Abstract
Diabetes mellitus is associated with a high risk of developing gastric cancer (GC). Metformin, which is conventionally used to treat type 2 diabetes, induces AMP-activated protein kinase signaling and suppresses gluconeogenesis. Recent studies have reported that metformin is associated with beneficial effects in cancer prevention and treatment owing to its anti-tumor effects. This makes metformin a potential medication for GC therapy. However, contradicting reports have emerged regarding the efficacy of metformin in reducing the risk of GC. This review summarizes the impact of metformin on mitigating GC risk by analyzing clinical databases. The mechanism underlying the anti-tumor effect of metformin on GC is also discussed.
1. Introduction
Gastric cancer (GC) is a global health issue with over a million cases and 700,000 deaths worldwide by 2020 according to GLOBOCAN [1]. Risk factors for the disease include age, sex, genetics, tobacco smoking, and high nitrate or nitrite diet [2]. Nevertheless, Helicobacter pylori (H. pylori) infection remains the primary risk factor for the onset of GC in more than 89% of cases worldwide [3]. H. pylori is usually acquired at a young age; if left untreated it persists lifelong. Advances in adjuvant and neoadjuvant chemotherapy, targeted therapy, and immunotherapy have also improved the outcomes of advanced GC. Despite the decline in the incidence and mortality rates of GC, various treatment options need to be explored as the currently available treatments are flawed. Surgical resection of gastric tumor is either invasive or unviable if the diagnosis is made at a late stage when the tumor has metastasized—as is often the case [4]. In such cases, chemoradiation and cytotoxic therapy are the only options available; however, they are associated with adverse effects [5].
Similar to GC, diabetes mellitus (DM) is an increasingly prevalent global disease [6]. Previous studies have demonstrated that DM predisposes patients to a higher risk of cardiovascular disease and cancer development [7]. The association between GC and DM remains unclear because contradictory results have been reported. A previous study reported that DM increases the incidence of GC by 67% [8]. DM is correlated with higher mortality rates in patients with GC [7]. Several mechanisms through which DM promotes tumor development such as hyperglycemia-induced DNA damage, increased production of reactive oxygen species (ROS), and stimulation of cell proliferation and angiogenesis due to altered glucose metabolism have been reported [7,8].
Metformin, a guanidine derivative, is commonly used for the treatment of type 2 diabetes (T2D) [9]. The inhibition of mitochondrial complex I and activation of AMP-activated protein kinase (AMPK) enables metformin to lower glucose levels with minimal adverse effects [10]. Its anti-tumor properties include decreasing ROS generation and inducing autophagy [10]. Additionally, metformin restores the microbiome diversity, which is closely linked to H. pylori colonization and GC progression [9]. Thus, metformin needs to be investigated as a potential intervention for GC, and its underlying mechanism in treating GC needs to be elucidated.
2. Metformin Mechanism of Action and Diabetes Mellitus
Although metformin is the drug of choice to initiate diabetes mellitus treatment, how it lowers hepatic glucose production to benefit diabetes is still not fully understood [11]. Metformin inhibits mitochondrial respiratory chain complex 1, leading to the suppression of gluconeogenesis, followed by alteration of hepatic energy and AMPK activation [12]. Reduced electron transport chain activity decreases ATP generation [13] as well as the ATP/ADP and ATP/AMP ratios. AMPK is a cellular energy sensor that is directly phosphorylated and activated by liver kinase B1 (LKB1) or calcium/calmodulin-dependent protein kinase 2 [14,15]. Additionally, AMPK was activated by an increase in the ADP/ATP and AMP/ATP ratios (Figure 1). Although the energy stress mechanism is assumed to be canonical, evidence of other ATP level-independent mechanisms is accumulating. Moreover, higher AMP levels in cells may also inhibit the key gluconeogenesis enzyme, fructose 1,6-phosphate (FBP1).
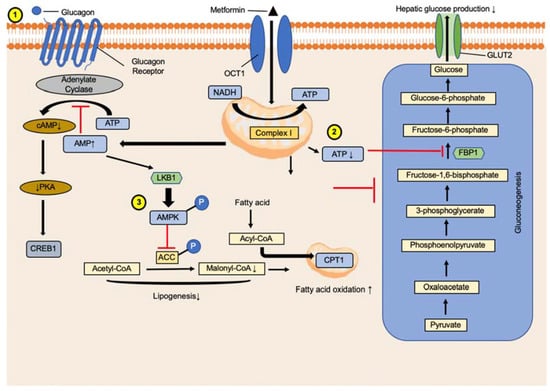
Figure 1.
