Targeted Disruption of Lats1 and Lats2 in Mice Impairs Testis Development and Alters Somatic Cell Fate
Abstract
1. Introduction
2. Results
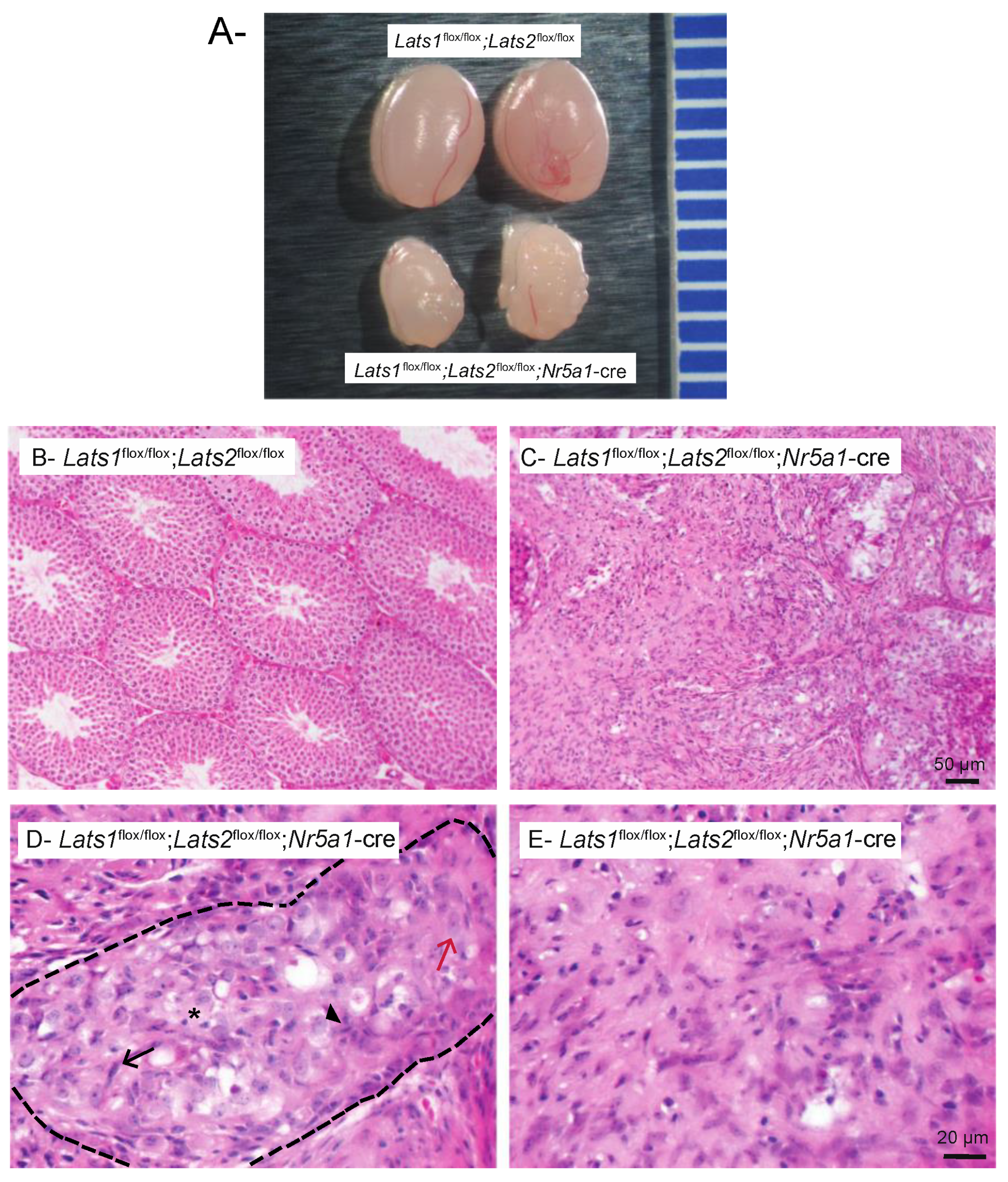
2.1. Testicular Dysgenesis Is Observed following Concomitant Deletion of Lats1 and Lats2 in Testis Somatic Cells
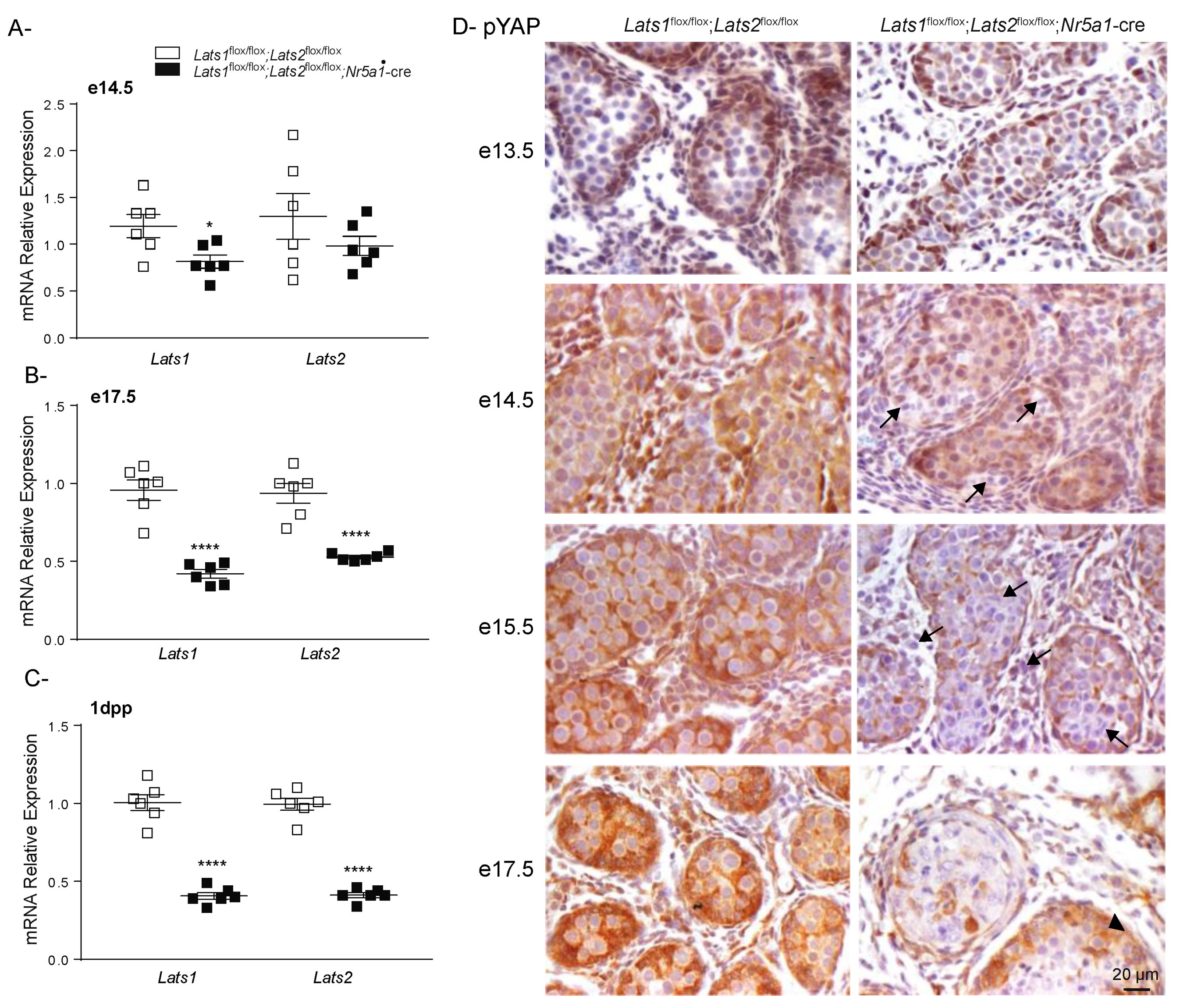
2.2. Testicular Dysgenesis Is First Observed at e14.5 in Lats1flox/flox;Lats2flox/flox;Nr5a1-cre Mice
2.3. Hippo Signaling Is Inactivated in Late Stages of Testicular Differentiation
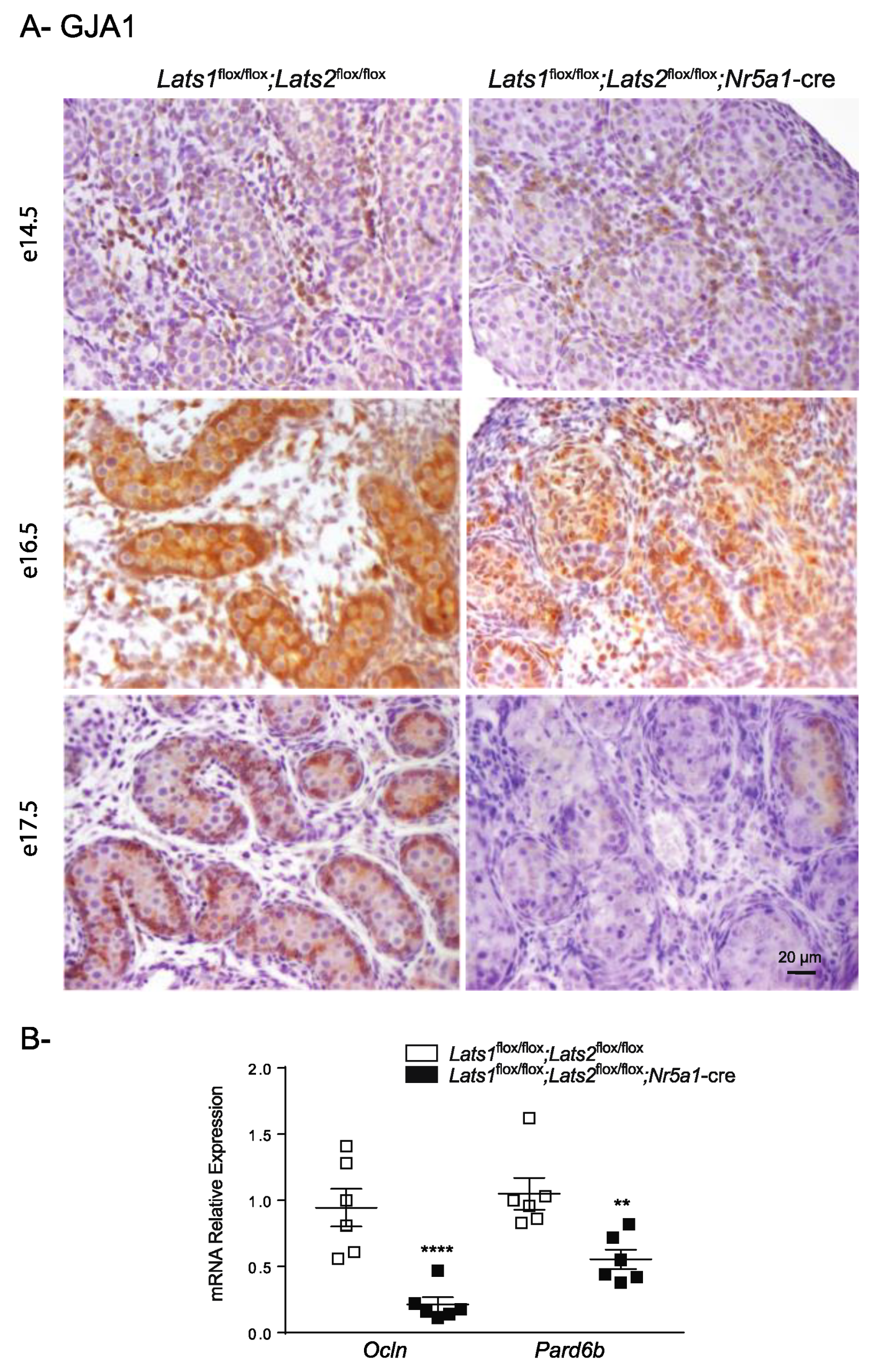
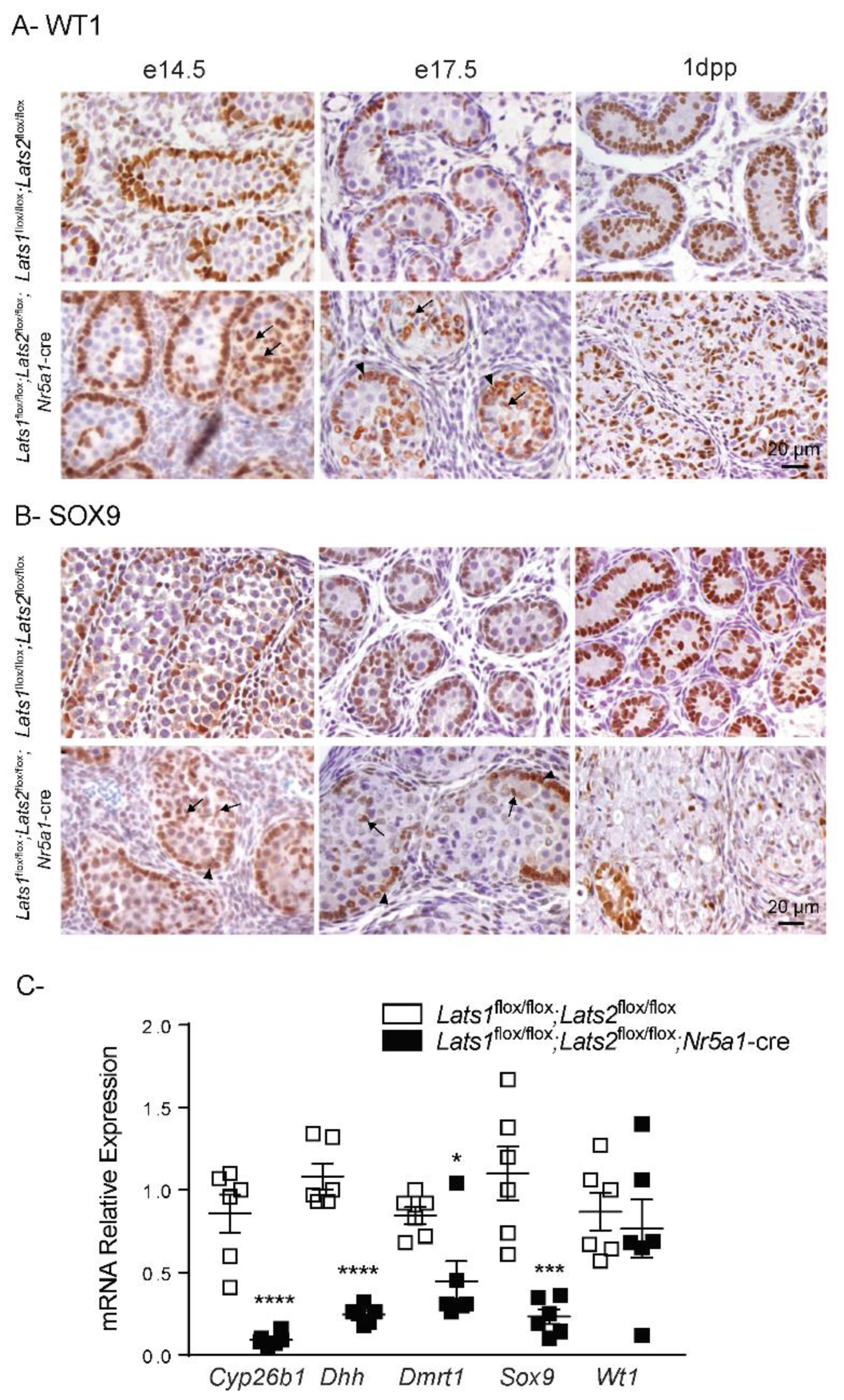
2.4. Disruption of Lats1 and Lats2 Alters the Epithelization and the Differentiation of the Sertoli Cells during Testis Development
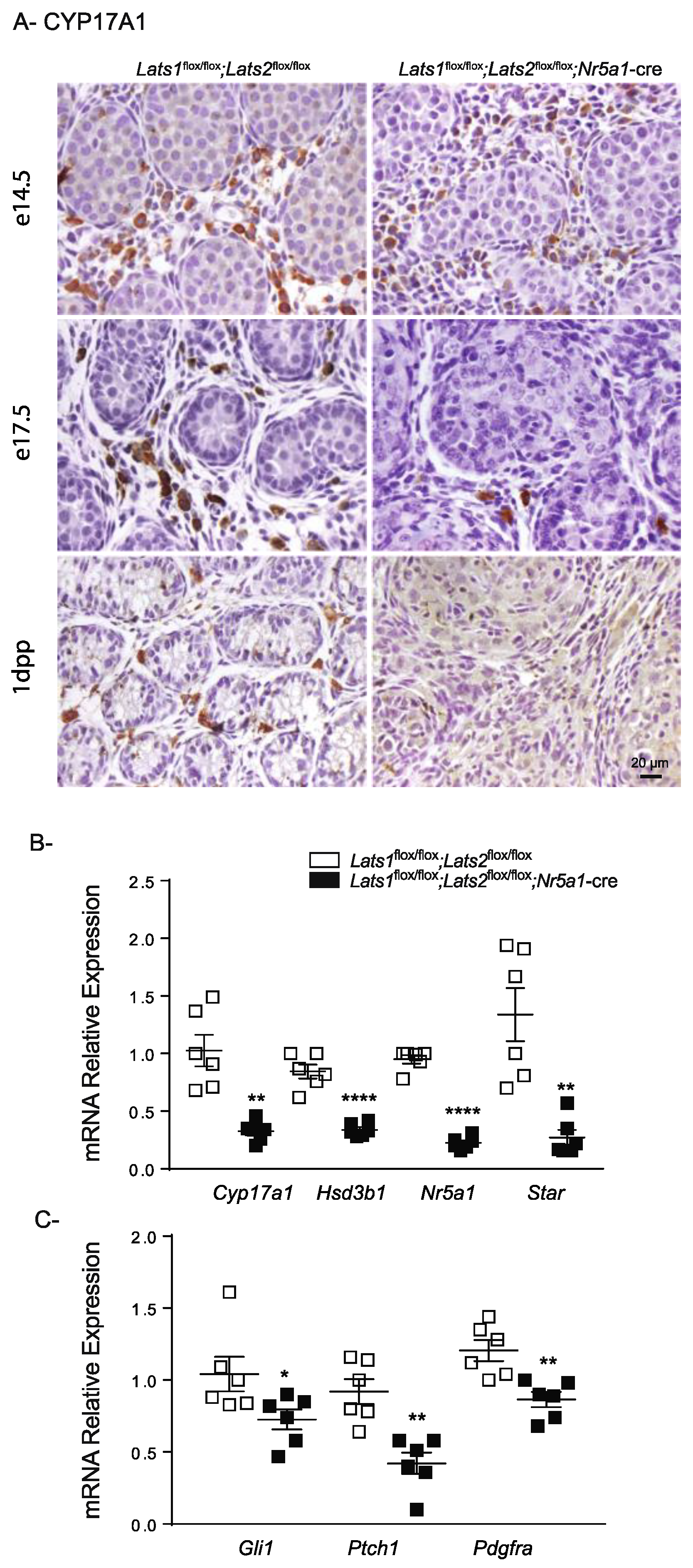
2.5. Disruption of Lats1 and Lats2 Causes the Loss of Steroidogenic Markers and Leads to Fibrosis of the Testis Interstitium
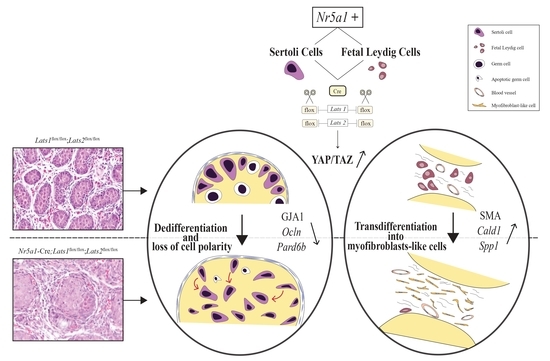
3. Discussion
4. Material and Methods
4.1. Ethics
4.2. Transgenic Mouse Strains
4.3. Tissue Collection
4.4. Histopathology and Immunohistochemistry
4.5. Reverse-Transcription-Quantitative PCR
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatano, O.; Takakusu, A.; Nomura, M.; Morohashi, K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1996, 1, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, R.; Sacco, S.; Vidal, V.P.; Chaboissier, M.C.; Schedl, A. Steroidogenic organ development and homeostasis: A WT1-centric view. Mol. Cell. Endocrinol. 2015, 408, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Val, P.; Martinez-Barbera, J.P.; Swain, A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 2007, 134, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.H.; Eicher, E.M. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 2001, 240, 92–107. [Google Scholar] [CrossRef]
- Schmahl, J.; Eicher, E.M.; Washburn, L.L.; Capel, B. Sry induces cell proliferation in the mouse gonad. Development 2000, 127, 65–73. [Google Scholar] [CrossRef]
- Chaboissier, M.C.; Kobayashi, A.; Vidal, V.I.; Lützkendorf, S.; van de Kant, H.J.; Wegner, M.; de Rooij, D.G.; Behringer, R.R.; Schedl, A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 2004, 131, 1891–1901. [Google Scholar] [CrossRef]
