Trichostatin A-Mediated Epigenetic Modulation Predominantly Triggers Transcriptomic Alterations in the Ex Vivo Expanded Equine Chondrocytes
Abstract
1. Introduction
2. Results
2.1. Efficiency Alignment of NGS Reads
2.2. Differentially Expressed Genes (DEGs) Obtained Using 3′ mRNA-Seq
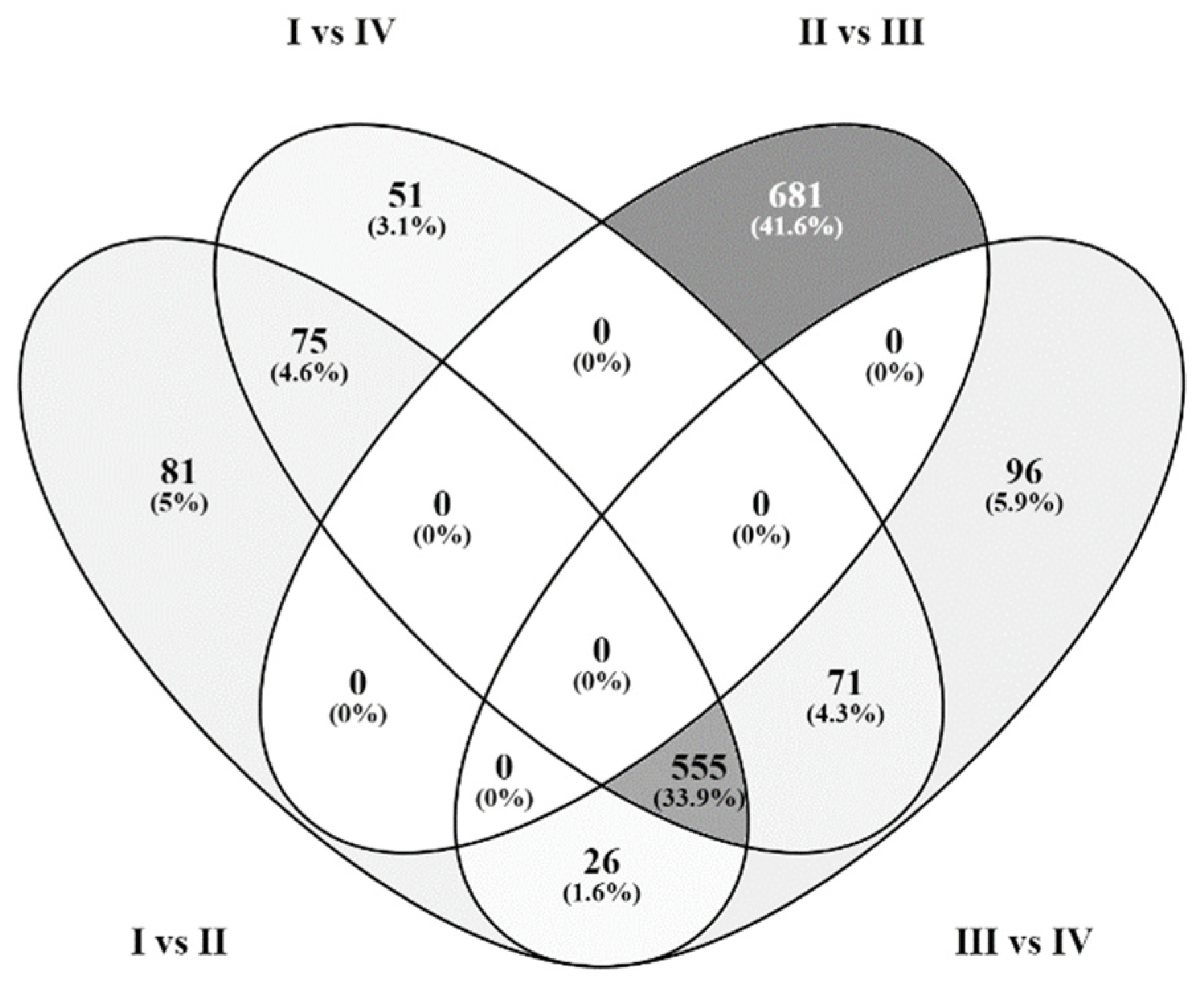
2.3. General Description of Differentially Expressed Genes upon Applying Chondrocyte Stimulation in Monolayer Culture
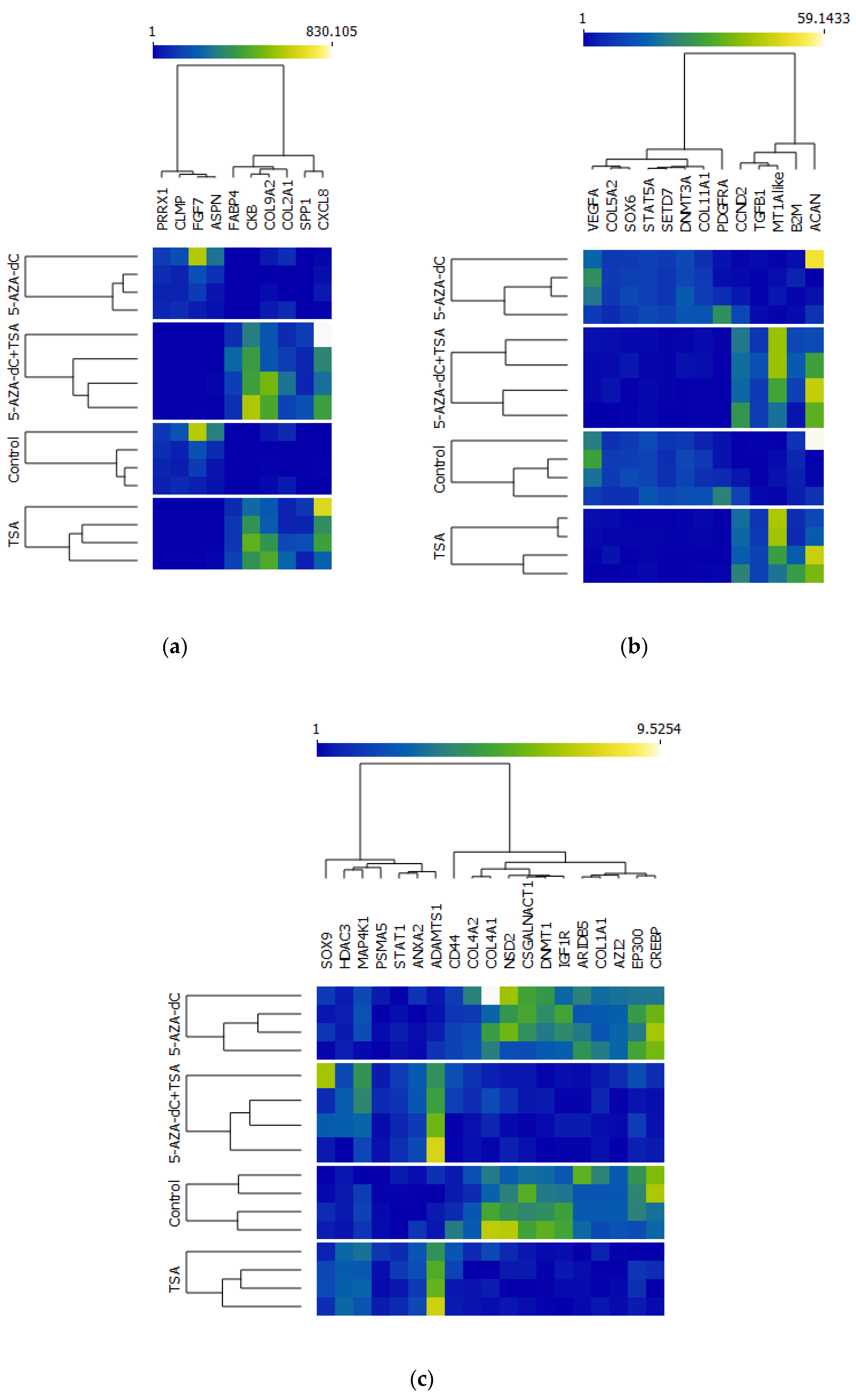
2.4. Results of Functional Overrepresentation of DEGs Using DAVID Annotation Tools
2.5. Validation in RNA-Seq Results
3. Discussion
4. Materials and Methods
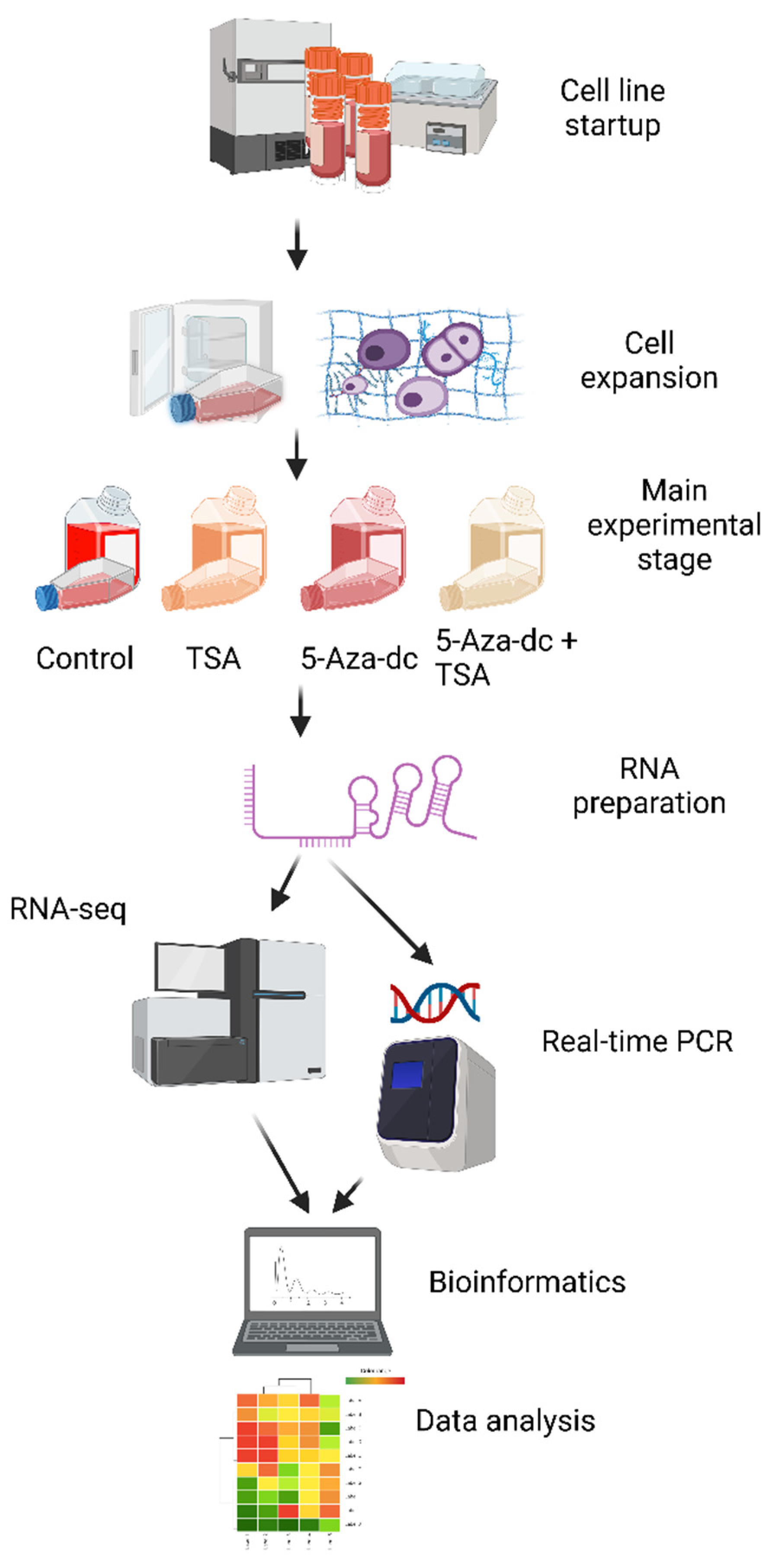
4.1. Short Description of the Research
4.2. Chondrocyte Culture Conditions and Applied Stimulations
4.3. Preparation of RNA and 3′ RNA-seq Libraries, and Next-Generation Sequencing
4.4. Trimming, Filtering, Quantification, and Mapping of Demultiplexed NGS Reads, and Differential Analysis
4.5. DEGs’ Functional Annotation in KEGG Pathways
4.6. Real-Time PCR
5. Conclusions and Future Goals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortved, K.F.; Nixon, A.J. Cell-based cartilage repair strategies in the horse. Vet. J. 2016, 208, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lipa, K.E.; Alexander, P.G.; Clark, K.L.; Lin, H. Potential Methods of Targeting Cellular Aging Hallmarks to Reverse Osteoarthritic Phenotype of Chondrocytes. Biology 2022, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef]
- Buhrmann, C.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: Comparison with TNF-α. PLoS ONE 2017, 12, e0186993. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef] [PubMed]
- Hata, K. Epigenetic regulation of chondrocyte differentiation. Jpn. Dent. Sci. Rev. 2015, 51, 105–113. [Google Scholar] [CrossRef]