Mechanism deciphering the role of metformin in glucagon signaling. (1) Metformin acts by suppressing glucagon signaling, leading to decreased cAMP production in hepatocytes and lower glucagon-dependent glucose output from hepatocytes. Reduced cAMP synthesis lowers the activity of gluconeogenic gene phosphorylation regulators cAMP responsive element binding protein 1 (CREB1) and triiodide (I3PR). (2) ATP reduction lowers gluconeogenesis and increases AMP levels, which inhibits the key gluconeogenic enzyme fructose-bisphosphatase 1 (FBP1). (3) The acetyl-CoA carboxylase (ACC) was phosphorylated and inhibited malonyl-CoA and acyl-CoA synthesis, increasing acyl-CoA content entering mitochondria and leading to the oxidation of fatty acid.
Shaw et al. indicated that in high-fat diet-fed mice, the deletion of LKB1 in the liver inhibited AMPK activity and led to hyperglycemia [16]. This study also showed that the LKB1-AMPK signaling pathway might repress the gluconeogenic transcriptional pathway by the phosphorylation and nuclear exclusion of the CREB-regulated transcription coactivator 2. However, a later study challenged the mechanism of transcriptional inhibition by AMPK activation. The results showed that metformin inhibition of gluconeogenesis was amplified in both LKB1- and AMPK-deficient hepatocytes [17]. Metformin affects the hepatic energy state and changes the NADH/NAD+ ratio, inhibiting glucose production.
Furthermore, Miller et al. suggested that metformin acts by suppressing glucagon signaling [12]. They reported that metformin could decrease cyclic 3′,5′-adenosine monophosphate (cAMP) production after glucagon stimulation in hepatocytes, thus blocking glucagon-dependent glucose output from hepatocytes. However, the relevance of glucagon in the mechanism of action of metformin is doubtful because of the very high dose used in the study (500 mg/kg).
Metformin-induced activation of AMPK in hepatocytes reduces acetyl-CoA carboxylase (ACC) activity and suppresses lipogenic transcription factor, sterol regulatory element-binding transcription factor 1 (SREBF1) expression, which may be linked to a reduced fatty liver [18]. Phosphorylation of ACC by AMPK reduces lipogenesis and promotes hepatic mitochondrial oxidation of fatty acids, resulting in a lipid-lowering and insulin-sensitizing effect exerted by metformin [19]. Therefore, AMPK is essential for metformin for the inhibition of glucose production by metformin in the hepatocytes.
Metformin has been demonstrated to inhibit small intestinal glucose absorption in T2D patients [20]. This action may be relevant to enhanced secretion of glucagon-like peptide 1 (GLP1) and regulation of gut microbiota [21]. Metformin inhibits glucose absorption in the proximal small intestine and increases glucose utilization in intestinal enterocytes via the recruitment of glucose transporter 2 to the apical membrane [22]. Metformin also, directly and indirectly, stimulates the secretion of GLP1 from L cells in the distal small intestinal mucosa. GLP1 is an incretin that acts through the gut–brain–liver neuronal axis to reduce hepatic glucose production. Furthermore, GLP1 enhanced glucose-stimulated insulin secretion and inhibited pancreatic glucagon secretion [11]. Through the reduction in ileal absorption, metformin increases the intestinal bile acid pool, which contributes to cholesterol homeostasis, glucose homeostasis, and elevated GLP1 levels [23,24]. In addition, metformin can slow gastric emptying, which plays an important role in the glucose-lowering effect after a meal [25]. It is well known that bile acids are key regulators of glucose metabolism. Metformin reduces gut bile acid resorption substantially and then exerts its glucose-lowering activity [26].
3. Clinical Use of Metformin
3.1. Metformin Use Reduces GC Risk in Patients with Diabetes
Diabetes and GC are often associated with each other owing to shared risk factors, such as hyperglycemia, hyperinsulinemia, insulin resistance, high salt intake, and cigarette smoking [27]. GC diagnosis leads to a poorer prognosis in patients with diabetes than those without diabetes [27]. In addition to diabetes, H. pylori infection is a critical risk factor for GC. Previous research has shown that patients with diabetes exhibit a higher infection rate and lower H. pylori eradication rate [28], further strengthening the link between diabetes and GC. Metformin is conventionally used to treat T2D; however, its antitumor effects make it a potential drug for treating GC. Alleviation of the adverse effects of diabetes reduces risk factors for GC. Thus, controlling diabetes may suppress the progression of GC.