- Kim, Y.; Kobayashi, A.; Sekido, R.; DiNapoli, L.; Brennan, J.; Chaboissier, M.C.; Poulat, F.; Behringer, R.R.; Lovell-Badge, R.; Capel, B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006, 4, e187. [Google Scholar] [CrossRef]
- Schmahl, J.; Kim, Y.; Colvin, J.S.; Ornitz, D.M.; Capel, B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 2004, 131, 3627–3636. [Google Scholar] [CrossRef]
- Clark, A.M.; Garland, K.K.; Russell, L.D. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 2000, 63, 1825–1838. [Google Scholar] [CrossRef]
- Pierucci-Alves, F.; Clark, A.M.; Russell, L.D. A developmental study of the Desert hedgehog-null mouse testis. Biol. Reprod. 2001, 65, 1392–1402. [Google Scholar] [CrossRef]
- Yao, H.H.; Whoriskey, W.; Capel, B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002, 16, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rodriguez, K.; Yao, H.H. Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development 2016, 143, 3700–3710. [Google Scholar] [CrossRef] [PubMed]
- Stévant, I.; Neirijnck, Y.; Borel, C.; Escoffier, J.; Smith, L.B.; Antonarakis, S.E.; Dermitzakis, E.T.; Nef, S. Deciphering Cell Lineage Specification during Male Sex Determination with Single-Cell RNA Sequencing. Cell Rep. 2018, 22, 1589–1599. [Google Scholar] [CrossRef]
- Stévant, I.; Kühne, F.; Greenfield, A.; Chaboissier, M.C.; Dermitzakis, E.T.; Nef, S. Dissecting Cell Lineage Specification and Sex Fate Determination in Gonadal Somatic Cells Using Single-Cell Transcriptomics. Cell Rep. 2019, 26, 3272–3283.e3273. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, R.; Fraizer, G.C.; Trapman, J.; Lau Yf, C.; Saunders, G.F. The Wilms’ tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Müllerian-inhibiting substance, and the androgen receptor. Clin. Cancer Res. 1997, 3, 2571–2580. [Google Scholar]
- Bradford, S.T.; Wilhelm, D.; Bandiera, R.; Vidal, V.; Schedl, A.; Koopman, P. A cell-autonomous role for WT1 in regulating Sry in vivo. Hum. Mol. Genet. 2009, 18, 3429–3438. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Zheng, Q.S.; Li, X.X.; Hu, Z.Y.; Gao, F.; Cheng, C.Y.; Liu, Y.X. Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1131–E1143. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Wen, Q.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Cui, X.; Yang, L.; Huff, V.; et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc. Natl. Acad. Sci. USA 2015, 112, 4003–4008. [Google Scholar] [CrossRef]
