Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−.Leiden Mice
Abstract
:1. Introduction
2. Results
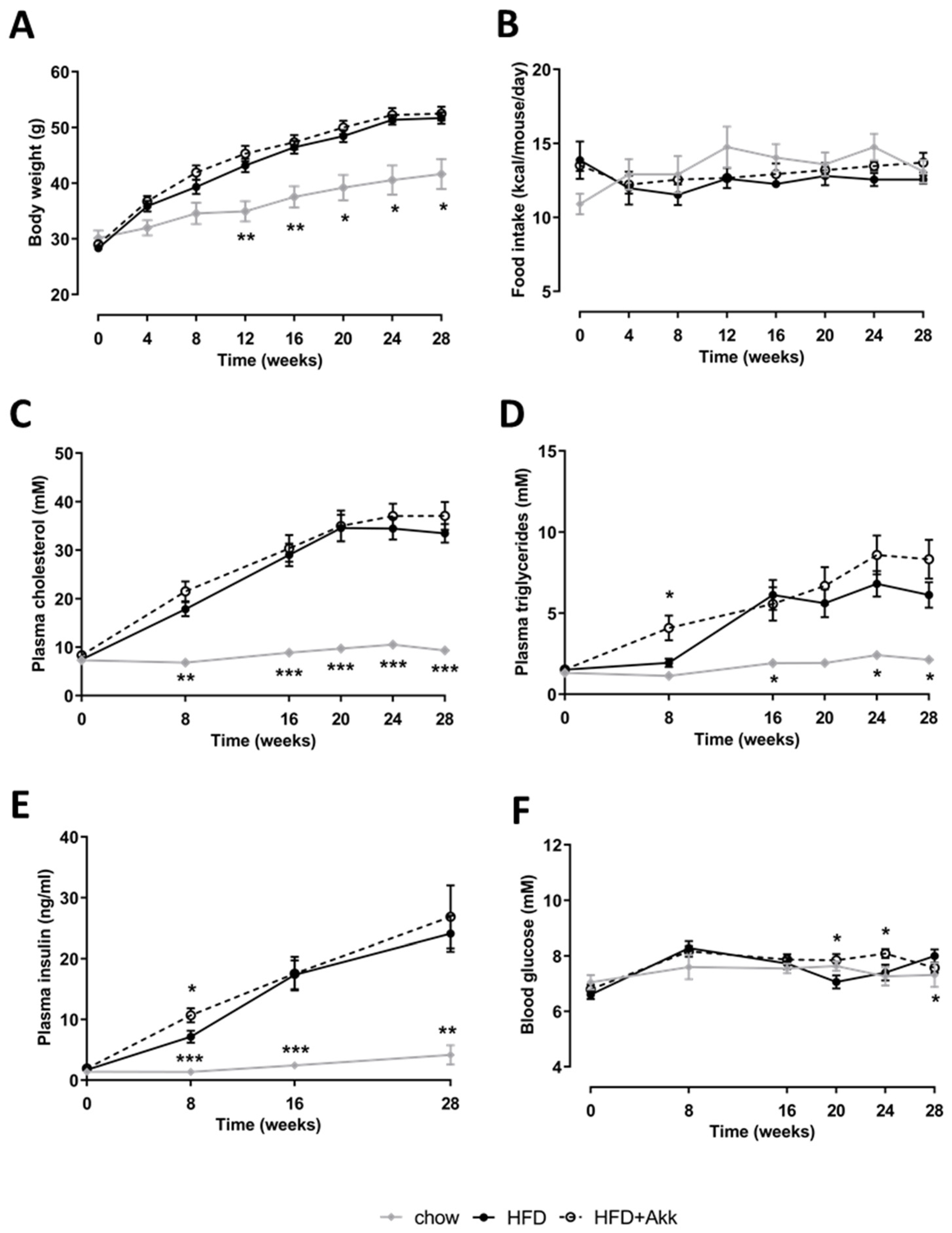
2.1. Heat-Inactivated A. muciniphila Did Not Affect Development of Obesity or Associated Metabolic Risk Factors
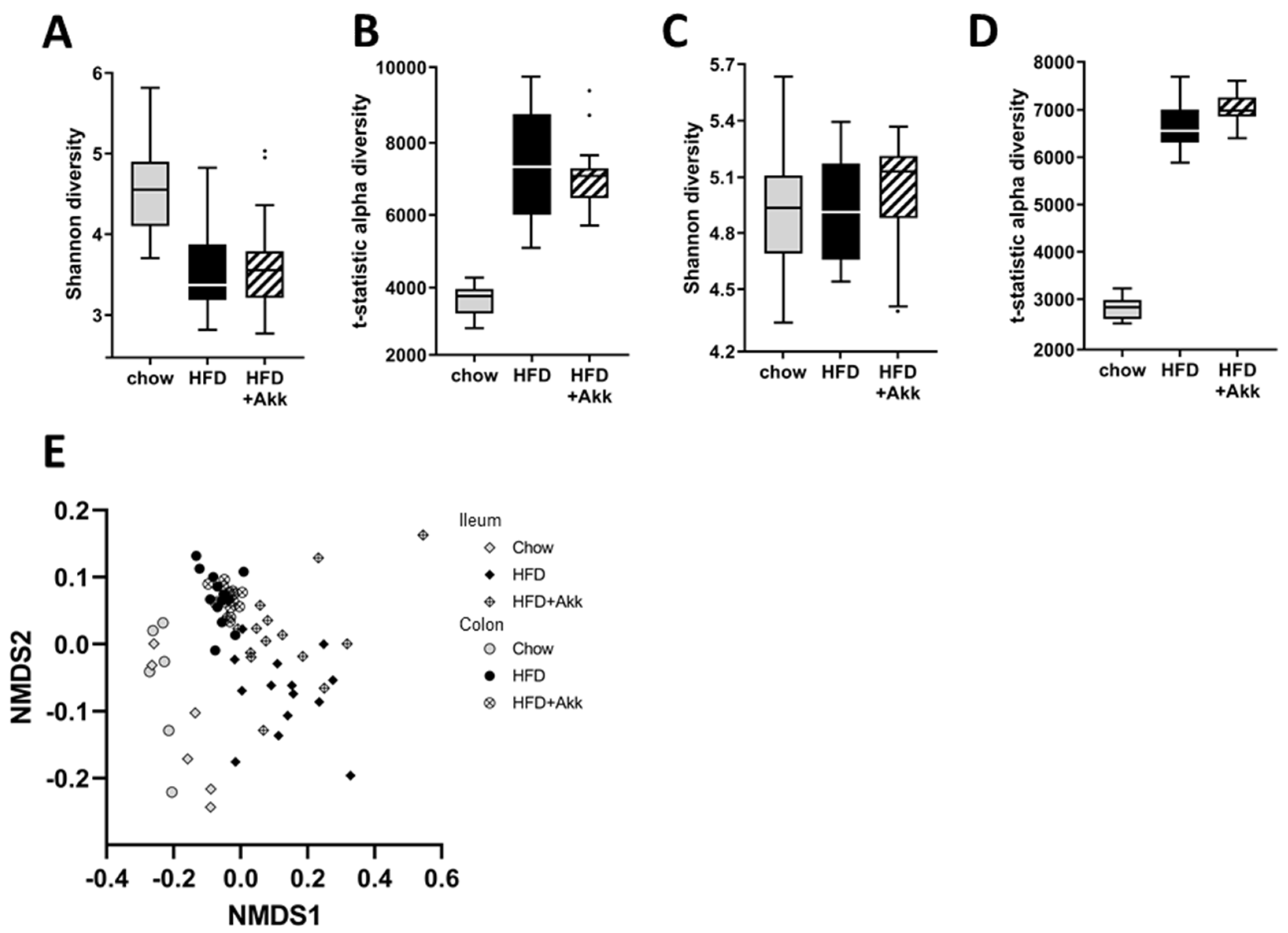
2.2. Heat-Inactivated A. muciniphila Had Minor Effects on Gut Microbiota in Ileum and Colon Mucosa and Lowered Circulating SCFA Valeric Acid and Caproic Acid
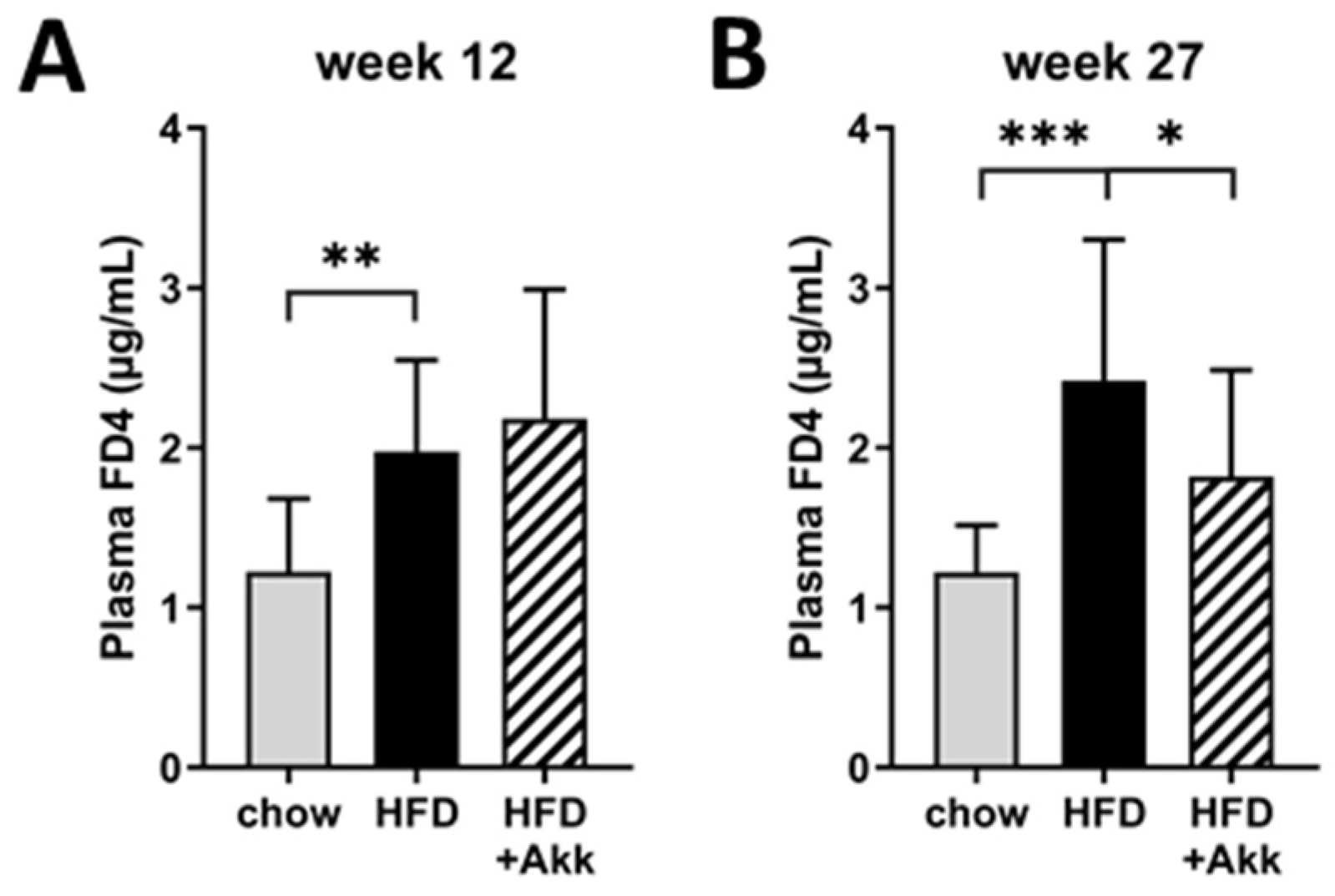
2.3. Heat-Inactivated A. muciniphila Lowered Gut Permeability at the End of the Study and Had Minor Effects on Inflammation in Ileum and Colon
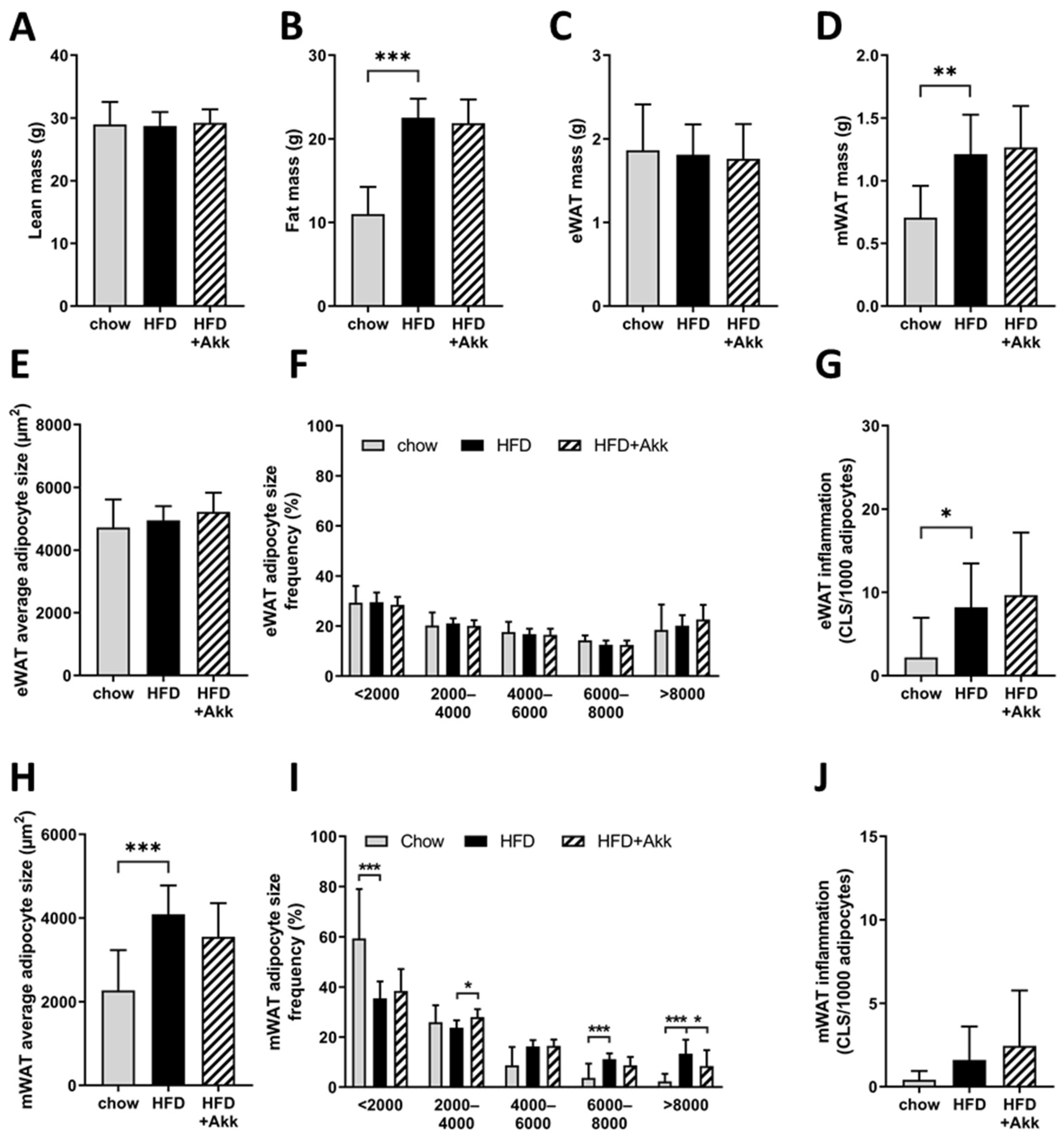
2.4. Heat-Inactivated A. muciniphila Did Not Affect Adiposity but Did Improve Adipocyte Hypertrophy in the Mesenteric Depot
2.5. Heat-Inactivated A. muciniphila Does Not Affect Development of Non-Alcoholic Steatohepatitis or Hepatic Fibrosis
2.6. Heat-Inactivated A. muciniphila Improves the Expression of Biomarkers Associated with Matrix Remodelling and Gut Permeability
3. Discussion
4. Materials and Methods
4.1. Culture and Pasteurization of Akkermansia muciniphila
4.2. Animal Study
4.3. Plasma Biochemistry
4.4. Gut Microbiota Analyses
4.5. Gut Chemokine and Cytokine Analyses
4.6. Adipose Tissue Histology
4.7. Liver Histology and Biochemical Analysis of Collagen
4.8. Determination of Short-Chain Fatty Acids and Bile Acids in Plasma
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Non-Alcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology 2015, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.-K.K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H. Multiple organs involved in the pathogenesis of non-alcoholic fatty liver disease. Cell Biosci. 