3.2. Metformin and GC Prevention
The efficacy of metformin in preventing GC was evaluated by analyzing the incidence of GC between the ever-users and non-users of metformin in T2D patients based on the National Taiwan Insurance database. The statistical results showed that the incidence rate was 0.26% and 0.55%, respectively, with a hazard ratio of 0.448, suggesting a significantly lower risk among ever-users [29] (Table 1). Additionally, the data presented a time-dependent factor. The patients prescribed metformin for more than two years exhibited a significant reduction in GC risk, and long-term treatment with metformin lowered the morbidity of GC compared with diabetes treatment without metformin. Different models of metformin use based on variables, such as combined use with other antidiabetic drugs, statins, and H. pylori infection, were studied to investigate the joint effect of other drugs and potential risk factors. The results showed that metformin significantly reduced the risk of GC, independent of the presence of H. pylori infection and the use of other antidiabetic drugs [29]. H. pylori was validated as a risk factor for GC by a prominent increase in the hazard ratio in the model of metformin use with H. pylori infection compared with that in the other models.

Table 1.
The effects of metformin use on GC patients.
A similar trend was detected for GC inhibition by metformin on analysis of the statistics pertaining to patients with T2D in the Korean National Health Insurance Database. Metformin use for more than three years was associated with a significant reduction in GC risk by 43% among those who used metformin compared with those who did not [30]. The duration of metformin use was positively correlated with a reduced risk of GC; however, the difference was not significant for insulin users. In fact, the risk of GC doubled in insulin users compared with that in non-users, independent of metformin use. According to some previous population-based observational studies, insulin may be related to an increase in cancer risk owing to the increased expression of IGFR and the corresponding signaling pathway, leading to stimulation of cell proliferation [41]. Another possible explanation for the association between insulin and GC risk may be H. pylori infection. The statistics from the Korean National Health Insurance Database do not present information on whether the patients had H. pylori infection.
As insulin can be used to eradicate H. pylori, it is often prescribed to patients with a more serious case of H. pylori infection, which in itself is a risk factor for GC. The association between H. pylori infection and glycemic control in those with GC and had been prescribed metformin was analyzed using the Clinical Data Analysis and Reporting System (CDARS) of the Hong Kong Hospital Authority. In patients receiving H. pylori eradication therapy, metformin use exhibited a 51% reduction in GC risk, whereas the sole use of H. pylori eradication therapy reduced GC risk by only 40%. Metformin demonstrated remarkable efficacy in reducing risk and was strongly associated with dose and duration gradients [34]. These results also indicate another potential therapeutic approach to preventing GC by combining H. pylori eradication and metformin prescription. Interestingly, the results showed that the efficacy of metformin was independent of HbA1c levels in H. pylori-eradicated patients [34], indicating that glycemic control has little effect on metformin treatment.
As discussed earlier, hyperinsulinemia is related to cancer risk, whereas metformin is an insulin-sensitizing drug. An Italian population-based cohort study found that metformin reduced the incidence of most types of digestive cancers, including GC. The study analyzed different types of drugs for diabetes treatment; however, metformin was the only medication that showed a significant association with a decreased risk for most types of digestive cancers [31]. However, the mechanism underlying the anti-tumor action of metformin remains elusive. Additional studies are required to establish a biochemical model for analyzing the molecular mechanism underlying GC prevention by metformin.
However, some studies that used different databases did not find a significant relationship between metformin and the reduction in GC risk. Two cohort studies were conducted using the Swedish Prescribed Drugs and Health Cohort (SPREDH). One of the studies included individuals with diabetes who were medicated with anti-diabetic drugs other than metformin. The other study included common-medication users, setting metformin users as the exposed group [37]. The results from both cohort studies showed that metformin did not reduce GC risk.
Another study conducted in the Netherlands that investigated the association between metformin and GC also arrived at a similar conclusion. The Netherlands Cancer Registry (NCR) PHARMO database was analyzed, and metformin users were compared with users of other non-insulin antidiabetic drugs (NIADs). The risk of GC was not significantly different between metformin users and other NIADs users [33]. However, the sample size of the GC arm was only 53 patients, which may not be sufficiently representative.