- Luo, X.; Ikeda, Y.; Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994, 77, 481–490. [Google Scholar] [CrossRef]
- Tevosian, S.G.; Albrecht, K.H.; Crispino, J.D.; Fujiwara, Y.; Eicher, E.M.; Orkin, S.H. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 2002, 129, 4627–4634. [Google Scholar] [CrossRef]
- Hu, Y.C.; Okumura, L.M.; Page, D.C. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013, 9, e1003629. [Google Scholar] [CrossRef] [PubMed]
- Viger, R.S.; de Mattos, K.; Tremblay, J.J. Insights Into the Roles of GATA Factors in Mammalian Testis Development and the Control of Fetal Testis Gene Expression. Front. Endocrinol. 2022, 13, 902198. [Google Scholar] [CrossRef] [PubMed]
- Nef, S.; Verma-Kurvari, S.; Merenmies, J.; Vassalli, J.D.; Efstratiadis, A.; Accili, D.; Parada, L.F. Testis determination requires insulin receptor family function in mice. Nature 2003, 426, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, J.L.; Calvel, P.; Romero, Y.; Conne, B.; Truong, V.; Papaioannou, M.D.; Schaad, O.; Docquier, M.; Herrera, P.L.; Wilhelm, D.; et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013, 9, e1003160. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Tanaka, S.S.; Yamaguchi, Y.L.; Kobayashi, H.; Kuroki, S.; Tachibana, M.; Shinomura, M.; Kanai, Y.; Morohashi, K.; Kawakami, K.; et al. Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev. Cell 2013, 26, 416–430. [Google Scholar] [CrossRef]
- Garcia-Moreno, S.A.; Lin, Y.T.; Futtner, C.R.; Salamone, I.M.; Capel, B.; Maatouk, D.M. CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet. 2019, 15, e1007895. [Google Scholar] [CrossRef]
- Katoh-Fukui, Y.; Miyabayashi, K.; Komatsu, T.; Owaki, A.; Baba, T.; Shima, Y.; Kidokoro, T.; Kanai, Y.; Schedl, A.; Wilhelm, D.; et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology 2012, 153, 913–924. [Google Scholar] [CrossRef]
- Maugeri-Sacca, M.; De Maria, R. The Hippo pathway in normal development and cancer. Pharmacol. Ther. 2018, 186, 60–72. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Yuan, H.X.; Plouffe, S.W.; Moon, S.; Kim, W.; Jho, E.H.; Guan, K.L. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 2017, 18, 72–86. [Google Scholar] [CrossRef]
