MS/MS Molecular Networking Unveils the Chemical Diversity of Biscembranoid Derivatives, Neutrophilic Inflammatory Mediators from the Cultured Soft Coral Sarcophyton trocheliophorum
Abstract
1. Introduction
2. Results and Discussion
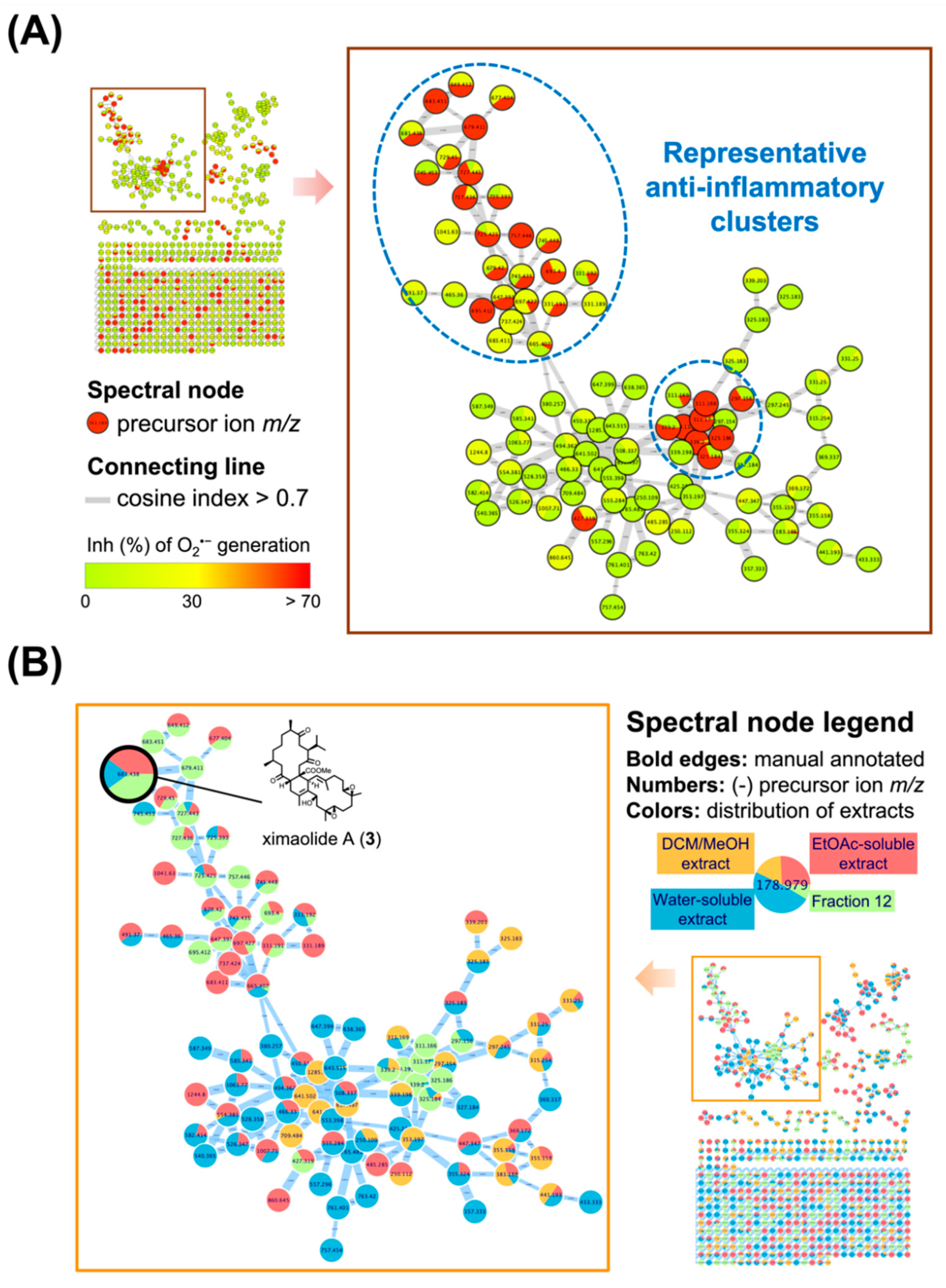
2.1. Characterizing the Distribution of Anti-Inflammatory Biscembranoids Using Multi-informative Molecular Networking (MIMN)
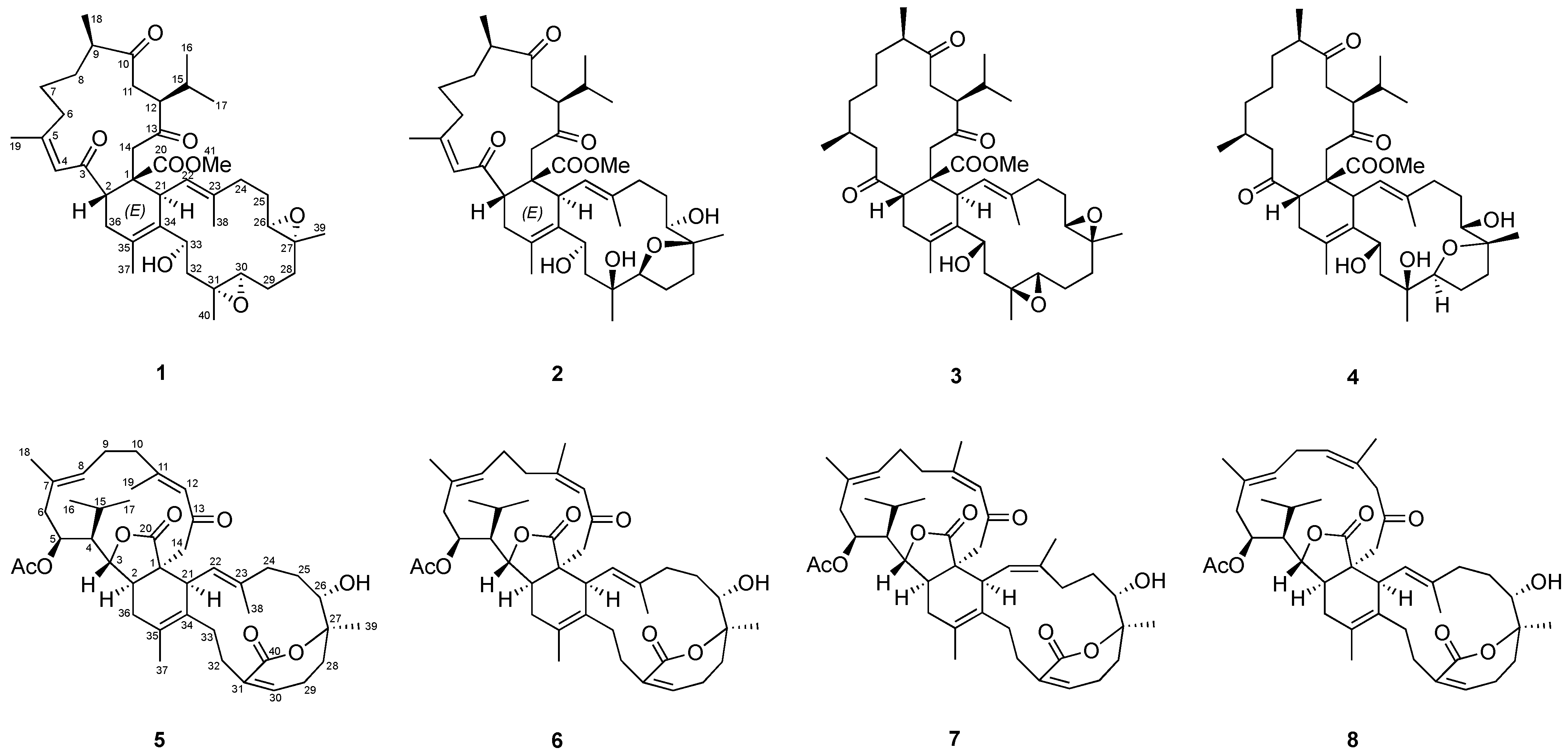
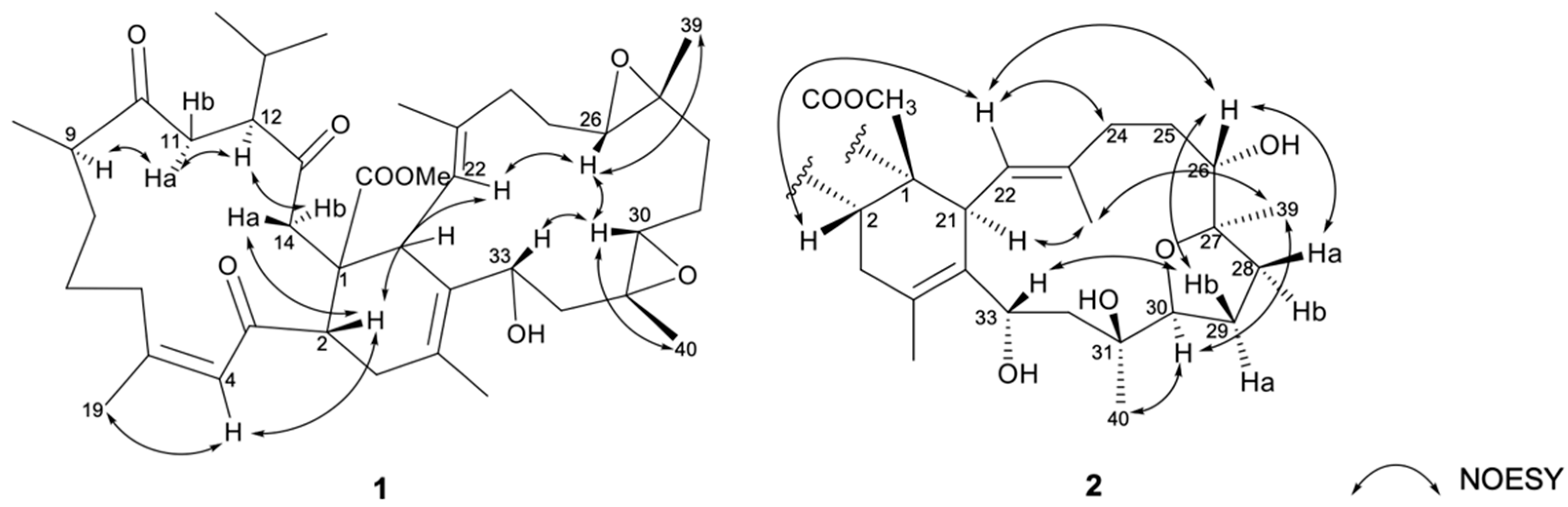
2.2. Chemical Identification of Isolated Compounds
2.3. Bioactivities of the Biscembranoids
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Non-Targeted Fragment Ions Collection Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
3.3. GNPS-Based Molecular Networking Analysis
3.4. Animal Material, Extraction, and Isolation
3.5. Preparation of Human Neutrophils
3.6. Determination of Superoxide Anion (O2•−) Generation
3.7. Measurement of Elastase Release
3.8. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, J.; Long, K.; Pang, T.; He, C.H.; Clardy, J. The structure of methyl isosartortuoate, a novel tetracyclic tetraterpenoid from the soft coral Sarcophyton tortuosum. J. Am. Chem. Soc. 1986, 108, 177–178. [Google Scholar] [CrossRef]
- Kusumi, T.; Igari, M.; Ishitsuka, M.O.; Ichikawa, A.; Itezono, Y.; Nakayama, N.; Kakisawa, H. A novel chlorinated biscembranoid from the marine soft coral Sarcophyton glaucum. J. Org. Chem. 1990, 55, 6286–6289. [Google Scholar] [CrossRef]
- Leone, P.A.; Bowden, B.F.; Carroll, A.R.; Coll, J.C.; Meehan, G.V. Studies of Australian soft corals, XLIX. a new biscembranoid and its probable biosynthetic precursors from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 1993, 56, 521–526. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Tseng, H.-K.; Sheu, J.-H. A novel cytotoxic biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 1998, 39, 7121–7122. [Google Scholar] [CrossRef]