3.3. Metformin Use in GC Treatment
In addition to the potential use of metformin in GC prevention, another potential clinical application could be as a drug to reduce mortality. A study based at Chang Gung Memorial Hospital in Taiwan analyzed patients with GC and DM post-gastrectomy. The results showed that among patients with stage III GC, metformin users had significantly prolonged cancer-specific survival; the results were not significant in stages I and II GC [38]. Apart from improving survival, metformin also decreased the risk of tumor recurrence, confirming metformin as a potential adjuvant drug in the treatment of gastrectomy. A Korean study in GC patients with diabetes who had undergone gastrectomy showed that medication with metformin for longer than 6 months significantly decreased the risk of recurrence, cancer-specific mortality, and all-cause mortality. In addition, survival rates were comparable to those of patients without diabetes [32]. According to a previous study, metformin is especially beneficial for GC patients with diabetes by successfully reducing mortality.
Compared with studies undertaken in Taiwan and Korea, the results of a Belgian population-based study showed that despite reduced all-cause mortality in GC patients with diabetes, cancer-specific mortality did not improve [35]. In addition, the relationship between metformin and survival was dose independent. The possible reason for the reduced mortality needs to be clarified by investigating the mechanism underlying the anti-tumor effect of metformin.
Although the results of these studies differ regarding the efficacy of metformin in reducing mortality in GC patients, the consensus is that metformin is efficacious in GC treatment. However, the statistics from the Lithuanian Cancer Registry and the National Health Insurance Fund Database estimating the survival of patients with GC while taking various medications for T2D did not show a significant association between the reduction in GC mortality and treatment with metformin. In other studies, insulin was found to be related to increased GC risk. However, the mortality rate of GC patients is not different from that of other anti-hyperglycemic drugs or non-diabetic patients [36]. The detailed mechanism that leads to different tendencies in morbidity and mortality in patients with GC needs further exploration.
4. Repositioning (Repurposing) of Metformin for Cancer Inhibition
In addition to its effect on gluconeogenesis and insulin sensitivity, LKB1 functions as a tumor suppressor [42]. The anti-tumor effect exerted by metformin may be attributed to its interaction with LKB1, which is most pronounced in the regulation of cell growth. Metformin inhibits the proliferation of several human GC cell lines including MKN1, MKN45, and MKN74 [43]. High concentrations of metformin strongly inhibited cancer cell growth in dose- and time-dependent manners. Metformin inhibits cancer cell proliferation by decreasing the expression of cyclins, such as cyclin-dependent kinase (Cdk) 4, Cdk6, cyclin E, and Cdk2, and reducing the level of phosphorylated retinoblastoma protein. Loss of cyclin D1 prevents cells from transitioning from the G0 to G1 phase, suppressing normal cell cycle progression [43]. The results of in vivo studies showed that metformin inhibits cell proliferation. In experiments where MKN74 cells (a gastric adenocarcinoma cell line) and metformin were injected into nude mice [43], a decrease in tumor growth was observed as a result of G1 cell cycle arrest. Additionally, the tumor volume dramatically decreased as the metformin dosage increased. Metformin also reduced the expression of phosphorylated epidermal growth factor receptor (p-EGFR) and phosphorylated insulin-like growth factor-1 receptor (p-IGF-1R) in vitro and in vivo [43]. In addition, metformin regulates the mTOR pathway and decreases the expression of surviving [44], which is involved in apoptosis inhibition and cell cycle regulation [45]. Furthermore, metformin downregulates hepatocyte nuclear factor 4 alpha (HNF4α) by activating AMPKα, leading to cyclin downregulation, cell cycle arrest, and tumor growth inhibition [46].
EGFR, a receptor tyrosine kinase of the ErbB family, is actively expressed in a variety of solid tumors [47]. IGF-1R has long been recognized for its role in tumorigenesis [48]. Both EGFR and IGF-1R are involved in tumor cell proliferation. Metformin inhibits cancer cell proliferation by reducing the expression of EGFR and blocking signaling through IGF-1R [49,50,51]. Treatment with metformin modulates miRNA levels in diabetic patients and reduces cyclin levels in several cancer cell lines. For example, in the SGC-7901 GC cells, metformin increases the expression of miR-107, which inhibits tumor growth and invasion, induces apoptosis, and is associated with cell cycle arrest [52]. Metformin along with miR-365 promotes apoptosis in GC cells [53]. Additionally, the epithelial-to-mesenchymal transition and self-renewal properties induced in cancer stem cells by the Wnt/β-catenin pathway are reversed by metformin in GC [54,55]. Long noncoding RNAs have been implicated to be possibly oncogenic in several cancers. A higher expression level of Loc100506691 RNA was associated with poor survival in patients with GC. However, metformin was shown to decrease the Loc100506691 RNA level in GC cells [56] (Table 2).

Table 2.
