The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS)
Abstract
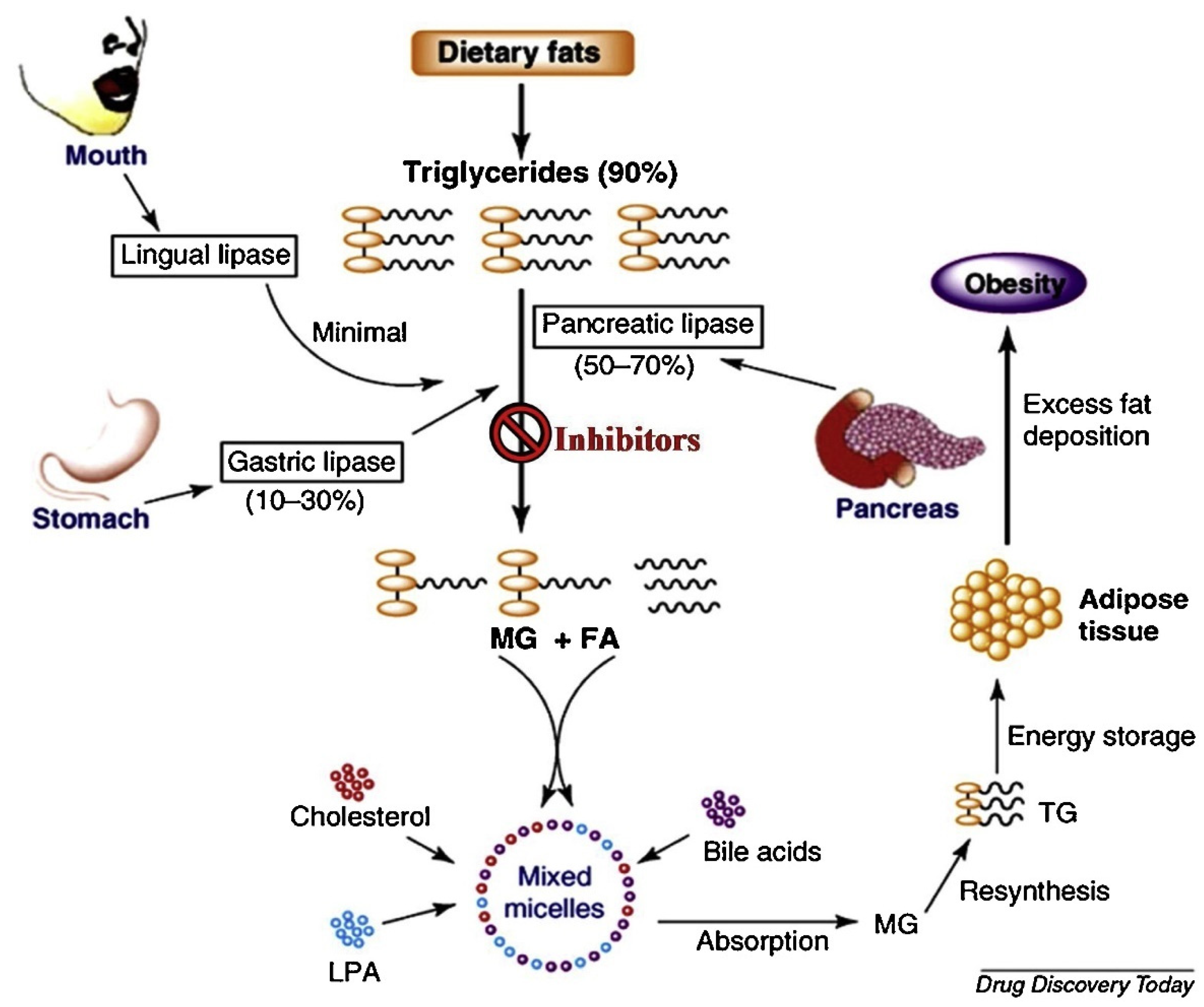
1. Introduction
2. Results and Discussion
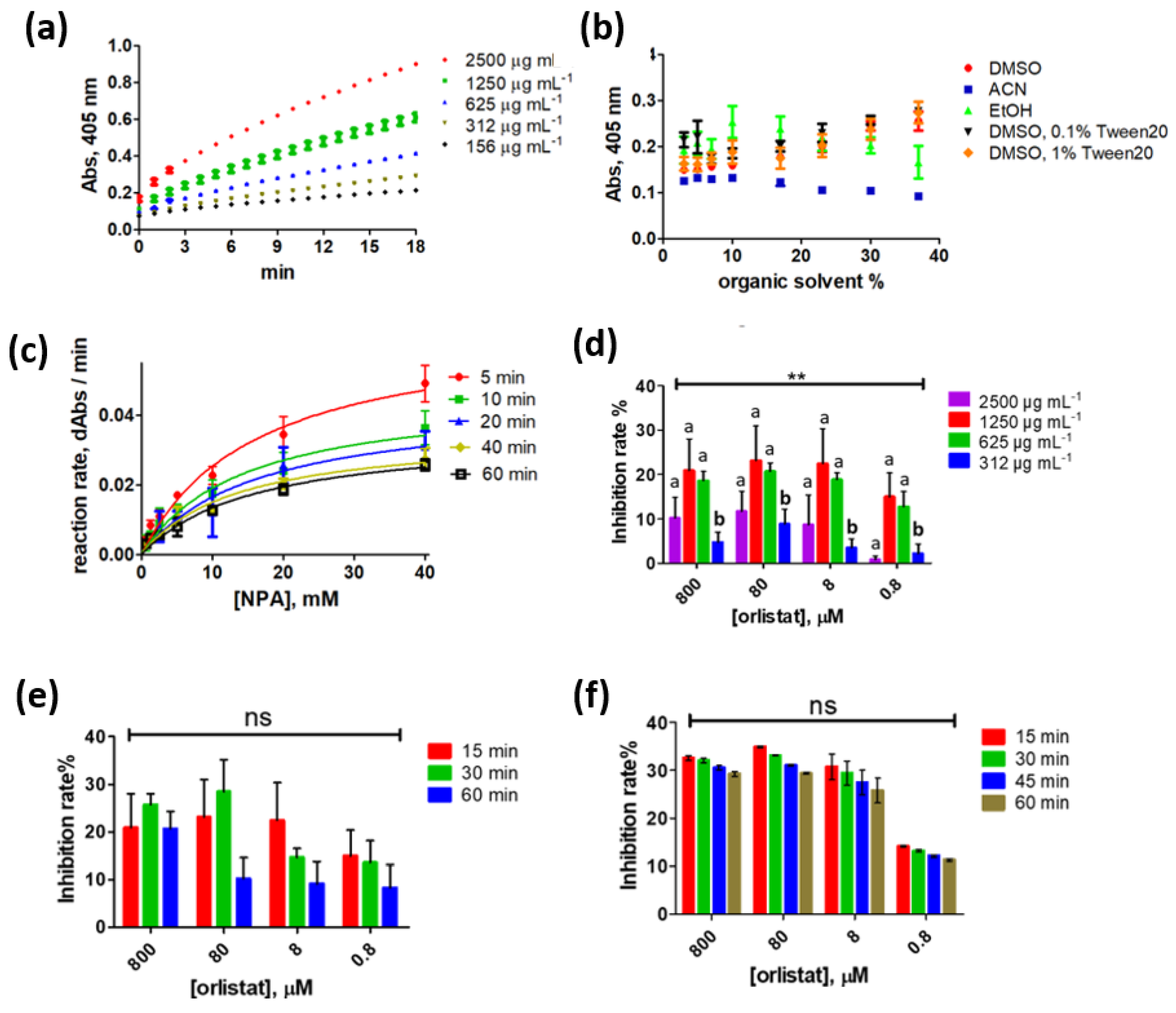
2.1. Testing of Different Substrates Resulting in Coloured and Fluorescent Products
2.2. PNLIP Optimization Using NPA as the Substrate
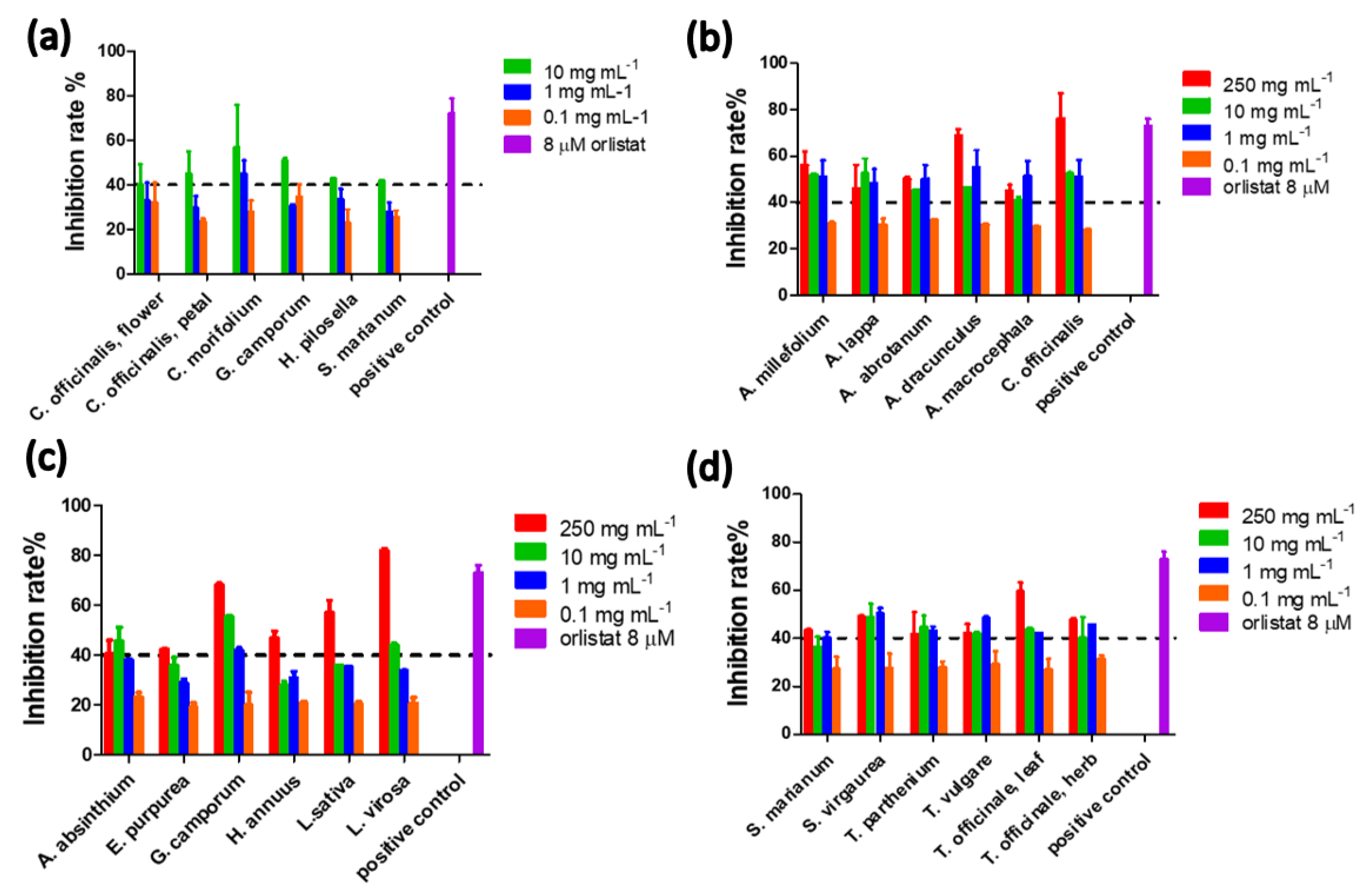
2.3. Screening of the Inhibitory Effect of the Studied Plants on PNLIP
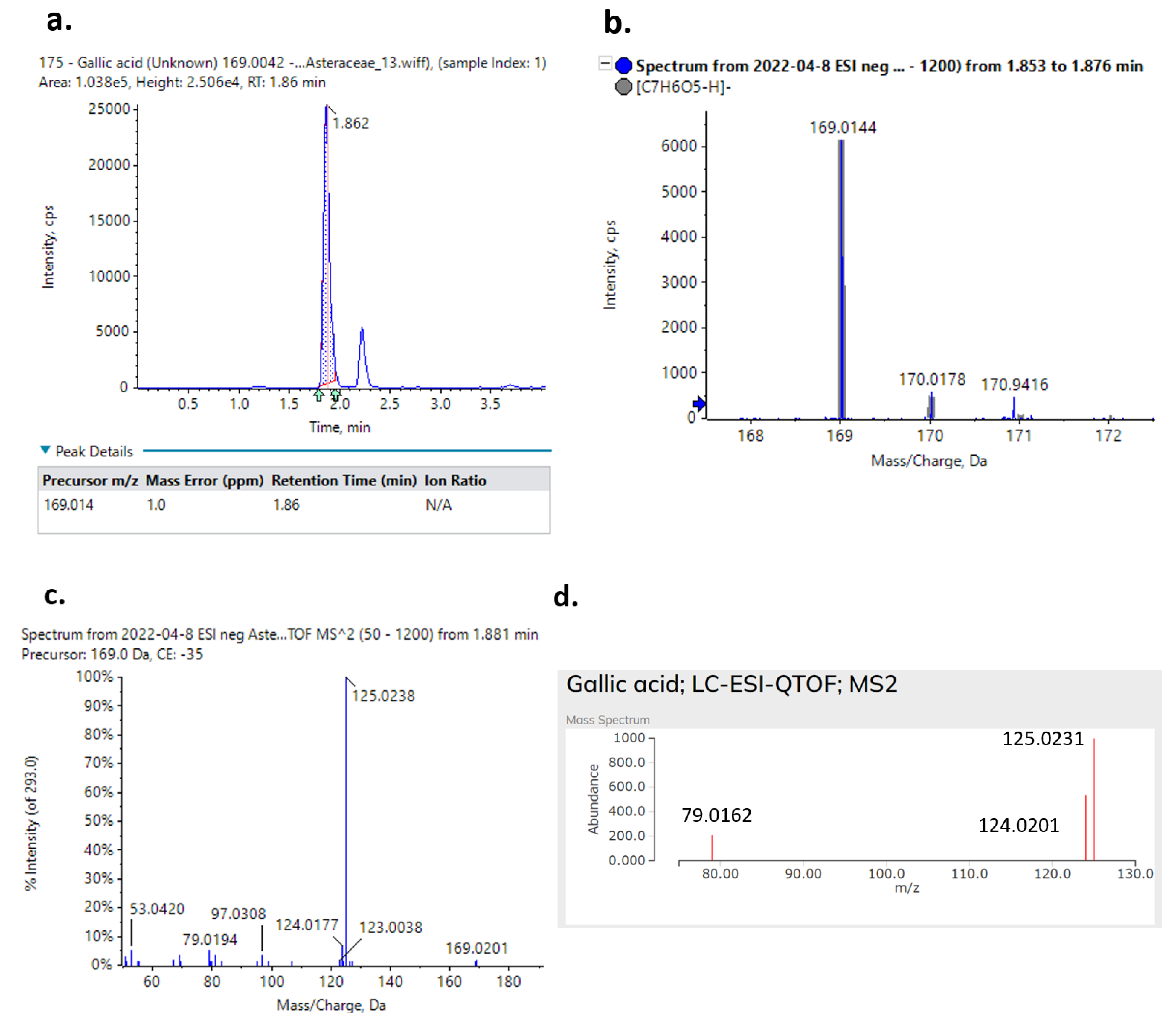
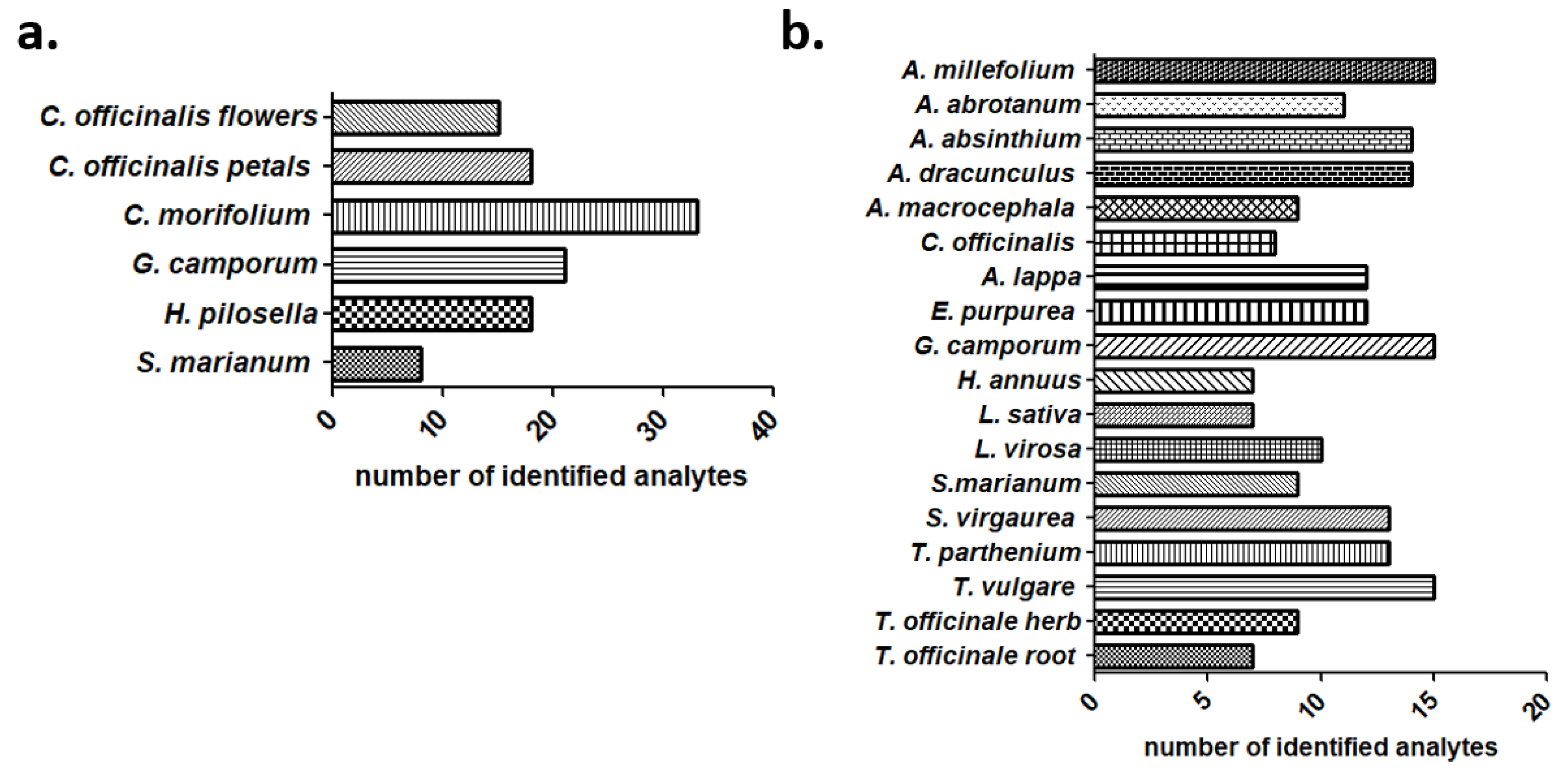
2.4. Tentative Identification of the Tested Extract Metabolites through Suspect Screening
3. Materials and Methods
3.1. Chemicals
3.2. PNLIP Assay Substrate Selection
3.3. In-Vitro PNLIP Assay
3.4. Tested Plant Extracts and Extract Preparation
3.5. In Vitro PNLIP Assay Data Processing and Handling
3.6. UHPLC-q-TOF-MS Analysis
3.7. Suspect Screening Workflow to Tentatively Identify the Selected Extract Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Coulter, A.A.; Rebello, C.J.; Greenway, F.L. Centrally acting agents for obesity: Past, present, and future. Drugs 2018, 78, 1113–1132. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Chapter 20—Phyto-Pharmaceuticals, Nutraceuticals and Their Evaluation; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 707–722. ISBN 978-0-12-813374-3. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Gondoin, A.; Grussu, D.; Stewart, D.; McDougall, G.J. White and green tea polyphenols inhibit pancreatic lipase in vitro. Food Res. Int. 2010, 43, 1537–1544. [Google Scholar] [CrossRef]
- Vitalini, S.; Garzoli, S.; Sisto, F.; Pezzani, R.; Argentieri, M.P.; Scarafoni, A.; Ciappellano, S.; Zorzan, M.; Capraro, J.; Collazuol, D. Digestive and gastroprotective effects of Achillea erba-rotta subsp. moschata (Wulfen) I. Richardson (syn. A. moschata Wulfen) (Asteraceae): From traditional uses to preclinical studies. J. Ethnopharmacol. 2022, 298, 115670. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Koc, S.; Isgor, B.S.; Isgor, Y.G.; Shomali Moghaddam, N.; Yildirim, O. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharm. Biol. 2015, 53, 746–751. [Google Scholar] [CrossRef]
- Akbar, S. Chamaemelum nobile (L.) all. (asteraceae/aompositae). In Handbook of 200 Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 593–599. [Google Scholar]
- Grauso, L.; Emrick, S.; de Falco, B.; Lanzotti, V.; Bonanomi, G. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Pohanka, M. Biosensors and bioassays based on lipases, principles and applications, a review. Molecules 2019, 24, 616. [Google Scholar] [CrossRef]
- Lankatillake, C.; Luo, S.; Flavel, M.; Lenon, G.B.; Gill, H.; Huynh, T.; Dias, D.A. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 2021, 17, 3. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Park, J.-Y.; Ha, J.; Choi, Y.; Chang, P.-S.; Park, K.-M. Optimization of Spectrophotometric and Fluorometric Assays Using Alternative Substrates for the High-Throughput Screening of Lipase Activity. J. Chem. 2021, 2021, 3688124. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An overview of existing individual unit operations. Biorefineries 2014, 3–36. [Google Scholar] [CrossRef]