- Moon, S.; Kim, W.; Kim, S.; Kim, Y.; Song, Y.; Bilousov, O.; Kim, J.; Lee, T.; Cha, B.; Kim, M.; et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017, 18, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wehling, L.; Keegan, L.; Fernández-Palanca, P.; Hassan, R.; Ghallab, A.; Schmitt, J.; Schirmacher, P.; Kummer, U.; Hengstler, J.G.; Sahle, S.; et al. Spatial modeling reveals nuclear phosphorylation and subcellular shuttling of YAP upon drug-induced liver injury. bioRxiv 2022. [Google Scholar] [CrossRef]
- Li, W.; Cooper, J.; Zhou, L.; Yang, C.; Erdjument-Bromage, H.; Zagzag, D.; Snuderl, M.; Ladanyi, M.; Hanemann, C.O.; Zhou, P.; et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 2014, 26, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014, 141, 1614–1626. [Google Scholar] [CrossRef]
- Sen Sharma, S.; Majumdar, S.S. Transcriptional co-activator YAP regulates cAMP signaling in Sertoli cells. Mol. Cell. Endocrinol. 2017, 450, 64–73. [Google Scholar] [CrossRef]
- Sen Sharma, S.; Vats, A.; Majumdar, S. Regulation of Hippo pathway components by FSH in testis. Reprod. Biol. 2019, 19, 61–66. [Google Scholar] [CrossRef]
- Badr, G.; Abdel-Tawab, H.S.; Ramadan, N.K.; Ahmed, S.F.; Mahmoud, M.H. Protective effects of camel whey protein against scrotal heat-mediated damage and infertility in the mouse testis through YAP/Nrf2 and PPAR-gamma signaling pathways. Mol. Reprod. Dev. 2018, 85, 505–518. [Google Scholar] [CrossRef]
- Levasseur, A.; Paquet, M.; Boerboom, D.; Boyer, A. Yes-associated protein and WW-containing transcription regulator 1 regulate the expression of sex-determining genes in Sertoli cells, but their inactivation does not cause sex reversal. Biol. Reprod. 2017, 97, 162–175. [Google Scholar] [CrossRef]
- Tsoi, M.; Morin, M.; Rico, C.; Johnson, R.L.; Paquet, M.; Gévry, N.; Boerboom, D. Lats1 and Lats2 are required for ovarian granulosa cell fate maintenance. FASEB J. 2019, 33, 10819–10832. [Google Scholar] [CrossRef]
- Chi, X.; Luo, W.; Song, J.; Li, B.; Su, T.; Yu, M.; Wang, T.; Wang, Z.; Liu, C.; Li, Z.; et al. Kindlin-2 in Sertoli cells is essential for testis development and male fertility in mice. Cell Death Dis. 2021, 12, 604. [Google Scholar] [CrossRef]