- Michalowsky, L.A.; Jones, P.A. Differential nuclear protein binding to 5-azacytosine-containing DNA as a potential mechanism for 5-aza-2’-deoxycytidine resistance. Mol. Cell Biol. 1987, 7, 3076–3083. [Google Scholar]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Kirpotin, D.B.; Guo, Z.; Scott, G.K.; Benz, C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 495–528. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar]
- Haq, S.H. 5-Aza-2’-deoxycytidine acts as a modulator of chondrocyte hypertrophy and maturation in chick caudal region chondrocytes in culture. Anat. Cell Biol. 2016, 49, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kadler, S.; Vural, Ö.; Rosowski, J.; Reiners-Schramm, L.; Lauster, R.; Rosowski, M. Effects of 5-aza-2’-deoxycytidine on primary human chondrocytes from osteoarthritic patients. PLoS ONE 2020, 15, e0234641. [Google Scholar] [CrossRef]
- Young, D.A.; Lakey, R.L.; Pennington, C.J.; Jones, D.; Kevorkian, L.; Edwards, D.R.; Cawston, T.E.; Clark, I.M. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res. Ther. 2005, 7, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Leoni, F.; Zaliani, A.; Bertolini, G.; Porro, G.; Pagani, P.; Pozzi, P.; Donà, G.; Fossati, G.; Sozzani, S.; Azam, T.; et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl. Acad. Sci. USA 2002, 99, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Im, G.I. Effects of Trichostatin A on the Chondrogenesis from Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 403–410. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, F.; Yao, H.; Li, H.; Tuan, R.S. Histone Modifications and Chondrocyte Fate: Regulation and Therapeutic Implications. Front. Cell Dev. Biol. 2021, 9, 626708. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, J.; Muguruma, M.; Aoto, H.; Suetake, I.; Nakamura, M.; Tajima, S. Isolation of the novel cDNA of a gene of which expression is induced by a demethylating stimulus. Gene 1999, 240, 289–295. [Google Scholar] [CrossRef]
- Goldring, M.B. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Med. 2005, 107, 69–95. [Google Scholar] [PubMed]