- Feller, M.; Rudi, A.; Berer, N.; Goldberg, I.; Stein, Z.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Isoprenoids of the soft coral Sarcophyton glaucum: Nyalolide, a new biscembranoid, and other terpenoids. J. Nat. Prod. 2004, 67, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-M.; Lan, W.-J.; Su, J.-Y.; Zhang, G.-W.; Feng, X.-L.; Liang, Y.-J.; Yang, X.-P. Two new cytotoxic tetracyclic tetraterpenoids from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 2004, 67, 1915–1918. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hashimoto, K.; Okamura, H.; Kurawaki, J.-I.; Nakatani, M.; Hou, D.-X.; Fujii, M.; Doe, M.; Morimoto, Y.; Takemura, K. Biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2006, 69, 1130–1133. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic diels–alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.-W.; Chen, P.; Yang, Y.-M.; Mollo, E.; Gavagnin, M.; Cimino, G. Biscembranoids and their probable biogenetic precursor from the Hainan soft coral Sarcophyton tortuosum. J. Nat. Prod. 2007, 70, 1158–1166. [Google Scholar] [CrossRef]
- Yan, X.-H.; Gavagnin, M.; Cimino, G.; Guo, Y.-W. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett. 2007, 48, 5313–5316. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.-W.; Mollo, E.; Gavagnin, M.; Cimino, G. Further new bis-cembranoids from the Hainan soft coral Sarcophyton tortuosum. Helv. Chim. Acta 2008, 91, 2069–2074. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hashimoto, K.; Yokogawa, Y.; Okamura, H.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Cytotoxic biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2009, 72, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Huang, C.-Y.; Dai, C.-F.; Wen, Z.-H.; Sheu, J.-H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010, 51, 5764–5766. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum. Mar. Drugs 2010, 8, 2837–2848. [Google Scholar] [CrossRef]
- Yan, P.; Lv, Y.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones A-G, new isobiscembranoids from the soft coral Lobophytum pauciflorum. Org. Lett. 2010, 12, 2484–2487. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones U- Z1, biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Biodivers. 2011, 8, 1724–1734. [Google Scholar] [CrossRef]
