarra, R.; Grasselli, F. Melatonin potentially acts directly on swine ovary by modulating granulosa cell function and angiogenesis. Reprod. Fertil. Dev. 2017, 29, 2305–2312. [Google Scholar] [CrossRef]
- Yang, M.Y.; Fortune, J.E. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol. Reprod. Dev. 2007, 74, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Li, W.; Zhang, L.; Zhou, J.; Sun, M.; Zhou, J.; Yao, W.; Zhang, X.; Wang, H.; et al. The FSH-HIF-1alpha-VEGF Pathway Is Critical for Ovulation and Oocyte Health but Not Necessary for Follicular Growth in Mice. Endocrinology 2020, 161, 161. [Google Scholar] [CrossRef] [PubMed]
- Jozkowicz, A.; Huk, I.; Nigisch, A.; Weigel, G.; Weidinger, F.; Dulak, J. Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxid. Redox. Signal. 2002, 4, 577–585. [Google Scholar] [CrossRef] [PubMed]
| Antibody Name | Antibody Number | Corporation | Application |
|---|---|---|---|
| VEGFA | Orb11554 | Biorbyt | WB, IF |
| VEGFR2 | Orb11557 | Biorbyt | WB |
| FSHR | Ab75200 | Abcam | WB |
| CD34 | Ab81289 | Abcam | IF, IHC |
| HIF-1α | 36169S | Cell Signaling Technology | IF |
| TUBA1A | 2125S | Cell Signaling Technology | WB |
| Nrf2 | 16396-1 | Proteintech | WB |
| HO-1 | 66743-1 | Proteintech | WB, IF |
| Keap1 | 10503-2 | Proteintech | WB |
| Genes | Accession No. | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| CAT | NM_009804.2 | F:GTCCCTGCTGTCTCACGTTC R:GACATCAGGTCTCTGCGAGG | 128 |
| Gpx1 | NM_008160.6 | F:AGTCCACCGTGTATGCCTTCT R:GAGACGCGACATTCTCAATGA | 105 |
| Gpx3 | NM_008161.4 | F:CCTTTTAAGCAGTATGCAGGCA R:CAAGCCAAATGGCCCAAGTT | 120 |
| Glrx1 | NM_001360151.1 | F:TATAAAAGGGGTGGCAGAGTCCA R:GCCGCCTTGTTGAAAAATCCC | 132 |
| Glrx2 | NM_001038592.1 | F:ATCGTCGTTTTGGGGGAAGTC R:GGAACAGTAAGAGCAGGATGTTT | 109 |
| SOD1 | NM_011434.2 | F:AACCAGTTGTGTTGTCAGGAC R:CCACCATGTTTCTTAGAGTGAGG | 139 |
| SOD2 | NM_013671.3 | F:CAGACCTGCCTTACGACTATGG R:CTCGGTGGCGTTGAGATTGTT | 113 |
| Prdx3 | NM_007452.2 | F:GGTTGCTCGTCATGCAAGTG R:CCACAGTATGTCTGTCAAACAGG | 100 |
| Nrf2 | NM_010902.4 | F:GAAGCACGCTGAAGGCACAAT R:TTAGGGCCGTTCTGTTTGACA | 192 |
| keap1 | NM_016679.4 | F:TCTACGTCCTCGGAGGCTAT R:GCTCAGGTATTCCAAGTGCTTC | 199 |
| HO-1 | NM_010442.2 | F:CAGAGCCGTCTCGAGCATA R:CAAATCCTGGGGCATGCTGT | 108 |
| NQO1 | NM_008706.5 | F:TGTAGCCAGCCCTAAGGATCT R:GGCTCTTCTCGCCGCCAT | 126 |
| β-Actin | NM_007393.5 | F:TATAAAACCCGGCGGCGCA R:TCATCCATGGCGAACTGGTG | 117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Zhang, L.; Zhang, X.; Chen, Y.; Chen, Q.; Shen, M.; Liu, H.; Deng, S. Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis. Int. J. Mol. Sci. 2021, 22, 11262. https://doi.org/10.3390/ijms222011262
Tao J, Zhang L, Zhang X, Chen Y, Chen Q, Shen M, Liu H, Deng S. Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis. International Journal of Molecular Sciences. 2021; 22(20):11262. https://doi.org/10.3390/ijms222011262
Chicago/Turabian StyleTao, Jingli, Liangliang Zhang, Xuan Zhang, Yuanyuan Chen, Qianqian Chen, Ming Shen, Honglin Liu, and Shoulong Deng. 2021. "Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis" International Journal of Molecular Sciences 22, no. 20: 11262. https://doi.org/10.3390/ijms222011262
APA StyleTao, J., Zhang, L., Zhang, X., Chen, Y., Chen, Q., Shen, M., Liu, H., & Deng, S. (2021). Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis. International Journal of Molecular Sciences, 22(20), 11262. https://doi.org/10.3390/ijms222011262








