Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂
Abstract
1. Introduction
2. Results
2.1. Growth Performance and Feed Utilization
2.2. Nutritional Composition
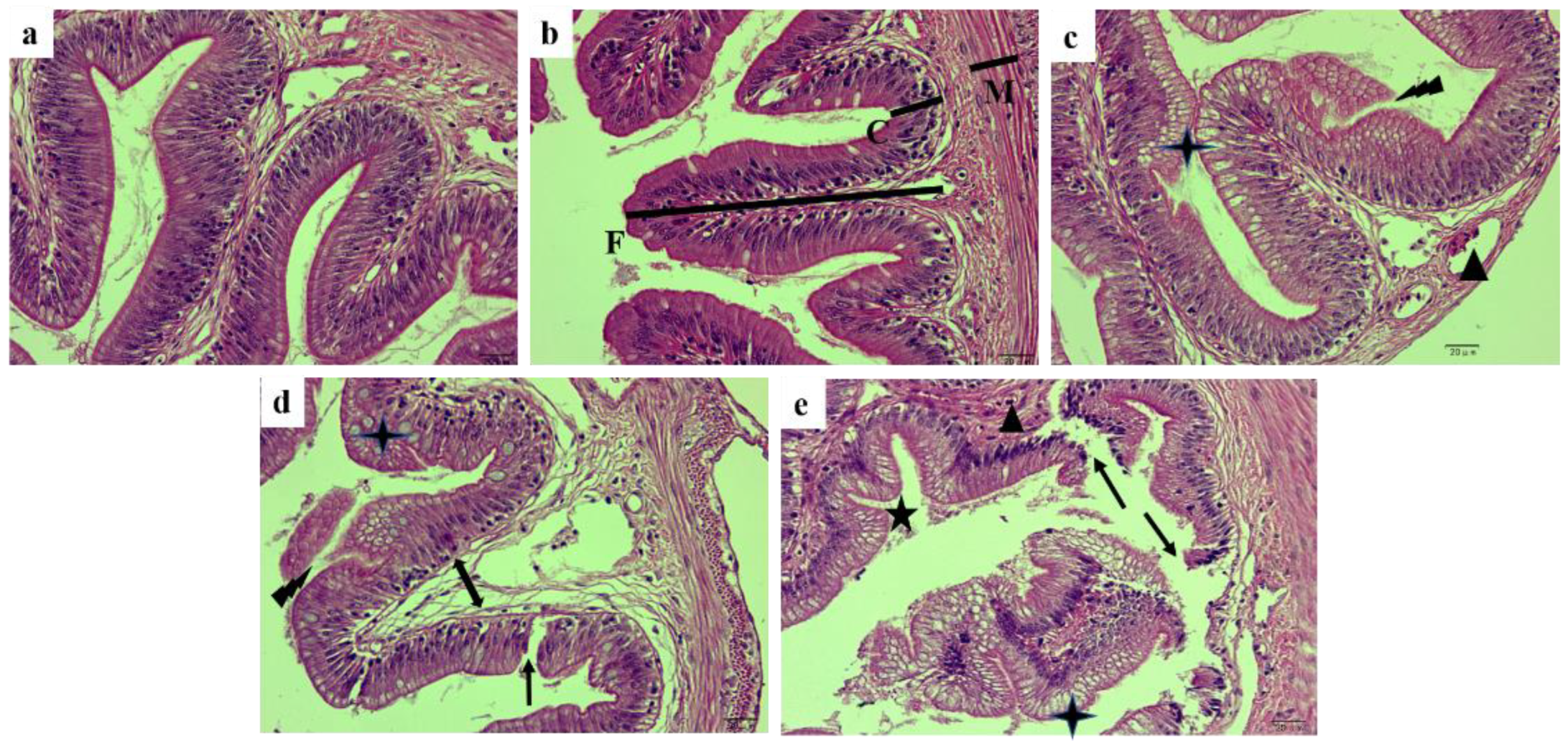
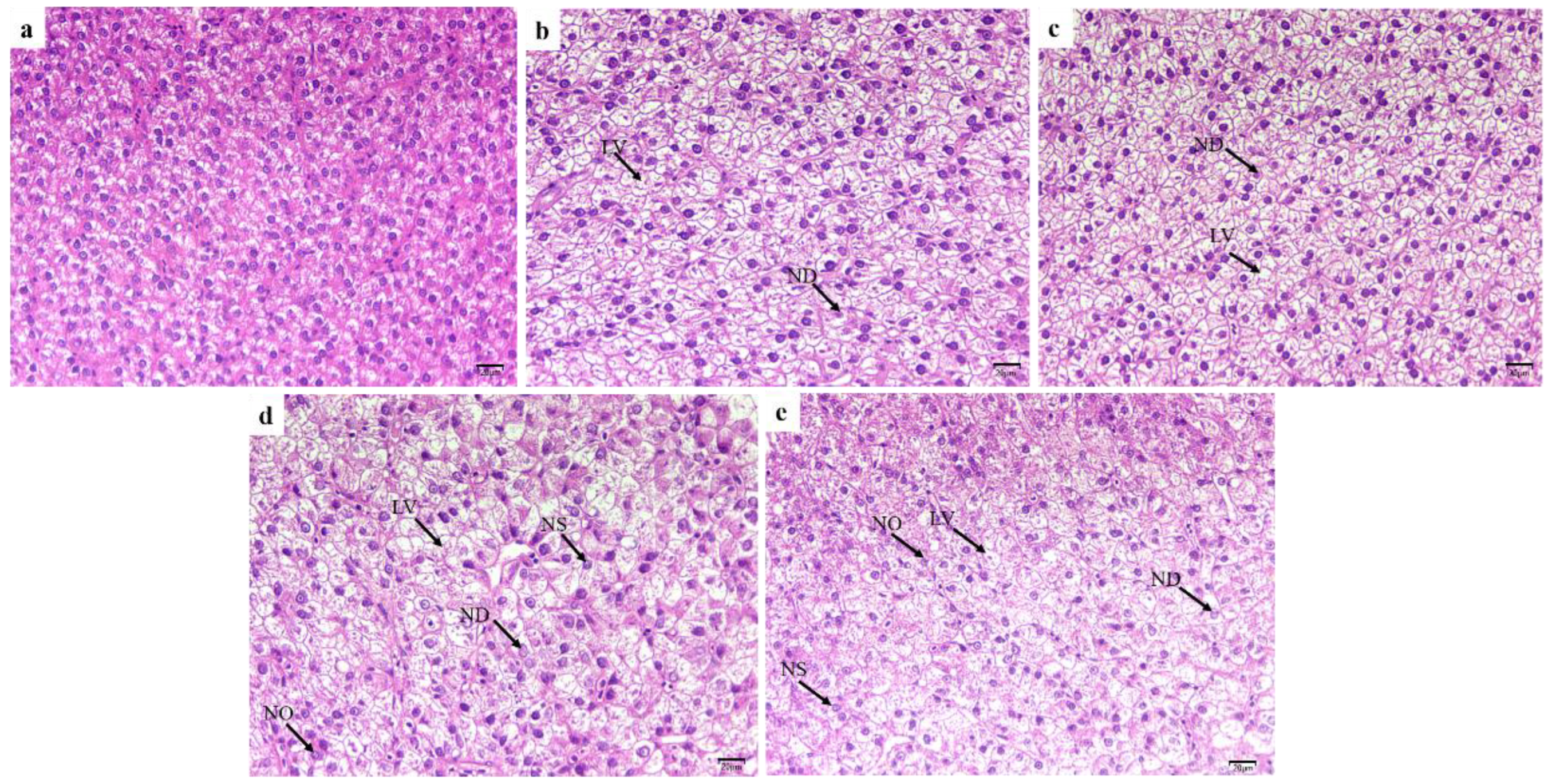
2.3. Morphology of Posterior Intestine and Liver
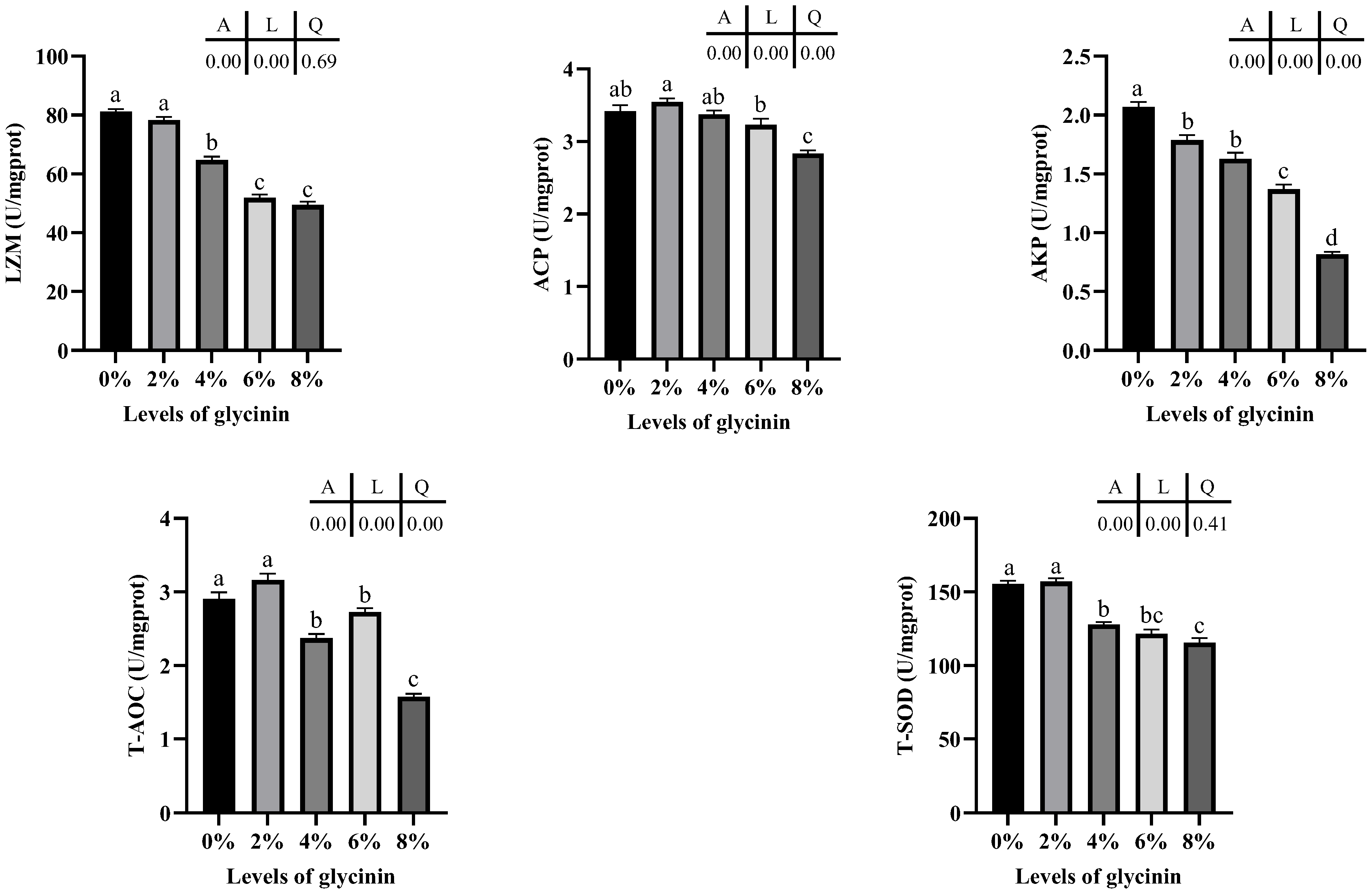
2.4. Intestinal Nonspecific Immune and Antioxidant Enzyme Activity
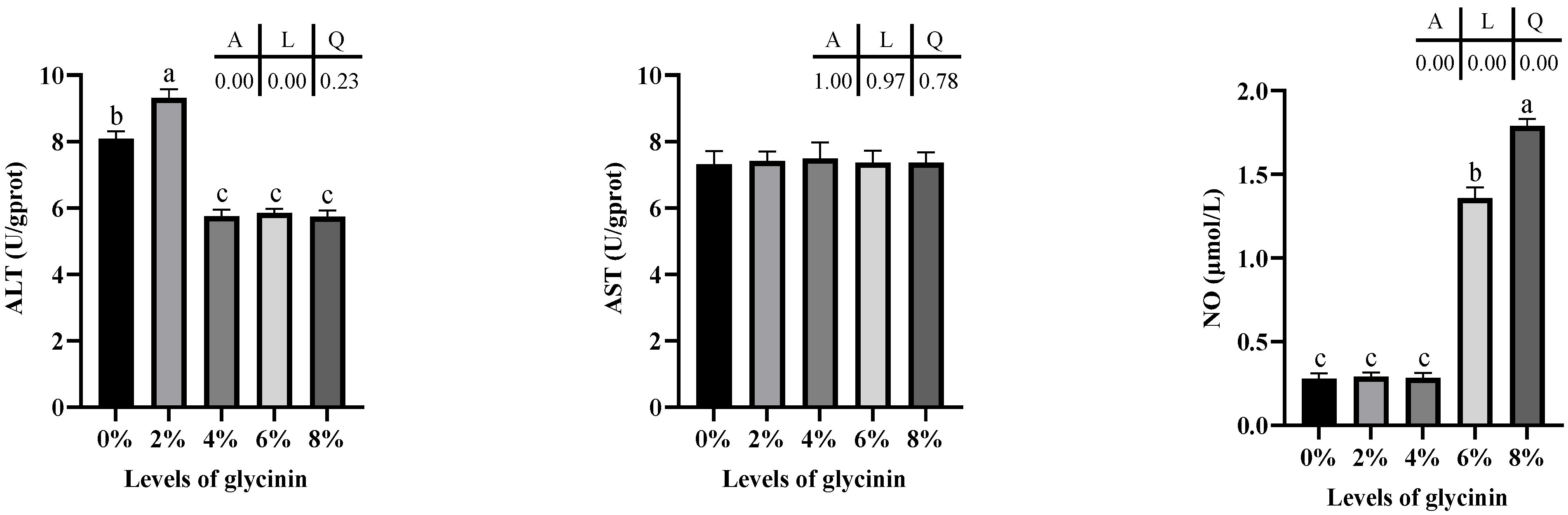
2.5. Protein metabolic Enzyme Activity of Liver
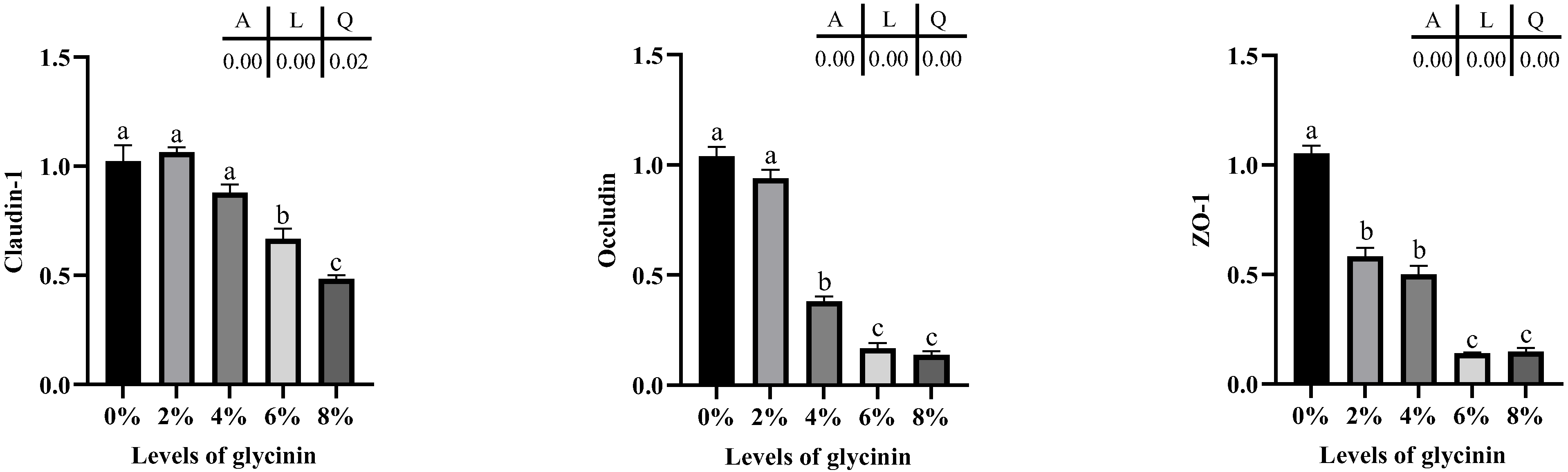
2.6. Tight Junction mRNA Expression Levels of Posterior Intestine
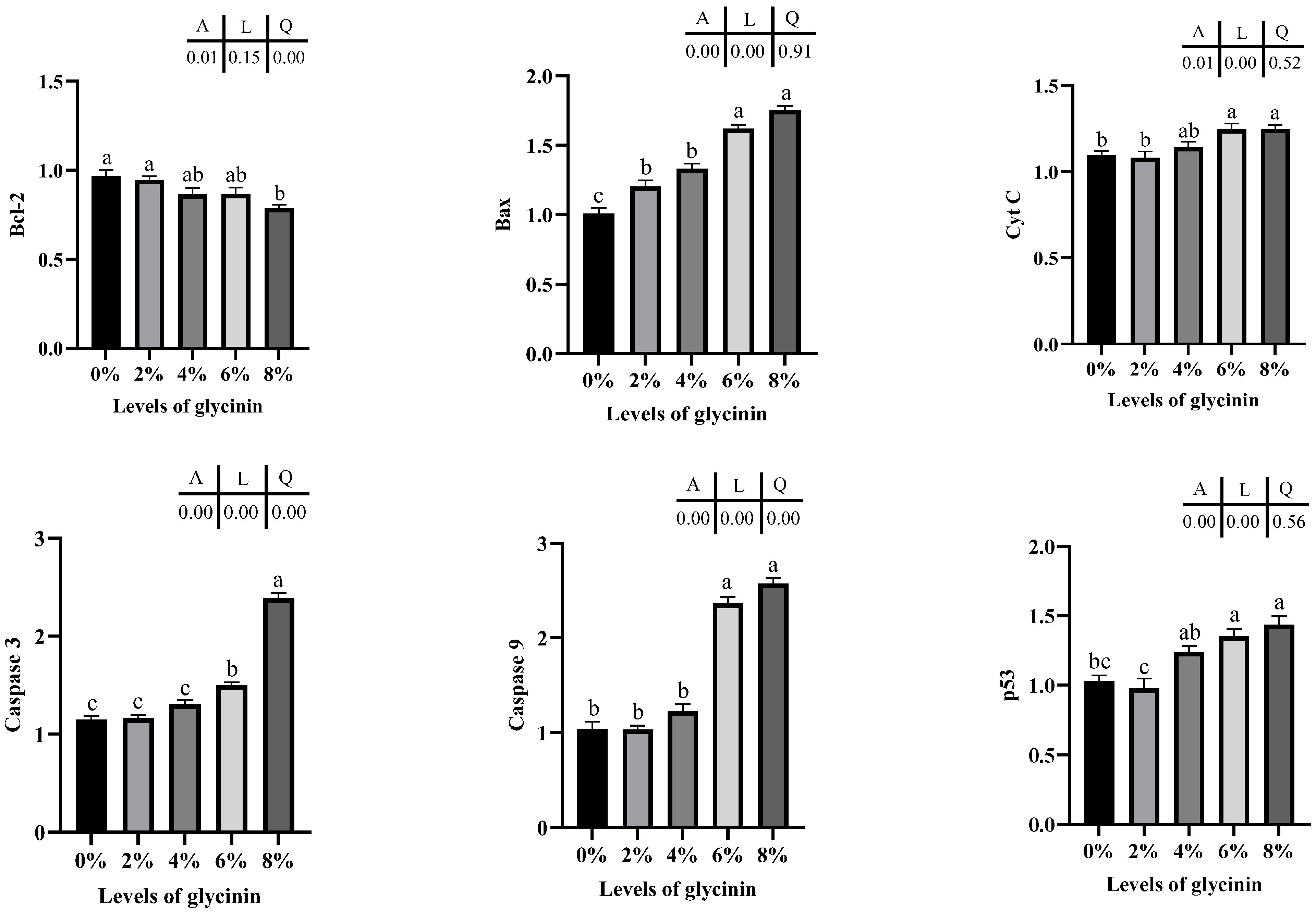
2.7. Apoptosis mRNA Expression Levels in Posterior Intestine
3. Discussion
4. Materials and Methods
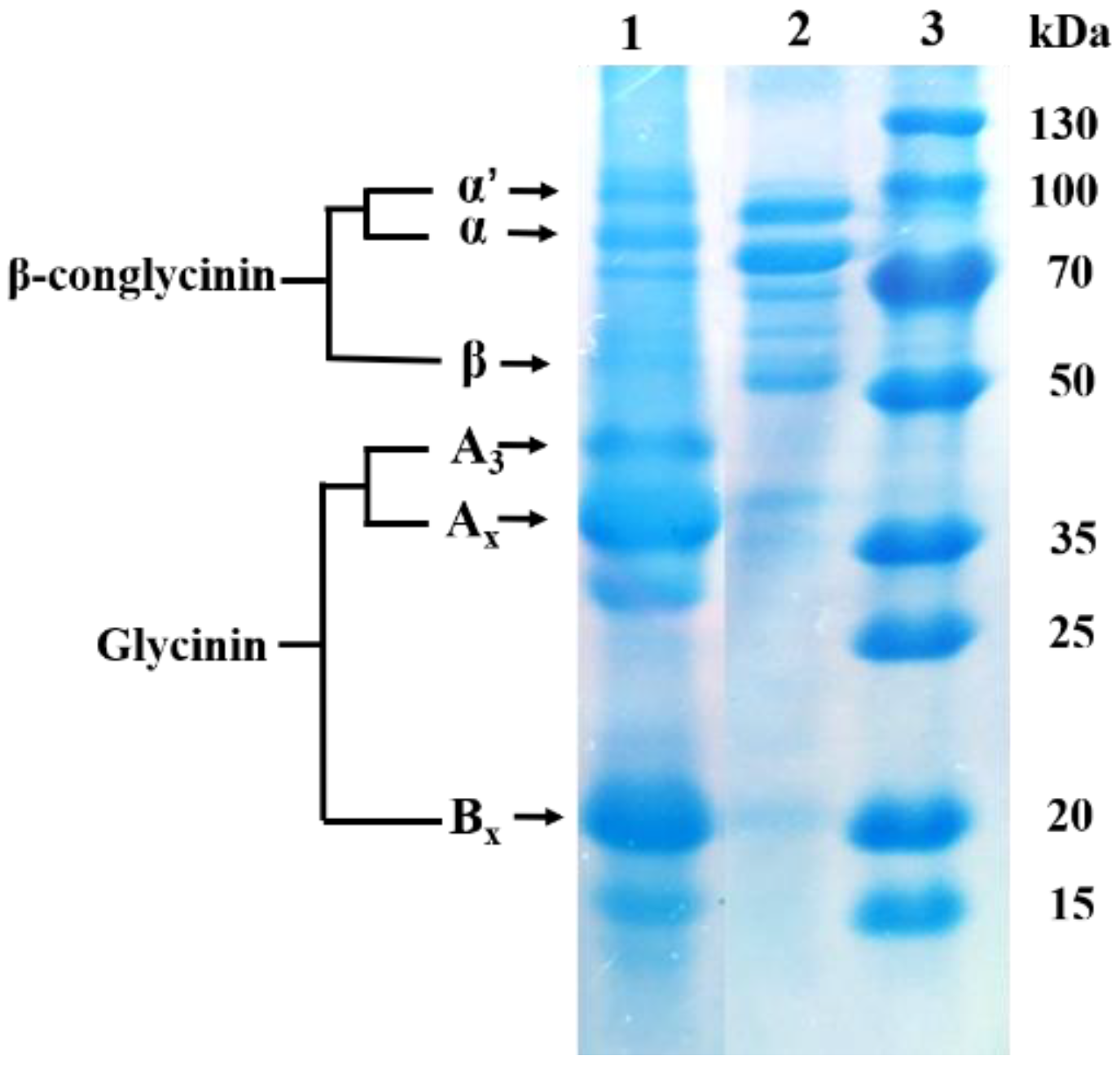
4.1. Glycinin and Diet Preparation
4.2. Growth Trial and Rearing Facilities
4.3. Sample Collection
4.4. Calculation Formula of Growth Indexes
4.5. Nutritive Composition Analysis
4.6. Histological Assessment
4.7. Enzyme Activity Determination
4.8. Analysis of Gene Expression in Posterior Intestine
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M.; Islam, J.; Maekawa, M.; Fujieda, T. Substitution of dietary fishmeal by soybean meal with inosine administration influences growth, digestibility, immunity, stress resistance and gut morphology of juvenile amberjack. Seriola Dumerili. Aquac. 2018, 488, 174–188. [Google Scholar] [CrossRef]
- Gu, M.; Bai, N.; Zhang, Y.Q.; Krogdahl, Å. Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 2016, 464, 286–295. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, S.; An, M.; Wang, Q.; Sun, J.; Fang, Z.; Xing, K. Effects of replacing fish meal with soybean meal, with or without dietary arginine, on growth performance, immune indices and intestinal morphology of grouper. Epinephelus Malabaricus. Aquac. Res. 2018, 49, 2954–2964. [Google Scholar] [CrossRef]
- Peng, K.S.; Wu, N.; Cui, Z.W.; Zhang, X.Y.; Lu, X.B.; Wang, Z.X.; Zhang, Y.A. Effect of the complete replacement of dietary fish meal by soybean meal on histopathology and immune response of the hindgut in grass carp (Ctenopharyngodon idellus). Vet. Immunol. Immunopathol. 2020, 221, 110009. [Google Scholar] [CrossRef]
- Zhu, R.; Li, L.; Li, M.; Yu, Z.; Wang, H.; Wu, L. The effects of substituting fish meal with soy protein concentrate on growth performance, antioxidant capacity and intestinal histology in juvenile golden crucian carp. Cyprinus Carpio× Carassius Auratus. Aquac. Rep. 2020, 18, 100435. [Google Scholar]