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Welch, A.A.; Ocké, M.; Amiano, P.; Agnoli, C.; Ferrari, P.; Sonestedt, E.; Chajès, V.; Bueno-De-Mesquita, H.B.; Kaaks, R.; et al. Dietary fat intake in the european prospective investigation into cancer and nutrition: Results from the 24-h dietary recalls. Eur. J. Clin. Nutr. 2009, 63, S61–S80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harika, R.K.; Eilander, A.; Alssema, M.; Osendarp, S.J.M.; Zock, P.L. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann. Nutr. Metab. 2013, 63, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.M.; Kleemann, R.; Gart, E.; van Duyvenvoorde, W.; Verschuren, L.; Caspers, M.; Menke, A.; Krömmelbein, N.; Salic, K.; Burmeister, Y.; et al. Cholesterol Accumulation as a Driver of Hepatic Inflammation Under Translational Dietary Conditions Can Be Attenuated by a Multicomponent Medicine. Front. Endocrinol. 2021, 12, 601160. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Salic, K.; Verheij, J.; Liang, W.; van Duyvenvoorde, W.; Menke, A.; Kooistra, T.; Kleemann, R.; Wielinga, P.Y. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis (NASH) and hepatic fibrosis in LDLR-/-.Leiden mice. Int. J. Obes. 2016, 40, 1416–1423. [Google Scholar] [CrossRef] [Green Version]
- Gart, E.; Lima, E.S.; Schuren, F.; de Ruiter, C.G.F.; Attema, J.; Verschuren, L.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R.; et al. Diet-Independent Correlations between Bacteria and Dysfunction of Gut, Adipose Tissue, and Liver: A Comprehensive Microbiota Analysis in Feces and Mucosa of the Ileum and Colon in Obese Mice with NAFLD. Int. J. Mol. Sci. 2019, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Tengeler, A.C.; Gart, E.; Wiesmann, M.; Arnoldussen, I.A.C.; van Duyvenvoorde, W.; Hoogstad, M.; Dederen, P.J.; Verweij, V.; Geenen, B.; Kozicz, T.; et al. Propionic acid and not caproic acid, attenuates nonalcoholic steatohepatitis and improves (cerebro) vascular functions in obese Ldlr−/−.Leiden mice. FASEB J. 2020, 34, 9575–9593. [Google Scholar] [CrossRef]