Mechanism of metformin in the inhibition of cancer cells.
5. Metformin Use Alters Microbiota Composition
The gut microbiota contributes to enteric protection against pathogens, metabolic regulation of nutrients and drugs [60], and the maintenance of the intestinal barrier [61]. Many diseases, such as diabetes and obesity, are associated with alterations in the composition of the gut microbiota, and H. pylori infections are known to reduce gastric bacterial diversity [60]. Gut microbiota contributes to carcinogenesis through metabolite production. For instance, certain bacteria produce butyrate, which promotes the proliferation of abnormal epithelial cells [62]. N-nitroso compounds (NOCs) produced by E. coli, Lactobacillus, and Nitrospirae [63] promote mutagenesis, angiogenesis, of proto-oncogene expression, and apoptosis inhibition [64].
One of the anti-tumorigenic properties exhibited by metformin has been the restoration of gastric bacterial diversity by directly inhibiting bacterial growth and modifying gastrointestinal physiology [60]. For example, metformin enhances intestinal bile acid secretion, glucose release [60], and lipopolysaccharide production [65]. These changes in the gastric microenvironment consequently affect the bacterial communities.
In murine models, metformin treatment has increased the relative abundance of many short-chain fatty acid (SCFA)-producing bacteria, such as Bacteroides and Butynococcus [9]. Metformin also increases the abundance of probiotic organisms such as Akkermansia spp. [9], Faecalibaculum, and Bifidobacterium [66]. Akkermansia spp. attenuate cancer development by regulating glucose metabolism [67], while Bifidobacterium spp. produce metabolites that create an anti-tumorigenic environment by lowering gastric pH [68] and inflammatory responses [60]. Metformin decreased the abundance of the tumorigenic bacteria Lactobacillus and Clostridium, the latter of which contributes to gastric tumorigenesis by producing carcinogenic factors [69]. It also effectively decreased the relative abundance of Firmicutes in H. pylori-infected murine models [60].
6. Metformin and Adverse Effects
Metformin’s most common adverse effects are gastrointestinal symptoms, including nausea, diarrhea, vomiting, and intestinal discomfort [70]. Vitamin B12 is involved in red blood cell formation, and long-term metformin use has been reported to cause low vitamin B12 levels, leading to anemia and peripheral neuropathy [71]. Because metformin reduces hepatic gluconeogenesis and glucose uptake, metformin overdose can result in severe hypoglycemia [72]. Lactic acidosis and acute pancreatitis have been reported in patients with renal insufficiency because unmetabolized metformin cannot be properly excreted. Metformin inhibits mitochondrial respiration in the liver, leading to increased plasma lactate concentrations [73,74]. Although the incidence of metformin-induced lactic acidosis is infrequent, it is associated with high mortality and should be considered before the prescription. Hepatotoxicity is a rare adverse effect reported in metformin-treated patients [75].
7. Conclusions and Perspectives
Metformin has been widely prescribed as a T2D drug for decades, and its effects and mechanisms have been extensively studied. Mounting evidence has revealed its antitumor properties and potential as a GC prevention drug and medication. Apart from its biochemical effects, metformin also contributes to GC treatment by altering the gut microbiome. In this study, we have summarized the mechanisms by which metformin inhibits cancer cells and comprehensively discussed the efficacy of metformin in GC prevention by analyzing databases from different countries. The adverse effects of metformin have also been discussed, and it is considered a safe drug with low risks.
The repositioning or repurposing of metformin for the inhibition of several types of cancer has been reported elsewhere. Although the mechanism underlying the antitumor effect of metformin on GC has been investigated in cell-based studies, the information available from in vivo studies is currently insufficient. Additionally, analyses based on databases from different countries have yielded conflicting results. The role of metformin in the prevention or treatment of T2D in patients with GC remains controversial. Long-term follow-up studies with large-scale prospective trials on the effects of metformin in GC treatment are warranted to elucidate the precise mechanism by which metformin exerts its antitumor effect.