- Yu, X.; Wu, Z.; Guo, J.; Fu, Y.; Luo, K.; Guo, Y.; Zhang, W.; Mai, K. Replacement of dietary fish meal by soybean meal on growth performance, immunity, anti-oxidative capacity and mTOR pathways in juvenile abalone Haliotis discus hannai Ino. Aquaculture 2022, 551, 737914. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, L.; Zhang, C.X.; Song, K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquac. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- Gizzarelli, F.; Corinti, S.; Barletta, B.; Iacovacci, P.; Brunetto, B.; Butteroni, C.; Afferni, C.; Onori, R.; Miraglia, M.; Panzini, G.; et al. Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clin. Exp. Allergy 2006, 36, 238–248. [Google Scholar] [CrossRef]
- Yasothai, R. Antinutritional factors in soybean meal and its deactivation. Int. J. Sci. Environ. Technol. 2016, 5, 3793–3797. [Google Scholar]
- Hou, D.H.J.; Chang, S.K.C. Structural characteristics of purified glycinin from soybeans stored under various conditions. J. Agric. Food Chem. 2004, 52, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Robert, W.Y. Beta-conglycinin and glycinin in high-protein seeds. J. Agric. Food Chem. 2001, 49, 729–735. [Google Scholar]
- Barratt, M.E.; Strachan, P.J.; Porter, P. Antibody mechanisms implicated in digestive disturbances following ingestion of soya protein in calves and piglets. Clin. Exp. Immunol. 1978, 31, 305. [Google Scholar] [PubMed]
- Li, D.F.; Nelssen, J.L.; Reddy, P.G.; Blecha, F.; Klemm, R.; Goodband, R.D. Interrelationship between hypersensitivity to soybean proteins and growth performance in early-weaned pigs. J. Anim. Sci. 1991, 69, 4062–4069. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Oral allergy syndrome and anaphylactic reactions in BALB/c mice caused by soybean glycinin and β-conglycinin. Clin. Exp. Allergy 2008, 38, 350–356. [Google Scholar] [CrossRef]
- Wu, L.F.; Lai, H.E.; Yang, H.; Xing, X.P.; Wang, H.H.; Zhang, D.M.; Qin, G.X. Effects of soybean antigen protein glycinin on activities of protease and amylase in larval and juvenile common carps (Cyprinus carpio). J. Northw. A F Univ-Nat. Sci. Edn. 2013, 41, 30–36. [Google Scholar]
- Jiang, W.D.; Hu, K.; Zhang, J.X.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Soyabean glycinin depresses intestinal growth and function in juvenile Jian carp (Cyprinus carpio var Jian): Protective effects of glutamine. Br. J. Nutr. 2015, 114, 1569–1583. [Google Scholar] [CrossRef]
- Li, Y.X.; Yang, P.; Zhang, Y.J.; Ai, Q.H.; Xu, W.; Zhang, W.B.; Mai, K.S. Effects of dietary glycinin on the growth performance, digestion, intestinal morphology and bacterial community of juvenile turbot. Scophthalmus maximus L. Aquaculture 2017, 479, 125–133. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Kong, Y.D.; Zhu, R.; Yu, Z.; Wang, J.Y.; Duan, J.; Wu, L.F. Effects of glycinin on growth performance, immunity and antioxidant capacity in juvenile golden crucian carp, Cyprinus carpio× Carassius auratus. Aquac. Res. 2020, 51, 465–479. [Google Scholar] [CrossRef]
