FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity
Abstract
1. Introduction
2. Results
2.1. FSH Downregulates, in a Concentration-Dependent Manner, mRNA Levels for CTGF and Other Classic YAP-TEAD Transcriptional Target Genes
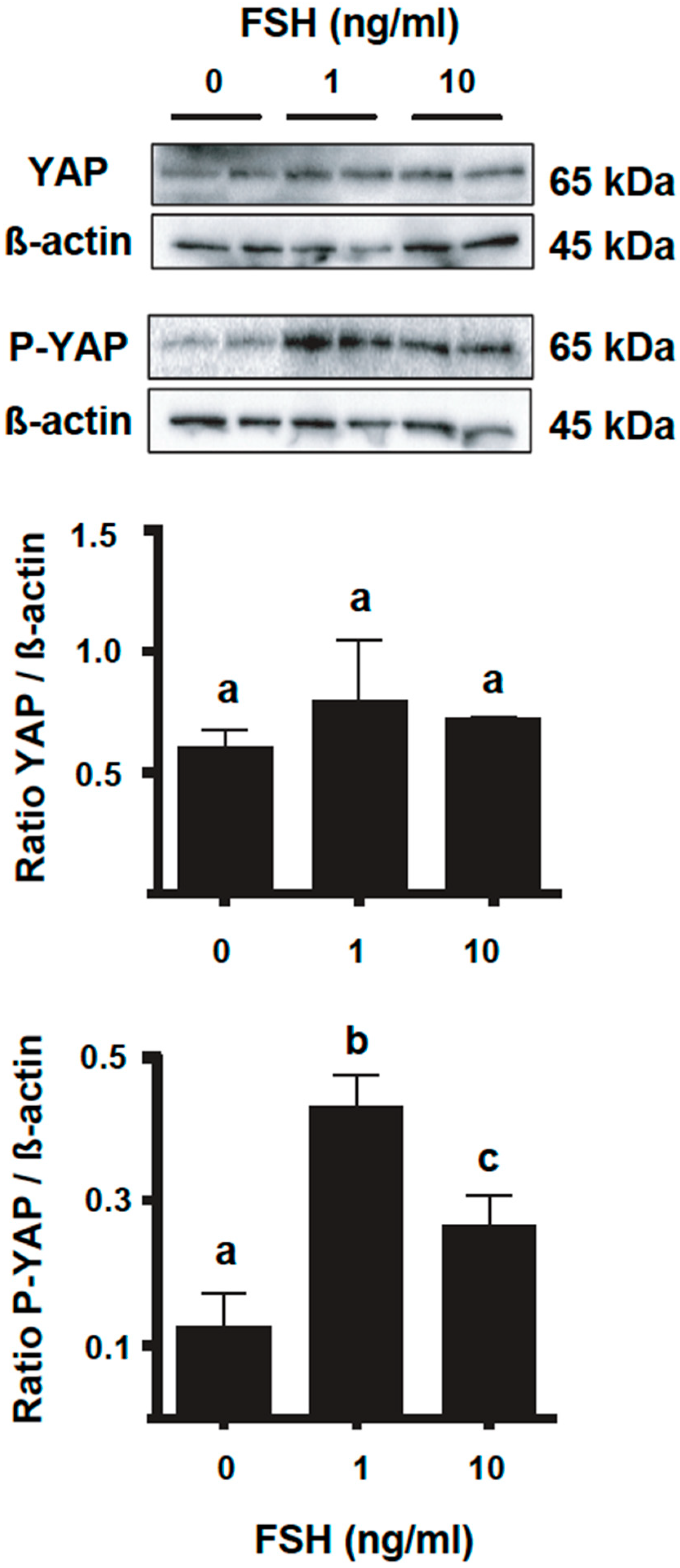
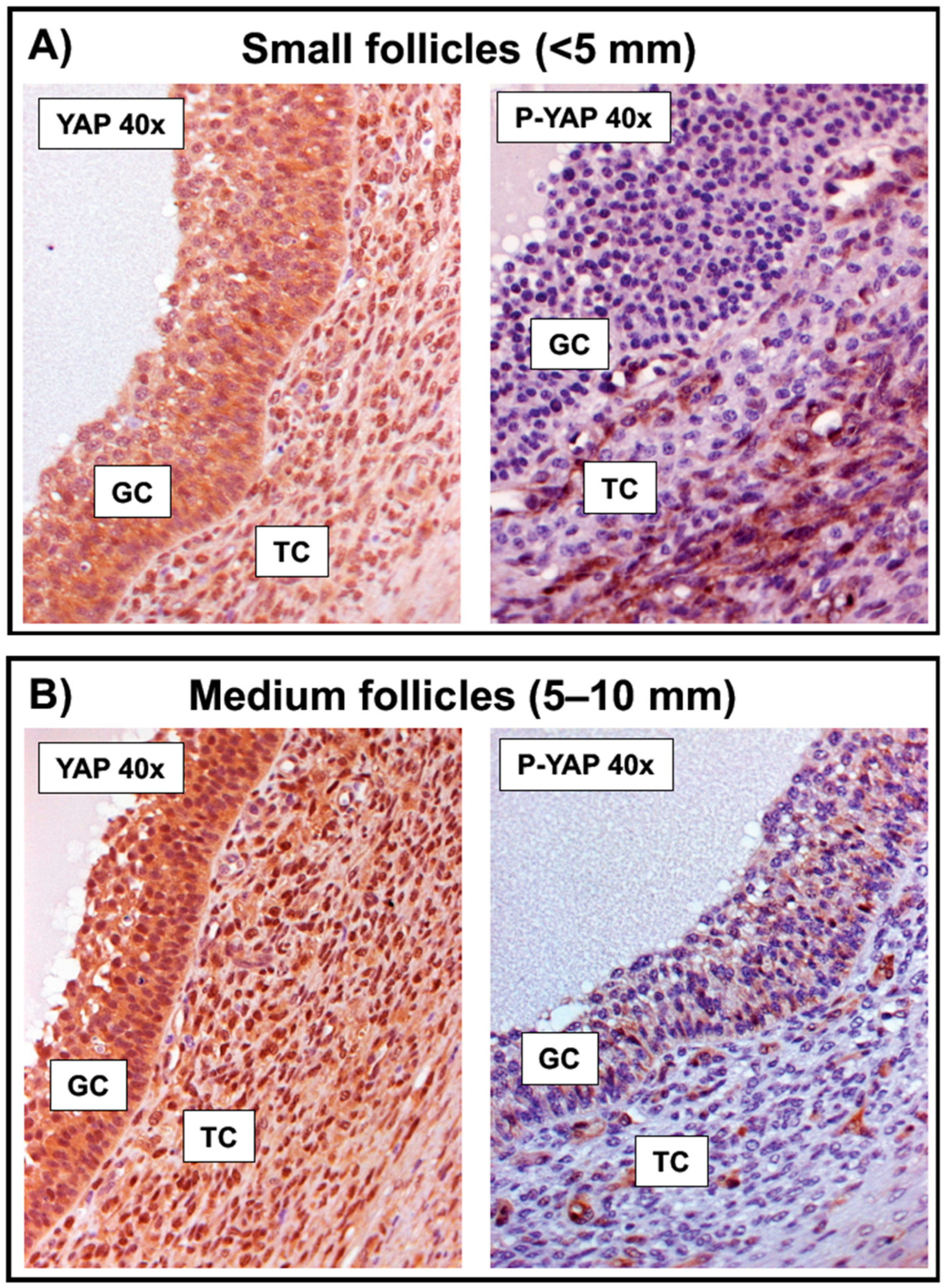
2.2. YAP Phosphorylation in Granulosa Cells Increases following FSH Challenge and along the Bovine Follicle Growth
2.3. The mRNA Abundance of CTGF, ANKRD1, and CYR61 Is Higher in Subordinate Follicles following the Follicular Divergence
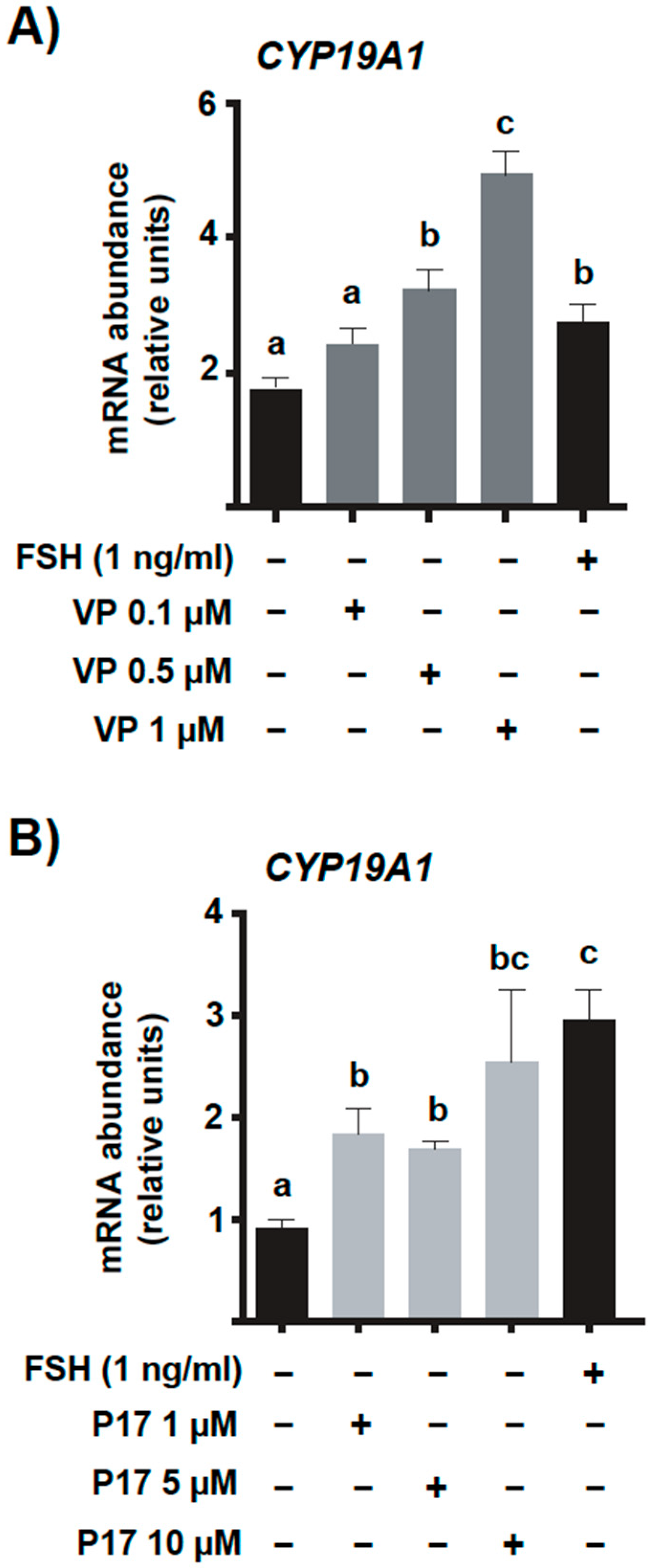
2.4. Pharmacological Inhibition of YAP-TEAD Interaction In Vitro Increases Basal Levels for mRNA Encoding CYP19A1
3. Discussion
4. Material and Methods
4.1. In Vitro Studies
4.1.1. Steroid Assay
4.1.2. Western Blotting
4.2. Ex Vivo Study
4.2.1. Tissue Sampling
4.2.2. Immunohistochemistry
4.3. In Vivo Study
4.4. RNA Extraction, Reverse Transcription, and Quantitative PCR (qPCR) for In Vitro and In Vivo Studies
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, P.S.P.; Folger, J.K.; Rajput, S.K.; Lv, L.; Yao, J.; Ireland, J.J.; Smith, G.W. Regulation and Regulatory Role of WNT Signaling in Potentiating FSH Action during Bovine Dominant Follicle Selection. PLoS ONE 2014, 9, e0100201. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.-A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of Folliculogenesis and the Determination of Ovulation Rate in Ruminants. Reprod. Fertil. Dev. 2011, 23, 444. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.S.; Singh, J.; Adams, G.P. Developmental Pattern of Small Antral Follicles in the Bovine Ovary. Biol. Reprod. 2004, 71, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Jaiswal, R.; Singh, J.; Malhi, P. Progress in Understanding Ovarian Follicular Dynamics in Cattle. Theriogenology 2008, 69, 72–80. [Google Scholar] [CrossRef]
- Evans, A.C.O.; Fortune, J.E. Selection of the Dominant Follicle in Cattle Occurs in the Absence of Differences in the Expression of Messenger Ribonucleic Acid for Gonadotropin Receptors. Endocrinology 1997, 138, 2963–2971. [Google Scholar] [CrossRef][Green Version]