- Liu, C.F.; Samsa, W.E.; Zhou, G.; Lefebvre, V. Transcriptional control of chondrocyte specification and differentiation. Semin. Cell Dev. Biol. 2017, 62, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Im, H.J.; Richardson, B.; Lu, Q.; Chubinskaya, S. Methylation of the OP-1 promoter: Potential role in the age-related decline in OP-1 expression in cartilage. Osteoarthr. Cartil. 2009, 17, 513–517. [Google Scholar] [CrossRef]
- Rao, J.; Bhattacharya, D.; Banerjee, B.; Sarin, A.; Shivashankar, G.V. Trichostatin-A induces differential changes in histone protein dynamics and expression in HeLa cells. Biochem. Biophys. Res. Commun. 2007, 363, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef] [PubMed]
- Korogi, W.; Yoshizawa, T.; Karim, M.F.; Tanoue, H.; Yugami, M.; Sobuz, S.U.; Hinoi, E.; Sato, Y.; Oike, Y.; Mizuta, H.; et al. SIRT7 is an important regulator of cartilage homeostasis and osteoarthritis development. Biochem. Biophys. Res. Commun. 2018, 496, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, B.F.; Chan, W.Y. An epigenetic regulator: Methyl-CpG-binding domain protein 1 (MBD1). Int. J. Mol. Sci. 2015, 16, 5125–5140. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Lefebvre, V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015, 43, 8183–8203. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Itoi, E.; Otero, M.; Roach, H.I.; Goldring, M.B.; Oreffo, R.O. Association of reduced type IX collagen gene expression in human osteoarthritic chondrocytes with epigenetic silencing by DNA hypermethylation. Arthritis Rheumatol. 2014, 66, 3040–3051. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014, 2014, 648459. [Google Scholar] [CrossRef]
- Shin, H.; Lee, M.N.; Choung, J.S.; Kim, S.; Choi, B.H.; Noh, M.; Shin, J.H. Focal Adhesion Assembly Induces Phenotypic Changes and Dedifferentiation in Chondrocytes. J. Cell Physiol. 2016, 231, 1822–1831. [Google Scholar] [CrossRef]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Kita, K.; Kimura, T.; Nakamura, N.; Yoshikawa, H.; Nakano, T. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells. 2008, 13, 839–850. [Google Scholar] [CrossRef]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 2015, 10, e0120857. [Google Scholar] [CrossRef] [PubMed]