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; McGovern, R.A.; Tang, V.; Kenny, C.D.; Christiansen, L.M.; White, R.D.; Edelstein, E.A.; et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006, 49, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ménard, A.; Abou Nader, N.; Levasseur, A.; St-Jean, G.; Le Roy, M.L.G.; Boerboom, D.; Benoit-Biancamano, M.O.; Boyer, A. Targeted Disruption of Lats1 and Lats2 in Mice Impairs Adrenal Cortex Development and Alters Adrenocortical Cell Fate. Endocrinology 2020, 161, bqaa052. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Shen, W.H.; Ingraham, H.A.; Parker, K.L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Carette, D.; Weider, K.; Gilleron, J.; Giese, S.; Dompierre, J.; Bergmann, M.; Brehm, R.; Denizot, J.P.; Segretain, D.; Pointis, G. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev. Biol. 2010, 346, 54–67. [Google Scholar] [CrossRef]
- Sridharan, S.; Simon, L.; Meling, D.D.; Cyr, D.G.; Gutstein, D.E.; Fishman, G.I.; Guillou, F.; Cooke, P.S. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 2007, 76, 804–812. [Google Scholar] [CrossRef]
- Inoue, M.; Shima, Y.; Miyabayashi, K.; Tokunaga, K.; Sato, T.; Baba, T.; Ohkawa, Y.; Akiyama, H.; Suyama, M.; Morohashi, K. Isolation and Characterization of Fetal Leydig Progenitor Cells of Male Mice. Endocrinology 2016, 157, 1222–1233. [Google Scholar] [CrossRef]
- McClelland, K.S.; Bell, K.; Larney, C.; Harley, V.R.; Sinclair, A.H.; Oshlack, A.; Koopman, P.; Bowles, J. Purification and Transcriptomic Analysis of Mouse Fetal Leydig Cells Reveals Candidate Genes for Specification of Gonadal Steroidogenic Cells. Biol. Reprod. 2015, 92, 145. [Google Scholar] [CrossRef]
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 17, 800–810. [Google Scholar] [CrossRef]
- Karl, J.; Capel, B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol. 1998, 203, 323–333. [Google Scholar] [CrossRef]
- Bingham, N.C.; Verma-Kurvari, S.; Parada, L.F.; Parker, K.L. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 2006, 44, 419–424. [Google Scholar] [CrossRef]