- Beg, M.A.; Bergfelt, D.R.; Kot, K.; Ginther, O.J. Follicle Selection in Cattle: Dynamics of Follicular Fluid Factors during Development of Follicle Dominance 1. Biol. Reprod. 2002, 126, 120–126. [Google Scholar] [CrossRef]
- Ginther, O.J.; Kot, K.; Kulick, L.J.; Wiltbank, M.C. Emergence and Deviation of Follicles during the Development of Follicular Waves in Cattle. Theriogenology 1997, 48, 75–87. [Google Scholar] [CrossRef]
- Ferreira, R.; Gasperin, B.; Santos, J.; Rovani, M.; Santos, R.A.; Gutierrez, K.; Oliveira, J.F.; Reis, A.M.; Gonçalves, P.B. Angiotensin II Profile and MRNA Encoding RAS Proteins during Bovine Follicular Wave. JRAAS—J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 475–482. [Google Scholar] [CrossRef]
- Conley, A.J.; Bird, I.M. The Role of Cytochrome P450 17α-Hydroxylase and 3β-Hydroxysteroid Dehydrogenase in the Integration of Gonadal and Adrenal Steroidogenesis via the Δ5 and Δ4 Pathways of Steroidogenesis in Mammals. Biol. Reprod. 1997, 56, 789–799. [Google Scholar] [CrossRef]
- Lapointe, E.; Boerboom, D. WNT Signaling and the Regulation of Ovarian Steroidogenesis. Front. Biosci.—Sch. 2011, 3, 276–285. [Google Scholar] [CrossRef]
- Silva, J.M.; Price, C.A. Effect of Follicle-Stimulating Hormone on Steroid Secretion and Messenger Ribonucleic Acids Encoding Cytochromes P450 Aromatase and Cholesterol Side-Chain Cleavage in Bovine Granulosa Cells In Vitro. Biol. Reprod. 2000, 62, 186–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, J.M.; Hamel, M.; Sahmi, M.; Price, C.A. Control of Oestradiol Secretion and of Cytochrome P450 Aromatase Messenger Ribonucleic Acid Accumulation by FSH Involves Different Intracellular Pathways in Oestrogenic Bovine Granulosa Cells in Vitro. Reproduction 2006, 132, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Johnson, R.L. Hippo Signaling: Growth Control and Beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo Pathway Regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Lv, X.; He, C.; Huang, C.; Wang, H.; Hua, G.; Wang, Z.; Zhou, J.; Chen, X.; Ma, B.; Timm, B.K.; et al. Timely Expression and Activation of YAP1 in Granulosa Cells Is Essential for Ovarian Follicle Development. FASEB J. 2019, 39, 10049–10064. [Google Scholar] [CrossRef]