- Kamal, M.Z.; Yedavalli, P.; Deshmukh, M.V.; Rao, N.M. Lipase in aqueous-polar organic solvents: Activity, structure, and stability. Protein Sci. 2013, 22, 904–915. [Google Scholar] [CrossRef]
- Kim, G.-N.; Shin, M.-R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.-I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H.; et al. Study of Antiobesity Effect through Inhibition of Pancreatic Lipase Activity of Diospyros kaki Fruit and Citrus unshiu Peel. Biomed Res. Int. 2016, 2016, 1723042. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, M.-J.; Kim, M.-J.; Kim, M.-E.; Song, J.-H.; Lee, Y.-M.; Kim, J.-I. Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr. Res. Pract. 2008, 2, 200–203. [Google Scholar] [CrossRef]
- Hou, X.-D.; Ge, G.-B.; Weng, Z.-M.; Dai, Z.-R.; Leng, Y.-H.; Ding, L.-L.; Jin, L.-L.; Yu, Y.; Cao, Y.-F.; Hou, J. Natural constituents from Cortex Mori Radicis as new pancreatic lipase inhibitors. Bioorg. Chem. 2018, 80, 577–584. [Google Scholar] [CrossRef]
- Dechakhamphu, A.; Wongchum, N. Investigation of the kinetic properties of Phyllanthus chamaepeuce Ridl. extracts for the inhibition of pancreatic lipase activity. J. Herb. Med. 2022, 32, 100508. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Szewczyk, A.; Kisiel, W. Caffeic acid derivatives from a hairy root culture of Lactuca virosa. Acta Physiol. Plant. 2012, 34, 291–298. [Google Scholar] [CrossRef][Green Version]
- Hernández-Saavedra, D.; Pérez-Ramírez, I.F.; Ramos-Gómez, M.; Mendoza-Díaz, S.; Loarca-Pina, G.; Reynoso-Camacho, R. Phytochemical characterization and effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis infusions on obesity-associated cardiovascular risk. Med. Chem. Res. 2016, 25, 163–172. [Google Scholar] [CrossRef]
- Zaki, A.; Ashour, A.; Mira, A.; Kishikawa, A.; Nakagawa, T.; Zhu, Q.; Shimizu, K. Biological activities of oleanolic acid derivatives from Calendula officinalis seeds. Phyther. Res. 2016, 30, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, V.K.; Seo, S.-Y. Screening of some medicinal plants for anti-lipase activity. J. Ethnopharmacol. 2005, 97, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Danış, O.; Ogan, A.; Anbar, D.; Basak Yuce, D.; Demir, S.; Salan, U. Inhibition of pancreatic lipase by culinary plant extracts. Int. J. Plant Biol. Res. 2015, 3, 1038. [Google Scholar]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Rahim, A.T.M.A.; Takahashi, Y.; Yamaki, K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res. Int. 2015, 75, 289–294. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Dong, S.; Liu, Y.; Liu, Y. Molecular interactions between (−)-epigallocatechin gallate analogs and pancreatic lipase. PLoS ONE 2014, 9, e111143. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory Effects of Oolong Tea Polyphenols on Pancreatic Lipase in Vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef]
- Hu, B.; Cui, F.; Yin, F.; Zeng, X.; Sun, Y.; Li, Y. Caffeoylquinic acids competitively inhibit pancreatic lipase through binding to the catalytic triad. Int. J. Biol. Macromol. 2015, 80, 529–535. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharm. 2021, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janeš, D.; Štrukelj, B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phyther. Res. Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 874–877. [Google Scholar] [CrossRef]