- Jia, R.; Kurtan, T.; Mandi, A.; Yan, X.H.; Zhang, W.; Guo, Y.W. Biscembranoids formed from an alpha,beta-unsaturated gamma-lactone ring as a dienophile: Structure revision and establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J. Org. Chem. 2013, 78, 3113–3119. [Google Scholar] [CrossRef]
- Li, Y.F.; He, L.L.; Liu, H.L.; Liang, L.F.; Zhang, H.B.; Guo, Y.W. Structural revision of methyl tortuoate D, a bis-cembranoid from Hainan Sarcophyton tortuosum and its absolute stereochemistry. J. Asian Nat. Prod. Res. 2013, 15, 566–573. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Van Quang, N.; Van Minh, C.; Thuy Hang, D.T.; Le Tuan Anh, H.; Tai, B.H.; Yen, P.H.; Hoai, N.T.; Thung, D.C.; Van Kiem, P. Biscembranoids from the marine sponge Petrosia nigricans. Nat. Prod. Commun. 2013, 8, 1209–1212. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef]
- Nam, N.H.; Tung, P.T.; Ngoc, N.T.; Hanh, T.T.H.; Thao, N.P.; Thanh, N.V.; Cuong, N.X.; Thao, D.T.; Huong, T.T.; Thung, D.C.; et al. Cytotoxic biscembranoids from the soft coral Sarcophyton pauciplicatum. Chem. Pharm. Bull. 2015, 63, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.; Hertzer, C.; Kehraus, S.; Nietzer, S.; Rohde, S.; Schupp, P.J.; Wagele, H.; Konig, G.M. Secondary metabolome and its defensive role in the aeolidoidean Phyllodesmium longicirrum, (Gastropoda, Heterobranchia, Nudibranchia). Beilstein J. Org. Chem. 2017, 13, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, Y.H.; Ge, M.X.; Lou, L.L.; Xu, Y.S.; Ahmed, A.; Chen, Y.Y.; Zhang, J.S.; Tang, G.H.; Yin, S. Biscembranoids and cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2017, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Huang, C.Y.; Ahmed, A.F.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef]