- Harlow, C.R.; Davidson, L.; Burns, K.H.; Changning, Y.; Matzuk, M.M.; Hillier, S.G. FSH and TGF-β Superfamily Members Regulate Granulosa Cell Connective Tissue Growth Factor Gene Expression in Vitro and in Vivo. Endocrinology 2002, 143, 3316–3325. [Google Scholar] [CrossRef]
- Han, P.; Relav, L.; Price, C.A. Regulation of the Early Growth Response-1 Binding Protein NAB2 in Bovine Granulosa Cells and Effect on Connective Tissue Growth Factor Expression. Mol. Cell. Endocrinol. 2020, 518, 111041. [Google Scholar] [CrossRef]
- Zamberlam, G.; Portela, V.; de Oliveira, J.F.C.; Gonçalves, P.B.D.; Price, C.A. Regulation of Inducible Nitric Oxide Synthase Expression in Bovine Ovarian Granulosa Cells. Mol. Cell. Endocrinol. 2011, 335, 189–194. [Google Scholar] [CrossRef]
- Cao, M.; Nicola, E.; Portela, V.M.; Price, C.A. Regulation of Serine Protease Inhibitor-E2 and Plasminogen Activator Expression and Secretion by Follicle Stimulating Hormone and Growth Factors in Non-Luteinizing Bovine Granulosa Cells in Vitro. Matrix Biol. 2006, 25, 342–354. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin Inhibits YAP Function through Up-Regulating 14-3-3σ Sequestering YAP in the Cytoplasm. Am. J. Cancer Res. 2016, 6, 27. [Google Scholar]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and Pharmacological Disruption of the TEAD–YAP Complex Suppresses the Oncogenic Activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gou, J.; Jia, J.; Yi, T.; Cui, T.; Li, Z. Verteporfin, a Suppressor of YAP–TEAD Complex, Presents Promising Antitumor Properties on Ovarian Cancer. Onco. Targets. Ther. 2016, 9, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, Z.; Zhou, Z.; Shen, H.C.; Yan, S.F.; Mayweg, A.V.; Xu, Z.; Qin, N.; Wong, J.C.; Zhang, Z.; et al. Structure-Based Design and Synthesis of Potent Cyclic Peptides Inhibiting the YAP-TEAD Protein-Protein Interaction. ACS Med. Chem. Lett. 2014, 5, 993–998. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, T.; Xu, Z.; Lin, Z.; Zhang, Z.; Feng, T.; Zhu, L.; Rong, Y.; Shen, H.; Luk, J.M.; et al. Targeting Hippo Pathway by Specific Interruption of YAP-TEAD Interaction Using Cyclic YAP-like Peptides. FASEB J. 2015, 29, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Hou, X.; Zhang, P.; Wang, C.; Davis, J.S. Yes-Associated Protein (YAP1) Is Required for Proliferation and Function of Bovine Granulosa Cells in Vitro. Biol. Reprod. 2019, 101, 1001–1017. [Google Scholar] [CrossRef]