Author Contributions
Conception or design of this work: C.-J.K. and C.-H.L.; literature analysis and interpretation: W.-H.L., T.-Y.L., J.-A.Y., C.-L.F., J.-T.H. and H.-J.L.; writing the manuscript: W.-H.L., T.-Y.L., J.-A.Y., C.-L.F., J.-T.H., H.-J.L., C.-J.K., and C.-H.L.; W.-H.L. and T.-Y.L. were equally contributed to this work; final approval: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science and Technology Council (109-2320-B-182-025-MY3, 109-2320-B-182-029-MY3, and 109-2622-B-182-002), Chang Gung Memorial Hospital (CMRPD1K0361, CMRPD1L0321, CMRPD1M0491-2, CORPD1M0021, and CORPG3L0191), and Tomorrow Medical Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the editor and reviewers for the editorial assistance and their valuable comments.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Šterbenc, A.; Jarc, E.; Poljak, M.; Homan, M. Virulence genes. World J. Gastroenterol. 2019, 25, 4870–4884. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, S. Diabetes mellitus carries a risk of gastric cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 6902–6910. [Google Scholar] [CrossRef]
- Zhou, X.L.; Xue, W.H.; Ding, X.F.; Li, L.F.; Dou, M.M.; Zhang, W.J.; Lv, Z.; Fan, Z.R.; Zhao, J.; Wang, L.X. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: A meta-analysis of cohort studies. Oncotarget 2017, 8, 55622–55631. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Diabetes increases risk of gastric cancer after. Diabetes Care 2019, 42, 1769–1775. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An old drug with new applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic amp. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-induced mitochondrial complex i inhibition: Facts, uncertainties, and consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Oakhill, J.S.; Scott, J.W.; Kemp, B.E. Ampk functions as an adenylate charge-regulated protein kinase. Trends Endocrinol. Metab. 2012, 23, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. Lkb1 is the upstream kinase in the amp-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase lkb1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the lkb1/ampk pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of amp-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in acc1 and acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Wu, T.; Xie, C.; Wu, H.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 290–293. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Koffert, J.P.; Mikkola, K.; Virtanen, K.A.; Andersson, A.D.; Faxius, L.; Hällsten, K.; Heglind, M.; Guiducci, L.; Pham, T.; Silvola, J.M.U.; et al. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res. Clin. Pract. 2017, 131, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Sun, J.; Lim, G.E.; Fantus, I.G.; Brubaker, P.L.; Jin, T. Cross talk between the insulin and wnt signaling pathways: Evidence from intestinal endocrine l cells. Endocrinology 2008, 149, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Beysen, C.; Murphy, E.J.; Deines, K.; Chan, M.; Tsang, E.; Glass, A.; Turner, S.M.; Protasio, J.; Riiff, T.; Hellerstein, M.K. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: A randomised controlled study. Diabetologia 2012, 55, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.J.; Bound, M.; Grivell, J.; Sun, Z.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Comparative effects of proximal and distal small intestinal administration of metformin on plasma glucose and glucagon-like peptide-1, and gastric emptying after oral glucose, in type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 640–647. [Google Scholar] [CrossRef]
- Sansome, D.J.; Xie, C.; Veedfald, S.; Horowitz, M.; Rayner, C.K.; Wu, T. Mechanism of glucose-lowering by metformin in type 2 diabetes: Role of bile acids. Diabetes Obes. Metab. 2020, 22, 141–148. [Google Scholar] [CrossRef]
- Tseng, C.H. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: An extensive review of the literature. Biomolecules 2021, 11, 1022. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tseng, F.-H. Diabetes and gastric cancer: The potential links. World J. Gastroenterol. 2014, 20, 1701–1711. [Google Scholar] [CrossRef]
- Tseng, C.-H. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging 2016, 8, 1636. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.Y.; Cho, S.J.; Park, J.H.; Choi, I.J.; Lee, Y.J.; Lee, E.K.; Kook, M.C.; Kim, C.G.; Ryu, K.W.; et al. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: A nationwide cohort study. Aliment. Pharmacol. Ther. 2014, 39, 854–863. [Google Scholar] [CrossRef]