- Xie, D.; Cui, J.; Xia, T.; Jia, Z.; Wang, L.; Wei, W.; Zhu, A.; Gao, Y.; Xie, K.; Quan, M. Hippo transducer TAZ promotes epithelial mesenchymal transition and supports pancreatic cancer progression. Oncotarget 2015, 6, 35949–35963. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Zhang, R.; Liang, Z.; Liao, W.; Du, Z.; Gao, C.; Liu, F.; Fan, Y.; Hong, H. Hippo pathway contributes to cisplatin resistant-induced EMT in nasopharyngeal carcinoma cells. Cell Cycle 2017, 16, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef]
- Shao, D.D.; Xue, W.; Krall, E.B.; Bhutkar, A.; Piccioni, F.; Wang, X.; Schinzel, A.C.; Sood, S.; Rosenbluh, J.; Kim, J.W.; et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 2014, 158, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, K.; Hu, Y.; Guo, X.; Wang, D.; Shi, W.; Li, J.; Song, J. YAP modulates TGF-beta1-induced simultaneous apoptosis and EMT through upregulation of the EGF receptor. Sci. Rep. 2017, 7, 45523. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, D.H.; Shah, S.R.; Kim, H.N.; Kshitiz; Kim, P.; Quinones-Hinojosa, A.; Levchenko, A. Switch-like enhancement of epithelial-mesenchymal transition by YAP through feedback regulation of WT1 and Rho-family GTPases. Nat. Commun. 2019, 10, 2797. [Google Scholar] [CrossRef] [PubMed]
- Nel-Themaat, L.; Jang, C.W.; Stewart, M.D.; Akiyama, H.; Viger, R.S.; Behringer, R.R. Sertoli cell behaviors in developing testis cords and postnatal seminiferous tubules of the mouse. Biol. Reprod. 2011, 84, 342–350. [Google Scholar] [CrossRef]
- Armstrong, J.F.; Pritchard-Jones, K.; Bickmore, W.A.; Hastie, N.D.; Bard, J.B. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 1993, 40, 85–97. [Google Scholar] [CrossRef]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 is required for early kidney development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Reginensi, A.; Scott, R.P.; Gregorieff, A.; Bagherie-Lachidan, M.; Chung, C.; Lim, D.S.; Pawson, T.; Wrana, J.; McNeill, H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013, 9, e1003380. [Google Scholar] [CrossRef] [PubMed]