- van Koppen, A.; Verschuren, L.; van den Hoek, A.M.; Verheij, J.; Morrison, M.C.; Li, K.; Nagabukuro, H.; Costessi, A.; Caspers, M.P.M.; van den Broek, T.J.; et al. Uncovering a Predictive Molecular Signature for the Onset of NASH-Related Fibrosis in a Translational NASH Mouse Model. Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 83–98.e10. [Google Scholar] [CrossRef] [Green Version]
- Morrison, M.C.; Verschuren, L.; Salic, K.; Verheij, J.; Menke, A.; Wielinga, P.Y.; Iruarrizaga-Lejarreta, M.; Gole, L.; Yu, W.W.-M.; Turner, S.; et al. Obeticholic Acid Modulates Serum Metabolites and Gene Signatures Characteristic of Human NASH and Attenuates Inflammation and Fibrosis Progression in Ldlr−/−.Leiden Mice. Hepatol. Commun. 2018, 2, 1513–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, M.C.; Kleemann, R.; van Koppen, A.; Hanemaaijer, R.; Verschuren, L. Key Inflammatory Processes in Human NASH Are Reflected in Ldlr−/−.Leiden Mice: A Translational Gene Profiling Study. Front. Physiol. 2018, 9, 132. [Google Scholar] [CrossRef]
- van den Hoek, A.M.; Verschuren, L.; Worms, N.; van Nieuwkoop, A.; de Ruiter, C.; Attema, J.; Menke, A.L.; Caspers, M.P.M.; Radhakrishnan, S.; Salic, K.; et al. A Translational Mouse Model for NASH with Advanced Fibrosis and Atherosclerosis Expressing Key Pathways of Human Pathology. Cells 2020, 9, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; Salic, K.; Morrison, M.C.; Caspers, M.; van Duyvenvoorde, W.; Heijnk, M.; Giera, M.; Bobeldijk-Pastorova, I.; Keijer, J.; Storsve, A.B.; et al. Krill Oil Treatment Increases Distinct PUFAs and Oxylipins in Adipose Tissue and Liver and Attenuates Obesity-Associated Inflammation via Direct and Indirect Mechanisms. Nutrients 2021, 13, 2836. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Gurchenkov, V.; Vignjevic, D.M. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adhes. Migr. 2014, 8, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Mantaj, J.; Abu-Shams, T.; Enlo-Scott, Z.; Swedrowska, M.; Vllasaliu, D. Role of the Basement Membrane as an Intestinal Barrier to Absorption of Macromolecules and Nanoparticles. Mol. Pharm. 2018, 15, 5802–5808. [Google Scholar] [CrossRef] [Green Version]