- Navratilova, K.; Hurkova, K.; Hrbek, V.; Uttl, L.; Tomaniova, M.; Valli, E.; Hajslova, J. Metabolic fingerprinting strategy: Investigation of markers for the detection of extra virgin olive oil adulteration with soft-deodorized olive oils. Food Control 2022, 134, 108649. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey Phenolic Compound Profiling and Authenticity Assessment Using HRMS Targeted and Untargeted Metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef] [PubMed]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.-L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography—High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Abdossi, V.; Kazemi, M. Bioactivities of Achillea millefolium essential oil and its main terpenes from Iran. Int. J. Food Prop. 2016, 19, 1798–1808. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef]
- Lusa, M.G.; Martucci, M.E.P.; Loeuille, B.F.P.; Gobbo-Neto, L.; Appezzato-da-Glória, B.; Da Costa, F.B. Characterization and evolution of secondary metabolites in Brazilian Vernonieae (Asteraceae) assessed by LC-MS fingerprinting. Bot. J. Linn. Soc. 2016, 182, 594–611. [Google Scholar] [CrossRef]
- Burlec, A.F.; Arsene, C.; Gille, E.; Hăncianu, M.; Cioancă, O. Ornamental Asteraceae species as new sources of secondary metabolites. Indian J Pharma Educ Res 2017, 51, S425–S428. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Bigović, D.; Janković, T.; Jelačić, S.; Šavikin, K. Sandy everlasting (Helichrysum arenarium (L.) Moench): Botanical, chemical and biological properties. Front. Plant Sci. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Zengin, G.; Ceylan, R.; Fawzi Mahomoodally, M.; Jugreet, S.; Mollica, A.; Stefanucci, A. Chemical composition and biological activities of essential oils from Calendula officinalis L. flowers and leaves. Flavour Fragr. J. 2021, 36, 554–563. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012, 6, 1–7. [Google Scholar] [CrossRef]
- El Moghazy, A.M.; Darwish, F.M.; El Khayat, E.S.; Mohamed, M.O.; Wink, M.; El Readi, M.Z. New Hexadecan-2-ol and 3-Hydroxymethyloctadecanoate with Hepatoprotective Activity and Cytotoxicity Activity from Grindelia camporum Greene (Asteraceae). Planta Med. 2011, 77, PG91. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Corchete, P. Silybum marianum (L.) Gaertn: The source of silymarin. In Bioactive Molecules and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 123–148. [Google Scholar]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phyther. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zameer, S.; Yaqoob, M. Ethnobotanical, phytochemical and pharmacological properties of Galinsoga parviflora (Asteraceae): A review. Trop. J. Pharm. Res. 2017, 16, 3023–3033. [Google Scholar]
- Ayaz, F.A.; Ozcan, M.; Kurt, A.; Karayigit, B.; Ozogul, Y.; Glew, R.; Ozogul, F. Fatty acid composition and antioxidant capacity of cypselas in Centaurea s.l. taxa (Asteraceae, Cardueae) from NE Anatolia. S. Afr. J. Bot. 2017, 112, 474–482. [Google Scholar] [CrossRef]