- Valent, F. Diabetes mellitus and cancer of the digestive organs: An italian population-based cohort study. J. Diabetes Complicat. 2015, 29, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Jung, M.; Jung, I.; Heo, S.J.; Jeong, Y.H.; An, J.Y.; Kim, H.-I.; Cheong, J.-H.; Hyung, W.J.; Noh, S.H.; et al. Cumulative metformin use and its impact on survival in gastric cancer patients after gastrectomy. Ann. Surg. 2016, 263, 96–102. [Google Scholar] [CrossRef]
- De Jong, R.G.; Burden, A.M.; de Kort, S.; van Herk-Sukel, M.P.; Vissers, P.A.; Janssen, P.K.; Haak, H.R.; Masclee, A.A.; de Vries, F.; Janssen-Heijnen, M.L. No decreased risk of gastrointestinal cancers in users of metformin in the netherlands; a time-varying analysis of metformin exposure. Cancer Prev. Res. 2017, 10, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Metformin use and gastric cancer risk in diabetic patients after helicobacter pylori eradication. J. Natl. Cancer Inst. 2018, 111, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, O.; Couttenier, A.; Vaes, E.; Cardwell, C.R.; De Schutter, H.; Robert, A. Impact of metformin on gastric adenocarcinoma survival: A belgian population based study. Cancer Epidemiol. 2018, 53, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dulskas, A.; Patasius, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Smailyte, G. A cohort study of antihyperglycemic medication exposure and survival in patients with gastric cancer. Aging 2019, 11, 7197–7205. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, S.-H.; Santoni, G.; Lagergren, J. Metformin use and risk of gastric adenocarcinoma in a swedish population-based cohort study. Br. J. Cancer 2019, 121, 877–882. [Google Scholar] [CrossRef]
- Chung, W.-S.; Le, P.-H.; Kuo, C.-J.; Chen, T.-H.; Kuo, C.-F.; Chiou, M.-J.; Chou, W.-C.; Yeh, T.-S.; Hsu, J.-T. Impact of metformin use on survival in patients with gastric cancer and diabetes mellitus following gastrectomy. Cancers 2020, 12, 2013. [Google Scholar] [CrossRef]
- Cho, M.H.; Yoo, T.G.; Jeong, S.-M.; Shin, D.W. Association of aspirin, metformin, and statin use with gastric cancer incidence and mortality: A nationwide cohort study. Cancer Prev. Res. 2021, 14, 95–104. [Google Scholar] [CrossRef]
- MacArthur, T.A.; Harmsen, W.S.; Mandrekar, J.; Abraha, F.; Grotz, T.E. Association of common medications and the risk of early-onset gastric cancer: A population-based matched study. J. Cancer Epidemiol. 2021, 2021, 2670502. [Google Scholar] [CrossRef]
- McFarland, M.S.; Cripps, R. Diabetes mellitus and increased risk of cancer: Focus on metformin and the insulin analogs. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1159–1178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, R.; Yu, J.; Li, N.; Ke, R.; Luo, L.; Zou, J.; Zhang, J.; Zhang, K.; Lu, N.; et al. Clinical significance and role of lkb1 in gastric cancer. Mol. Med. Rep. 2016, 13, 249–256. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Gong, J.; Iwama, H.; Kitanaka, A.; Tani, J.; Miyoshi, H.; Nomura, K.; Mimura, S.; Kobayashi, M.; Aritomo, Y.; et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Gong, H.; Wang, Y.; Guo, S.; Liu, K. Ampk/mtor-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol. Ther. 2015, 16, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; O’Donovan, N.; Duffy, M.J. Survivin: A new target for anti-cancer therapy. Cancer Treat. Rev. 2009, 35, 553–562. [Google Scholar] [CrossRef]
- Chang, H.R.; Nam, S.; Kook, M.C.; Kim, K.T.; Liu, X.; Yao, H.; Jung, H.R.; Lemos, R., Jr.; Seo, H.H.; Park, H.S.; et al. Hnf4α is a therapeutic target that links ampk to wnt signalling in early-stage gastric cancer. Gut 2016, 65, 19–32. [Google Scholar] [CrossRef]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Chen, H.X.; Sharon, E. Igf-1r as an anti-cancer target--trials and tribulations. Chin. J. Cancer 2013, 32, 242–252. [Google Scholar] [CrossRef]
- Sarfstein, R.; Friedman, Y.; Attias-Geva, Z.; Fishman, A.; Bruchim, I.; Werner, H. Metformin downregulates the insulin/igf-i signaling pathway and inhibits different uterine serous carcinoma (usc) cells proliferation and migration in p53-dependent or -independent manners. PLoS ONE 2013, 8, e61537. [Google Scholar] [CrossRef]