- Han, F.; Wang, X.; Guo, J.; Qi, C.; Xu, C.; Luo, Y.; Li, E.; Qin, J.G.; Chen, L. Effects of glycinin and β-conglycinin on growth performance and intestinal health in juvenile Chinese mitten crabs (Eriocheir sinensis). Fish Shellfish. Immunol. 2019, 84, 269–279. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Duan, X.D.; Jiang, W.D.; Feng, L.; Wu, P.; Liu, Y.; Zhou, X.Q. Soybean glycinin decreased growth performance, impaired intestinal health, and amino acid absorption capacity of juvenile grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2019, 45, 1589–1602. [Google Scholar] [CrossRef]
- Zhu, R.; Li, L.; Li, M.; Yu, Z.; Wang, H.H.; Quan, Y.N.; Wu, L.F. Effects of dietary glycinin on the growth performance, immunity, hepatopancreas and intestinal health of juvenile Rhynchocypris lagowskii Dybowski. Aquaculture 2021, 544, 737030. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Myklebust, R.; Whybrow, A.; Jenkins, R. Invited Reviews-Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol. Histopathol. 1999, 14, 257–268. [Google Scholar] [PubMed]
- Zhang, Y.L.; Duan, X.D.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Soybean glycinin disrupted intestinal structural integrity related to aggravation of apoptosis and downregulated transcription of tight junction proteins in the intestine of juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2021, 531, 735909. [Google Scholar] [CrossRef]
- Refstie, S.; Storebakken, T.; Baeverfjord, G.; Roem, A.J. Long-term protein and lipid growth of Atlantic salmon (Salmo salar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level. Aquaculture 2001, 193, 91–106. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Coves, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Rumsey, G.L.; Siwicki, A.K.; Anderson, D.P.; Bowser, P.R. Effect of soybean protein on serological response, non-specific defense mechanisms, growth, and protein utilization in rainbow trout. Vet. Immunol. Immunopathol. 1994, 41, 323–339. [Google Scholar] [CrossRef]
- Yu, G.; Ou, W.; Liao, Z.; Xu, H.; Liang, M.; Zhang, Y.; Mai, K. Intestinal homeostasis of juvenile tiger puffer Takifugu rubripes was sensitive to dietary arachidonic acid in terms of mucosal barrier and microbiota. Aquaculture 2019, 502, 97–106. [Google Scholar] [CrossRef]