- Soetardjo, S.; Jong, P.C.; Noor, A.M.; Lachimanan, Y.L.; Sreenivasan, S. Chemical composition and biological activity of the Centipeda minima (Asteraceae). Malays. J. Nutr. 2007, 13, 81–87. [Google Scholar]
- Kuczmannová, A.; Gál, P.; Varinská, L.; Treml, J.; Kováč, I.; Novotný, M.; Vasilenko, T.; Dall’Acqua, S.; Nagy, M.; Mučaji, P. Agrimonia eupatoria L. and Cynara cardunculus L. water infusions: Phenolic profile and comparison of antioxidant activities. Molecules 2015, 20, 20538–20550. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Salomon, L.; Lorenz, P.; Bunse, M.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L. Molecules 2021, 26, 1530. [Google Scholar] [CrossRef]
- Palić, R.; Stojanović, G.; Ranđelović, N.; Ranđelović, V.; Veličković, J. The fatty acids from plants of the genus Achillea. Facta Univ. Phys. Chem. Technol. 2000, 2, 101–104. [Google Scholar]
- Chen, S.; Liu, J.; Dong, G.; Zhang, X.; Liu, Y.; Sun, W.; Liu, A. Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat flowers: A potentially rich source of bioactive compounds. Food Chem. 2021, 344, 128733. [Google Scholar] [CrossRef]
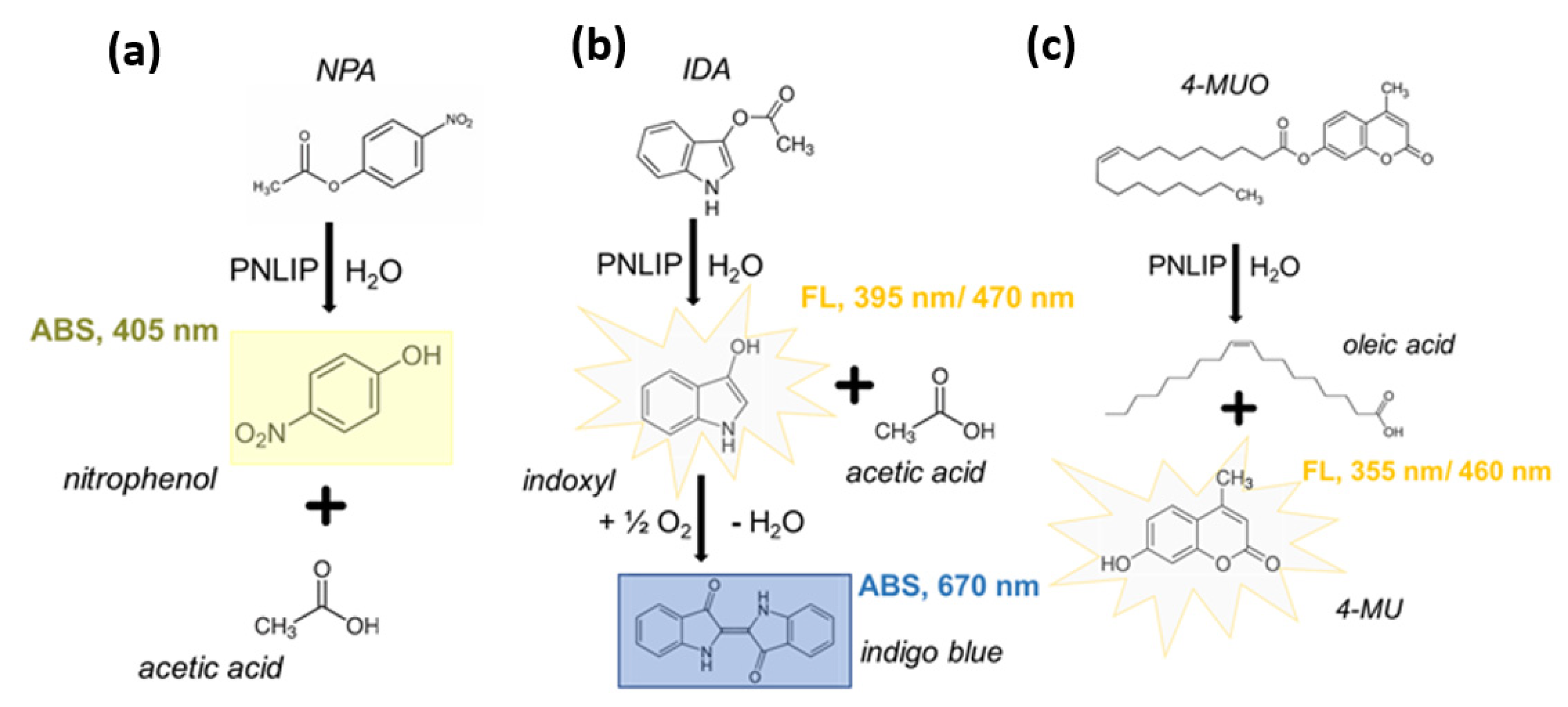
| Assay Characteristic | NPA | IDA | 4-MUO | |
|---|---|---|---|---|
| optical detection | absorbance, yellow product (405 nm) | absorbance, blue product (670 nm) | fluorescence, λexc = 395 nm & λem = 470 nm | fluorescence, λexc = 355 nm & λem = 460 nm |
| substrate concentration range | 1.25–20 mM | 0.625–10 mM | ||
| substrate cost per g * | 60 EUR | 150 EUR | 3000 EUR | |
| total assay time ** | 30 min | 75 min | 55 min | 45 min |
| Species | Common Name | Plant Part | Aqueous Extract Inhibition Rate (%) | SD | DCM Extract Inhibition Rate (%) | SD |
|---|---|---|---|---|---|---|
| Achillea millefolium | Yarrow | Leaf | 30 | 3.7 | 56 | 6.3 |
| Arctium lappa | Burdock | Leaf | 29 | 1.4 | 46 | 10 |
| Arctium lappa | Burdock | Root | 16 | 4.00 | 39 | 8.3 |
| Artemisia abrotanum | Southernwood herb | Leaf | 20 | 1.4 | 50 | 0.90 |
| Artemisia absinthium | Wormwood | Aerial part | 28 | 0.799 | 41 | 5.5 |
| Artemisia annua | Sweet wormwood (Qing Hao) | Stem | 11 | 20 | 33 | 1.1 |
| Artemisia dracunculus | Tarragon | Leaf | 2.3 | 20 | 69 | 2.6 |
| Anthemis nobilis | Chamomile | Flower | 43 | 0.88 | 45 | 1.6 |
| Artemisia vulgaris | Mugwort herb | Aerial part | 34 | 16 | 39 | 1.4 |
| Atractylodes macrocephala | Atractylodes | Rhizome | 22 | 35 | 45 | 2.5 |
| Calendula officinalis | Marigold | Petal | 45 | 10 | 76 | 25 |
| Calendula officinalis | Marigold | Flower | 40 | 9.4 | 36 | 0.46 |
| Cichorium intybus | Chicory root | Root | 27 | 0.21 | 35 | 3.2 |
| Cnicus benedictus | Holythistle | Aerial part | 21 | 0.46 | 39 | 1.5 |
| Cynara cardunculus | Artichoke | Leaf of stem | 28 | 0.39 | 34 | 2.2 |
| Eclipta alba | Bhringaraj root | Root | 20 | 0.47 | 22 | 11 |
| Echinacea angustifolia | Narrow-leaved purple coneflower | Root | 27 | 6.2 | 36 | 4.3 |
| Echinacea purpurea | Purple coneflower | Root | 29 | 0.26 | 42 | 0.15 |
| Eupatorium perfoliatum | Boneset | Leaf | 23 | 7.4 | 27 | 15 |
| Eupatorium purpureum | Gravel root | Root | 19 | 3.4 | 4.8 | 3.1 |