- Rokutanda, S.; Fujita, T.; Kanatani, N.; Yoshida, C.A.; Komori, H.; Liu, W.; Mizuno, A.; Komori, T. Akt regulates skeletal development through GSK3, mTOR, and FoxOs. Dev. Biol. 2009, 328, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Stanton, L.A.; Underhill, T.M.; Beier, F. MAP kinases in chondrocyte differentiation. Dev. Biol. 2003, 263, 165–175. [Google Scholar] [CrossRef]
- Hollander, J.M.; Zeng, L. The Emerging Role of Glucose Metabolism in Cartilage Development. Curr. Osteoporos. Rep. 2019, 17, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, P.; Lee, N.H.; Goldring, M.B.; Konstantopoulos, K. Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PLoS ONE 2010, 5, e15174. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.Y.; Ballock, R.T. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J. Bone Min. Res. 2010, 25, 1138–1146. [Google Scholar] [CrossRef]
- Tseng, C.C.; Chen, Y.J.; Chang, W.A.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; Sung, W.Y.; Yen, J.H.; Kuo, P.L. Dual Role of Chondrocytes in Rheumatoid Arthritis: The Chicken and the Egg. Int. J. Mol. Sci. 2020, 21, 1071. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombos-pondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Legeai-Mallet, L.; Benoist-Lasselin, C.; Munnich, A.; Bonaventure, J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 correlates with phenotypic severity and defective chondrocyte differentiation in FGFR3-related chondrodysplasias. Bone 2004, 34, 26–36. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef]
- Edwards, R.B.; Lu, Y.; Cole, B.J.; Muir, P.; Markel, M.D. Comparison of radiofrequency treatment and mechanical debride-ment of fibrillated cartilage in an equine model. Vet. Comp. Orthop. Traumatol. 2008, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.B., 3rd; Lu, Y.; Uthamanthil, R.K.; Bogdanske, J.J.; Muir, P.; Athanasiou, K.A.; Markel, M.D. Comparison of me-chanical debridement and radiofrequency energy for chondroplasty in an in vivo equine model of partial thickness carti-lage injury. Osteoarthr. Cartil. 