- Sun, P.; Cai, F.Y.; Lauro, G.; Tang, H.; Su, L.; Wang, H.L.; Li, H.H.; Mandi, A.; Kurtan, T.; Riccio, R.; et al. Immunomodulatory biscembranoids and assignment of their relative and absolute configurations: Data set modulation in the density functional theory/nuclear magnetic resonance approach. J. Nat. Prod. 2019, 82, 1264–1273. [Google Scholar] [CrossRef]
- Huang, T.Y.; Huang, C.Y.; Chao, C.H.; Lin, C.C.; Dai, C.F.; Su, J.H.; Sung, P.J.; Wu, S.H.; Sheu, J.H. New biscembranoids sardigitolides A-D and known cembranoid-related compounds from sarcophyton digitatum: Isolation, structure elucidation, and bioactivities. Mar. Drugs 2020, 18, 452. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Cuadrado, C.; Gao, C.; Wu, Q.; Li, X.; Pang, T.; Daranas, A.H.; Guo, Y.; Li, X. Polyoxygenated anti-inflammatory biscembranoids from the soft coral Sarcophyton tortuosum and their stereochemistry. Chin. Chem. Lett. 2021, 32, 271–276. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones H-N, biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Pharm. Bull. 2010, 58, 1591–1595. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Peng, B.-R.; Lai, G.-Y.; Weng, H.-J.; El-Shazly, M.; Su, C.-H.; Su, J.-H.; Sung, P.-J.; Liao, C.-P.; Lai, K.-H. Chemometric-guided exploration of marine anti-neurofibroma leads. Front. Mar. Sci. 2022, 9, 930736. [Google Scholar] [CrossRef]
- Lai, K.-H.; Chen, P.-J.; Chen, C.-C.; Yang, S.-H.; El-Shazly, M.; Chang, Y.-C.; Wu, Y.-H.; Wu, Y.-H.; Wang, Y.-H.; Hsieh, H.-L.; et al. Lophatherum gracile Brongn. attenuates neutrophilic inflammation through inhibition of JNK and calcium. J. Ethnopharmacol. 2021, 264, 113224. [Google Scholar] [CrossRef]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.R.; Lu, M.C.; El-Shazly, M.; Wu, S.L.; Lai, K.H.; Su, J.H. Aquaculture Soft Coral Lobophytum crassum as a Producer of Anti-Proliferative Cembranoids. Mar. Drugs 2018, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.R.; Lai, K.H.; Lee, G.H.; Yu, S.S.; Duh, C.Y.; Su, J.H.; Zheng, L.G.; Hwang, T.L.; Sung, P.J. Scalarane-type sesterterpenoids from the marine sponge Lendenfeldia sp. Alleviate inflammation in human neutrophils. Mar. Drugs 2021, 19, 561. [Google Scholar] [CrossRef]