| Grindelia camporum | Grindelia herb | Aerial part | 51 | 1.1 | 68 | 0.65 |
| Helianthus annuus | Sunflower seed | Seed | 0 | 0 | 47 | 2.5 |
| Hieracium pilosella | Mousear, hawkweed | Aerial part | 43 | 0.22 | 35 | 6.5 |
| Chrysanthemum morifolium | Chrysanthemum flowers | Flower | 57 | 19 | 29 | 10 |
| Inula helenium | Elecampane Root | Root | 6.6 | 4.1 | 38 | 5.7 |
| Lactuca sativa | Lettuce | Leaf | 6 | 7.1 | 57 | 5.0 |
| Lactuca virosa | Wild lettuce | Leaf | 11 | 13 | 82 | 0.71 |
| Matricaria recutita | German chamomile | Flower | 38 | 0.17 | 29 | 0.41 |
| Silybum marianum | Milk thistle seed | Seed | 43 | 0.25 | 43 | 0.55 |
| Solidago virgaurea | Golden rod | Aerial part | 29 | 5.2 | 49 | 0.40 |
| Stevia rebaudiana | Stevia leaf | Leaf | 24 | 0.57 | 4.8 | 2.1 |
| Tanacetum parthenium | Feverfew herb | Aerial part | 26 | 2.9 | 42 | 9.4 |
| Tanacetum vulgare | Tansy herb | Aerial part | 31 | 1.5 | 42 | 3.8 |
| Taraxacum officinale | Dandelion herb | Leaf | 38 | 12 | 60 | 3.6 |
| Taraxacum officinale | Dandelion root | Root | 12 | 2.09 | 48 | 0.79 |
| Tussilago farfara | Coltsfoot | Aerial part | 33 | 0.13 | 31 | 13 |
| Class | Compounds | Detected Ion | Molecular Formula | Measured m/z | Δ ppm | tR (min) | Fragment Ions (m/z) | Tentatively Identified in |
|---|---|---|---|---|---|---|---|---|
| flavanols | (−)-Catechin 3-O-gallate/Epicatechin 3-O-gallate | [M-H]− | C22H18O10 | 441.0824 | −0.8 | 4.77 | 125.0243, 169.0139, 245.0842, 289.0723 | C. officinalis flowers and petals |
| (−)-Epigallocatechin 3-O-gallate/(−)-Gallocatechin 3-O-gallate | [M-H]− | C22H18O11 | 457.0769 | −1.6 | 3.99 | 125.0245, 169.0149, 193.0123, 292.8134 | C. officinalis flowers and petals | |
| flavanones | Eriocitrin | [M-H]− | C27H32O15 | 595.1666 | −0.4 | 5.13 | 151.0032, 287.0556 | C. morifolium |
| Eriodictyol | [M-H]− | C15H12O6 | 287.0559 | −0.9 | 5.14 | 107.0170, 135.0449, 151.0083, 287.0560 | C. morifolium | |
| Eriodictyol 7-O-glucoside | [M-H]− | C21H22O11 | 449.1093 | 0.7 | 4.98 | 107.0132, 135.0450, 151.0031, 175.0023, 287.0561 | C. morifolium | |
| flavones | Apigenin | [M-H]− | C15H10O5 | 269.0461 | 2.1 | 6.72 | 117.0351, 151.007, 269.0469 | C. morifolium, H. pilosella |
| Apigenin 7-O-D-glucoronide | [M-H]− | C21H18O11 | 445.0779 | 0.5 | 6.1 | 269.0527 | C. morifolium, G. camporum, H. pilosella | |
| Apigenin 7-O-glucoside | [M-H]− | C21H20O10 | 431.098 | −0.7 | 6.18 | 268.0377, 269.0456 | C. morifolium, G. camporum, H. pilosella | |
| Apigenin 7-O-rutinoside | [M-H]− | C27H30O14 | 577.1565 | 0.4 | 6.08 | 269.0456 | C. morifolium, H. pilosella | |
| Aromadendrin | [M-H]− | C15H12O6 | 289.0692 | −5.1 | 5.85 | 107.0468, 121.0259, 149.0206, 153.0156 | S. marianum | |
| Chrysoeriol/Hispidulin/Diosmetin | [M-H]− | C16H12O6 | 299.0561 | −0.1 | 6.82 | 284.0239, 285.0280 | C. morifolium, G. camporum | |
| Diosmetin 7-O-6″-acetylglucoside | [M-H]− | C24H24O12 | 503.1192 | −0.7 | 6.81 | 284.0327, 299.0568 | C. morifolium | |
| Diosmetin 7-O-glucoronide | [M-H]− | C22H20O12 | 475.0881 | −0.2 | 6.13 | 284.0329, 299.0571 | G. camporum | |
| Diosmetin 7-O-glucoside | [M-H]− | C22H22O11 | 461.1088 | −0.3 | 6.35 | 284.0366, 299.0585 | C. morifolium | |