- Wen, H.; Feng, L.; Jiang, W.; Liu, Y.; Jiang, J.; Li, S.; Tang, L.; Zhang, Y.; Kuang, S.; Zhou, X. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2014, 40, 275–287. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, G.; Feng, Y.; Kong, C.; Ayisi, C.L.; Huang, X.; Hua, X. Dietary soybean antigen impairs growth and health through stress-induced non-specific immune responses in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2019, 84, 124–129. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsumura, I.; Ezoe, S.; Satoh, Y.; Sakamaki, T.; Albanese, C.; Machii, T.; Pestell, R.G. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-κB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell 2002, 9, 1017–1029. [Google Scholar] [CrossRef]
- Hu, Y.; Benedict, M.A.; Ding, L.; Núñez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 1999, 18, 3586–3595. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Walker, G.; Srinivasula, S.M.; Sun, X.M.; Butterworth, M.; Alnemri, E.S.; Cohen, G.M. Recruitment, activation and retention of caspases-9 and-3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001, 20, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. Optimal dietary protein level improved growth, disease resistance, intestinal immune and physical barrier function of young grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2016, 55, 64–87. [Google Scholar] [CrossRef]
- Dalmo, R.A.; BØgwald, J. Distribution of intravenously and perorally administered Aeromonas salmonicida lipopolysaccharide in Atlantic salmon, Salmo salar L. Fish Shellfish. Immunol. 1996, 6, 427–441. [Google Scholar] [CrossRef]
- Hu, W.; Chu, Z.; Xiong, Y.; Mei, J.; Lin, Q.; Ren, F.; Huang, P.; Guo, W. Defective germplasm assembly and germ cell development contribute to hybrid sterility in yellow catfish. Aquac. Res. 2021, 52, 4166–4175. [Google Scholar] [CrossRef]
- Yao, J.T.; Kong, C.; Hua, X.M.; Yang, J.F.; Liu, T.; Wang, G.; Shi, Y.H. T1R1 expression in obscure puffer (Takifugu fasciatus) is associated with effect of dietary soybean antigenic protein on intestinal health. Aquaculture 2019, 501, 202–212. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. J. Funct. Foods 2015, 14, 469–478. [Google Scholar] [CrossRef]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.; Giorgi, C. The role of mitochondria in inflammation: From cancer to neurodegenerative disorders. J. Clin. Med. 2020, 9, 740. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Wang, F.; Yang, Z.; Yue, J.; Shi, J.; Ke, F.; Xie, Z.; Fan, Y. An identified PfHMGB1 promotes microcystin-LR-induced liver injury of yellow catfish (Pelteobagrus fulvidraco). Ecotoxicol. Environ. Saf. 2021, 207, 111266. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability byintestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Jameson, B.J.; Jesaitis., L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity and gut microbiota community on rice field eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, T.; Zhou, X.Q.; Liu, Y.; Feng, L. Influence of glutamine and vitamin E on growth and antioxidant capacity of fish enterocytes. Aquacult. Nutr. 2009, 15, 409–414. [Google Scholar] [CrossRef]