- Vllasaliu, D.; Falcone, F.H.; Stolnik, S.; Garnett, M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp. Cell Res. 2014, 323, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Iruarrizaga-Lejarreta, M.; Martínez-Arranz, I.; Morrison, M.C.; Varela-Rey, M.; Ramos, D.F.; Delacruz-Villar, L.; Noureddin, M.; Martínez-Chantar, M.L.; Kleemann, R.; Alonso, C.; et al. Targeting the NAFLD metabolome and the shaping of precision medicine for patients with NASH. J. Hepatol. 2018, 68, S362–S363. [Google Scholar] [CrossRef]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Kelley, D.E.; Wing, R.R.; Meier, A.; Thaete, F.L. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 1999, 48, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; Morrison, M.C.; Wielinga, P.Y.; Van Duyvenvoorde, W.; Kooistra, T.; Kleemann, R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int. J. Obes. 2015, 40, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Tonini, G.; Mulder, P.; Kelder, T.; van Erk, M.; van den Hoek, A.M.; Mariman, R.; Wielinga, P.Y.; Baccini, M.; Kooistra, T.; et al. Coordinated and Interactive Expression of Genes of Lipid Metabolism and Inflammation in Adipose Tissue and Liver during Metabolic Overload. PLoS ONE 2013, 8, e75290. [Google Scholar] [CrossRef] [Green Version]
- Abe, N.; Kato, S.; Tsuchida, T.; Sugimoto, K.; Saito, R.; Verschuren, L.; Kleemann, R.; Oka, K. Longitudinal characterization of diet-induced genetic murine models of non-alcoholic steatohepatitis with metabolic, histological, and transcriptomic hallmarks of human patients. Biol. Open 2019, 8, bio041251. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, R.A.; Bijzet, J.; Meijers, W.C.; Yakala, G.K.; Kleemann, R.; Nguyen, T.Q.; de Boer, R.A.; Schalkwijk, C.G.; Hazenberg, B.P.C.C.; Tietge, U.J.F.F.; et al. Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci. Rep. 2015, 5, 16474. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Dong, C.X.; Zhao, W.; Solomon, C.; Rowland, K.J.; Ackerley, C.; Robine, S.; Holzenberger, M.; Gonska, T.; Brubaker, P.L. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology 2014, 155, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Nejdfors, P.; Ekelund, M.; Weström, B.R.; Willén, R.; Jeppsson, B. Intestinal permeability in humans is increased after radiation therapy. Dis. Colon Rectum 2000, 43, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokaria, R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450-11. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, J.; Xiao, H.; Zhang, S.; Wang, B.; Cui, M.; Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes 2020, 11, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Abdullah, T.S.; Mounajjed, T.; Hartono, S.; McConico, A.; White, T.; LeBrasseur, N.; Lanza, I.; Nair, S.; Gores, G.; et al. A longitudinal study of whole body, tissue, and cellular physiology in a mouse model of fibrosing NASH with high fidelity to the human condition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G666–G680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar]
- Leeming, D.J.; Nielsen, M.J.; Dai, Y.; Veidal, S.S.; Vassiliadis, E.; Zhang, C.; He, Y.; Vainer, B.; Zheng, Q.; Karsdal, M.A. Enzyme-linked immunosorbent serum assay specific for the 7S domain of collagen type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol. Res. 2012, 42, 482–493. [Google Scholar] [CrossRef]
- Sand, J.M.; Larsen, L.; Hogaboam, C.; Martinez, F.; Han, M.L.; Larsen, M.R.; Nawrocki, A.; Zheng, Q.; Karsdal, M.A.; Leeming, D.J.; et al. MMP Mediated Degradation of Type IV Collagen Alpha 1 and Alpha 3 Chains Reflects Basement Membrane Remodeling in Experimental and Clinical Fibrosis–Validation of Two Novel Biomarker Assays. PLoS ONE 2013, 8, e84934. [Google Scholar] [CrossRef]