| Linarin | [M-H]− | C28H32O14 | 591.1728 | 1.4 | 7.25 | 268.0395, 283.0628 | C. morifolium | |
| Vicenin 2 | [M-H]− | C27H30O15 | 593.1507 | −0.7 | 4.6 | 353.0680, 383.0799, 473.1068, 593.1549 | C. officinalis petals, C. morifolium, G. camporum, H. pilosella | |
| flavonols | Astragalin/Luteolin 3′-glucoside/Luteolin 7-O-glucoside/Trifolin | [M-H]− | C21H20O11 | 447.0937 | 1.0 | 5.73 | 284.0343, 285.0425, 447.0969 | C. morifolium, H. pilosella |
| Isoquercetin/Hyperoside/Quercetin 3-O-glucoside/Quercetin 7-O-galactoside/Quercetin 7-O-glucoside/Spiraein | [M-H]− | C21H20O12 | 463.0889 | 1.4 | 5.86 | 255.0272, 271.0319, 300.0327, 301.0401, 463.0844 | C. morifolium, S. marianum | |
| Isorhamnetin 3-O-glucoside | [M-H]− | C22H22O12 | 477.1041 | 0.4 | 6.4 | 271.0172, 285.0453, 314.0433, 315.0442, 477.1034 | C. morifolium | |
| Kaempferol/Luteolin | [M-H]− | C15H10O6 | 285.0405 | 0.1 | 6.41 | 107.0141, 133.0298, 151.0032, 175.0399, 285.0460 | C. morifolium | |
| Kaempferol 3-glucuronide/Luteolin 7-O-glucoronide | [M-H]− | C21H18O12 | 461.0725 | −0.1 | 5.17 | 285.043 | H. pilosella | |
| Luteolin 7-O-(6″-acetylglucoside) | [M-H]− | C23H22O12 | 489.1041 | 0.4 | 6.28 | 284.0329, 285.0398 | C. officinalis petals, C. morifolium, H. pilosella | |
| Luteolin 7-O-(6″-malonylglucoside) | [M-H]− | C24H22O14 | 533.0937 | 0.1 | 6.28 | 284.0323, 285.0401, 489.1043 | C. morifolium, H. pilosella | |
| Luteolin 7-O-rutinoside/Nicotiflorin | [M-H]− | C27H30O15 | 593.1512 | 0.1 | 5.65 | 285.0408, 593.1519 | C. officinalis flowers and petals, C. morifolium, 267 | |
| Quercetin | [M-H]− | C15H10O7 | 301.0352 | −0.7 | 7.06 | 63.0259, 65.0031, 83.0122, 108.0236, 134.0361, 145.0322, 149.0603, 151.003, 301.0001 | C. morifolium, G. camporum | |
| Quercetin 3-O-(6″-acetyl-glucoside) | [M-H]− | C23H22O13 | 505.0987 | −0.2 | 5.94 | 271.0228, 300.0290, 301.0356 | C. officinalis flowers and petals | |
| Quercetin 3-O-(6″-malonylglucoside) | [M-H]− | C24H22O15 | 549.0905 | 3.5 | 6.02 | 300.0253, 301.0368 | C. officinalis flowers and petals, C. morifolium | |
| Quercetin 3-O-glucoronide | [M-H]− | C21H18O13 | 477.0673 | −0.3 | 5.12 | 301.0351 | C. morifolium | |
| Rutin | [M-H]− | C27H30O16 | 609.1463 | 0.3 | 5.38 | 300.0277, 301.0354, 609.1475 | C. officinalis flowers and petals | |
| O-methylated flavone | Eupatilin/Nevadensin | [M-H]− | C18H16O7 | 343.0824 | 0.28 | 8.55 | 298.0113, 313.0346, 328.0580, 343.1569 | C. morifolium, G. camporum |
| O-methylated flavonol | Centaureidin | [M-H]− | C18H16O8 | 361.0908 | −2.8 | 7.95 | 285.0390, 303.0511, 328.0582, 345.0631, 361.0914 | C. morifolium, G. camporum |
| O-methylated isoflavone | Acacetin/Biochanin A/Genkwanin | [M-H]− | C16H12O5 | 283.0615 | 1.2 | 7.66 | 268.0371 | C. morifolium |
| dihydroflavonols | Taxifolin | [M-H]− | C15H12O7 | 303.0513 | 1.0 | 5.13 | 57.0342, 125.0250, 150.0315, 175.0395, 285.0389 | S. marianum |
| phenolic acid | 1.3-dicaffeoylquinic acid/1.5-di-O-Caffeoylquinic acid | [M-H]− | C25H24O12 | 515.1246 | 4.8 | 5.09 | 135.0446, 179.0350, 191.0561, 353.0895 | H. pilosella |