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lai, K.H.; Kumar, S.; Chen, P.J.; Wu, Y.H.; Lai, C.L.; Hsieh, H.L.; Sung, P.J.; Hwang, T.L. 1H NMR-Based Isolation of Anti-Inflammatory 9,11-Secosteroids from the Octocoral Sinularia leptoclados. Mar. Drugs 2020, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.B.A.; Chen, L.Y.; El-Shazly, M.; Peng, B.R.; Su, J.H.; Wu, H.C.; Lee, I.T.; Lai, K.H. Towards Sustainable Medicinal Resources through Marine Soft Coral Aquaculture: Insights into the Chemical Diversity and the Biological Potential. Mar. Drugs 2022, 20, 640. [Google Scholar] [CrossRef]

| Sample | Superoxide Anion | Elastase Release | ||
|---|---|---|---|---|
| IC50 (μg/mL) a | Inh% | IC50 (μg/mL) a | Inh% | |
| DCM/MeOH extract | 3.54 ± 1.96 | 38.95 ± 4.18 *** | ||
| EtOAc-soluble extract | 18.97 ± 4.17 * | 27.13 ± 8.44 * | ||
| Water-soluble extract b | 2.08 ± 1.90 | −0.48 ± 0.70 | ||
| Fraction 12 | 5.45 ± 0.66 | 70.40 ± 2.57 *** | 7.48 ± 0.99 | 61.14 ± 4.59 *** |
| Position | δH (J in Hz) a | δC b Mult. c | COSY | HMBC |
|---|---|---|---|---|
| 1. | 50.8, qC | |||
| 2 | 3.74, dd (8.3, 8.3) | 44.6, CH | H-36 | C-1, -3, -4, -6, -14, -20, -21, -35, -36 |
| 3 | 203.3, qC | |||
| 4 | 6.59, brs | 126.7, CH | H-19 | C-3, -6, -19 |
| 5 | 161.1, qC | |||
| 6 | 1.56–1.65, m; 3.25–3.31, m | 32.4, CH2 | C-4, -5, -7, -8, -19 | |
| 7 | 1.53–1.59, m; 1.24–1.28, m | 24.6, CH2 | H-8 | C-8, -9 |
| 8 | 1.63–1.71, m; 1.26–1.36, m | 30.9, CH2 | H-7, -9 | C-9, -10 |
| 9 | 2.73–2.81, m | 43.9, CH | H-8, -18 | C-7, -8, -10, 18 |
| 10 | 214.8, qC | |||
| 11 | 2.71–2.80, m; 2.05–2.11, m | 34.8, CH2 | H-12 | C-10, -12, -13, -15 |
| 12 | 2.95, ddd (10.0, 4.9, 2.8) | 53.2, CH | H-11, -15 | C-14, -15, -16 |
| 13 | 209.4, qC | |||
| 14 | 2.71–2.77, m; 3.05, d (18.6) | 47.0, CH2 | C-1, -2, -12, -13, -20, -21 | |
| 15 | 2.14–2.23, m | 29.2, CH | H-12, -16, -17 | C-11, -12, -13, -17 |