2007, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shakya, B.R.; Tiulpin, A.; Saarakkala, S.; Turunen, S.; Thevenot, J. Detection of experimental cartilage damage with acoustic. Equine Vet. J. 2020, 52, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Uthamanthil, R.K.; Edwards, R.B.; Lu, Y.; Manley, P.A.; Athanasiou, K.A.; Markel, M.D. In vivo study on the short-term effect of radiofrequency energy on chondromalacic patellar cartilage and its correlation with calcified cartilage pathology in an equine model. J. Orthop. Res. 2006, 24, 716–724. [Google Scholar] [CrossRef]
- Ryan, A.; Bertone, A.L.; Kaeding, C.C.; Backstrom, K.C.; Weisbrode, S.E. The effects of radiofrequency energy treatment on chondrocytes and matrix of fibrillated articular cartilage. Am. J. Sports Med. 2003, 31, 386–391. [Google Scholar] [CrossRef]
- Bodó, G.; Hangody, L.; Szabó, Z.; Peham, C.; Schinzel, M.; Girtler, D.; Sótonyi, P. Arthroscopic autologous osteochondral mosaicplasty for the treatment of subchondral cystic lesion in the medial femoral condyle in a horse. Acta Vet. Hung. 2000, 48, 343–354. [Google Scholar]
- Bodo, G.; Hangody, L.; Modis, L.; Hurtig, M. Autologous osteochondral grafting (mosaic arthroplasty) for treatment of subchondral cystic lesions in the equine stifle and fetlock joints. Vet. Surg. 2004, 33, 588–596. [Google Scholar] [CrossRef]
- Sparks, H.D.; Nixon, A.J.; Bogenrief, D.S. Reattachment of the articular cartilage component of type 1 subchondral cystic lesions of the medial femoral condyle with polydioxanone pins in 3 horses. J. Am. Vet. Med. Assoc. 2011, 238, 636–640. [Google Scholar] [CrossRef]
- Smith, M.A.; Walmsley, J.P.; Phillips, T.J.; Pinchbeck, G.L.; Booth, T.M.; Greet, T.R.; Richardson, D.W.; Ross, M.W.; Schramme, M.C.; Singer, E.R.; et al. Effect of age at presentation on outcome following arthroscopic debridement of sub-chondral cystic lesions of the medial femoral condyle: 85 horses (1993—2003). Equine Vet. J. 2005, 37, 175–180. [Google Scholar] [CrossRef]