| 1.4-dicaffeoyl quinic acid/3.4-Dicaffeoylquinic acid/3.5-Dicaffeoylquinic acid/4.5-Dicaffeoylquinic acid | [M-H]− | C25H24O12 | 515.1193 | −0.4 | 5.13 | 179.0359, 191.0560, 353.0867 | C. officinalis flowers and petals, C. morifolium, G. camporum, H. pilosella | |
| 1-O-caffeoylquinic acid/3-O-caffeoylquinic acid/4-O-caffeoylquinic acid | [M-H]− | C16H18O9 | 353.0879 | 0.2 | 3.99 | 191.0563 | C. officinalis flowers and petals, C. morifolium, G. camporum, H. pilosella, S. marianum | |
| Caffeic acid | [M-H]− | C9H8O4 | 179.0348 | −0.8 | 4.3 | 134.0343, 135.0460 | C. officinalis flowers and petals, C. morifolium, G. camporum, H. pilosella | |
| Gallic acid | [M-H]− | C7H6O5 | 169.0144 | 1.2 | 1.86 | 51.0229, 79.0185, 124.0130, 125.0253 | Calendula officinalis petals, C. morifolium, G. camporum, H. pilosella | |
| m-Coumaric acid/o-Coumaric acid/p-Coumaric acid | [M-H]− | C9H8O3 | 163.0399 | −0.7 | 5 | 93.0335, 119.0493 | G. camporum | |
| Quinic acid | [M-H]− | C7H12O6 | 191.0564 | 1.5 | 0.7 | 85.0293, 93.0334, 99.0463, 127.0404, 191.0548 | C. officinalis flowers and petals, C. morifolium, G. camporum, H. pilosella, S. marianum | |
| Syringic Acid | [M-H]− | C9H10O5 | 197.0455 | −0.5 | 4.39 | 89.0047, 123. 0089 | C. officinalis flowers and petals | |
| Vanillic Acid | [M-H]− | C8H8O4 | 167.035 | 0.3 | 4.37 | 108.0211, 152.0153 | C. officinalis flowers, G. camporum | |
| phenolic aldehyde | Protocatechualdehyde | [M-H]− | C7H6O3 | 137.0245 | 0.5 | 3.37 | 108.0204, 109.0283, 119.0137, 136.0161, 137.0232 | C. officinalis flowers, C. morifolium, G. camporum, H. pilosella, S. marianum |
| flavonolignans | isosilybin A/isosilybin B/silybin A/silybin B/silydianin | [M-H]− | C25H22O10 | 481.1143 | 0.6 | 7.11 | 125.0245, 152.0112, 178.9968, 180.0065, 301.0361, 481.1141 | S. marianum |
| silychristin | [M-H]− | C25H22O10 | 481.1143 | 0.6 | 6.01 | 125.0240, 151.0029, 178.9984, 325.0713 | S. marianum | |
| hydroxycoumarins | scopoletin | [M-H]− | C10H8O4 | 191.0351 | 0.8 | 3.7 | 104.0284, 120.0221, 148.0153 | C. officinalis flowers and petals, G. camporum |
| Class | Compound | Detected Ion | Molecular Formula | Measured m/z | Δ ppm | tR (min) | Fragment Ions (m/z) | Tentatively Identified in |
|---|---|---|---|---|---|---|---|---|
| flavanones | Naringenin | [M-H]− | C15H12O5 | 271.0611 | −0.3 | 4.56 | 107.0154, 119.0511, 271.0597 | A. millefolium, A. abrotanum, A. absinthium, A. dracunculus, A. macrocephala, C. officinalis petals, A. lappa leaf, E.purpurea, G. camporum, S. marianum, S. virgaurea, T. parthenium, T. vulgare |
| Eriodictyol | [M-H]− | C15H12O6 | 287.0558 | −1.2 | 3.79 | 107.0129, 135.0442, 151.0026, 287.0558 | A. dracunculus, A. lappa leaf, E.purpurea | |
| flavones | Apigenin | [M-H]− | C15H10O5 | 269.0453 | −0.9 | 5.1 | 117.0337, 151.0037, 269.0443 | A. millefolium, A. absinthium, A. dracunculus, A. macrocephala, A. lappa leaf, E.purpurea, G. camporum, L. sativa, L. virosa, T. parthenium, T. vulgare, T. officinale herb |
| Apigenin 7-O-glucoside | [M-H]− | C21H20O10 | 431.0976 | −1.72 | 3.2 | 268.0354, 431.0946 | A. millefolium | |
| Chrysoeriol/Hispidulin/Diosmetin | [M-H]− | C16H12O6 | 299.056 | −0.30 | 4.9 | 227.0366, 256.0363, 284.0308 | A. millefolium, A. absinthium, A. dracunculus, A. macrocephala, A. lappa leaf, E.purpurea, G. camporum, S. virgaurea, T. parthenium, T. vulgare | |