- Zavalova, L.L.; Yudina, T.G.; Artamonova, I.I.; Baskova, I.P. Antibacterial non-glycosidase activity of invertebrate destabilase-lysozyme and of its helical amphipathic peptides. Chemotherapy 2006, 52, 158–160. [Google Scholar] [CrossRef]
- Mann, J.K.; Ndungu, T. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Future Virol. 2020, 15, 609–624. [Google Scholar] [CrossRef]
- Yang, C.; Hao, R.; Deng, Y.; Liao, Y.; Wang, Q.; Sun, R.; Du, X. Effects of protein sources on growth, immunity and antioxidant capacity of juvenile pearl oyster Pinctada fucata martensii. Fish Shellfish. Immunol. 2017, 67, 411–418. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jiang, W.D.; Duan, X.D.; Feng, L.; Wu, P.; Liu, Y.; Zhou, X.Q. Soybean glycinin caused NADPH-oxidase-regulated ROS overproduction and decreased ROS elimination capacity in the mid and posterior intestine of juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2020, 516, 734651. [Google Scholar] [CrossRef]
- Marzo, I.; Brenner, C.; Zamzami, N.; Jürgensmeier, J.M.; Susin, S.A.; Vieira, H.L.; Prévost, M.C.; Xie, Z.; Matsuyama, S.; Reed, J.C.; et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 1998, 281, 2027–2031. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Zhang, B.; Wang, T. Effects of glycinin and β-conglycinin on enterocyte apoptosis, proliferation and migration of piglets. Food Agric. Immunol. 2010, 21, 209–218. [Google Scholar] [CrossRef]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000, 14, 34–44. [Google Scholar] [CrossRef]
- Szpotkowska, J.; Szpotkowski, K.; Ciesiołka, J. Structural Characteristics of the 5′-Terminal Region of Mouse p53 mRNA and Identification of Proteins That Bind to This mRNA Region. Int. J. Mol. Sci. 2022, 23, 9709. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.R.; Kumar, S.; Agarwal, G.G.; Ranjan, P. Blunt abdominal injury: Serum ALT-a marker of liver injury and a guide to assessment of its severity. Injury 2007, 38, 1069–1074. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mai, K.; Xu, W.; Deng, J.; Cheng, Z. Comparison of high-protein soybean meal and commercial soybean meal partly replacing fish meal on the activities of digestive enzymes and aminotransferases in juvenile Japanese seabass. Lateolabrax japonicus (Cuvier, 1828). Aquac. Res. 2014, 45, 1051–1060. [Google Scholar] [CrossRef]
- Billiar, T.R.; Curran, R.D.; Harbrecht, B.G.; Stuehr, D.J.; Demetris, A.J.; Simmons, R.A. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J. Leukoc. Biol. 1990, 48, 565–569. [Google Scholar] [CrossRef]