- Veidal, S.S.; Karsdal, M.A.; Vassiliadis, E.; Nawrocki, A.; Larsen, M.R.; Nguyen, Q.H.T.; Hägglund, P.; Luo, Y.; Zheng, Q.; Vainer, B.; et al. MMP mediated degradation of type VI collagen is highly associated with liver Fibrosis-Identification and validation of a novel biochemical marker assay. PLoS ONE 2011, 6, e24753. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.A.H.; Gart, E.; Vreeken, D.; Franx, B.A.A.; Wekking, L.; Verweij, V.G.M.; Worms, N.; Schoemaker, M.H.; Gross, G.; Morrison, M.C.; et al. Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice. Nutrients 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Van Den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobeldijk, I.; Hekman, M.; de Vries-van der Weij, J.; Coulier, L.; Ramaker, R.; Kleemann, R.; Kooistra, T.; Rubingh, C.; Freidig, A.; Verheij, E. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: Compound class targeting in a metabolomics workflow. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 306–313. [Google Scholar] [CrossRef]
- Salic, K.; Kleemann, R.; Wilkins-Port, C.; McNulty, J.; Verschuren, L.; Palmer, M. Apical sodium-dependent bile acid transporter inhibition with volixibat improves metabolic aspects and components of nonalcoholic steatohepatitis in Ldlr−/−.Leiden mice. PLoS ONE 2019, 14, e0218459. [Google Scholar] [CrossRef] [PubMed]

| Chow | HFD | HFD + Akk | |
|---|---|---|---|
| Acetic acid (µg/mL) | 2.87 ± 0.68 | 2.73 ± 0.82 | 2.54 ± 1.00 |
| Butyric acid (µg/mL) | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.03 |
| Valeric acid (µg/mL) | 0.006 ± 0.003 ** | 0.013 ± 0.005 | 0.008 ± 0.005 * |
| Caproic acid (µg/mL) | 0.10 ± 0.02 *** | 0.31 ± 0.13 | 0.17 ± 0.04 ** |
| Isobutyric acid (µg/mL) | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 |
| Methylbutyric acid (µg/mL) | 0.03 ± 0.01 * | 0.05 ± 0.01 | 0.04 ± 0.01 |