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell Signal. 2006, 18, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Ranta, T.; Knecht, M.; Darbon, J.-M.; Baukal, A.J.; Catt, K.J. Induction of Granulosa Cell Differentiation by Forskolin: Stimulation of Adenosine 3′,5′-Monophosphate Production, Progesterone Synthesis, and Luteinizing Hormone Receptor Expression. Endocrinology 1984, 114, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Nivet, A.L.; Vigneault, C.; Blondin, P.; Sirard, M.A. Changes in Granulosa Cells’ Gene Expression Associated with Increased Oocyte Competence in Bovine. Reproduction 2013, 145, 555–565. [Google Scholar] [CrossRef]
- Zhang, B.; Tsang, P.C.W.; Pate, J.L.; Moses, M.A. A Role for Cysteine-Rich 61 in the Angiogenic Switch during the Estrous Cycle in Cows: Regulation by Prostaglandin F2alpha. Biol. Reprod. 2011, 85, 261. [Google Scholar] [CrossRef]
- Vernon, R.K.; Spicer, L.J. Effects of Basic Fibroblast Growth Factor and Heparin on Follicle-Stimulating Hormone-Induced Steroidogenesis by Bovine Granulosa Cells. J. Anim. Sci. 1994, 72, 2696–2702. [Google Scholar] [CrossRef]
- Portela, V.M.; Dirandeh, E.; Guerrero-Netro, H.M.; Zamberlam, G.; Barreta, M.H.; Goetten, A.F.; Price, C.A. The Role of Fibroblast Growth Factor-18 in Follicular Atresia in Cattle. Biol. Reprod. 2015, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Matteri, R.L.; Kastelic, J.P.; Ko, J.C.; Ginther, O.J. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J Reprod Fertil. 1992, 94, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F. Morphological Classification of Bovine Ovarian Follicles. Reproduction 2010, 139, 309–318. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C. HipKAWAMURA, Kazuhiro e Colab. Hippo Signaling Disruption and Akt Stimulation of Ovarian Follicles for Infertility Treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Godin, P.; Tsoi, M.F.; Morin, M.; Gévry, N.; Boerboom, D. The Granulosa Cell Response to Luteinizing Hormone Is Partly Mediated by YAP1-Dependent Induction of Amphiregulin. Cell Commun. Signal. 