| 16 | 0.74, d (6.8) | 18.4, CH3 | H-15 | C-12, -15, -17 |
| 17 | 0.95, d (6.8) | 21.2, CH3 | H-15 | C-12, -15, -16 |
| 18 | 1.06, d (6.8) | 17.4, CH3 | H-9 | C-8, -9, -10 |
| 19 | 1.89, s | 26.3, CH3 | H-4 | C-3, -4, -5, -6, -7 |
| 20 | 174.6, qC | |||
| 21 | 3.26, d (11.1) | 43.2, CH | H-22 | C-1, -2, -20, -22, -23, -33, -34 |
| 22 | 5.10, d (11.1) | 126.7, CH | H-21, -38 | C-1, -21, -24, -34, -38 |
| 23 | 134.0, qC | |||
| 24 | 2.19–2.30, m; 2.03–2.12, m | 36.3, CH2 | H-25 | C-22, -23, -25, -26, -38 |
| 25 | 1.71–1.79, m; 1.47–1.58, m | 26.3, CH2 | H-24, -26 | C-23, -24, -26, -27 |
| 26 | 2.91, dd (6.1, 4.3) | 61.5, CH | H-25 | C-24, -25, -27, -28 |
| 27 | 59.4, qC | |||
| 28 | 2.03–2.12, m | 36.3, CH2 | H-29 | C-27, -29, -39 |
| 29 | 1.52–1.59, m; 1.60–1.66, m | 24.0, CH2 | H-28, -30 | C-28 |
| 30 | 2.29, dd (8.8, 4.1) | 60.8, CH | H-29 | C-28, -29, -32 |
| 31 | 60.0, qC | |||
| 32 | 1.95, dd (14.5, 10.0); 1.78–1.88, m | 39.7, CH2 | H-33 | C-30, -31, -33, -34, -40 |
| 33 | 4.79, dd (10.7, 2.1) | 65.1, CH | H-32 | C-21, -31, -32, -35 |
| 34 | 131.2, qC | |||
| 35 | 130.4, qC | |||
| 36 | 2.20–2.34, m | 33.2, CH2 | H-2 | C-1, -2, -3, -34, -35, -37 |
| 37 | 1.74, s | 18.7, CH3 | C-1, -2, -21, -34, -35, -36 | |
| 38 | 1.62, s | 17.3, CH3 | H-22 | C-1, -21, -22, -23, -24 |
| 39 | 1.24, s | 16.3, CH3 | C-26, -27, -28 | |
| 40 | 1.25, s | 18.7, CH3 | C-30, -31, -32 | |
| 41 | 3.50, s | 51.3, CH3 | C-20 |
| Position | δH (J in Hz) a | δC b Mult. c | COSY | HMBC |
|---|---|---|---|---|
| 1 | 50.5, qC | |||
| 2 | 3.45–3.49, m | 44.1, CH | H-36 | C-1, -3, -14, -20, -36 |
| 3 | 202.9, qC | |||
| 4 | 6.59, s | 126.4, CH | H-19 | C-3, -6, -19 |
| 5 | 161.4, qC | |||
| 6 | 1.59–1.67, m; 3.20–3.26, m | 33.0, CH2 | H-7 | C-5, -7, -19 |
| 7 | 1.22–1.29, m; 1.53–1.60, m | 25.0, CH2 | H-6, -8 | |
| 8 | 1.75–1.82, m; 1.27–1.34, m | 31.0, CH2 | H-7, -9 | |
| 9 | 2.87–2.94, m | 43.6, CH | H-8, -18 | C-8, -10, -18 |
| 10 | 214.7, qC | |||
| 11 | 2.74, dd (16.2, 9.7); 2.13–2.21, m | 34.6, CH2 | H-12 | C-10, -12, -13, -15 |