- Frazer, L.L.; Santschi, E.M.; Fischer, K.J. The impact of subchondral bone cysts on local bone stresses in the medial femoral condyle of the equine stifle joint. Med. Eng. Phys. 2017, 48, 158–167. [Google Scholar] [CrossRef]
- Russell, J.W.; Hall, M.S.; Kelly, G.M. Osteochondroma on the cranial aspect of the distal radial metaphysis causing teno-synovitis of the extensor carpi radialis tendon sheath in a horse. Aust. Vet. J. 2017, 95, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Olstad, K.; Østevik, L.; Carlson, C.S.; Ekman, S. Osteochondrosis Can Lead to Formation of Pseudocysts and True Cysts in the Subchondral Bone of Horses. Vet. Pathol. 2015, 52, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Secombe, C.J.; Anderson, B.H. Diagnosis and treatment of an osteochondroma of the distal tibia in a 3-year-old horse. Aust. Vet. J. 2000, 78, 16–18. [Google Scholar] [CrossRef]
- Ząbek, T.; Witarski, W.; Semik-Gurgul, E.; Szmatoła, T.; Kowalska, K.; Bugno-Poniewierska, M. Chondrogenic expression and DNA methylation patterns in prolonged passages of chondrocyte cell lines of the horse. Gene 2019, 707, 58–64. [Google Scholar] [CrossRef]
- Miao, Z.; Lu, Z.; Wu, H.; Liu, H.; Li, M.; Lei, D.; Zheng, L.; Zhao, J. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: A comparative study. J. Cell. Biochem. 2018, 119, 7924–7933. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, T.L.; Toh, W.S.; Pei, M. The role of laminins in cartilaginous tissues: From development to regeneration. Eur. Cell Mater. 2017, 34, 40–54. [Google Scholar] [CrossRef]
- Dodt, M.; Roehr, J.T.; Ahmed, R.; Dieterich, C. Flexbar—Flexible barcode and adapter processing for next-generation sequencing platforms. Biology 2012, 1, 895–905. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome. Biol. 2003, 4, P3. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.; Venny, C. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 8 August 2022).