2022, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.C.; Lalonde-Larue, A.; Antoniazzi, A.Q.; Barreta, M.H.; Price, C.A.; Dias Gonçalves, P.B.; Portela, V.M.; Zamberlam, G. YAP Signaling in Preovulatory Granulosa Cells Is Critical for the Functioning of the EGF Network during Ovulation. Mol. Cell. Endocrinol. 2022, 541, 111524. [Google Scholar] [CrossRef]
- Mazerbourg, S.; Bondy, C.A.; Zhou, J.; Monget, P. The Insulin-like Growth Factor System: A Key Determinant Role in the Growth and Selection of Ovarian Follicles? A Comparative Species Study. Reprod. Domest. Anim. 2003, 38, 247–258. [Google Scholar] [CrossRef]
- Spicer, L.J.; Aad, P.Y. Insulin-Like Growth Factor (IGF) 2 Stimulates Steroidogenesis and Mitosis of Bovine Granulosa Cells through the IGF1 Receptor: Role of Follicle-Stimulating Hormone and IGF2 Receptor. Biol. Reprod. 2007, 77, 18–27. [Google Scholar] [CrossRef]
- Portela, M.; Ph, D.; Zamberlam, G.; Sc, M.; Price, C.A. Cell Plating Density Alters the Ratio of Estrogenic to Progestagenic Enzyme Gene Expression in Cultured Granulosa Cells. Fertil. Steril. 2010, 93, 2050–2055. [Google Scholar] [CrossRef]
- Sahmi, M.; Nicola, E.S.; Silva, J.M.; Price, C.A. Expression of 17β- and 3β-Hydroxysteroid Dehydrogenases and Steroidogenic Acute Regulatory Protein in Non-Luteinizing Bovine Granulosa Cells in Vitro. Mol. Cell. Endocrinol. 2004, 223, 43–54. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Portela, V.M.; Dos Santos, E.C.; Missio, D.; de Andrade, L.G.; da Silva, Z.; Gasperin, B.G.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; Zamberlam, G. The Hippo Pathway Effectors YAP and TAZ Interact with EGF-like Signaling to Regulate Expansion-Related Events in Bovine Cumulus Cells in Vitro. J. Assist. Reprod. Genet. 2022, 39, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Portela, V.M.; Machado, M.; Jr, J.B.; Zamberlam, G.; Amorim, R.L.; Goncalves, P.; Price, C.A.; De Fisiologia, D.; Biocie, I. De Expression and Function of Fibroblast Growth Factor 18 in the Ovarian Follicle in Cattle 1. Biol. Reprod. 2010, 346, 339–346. [Google Scholar] [CrossRef] [PubMed]



| Name of Antibody | Manufacturer (Cat. No.) | Type | Dilution WB | Dilution IHC |
|---|---|---|---|---|