| flavonolignans | isosilybin A/isosilybin B/silybin A/silybin B/silydianin | [M-H]− | C25H22O10 | 481.1133 | −1.48 | 4.5 | 125.0238, 152.0124, 178.9981, 180.0058, 273.0404, 301.0362, 481.1176 | S. marianum |
| flavonols | Kaempferol/Luteolin | [M-H]− | C15H10O6 | 285.0404 | −0.07 | 4.4 | 107.0160, 133.0297, 151.0065, 175.0406, 285.0428 | A. millefolium, E.purpurea, T. vulgare |
| Isorhamnetin | [M-H]− | C16H12O7 | 315.0507 | −1.02 | 4.3 | 227.0322, 243.0332, 283.0360, 300.0283, 315.0528 | A. absinthium, A. dracunculus, T. vulgare | |
| Quercetin | [M-H]− | C15H10O7 | 301.0358 | 1.41 | 4.6 | 63.0243, 65.0031, 83.0138, 108.0221, 134.0384, 149.0601, 151.0030, 301.0732 | A. dracunculus | |
| hydroxycoumarins | umbelliferone | [M-H]− | C9H6O3 | 161.0243 | −0.80 | 1.8 | 133.0288, 161.0243 | A. millefolium, A. abrotanum, A. absinthium, A. dracunculus, A. lappa leaf, G. camporum, L. virosa, S. virgaurea, T. parthenium, T. vulgare |
| scopoletin | [M-H]− | C10H8O4 | 191.0353 | 1.44 | 1.8 | 120.0205, 148.0166, 191.0283 | A. abrotanum, A. absinthium, C. officinalis petals, G. camporum, S. virgaurea | |
| isoflavonoids | Formononetin | [M-H]− | C16H12O4 | 267.0662 | −0.43 | 5.6 | 135.0087, 195.0461, 223.0430, 252.0440 | A. millefolium, A. abrotanum, A. absinthium, G. camporum, H. annuus, T. parthenium, T. vulgare, T. officinale herb, T. officinale root |
| O-methylated flavone | Eupatilin/Nevadensin | [M-H]− | C18H16O7 | 343.0817 | −1.79 | 5.8 | 313.0331, 328.0562, 343.0828 | A. millefolium, A. abrotanum, A. absinthium, G. camporum, T. parthenium, T. vulgare, T. officinale herb |
| O-methylated isoflavone | Acacetin/Biochanin A/Genkwanin | [M-H]− | C16H12O5 | 283.0612 | 0.07 | 6.5 | 268.0375 | A. millefolium, A. abrotanum, A. absinthium, A. dracunculus, A. macrocephala, A. lappa leaf, E.purpurea, G. camporum, L. virosa, S. virgaurea, T. parthenium, T. vulgare, T. officinale herb, T. officinale root |
| phenolic aldehyde | Protocatechualdehyde | [M-H]− | C7H6O3 | 137.0243 | −0.82 | 1.06 | 108.0220, 109.0315, 136.0169, 137.0237 | E.purpurea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsagkaris, A.S.; Louckova, A.; Jaegerova, T.; Tokarova, V.; Hajslova, J. The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS). Int. J. Mol. Sci. 2022, 23, 11204. https://doi.org/10.3390/ijms231911204
Tsagkaris AS, Louckova A, Jaegerova T, Tokarova V, Hajslova J. The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS). International Journal of Molecular Sciences. 2022; 23(19):11204. https://doi.org/10.3390/ijms231911204
Chicago/Turabian StyleTsagkaris, Aristeidis S., Anna Louckova, Tereza Jaegerova, Viola Tokarova, and Jana Hajslova. 2022. "The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS)" International Journal of Molecular Sciences 23, no. 19: 11204. https://doi.org/10.3390/ijms231911204
APA StyleTsagkaris, A. S., Louckova, A., Jaegerova, T., Tokarova, V., & Hajslova, J. (2022). The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS). International Journal of Molecular Sciences, 23(19), 11204. https://doi.org/10.3390/ijms231911204