- Gao, C.; Cai, S.; He, Y. Metformin plus tyrosine kinase inhibitors in epidermal growth factor receptor-mutated non-small cell lung cancer. JAMA Oncol. 2020, 6, 782. [Google Scholar] [CrossRef]
- Wang, W.M.; Yang, S.S.; Shao, S.H.; Nie, H.Q.; Zhang, J.; Su, T. Metformin downregulates the expression of epidermal growth factor receptor independent of lowering blood glucose in oral squamous cell carcinoma. Front. Endocrinol. 2022, 13, 828608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, W.; Zhou, Y.; Fan, R.; Wu, Y.; Pei, W.; Sun, S.; Xu, X.; Jiang, H. Metformin up-regulated mir-107 expression and enhanced the inhibitory effect of mir-107 on gastric cancer growth. Transl. Cancer Res. 2020, 9, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Xiang, Y.; Li, T.; Huang, Y.; Wang, J.; Zhang, H.M.; Li, H.H.; Dai, Z.T.; Li, J.P.; Li, H.; et al. Metformin and mir-365 synergistically promote the apoptosis of gastric cancer cells via mir-365-pten-ampk axis. Pathol. Res. Pract. 2022, 230, 153740. [Google Scholar] [CrossRef] [PubMed]
- Chiurillo, M.A. Role of the wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J. Exp. Med. 2015, 5, 84–102. [Google Scholar] [CrossRef]
- Valaee, S.; Shamsara, M.; Yaghoobi, M.M. Metformin is a novel suppressor for vimentin in human gastric cancer cell line. Int. J. Mol. Cell. Med. 2021, 10, 200–206. [Google Scholar]
- Tseng, H.H.; Chen, Y.Z.; Chou, N.H.; Chen, Y.C.; Wu, C.C.; Liu, L.F.; Yang, Y.F.; Yeh, C.Y.; Kung, M.L.; Tu, Y.T.; et al. Metformin inhibits gastric cancer cell proliferation by regulation of a novel loc100506691-chac1 axis. Mol. Ther. Oncolytics 2021, 22, 180–194. [Google Scholar] [CrossRef]
- Zhou, J.; Zhi, X.; Wang, L.; Wang, W.; Li, Z.; Tang, J.; Wang, J.; Zhang, Q.; Xu, Z. Linc00152 promotes proliferation in gastric cancer through the egfr-dependent pathway. J. Exp. Clin. Cancer Res. 2015, 34, 135. [Google Scholar] [CrossRef]
- Courtois, S.; Durán, R.V.; Giraud, J.; Sifré, E.; Izotte, J.; Mégraud, F.; Lehours, P.; Varon, C.; Bessède, E. Metformin targets gastric cancer stem cells. Eur. J. Cancer 2017, 84, 193–201. [Google Scholar] [CrossRef]
- Zou, J.; Li, C.; Jiang, S.; Luo, L.; Yan, X.; Huang, D.; Luo, Z. Ampk inhibits smad3-mediated autoinduction of tgf-β1 in gastric cancer cells. J. Cell. Mol. Med. 2021, 25, 2806–2815. [Google Scholar] [CrossRef]
- Jauvain, M.; Courtois, S.; Lehours, P.; Bessède, E. Metformin modifies the gut microbiota of mice infected with. Pharmaceuticals 2021, 14, 329. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S. Gut microbial metabolism drives transformation of msh2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Vinasco, K.; Mitchell, H.M.; Kaakoush, N.O.; Castaño-Rodríguez, N. Microbial carcinogenesis: Lactic acid bacteria in gastric cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188309. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hong, X.; Wang, J.; Sun, T.; Yu, T.; Yu, Y.; Fang, J.; Xiong, H. Metformin elicits antitumour effect by modulation of the gut microbiota and rescues fusobacterium nucleatum-induced colorectal tumourigenesis. eBioMedicine 2020, 61, 103037. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Bahmani, S.; Azarpira, N.; Moazamian, E. Anti-colon cancer activity of bifidobacterium metabolites on colon cancer cell line sw742. Turk. J. Gastroenterol. 2019, 30, 835–842. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Liu, X.; Ling, Z.; Ji, F. Role of the gastric microbiome in gastric cancer: From carcinogenesis to treatment. Front. Microbiol. 2021, 12, 641322. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H. Long-term metformin use and vitamin b12 deficiency in the diabetes prevention program outcomes study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Al-Abri, S.; Hayashi, S.; Thoren, K.; Olson, K. Metformin overdose-induced hypoglycemia in the absence of other antidiabetic drugs. Clin. Toxicol. 2013, 51, 444–447. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Pasquel, F.J.; Hinedi, Z.; Umpierrez, G.E.; Klein, R.; Adigweme, A.; Coralli, R.; Pimentel, J.L.; Lopez, F.A. Metformin-associated lactic acidosis. Am. J. Med. Sci. 2015, 349, 263–267. [Google Scholar] [CrossRef]
- Miralles-Linares, F.; Puerta-Fernandez, S.; Bernal-Lopez, M.R.; Tinahones, F.J.; Andrade, R.J.; Gomez-Huelgas, R. Metformin-induced hepatotoxicity. Diabetes Care 2012, 35, e21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).