| 12 | 2.99–3.03, m | 53.9, CH | H-11, -15 | |
| 13 | 208.7, qC | |||
| 14 | 3.18, d (18.2); 2.58, d (18.2) | 46.0, CH2 | C-1, -2, -13, -20, -21 | |
| 15 | 2.32–2.38 m | 28.6, CH | H-12, -16, -17 | C-11, -12, -16, -17 |
| 16 | 0.74, d (6.5) | 18.2, CH3 | H-15 | C-12, -15, -17 |
| 17 | 0.98, d (6.9) | 21.4, CH3 | H-15 | C-12, -15, -16 |
| 18 | 1.07, d (6.6) | 17.9, CH3 | H-9 | C-8, -9, -10 |
| 19 | 1.89, s | 26.4, CH3 | H-4 | C-4, -5, -6, -7 |
| 20 | 174.9, qC | |||
| 21 | 3.67, d (10.8) | 42.9, CH | H-22 | C-1, -2, -14, -22, -23, -33, -34, -35 |
| 22 | 4.99, d (10.8) | 128.0, CH | H-21, -38 | C-24 |
| 23 | 137.2, qC | |||
| 24 | 2.08–2.17, m | 37.0, CH2 | H-25 | C-22, -23, -25, -26, -38 |
| 25 | 1.91–2.00, m; 1.26–1.33, m | 29.7, CH2 | H-24, -26 | C-23 |
| 26 | 3.24–3.30 m | 74.1, CH | H-25 | |
| 27 | 86.0, qC | |||
| 28 | 2.34–2.40, m; 1.64–1.71, m | 36.1, CH2 | H-29 | C-26, -27, -29, -30, -39 |
| 29 | 1.82–1.87, m; 1.54–1.60 m | 27.0, CH2 | H-28, -30 | C-27, -28, -31 |
| 30 | 3.96, dd (10.3, 6.3) | 88.4, CH | H-29 | C-31, -32, -40 |
| 31 | 76.3, qC | |||
| 32 | 2.19–2.26, m; 1.03–1.08, m | 39.5, CH2 | H-33 | C-31, -32, -40 |
| 33 | 5.05, d (11.2) | 67.5, CH | H-32 | C-21, -31, -32, -34, -35 |
| 34 | 125.3, qC | |||
| 35 | 132.2, qC | |||
| 36 | 2.41–2.51, m; 1.97–2.04, m | 33.4, CH2 | H-2, -37 | C-1, -2, -34, -35, 37 |
| 37 | 1.61, s | 18.1, CH3 | H-36 | C-21, -34, -35, -36 |
| 38 | 1.72, s | 16.4, CH3 | H-22 | C-1, -22, -23, -24 |
| 39 | 1.17, s | 20.6, CH3 | C-26, -27, -28 | |
| 40 | 1.19, s | 21.4, CH3 | C-30, -31, -32 | |
| 41 | 3.50, s | 51.1, CH3 | C-20 |
| Compound | Superoxide Anion | Elastase Release | ||
|---|---|---|---|---|
| IC50 (μM) a | Inh% | IC50 (μM) a | Inh% | |
| Sarcotrochelide A (1) | 16.92 ± 5.98 * | 13.86 ± 5.87 | ||
| Sarcotrochelide B (2) | 10.15 ± 2.39 * | 10.79 ± 4.60 | ||
| Ximaolide A (3) | 19.69 ± 5.00 * | 26.64 ± 5.02 ** | ||
| Methyl tortuoate D (4) | 17.61 ± 1.99 *** | 25.67 ± 5.27 ** | ||
| Glaucumolide A (5) | 5.46 ± 0.57 | 73.76 ± 3.84 *** | 6.22 ± 0.36 | 67.50 ± 1.73 *** |