| ß-actin (C4) | Santa Cruz (sc-47778 HRP) | CkM | 1:10,000 | |
| YAP (D8H1X) | Cell signaling (14074) | RbM | 1:1000 | 1:250 |
| Phospho-YAP (Ser127) (D9W2I) | Cell signaling (13008) | RbM | 1:1000 | 1:250 |
| Anti-Rabbit IgG–HRP Conjugate | Promega (W401B) | Rb | 1:1000 |
| Gene | Sequence 5′→3′ | Accession Number |
|---|---|---|
| ANKRD1 | F: ATCAGTGCGCGGGATAAGTT | NM_001034378.2 |
| R: GGGAGTATCTCCTTCCCGGT | ||
| CTGF | F: AGCTGAGCGAGTTGTGTACC | [42] |
| R: TCCGAAAATGTAGGGGGCAC | ||
| CYP19A1 | F: CTGAAGCAACAGGAGTCCTAAATGTACA | [43] |
| R: AATGAGGGGCCCAATTCCCAGA | ||
| CYR61 | F: GGCTCCCCGTTTTGGAATG | NM_001034340.2 |
| R: TCATTGGTAACGCGTGTGGA | ||
| GAPDH | F: GATTGTCAGCAATGCCTCCT | [36] |
| R: CGTTCTCTGCCTTGACTGTG | ||
| H2AFZ | F: GAGGAGCTGAACAAGCTGTTG | [43] |
| R: TTGTGGTGGCTCTCAGTCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, L.G.; Portela, V.M.; Dos Santos, E.C.; Aires, K.d.V.; Ferreira, R.; Missio, D.; da Silva, Z.; Koch, J.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; et al. FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity. Int. J. Mol. Sci. 2022, 23, 14160. https://doi.org/10.3390/ijms232214160
de Andrade LG, Portela VM, Dos Santos EC, Aires KdV, Ferreira R, Missio D, da Silva Z, Koch J, Antoniazzi AQ, Gonçalves PBD, et al. FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity. International Journal of Molecular Sciences. 2022; 23(22):14160. https://doi.org/10.3390/ijms232214160
Chicago/Turabian Stylede Andrade, Leonardo Guedes, Valério Marques Portela, Esdras Corrêa Dos Santos, Karine de Vargas Aires, Rogério Ferreira, Daniele Missio, Zigomar da Silva, Júlia Koch, Alfredo Quites Antoniazzi, Paulo Bayard Dias Gonçalves, and et al. 2022. "FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity" International Journal of Molecular Sciences 23, no. 22: 14160. https://doi.org/10.3390/ijms232214160
APA Stylede Andrade, L. G., Portela, V. M., Dos Santos, E. C., Aires, K. d. V., Ferreira, R., Missio, D., da Silva, Z., Koch, J., Antoniazzi, A. Q., Gonçalves, P. B. D., & Zamberlam, G. (2022). FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity. International Journal of Molecular Sciences, 23(22), 14160. https://doi.org/10.3390/ijms232214160