| Isovaleric acid (µg/mL) | 0.01 ± 0.00 * | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Chow | HFD | HFD + Akk | |
|---|---|---|---|
| Ileum | |||
| MIP-1a (pg/mg protein) | 6.99 ± 2.16 | 11.11 ± 4.22 | 6.80 ± 3.52 * |
| IP-10 (pg/mg protein) | 3.95 ± 1.09 | 2.93 ± 2.06 | 1.73 ± 1.40 |
| KC (pg/mg protein) | 6.83 ± 2.46 | 10.54 ± 3.66 | 9.25 ± 6.08 |
| RANTES (ng/mg protein) | 0.66 ± 0.64 | 0.52 ± 0.38 | 0.38 ± 0.16 |
| MIF (ng/mg protein) | 82.12 ± 42.45 | 74.93 ± 52.89 | 68.45 ± 55.98 |
| IL-10 (pg/mg protein) | 3.81 ± 2.25 | 1.90 ± 2.08 | 1.35 ± 1.37 |
| IL-17 (pg/mg protein) | 0.47 ± 0.27 | 0.88 ± 0.74 | 0.67 ± 0.63 |
| TNF-α (pg/mg protein) | 2.72 ± 1.31 | 1.84 ± 1.30 | 1.64 ± 1.26 |
| Colon | |||
| MIP-1a (pg/mg protein) | 8.64 ± 9.49 | 4.48 ± 1.19 | 3.90 ± 0.55 |
| IP-10 (pg/mg protein) | 4.80 ± 3.92 | 2.50 ± 0.75 | 2.66 ± 0.95 |
| KC (pg/mg protein) | 7.28 ± 1.93 | 7.94 ± 0.81 | 10.90 ± 3.14 ** |
| RANTES (ng/mg protein) | 0.71 ± 1.08 | 0.20 ± 0.06 | 0.20 ± 0.04 |
| MIF (ng/mg protein) | 84.53 ± 33.06 | 59.89 ± 39.90 | 74.63 ± 29.04 |
| IL-10 (pg/mg protein) | 4.50 ± 0.46 | 4.72 ± 2.65 | 3.44 ± 1.16 |
| IL-17 (pg/mg protein) | 1.18 ± 1.03 | 0.55 ± 0.53 | 0.43 ± 0.41 |
| TNF-α (pg/mg protein) | 3.81 ± 0.94 | 3.69 ± 1.36 | 4.22 ± 1.03 |
| Chow | HFD | HFD + Akk | ||
|---|---|---|---|---|
| Classical pathway | ||||
| Primary bile acids | ||||
| Cholic acid (µM) | 0.39 ± 0.36 | 0.25 ± 0.17 | 0.36 ± 0.43 | |
| Glycocholic acid (nM) | 2.67 ± 1.36 * | 9.92 ± 10.77 | 13.67 ± 11.98 | |
| Taurocholic acid (µM) | 0.10 ± 0.05 ** | 1.59 ± 1.82 | 2.06 ± 1.81 | |
| Secondary bile acids | ||||
| Deoxycholic acid (µM) | 0.29 ± 0.22 * | 0.53 ± 0.23 | 0.73 ± 0.39 | |
| Taurodeoxycholic acid (µM) | 0.03 ± 0.01 *** | 0.24 ± 0.19 | 0.24 ± 0.13 | |
| Alternative pathway | ||||
| Primary bile acids | ||||
| Chenodeoxycholic acid (nM) | 6.00 ± 6.48 | 12.92 ± 9.45 | 17.27 ± 17.81 | |
| Taurochenodeoxycholic acid (nM) | 4.40 ± 2.61 ** | 77.08 ± 61.25 | 96.33 ± 73.97 | |