- McNeill, H.; Reginensi, A. Lats1/2 Regulate Yap/Taz to Control Nephron Progenitor Epithelialization and Inhibit Myofibroblast Formation. J. Am. Soc. Nephrol. 2017, 28, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Miyabayashi, K.; Sato, T.; Suyama, M.; Ohkawa, Y.; Doi, M.; Okamura, H.; Suzuki, K. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development 2018, 145, dev169136. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Byun, J.; Del Re, D.P.; Zhai, P.; Ikeda, S.; Shirakabe, A.; Mizushima, W.; Miyamoto, S.; Brown, J.H.; Sadoshima, J. Yes-associated protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload. J. Biol. Chem. 2019, 294, 3603–3617. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Grahammer, F.; Hoppe, A.-K.; Kohli, P.; Hagmann, H.; Kretz, O.; Bertsch, S.; Höhne, M.; Göbel, H.; Bartram, M.P.; et al. YAP-mediated mechanotransduction determines the podocyte’s response to damage. Sci. Signal. 2017, 10, eaaf8165. [Google Scholar] [CrossRef]
- St-Jean, G.; Tsoi, M.; Abedini, A.; Levasseur, A.; Rico, C.; Morin, M.; Djordjevic, B.; Miinalainen, I.; Kaarteenaho, R.; Paquet, M.; et al. Lats1 and Lats2 are required for the maintenance of multipotency in the Mullerian duct mesenchyme. Development 2019, 146, dev180430. [Google Scholar] [CrossRef]
- Sonnylal, S.; Shi-Wen, X.; Leoni, P.; Naff, K.; Van Pelt, C.S.; Nakamura, H.; Leask, A.; Abraham, D.; Bou-Gharios, G.; de Crombrugghe, B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010, 62, 1523–1532. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Nakamura, M.; Lipson, K.E.; Miyake, T.; Kamikawa, Y.; Sagara, A.; Shinozaki, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017, 7, 5392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Z.; Liu, H.; Ren, Y.; Shao, D.; Zhang, W.; Lin, J.; Wolfram, J.; Wang, F.; Nie, S. Connective tissue growth factor stimulates the proliferation, migration and differentiation of lung fibroblasts during paraquat-induced pulmonary fibrosis. Mol. Med. Rep. 2015, 12, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Sonnylal, S.; Xu, S.; Jones, H.; Tam, A.; Sreeram, V.R.; Ponticos, M.; Norman, J.; Agrawal, P.; Abraham, D.; de Crombrugghe, B. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J. Cell Sci. 2013, 126, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Garrett, Q.; Khaw, P.T.; Blalock, T.D.; Schultz, G.S.; Grotendorst, G.R.; Daniels, J.T. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Grotendorst, G.R.; Rahmanie, H.; Duncan, M.R. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004, 18, 469–479. [Google Scholar] [CrossRef]