- Ng, Q.S.; Goh, V.; Milner, J.; Stratford, M.R.; Folkes, L.K.; Tozer, G.M.; Saunders, M.I.; Hoskin, P.J. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: A phase I study. Lancet Oncol. 2007, 8, 111–118. [Google Scholar] [CrossRef]
- Karatopuk, D.U.; Gökçımen, A. Effect of tenoxicam on rat liver tissue. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2010, 21, 146–152. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Rombenso, A.N.; Guerrero-Rentería, Y.; Nomura- Solís, M.; Gomez-Calle, V.; Mata-Sotres, J.A. Enteritis induction by soybean meal in Totoaba macdonaldi diets: Effects on growth performance, digestive capacity, immune response and distal intestine integrity. Aquaculture 2018, 495, 78–89. [Google Scholar] [CrossRef]
- Li, P.Y.; Wang, J.Y.; Song, Z.D.; Zhang, L.M.; Zhang, H.; Li, X.X.; Pan, Q. Evaluation of soy protein concentrate as a substitute for fishmeal in diets for juvenile starry flounder (Platichthys stellatus). Aquaculture 2015, 448, 578–585. [Google Scholar] [CrossRef]
- Lie, Ø.; Lied, E.; Lambertsen, G. Feed optimization in Atlantic cod (Gadus morhua): Fat versus protein content in the feed. Aquaculture 1988, 69, 333–341. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Baeza-Ariño, R.; Nogales-Mérida, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Carob seed germ meal as a partial substitute in gilthead sea bream (Sparus aurata) diets: Amino acid retention, digestibility, gut and liver histology. Aquaculture 2012, 338, 124–133. [Google Scholar] [CrossRef]
- Thanh, V.H.; Shibasaki, K. Major proteins of soybean seeds. A straightforward fractionation and their characterization. J. Agric. Food Chem. 1976, 24, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, K.R.; García-Ortega, A.; Trushenski, J.T. Partial fish meal replacement by soy protein concentrate, squid and algal meals in low fish-oil diets containing Schizochytrium limacinum for longfin yellowtail Seriola rivoliana. Aquaculture 2016, 452, 37–44. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Levels of Glycinin | Pr > F 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | ANOVA | Linear | Quadratic | |
| IW (g) | 1.03 ± 0.02 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.04 ± 0.01 | 1.01 ± 0.01 | 0.26 | 0.85 | 0.72 |
| FW (g) | 6.82 ± 0.37 a | 5.85 ± 0.11 b | 5.67 ± 0.06 b | 3.76 ± 0.29 c | 3.34 ± 0.20 c | 0.00 | 0.00 | 0.49 |
| FI (%/d) | 2.90 ± 0.03 a | 2.80 ± 0.02 ab | 2.78 ± 0.02 ab | 2.77 ± 0.05 b | 2.70 ± 0.02 b | 0.01 | 0.00 | 0.50 |
| WGR (%) | 562.27 ± 25.75 a | 475.09 ± 15.95 b | 458.27 ± 5.25 b | 261.02 ± 29.72 c | 229.89 ± 17.16 c | 0.00 | 0.00 | 0.40 |
| SGR (%/d) | 3.20 ± 0.06 a | 2.96 ± 0.05 a | 2.91 ± 0.04 a | 2.16 ± 0.14 b | 2.02 ± 0.08 b | 0.00 | 0.00 | 0.14 |