| β-Muricholic acid (µM) | 0.36 ± 0.21 * | 0.14 ± 0.08 | 0.25 ± 0.23 | |
| Secondary bile acids | ||||
| Ursodeoxycholic acid (nM) | 30.20 ± 15.99 | 35.23 ± 20.77 | 46.93 ± 33.16 | |
| Tauro-ursodeoxycholic acid (nM) | 14.50 ± 9.71 ** | 97.77 ± 68.33 | 123.40 ± 79.61 | |
| Hyodeoxycholic acid (nM) | 13.17 ± 13.23 | 20.64 ± 9.45 | 24.40 ± 17.71 | |
| Chow | HFD | HFD + Akk | |
|---|---|---|---|
| TIMP-1 (ng/mL) | 2.03 ± 0.43 ** | 4.45 ± 1.01 | 4.71 ± 1.97 |
| PRO-C3 (ng/mL) | 11.7 ± 3.77 | 16.24 ± 7.02 | 13.86 ± 6.02 |
| PRO-C4 (ng/mL) | 132.0 ± 21.27 | 182.0 ± 58.84 | 141.3 ± 38.64 * |
| C4M (ng/mL) | 4.23 ± 0.99 | 5.59 ± 1.59 | 4.53 ± 1.26 |
| C6M (ng/mL) | 4.04 ± 1.45 | 7.19 ± 3.29 | 5.89 ± 2.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison, M.C.; Gart, E.; Duyvenvoorde, W.v.; Snabel, J.; Nielsen, M.J.; Leeming, D.J.; Menke, A.; Kleemann, R. Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−.Leiden Mice. Int. J. Mol. Sci. 2022, 23, 2325. https://doi.org/10.3390/ijms23042325
Morrison MC, Gart E, Duyvenvoorde Wv, Snabel J, Nielsen MJ, Leeming DJ, Menke A, Kleemann R. Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−.Leiden Mice. International Journal of Molecular Sciences. 2022; 23(4):2325. https://doi.org/10.3390/ijms23042325
Chicago/Turabian StyleMorrison, Martine C., Eveline Gart, Wim van Duyvenvoorde, Jessica Snabel, Mette Juul Nielsen, Diana Julie Leeming, Aswin Menke, and Robert Kleemann. 2022. "Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−.Leiden Mice" International Journal of Molecular Sciences 23, no. 4: 2325. https://doi.org/10.3390/ijms23042325
APA StyleMorrison, M. C., Gart, E., Duyvenvoorde, W. v., Snabel, J., Nielsen, M. J., Leeming, D. J., Menke, A., & Kleemann, R. (2022). Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−.Leiden Mice. International Journal of Molecular Sciences, 23(4), 2325. https://doi.org/10.3390/ijms23042325