- Archambeault, D.R.; Yao, H.H. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 10526–10531. [Google Scholar] [CrossRef]
- Taylor, C.; Loomans, H.A.; Le Bras, G.F.; Koumangoye, R.B.; Romero-Morales, A.I.; Quast, L.L.; Zaika, A.I.; El-Rifai, W.; Andl, T.; Andl, C.D. Activin a signaling regulates cell invasion and proliferation in esophageal adenocarcinoma. Oncotarget 2015, 6, 34228–34244. [Google Scholar] [CrossRef]
- Torigata, K.; Daisuke, O.; Mukai, S.; Hatanaka, A.; Ohka, F.; Motooka, D.; Nakamura, S.; Ohkawa, Y.; Yabuta, N.; Kondo, Y.; et al. LATS2 Positively Regulates Polycomb Repressive Complex 2. PLoS ONE 2016, 11, e0158562. [Google Scholar] [CrossRef]
- Britschgi, A.; Duss, S.; Kim, S.; Couto, J.P.; Brinkhaus, H.; Koren, S.; De Silva, D.; Mertz, K.D.; Kaup, D.; Varga, Z.; et al. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature 2017, 541, 541–545. [Google Scholar] [CrossRef]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Nader, N.; Ménard, A.; Levasseur, A.; St-Jean, G.; Boerboom, D.; Zamberlam, G.; Boyer, A. Targeted Disruption of Lats1 and Lats2 in Mice Impairs Testis Development and Alters Somatic Cell Fate. Int. J. Mol. Sci. 2022, 23, 13585. https://doi.org/10.3390/ijms232113585
Abou Nader N, Ménard A, Levasseur A, St-Jean G, Boerboom D, Zamberlam G, Boyer A. Targeted Disruption of Lats1 and Lats2 in Mice Impairs Testis Development and Alters Somatic Cell Fate. International Journal of Molecular Sciences. 2022; 23(21):13585. https://doi.org/10.3390/ijms232113585
Chicago/Turabian StyleAbou Nader, Nour, Amélie Ménard, Adrien Levasseur, Guillaume St-Jean, Derek Boerboom, Gustavo Zamberlam, and Alexandre Boyer. 2022. "Targeted Disruption of Lats1 and Lats2 in Mice Impairs Testis Development and Alters Somatic Cell Fate" International Journal of Molecular Sciences 23, no. 21: 13585. https://doi.org/10.3390/ijms232113585
APA StyleAbou Nader, N., Ménard, A., Levasseur, A., St-Jean, G., Boerboom, D., Zamberlam, G., & Boyer, A. (2022). Targeted Disruption of Lats1 and Lats2 in Mice Impairs Testis Development and Alters Somatic Cell Fate. International Journal of Molecular Sciences, 23(21), 13585. https://doi.org/10.3390/ijms232113585