| Glaucumolide B (6) | 1.98 ± 0.32 | 98.52 ± 0.50 *** | 2.76 ± 0.47 | 101.94 ± 3.57 *** |
| Bistrochelide A (7) | 8.29 ± 0.48 | 56.19 ± 2.83 *** | 48.61 ± 0.96 *** | |
| Bistrochelide B (8) | 45.39 ± 4.30 *** | 38.67 ± 4.81 ** | ||
| LY294002 b | 1.62 ± 0.42 | 92.61 ± 3.81 *** | 2.22 ± 0.49 | 86.85 ± 6.37 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.B.A.; Chen, L.-Y.; Chen, P.-J.; El-Shazly, M.; Hwang, T.-L.; Su, J.-H.; Su, C.-H.; Yen, P.-T.; Peng, B.-R.; Lai, K.-H. MS/MS Molecular Networking Unveils the Chemical Diversity of Biscembranoid Derivatives, Neutrophilic Inflammatory Mediators from the Cultured Soft Coral Sarcophyton trocheliophorum. Int. J. Mol. Sci. 2022, 23, 15464. https://doi.org/10.3390/ijms232415464
Nguyen NBA, Chen L-Y, Chen P-J, El-Shazly M, Hwang T-L, Su J-H, Su C-H, Yen P-T, Peng B-R, Lai K-H. MS/MS Molecular Networking Unveils the Chemical Diversity of Biscembranoid Derivatives, Neutrophilic Inflammatory Mediators from the Cultured Soft Coral Sarcophyton trocheliophorum. International Journal of Molecular Sciences. 2022; 23(24):15464. https://doi.org/10.3390/ijms232415464
Chicago/Turabian StyleNguyen, Ngoc Bao An, Lo-Yun Chen, Po-Jen Chen, Mohamed El-Shazly, Tsong-Long Hwang, Jui-Hsin Su, Chun-Han Su, Pei-Tzu Yen, Bo-Rong Peng, and Kuei-Hung Lai. 2022. "MS/MS Molecular Networking Unveils the Chemical Diversity of Biscembranoid Derivatives, Neutrophilic Inflammatory Mediators from the Cultured Soft Coral Sarcophyton trocheliophorum" International Journal of Molecular Sciences 23, no. 24: 15464. https://doi.org/10.3390/ijms232415464
APA StyleNguyen, N. B. A., Chen, L.-Y., Chen, P.-J., El-Shazly, M., Hwang, T.-L., Su, J.-H., Su, C.-H., Yen, P.-T., Peng, B.-R., & Lai, K.-H. (2022). MS/MS Molecular Networking Unveils the Chemical Diversity of Biscembranoid Derivatives, Neutrophilic Inflammatory Mediators from the Cultured Soft Coral Sarcophyton trocheliophorum. International Journal of Molecular Sciences, 23(24), 15464. https://doi.org/10.3390/ijms232415464