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

| Group | 3rd Passage Chondrocyte Stimulation | 3’ mRNA-Seq Library | Total Reads after Filtering | Number of Uniquely Mapped Reads |
|---|---|---|---|---|
| I | 5-AZA-dc 1 | 2aza-1 | 3,114,172 | 2,541,510 (81.6%) |
| I | 5-AZA-dc | 3aza-1 | 2,685,011 | 2,216,276 (82.5%) |
| I | 5-AZA-dc | 4/DEO | 2,332,147 | 1,913,713 (82.1%) |
| I | 5-AZA-dc | 6/DEO | 2,603,715 | 2,137,736 (82.1%) |
| II | 5-AZA-dc+TSA 2 | 3tsaza-1 | 3,490,960 | 2,862,115 (82.0%) |
| II | 5-AZA-dc+TSA | 2tsaaza-1 | 2,915,748 | 2,336,842 (80.1%) |
| II | 5-AZA-dc+TSA | 4/Deo+TSA | 3,091,055 | 2,548,432 (82.4%) |
| II | 5-AZA-dc+TSA | 6/DEO+TSA | 2,821,472 | 2,329,453 (82.6%) |
| III | Control | 2k-1 | 2,361,866 | 1,954,158 (82.7%) |
| III | Control | 3k-1 | 3,062,350 | 2,511,373 (82.0%) |
| III | Control | 4/K | 3,312,867 | 2,732,432 (82.5%) |
| III | Control | 6/K | 3,207,522 | 2,627,829 (81.9%) |
| IV | TSA | 3tsa-1 | 3,699,592 | 3,054,305 (82.6%) |
| IV | TSA | 2tsa-1 | 2,753,414 | 2,261,816 (82.1%) |
| IV | TSA | 4/TSA | 3,158,031 | 2,620,954 (83.0%) |
| IV | TSA | 6/TSA | 2,858,574 | 2,361,644 (82.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ząbek, T.; Witarski, W.; Szmatoła, T.; Sawicki, S.; Mrozowicz, J.; Samiec, M. Trichostatin A-Mediated Epigenetic Modulation Predominantly Triggers Transcriptomic Alterations in the Ex Vivo Expanded Equine Chondrocytes. Int. J. Mol. Sci. 2022, 23, 13168. https://doi.org/10.3390/ijms232113168
Ząbek T, Witarski W, Szmatoła T, Sawicki S, Mrozowicz J, Samiec M. Trichostatin A-Mediated Epigenetic Modulation Predominantly Triggers Transcriptomic Alterations in the Ex Vivo Expanded Equine Chondrocytes. International Journal of Molecular Sciences. 2022; 23(21):13168. https://doi.org/10.3390/ijms232113168
Chicago/Turabian StyleZąbek, Tomasz, Wojciech Witarski, Tomasz Szmatoła, Sebastian Sawicki, Justyna Mrozowicz, and Marcin Samiec. 2022. "Trichostatin A-Mediated Epigenetic Modulation Predominantly Triggers Transcriptomic Alterations in the Ex Vivo Expanded Equine Chondrocytes" International Journal of Molecular Sciences 23, no. 21: 13168. https://doi.org/10.3390/ijms232113168
APA StyleZąbek, T., Witarski, W., Szmatoła, T., Sawicki, S., Mrozowicz, J., & Samiec, M. (2022). Trichostatin A-Mediated Epigenetic Modulation Predominantly Triggers Transcriptomic Alterations in the Ex Vivo Expanded Equine Chondrocytes. International Journal of Molecular Sciences, 23(21), 13168. https://doi.org/10.3390/ijms232113168