| FCR | 1.20 ± 0.01 b | 1.17 ± 0.02 b | 1.27 ± 0.01 b | 1.65 ± 0.08 a | 1.83 ± 0.18 a | 0.00 | 0.00 | 0.06 |
| PER (%) | 1.90 ± 0.02 a | 1.95 ± 0.03 a | 1.88 ± 0.02 a | 1.47 ± 0.05 b | 1.35 ± 0.04 b | 0.00 | 0.00 | 0.00 |
| SR (%) | 87.22 ± 0.96 a | 87.77 ± 1.93 a | 76.67 ± 1.28 b | 78.33 ± 2.25 b | 71.44 ± 2.75 b | 0.00 | 0.00 | 0.76 |
| VSI (%) | 8.02 ± 0.85 | 8.17 ± 0.77 | 8.25 ± 0.83 | 7.99 ± 0.81 | 9.09 ± 0.80 | 0.86 | 0.46 | 0.62 |
| HSI (%) | 2.11 ± 0.19 | 2.08 ± 0.18 | 2.02 ± 0.12 | 1.99 ± 0.19 | 2.04 ± 0.23 | 0.99 | 0.70 | 0.79 |
| CF (g/cm3) | 1.66 ± 0.04 | 1.70 ± 0.08 | 1.65 ± 0.04 | 1.56 ± 0.02 | 1.70 ± 0.08 | 0.46 | 0.72 | 0.54 |
| Levels of Glycinin | Pr > F 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | ANOVA | Linear | Quadratic | |
| Whole fish | ||||||||
| Moisture | 75.09 ± 0.24 d | 75.44 ± 0.26 cd | 76.30 ± 0.24 bc | 77.12 ± 0.22 ab | 77.54 ± 0.24 a | 0.00 | 0.00 | 0.92 |
| Ash | 3.54 ± 0.03 | 3.36 ± 0.06 | 3.35 ± 0.05 | 3.43 ± 0.14 | 3.31 ± 0.15 | 0.55 | 0.25 | 0.58 |
| Crude lipid | 5.65 ± 0.12 a | 5.20 ± 0.12 a | 4.50 ± 0.16 b | 3.79 ± 0.08 c | 3.79 ± 0.0.09 c | 0.00 | 0.00 | 0.07 |
| Crude protein | 15.10 ± 0.16 | 14.85 ± 0.32 | 14.84 ± 0.09 | 14.57 ± 0.34 | 14.61 ± 0.33 | 0.64 | 0.16 | 0.76 |
| Muscle | ||||||||
| Moisture | 79.86 ± 0.22 ab | 79.36 ± 0.21 b | 79.42 ± 0.26 b | 79.59 ± 0.12 b | 80.63 ± 0.24 a | 0.01 | 0.03 | 0.00 |
| Ash | 1.27 ± 0.05 | 1.33 ± 0.02 | 1.29 ± 0.01 | 1.33 ± 0.01 | 1.25 ± 0.03 | 0.26 | 0.66 | 0.17 |
| Crude lipid | 1.94 ± 0.09 b | 2.47 ± 0.09 a | 2.10 ± 0.05 b | 1.89 ± 0.06b | 1.20 ± 0.08 c | 0.00 | 0.00 | 0.00 |
| Crude protein | 16.33 ± 0.32 | 16.25 ± 0.18 | 16.48 ± 0.21 | 16.40 ± 0.29 | 15.97 ± 0.13 | 0.39 | 0.47 | 0.14 |
| Levels of Glycinin | Pr > F 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | ANOVA | Linear | Quadratic | |
| Relative height of fold (%) | 66.23 ± 0.43 a | 59.11 ± 0.55 b | 50.80 ± 0.53 c | 50.68 ± 0.63 c | 48.47 ± 0.71 d | 0.00 | 0.00 | 0.00 |
| Crypt depth (μm) | 31.69 ± 0.46 c | 28.88 ± 0.46 d | 38.09 ± 0.50 a | 39.40 ± 0.61 a | 33.74 ± 0.44 b | 0.00 | 0.00 | 0.00 |
| Thickness of muscle layer (μm) | 27.78 ± 0.36 | 27.59 ± 0.49 | 26.85 ± 0.46 | 27.53 ± 0.42 | 27.45 ± 0.64 | 0.70 | 0.63 | 0.37 |
| Ingredients (%) | Levels of Glycinin | ||||
|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | |
| White fishmeal | 40.00 | 32.00 | 24.00 | 16.00 | 8.00 |
| Chicken powder | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Corn gluten flour | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Glycinin | 0.00 | 2.08 | 4.16 | 6.24 | 8.32 |
| Casein | 0.00 | 2.72 | 5.44 | 8.16 | 10.88 |
| Soybean phospholipid oil | 5.00 | 5.60 | 6.20 | 6.80 | 7.40 |
| High gluten flour | 27.50 | 30.10 | 32.70 | 35.30 | 37.90 |
| Choline chloride | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Mineral premix 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Sodium alginate | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Antioxidant | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Anti-mildew agent | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Betaine | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Chromium trioxide | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Nutritional composition | |||||
| Moisture | 12.59 | 12.96 | 12.51 | 12.92 | 12.88 |
| Crude protein | 42.41 | 42.19 | 42.39 | 42.17 | 42.62 |
| Crude lipid | 9.23 | 9.14 | 9.73 | 9.13 | 9.38 |
| Ash | 8.80 | 8.92 | 8.71 | 8.30 | 8.14 |
| Gene Name | Primer Sequence (5′ to 3′) | GenBank Number | |
|---|---|---|---|
| Claudin-1 | Forward | ACGCTAACAACGGCTCAGA | XM_027148337.1 |
| Reverse | CCTTACATTCAGACACCACCTT | ||
| Occludin | Forward | CGAGCGAGAGACTACGACAC | XM_027141818.1 |
| Reverse | TCCAGGAATTGTGGGCTTCC | ||
| ZO-1 | Forward | GCGAACTCTCTGAACAGCCT | XM_027154070.1 |
| Reverse | TGTGTGTGTGCAGGAGGTTT | ||
| Bcl-2 | Forward | TTTAGACAAACGGGGCAGGG | XM_027170693.1 |
| Reverse | AGCTCCATCATCTGCCCCTA | ||
| Bax | Forward | TTTCTGGCCGGAGTTCTCAC | XM_027161178.2 |
| Reverse | TGGATCTGCCCCTTATTGCC | ||
| Cyt C | Forward | GCAGGATACGAGCAAGAT | XM_027159893.2 |
| Reverse | TACACGGATGCCAACAAG | ||
| Casepase 3 | Forward | GTCGTGAGGCAGTCGGTATT | XM_027134813.2 |
| Reverse | TAATGGCTCGGATGCAGGTC | ||
| Casepase 9 | Forward | GCTTCCCTGGAGCAGTTCAT | XM_047807446.1 |
| Reverse | TGCTGTACCTCATCGGGAGA | ||
| p53 | Forward | CTTCCTACAGGCTTTAGACAA | XM_047808967.1 |
| Reverse | GTAAGAAATCCAAGAACACCA | ||
| β-actin | Forward | CCACTTCCCCCACATCACTG | XM_027142941.1 |
| Reverse | AGAACCCCAGGAAAGCTCACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.; Liu, J.; Yang, H.; Mo, A.; Zhai, Y.; Wang, S.; Yuan, Y. Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂. Int. J. Mol. Sci. 2022, 23, 11198. https://doi.org/10.3390/ijms231911198
Yi L, Liu J, Yang H, Mo A, Zhai Y, Wang S, Yuan Y. Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂. International Journal of Molecular Sciences. 2022; 23(19):11198. https://doi.org/10.3390/ijms231911198
Chicago/Turabian StyleYi, Linyuan, Jingwen Liu, Huijun Yang, Aijie Mo, Yuxiang Zhai, Siru Wang, and Yongchao Yuan. 2022. "Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂" International Journal of Molecular Sciences 23, no. 19: 11198. https://doi.org/10.3390/ijms231911198
APA StyleYi, L., Liu, J., Yang, H., Mo, A., Zhai, Y., Wang, S., & Yuan, Y. (2022). Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂. International Journal of Molecular Sciences, 23(19), 11198. https://doi.org/10.3390/ijms231911198






