Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes
Abstract
1. Introduction
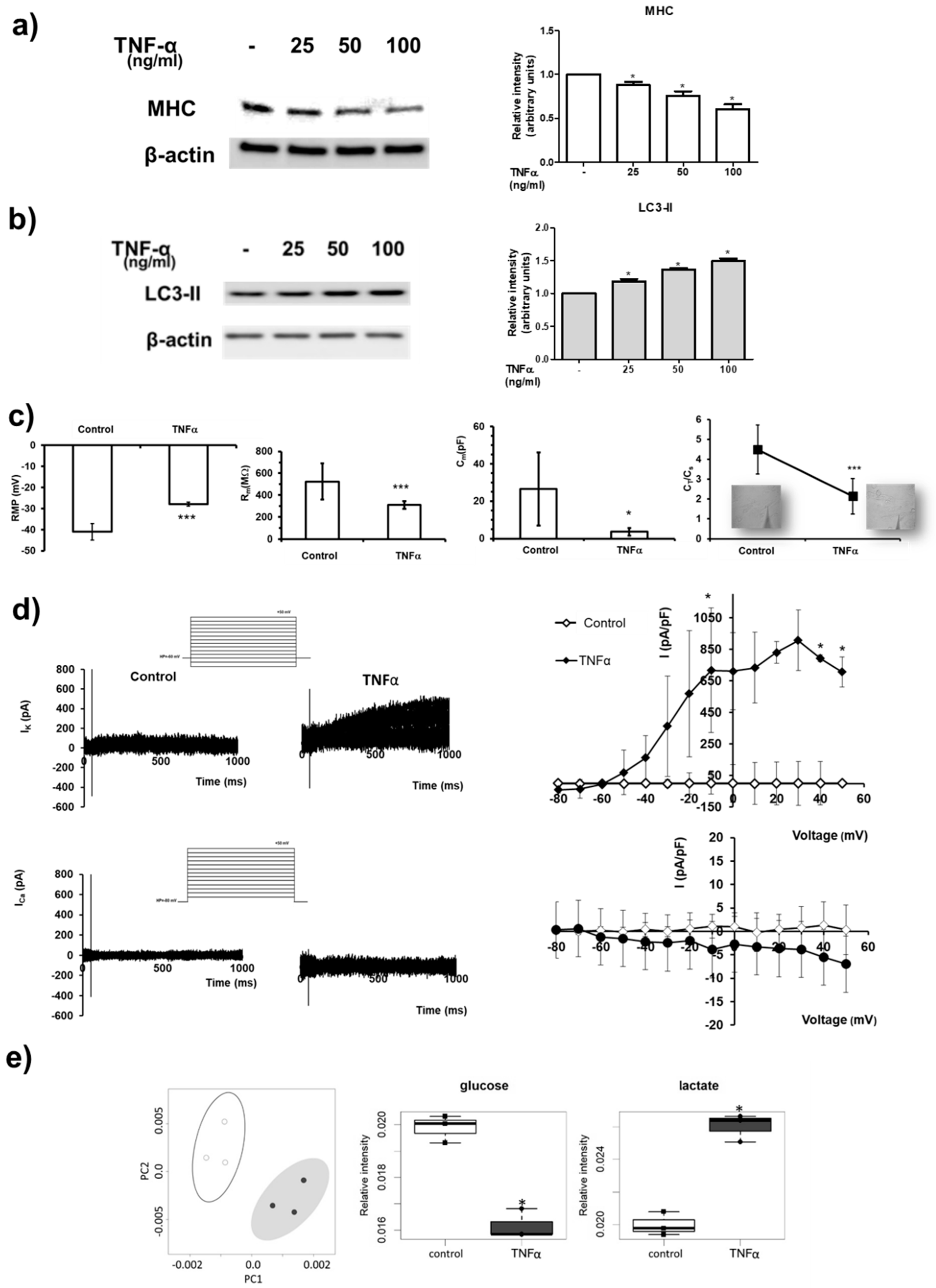
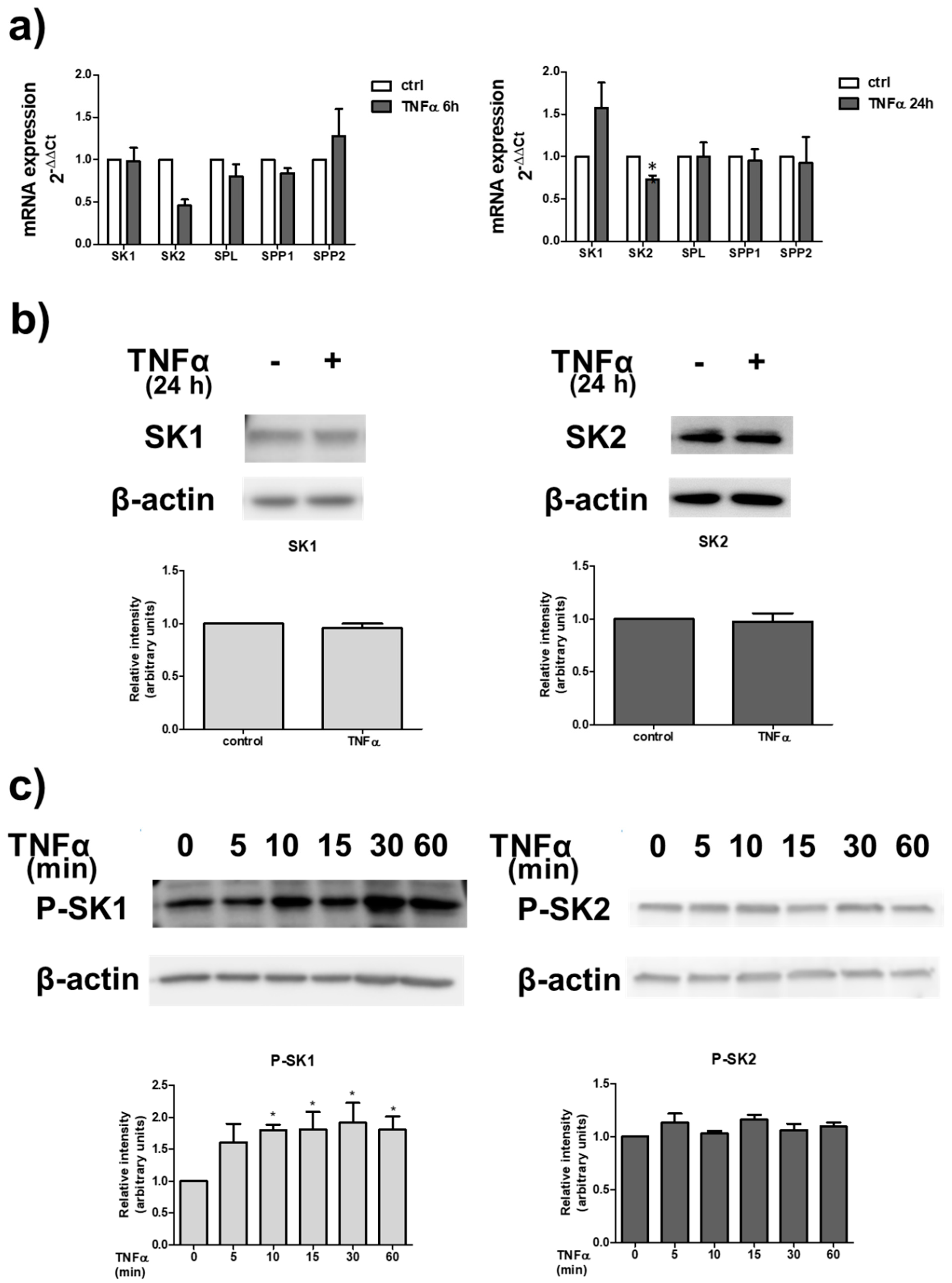
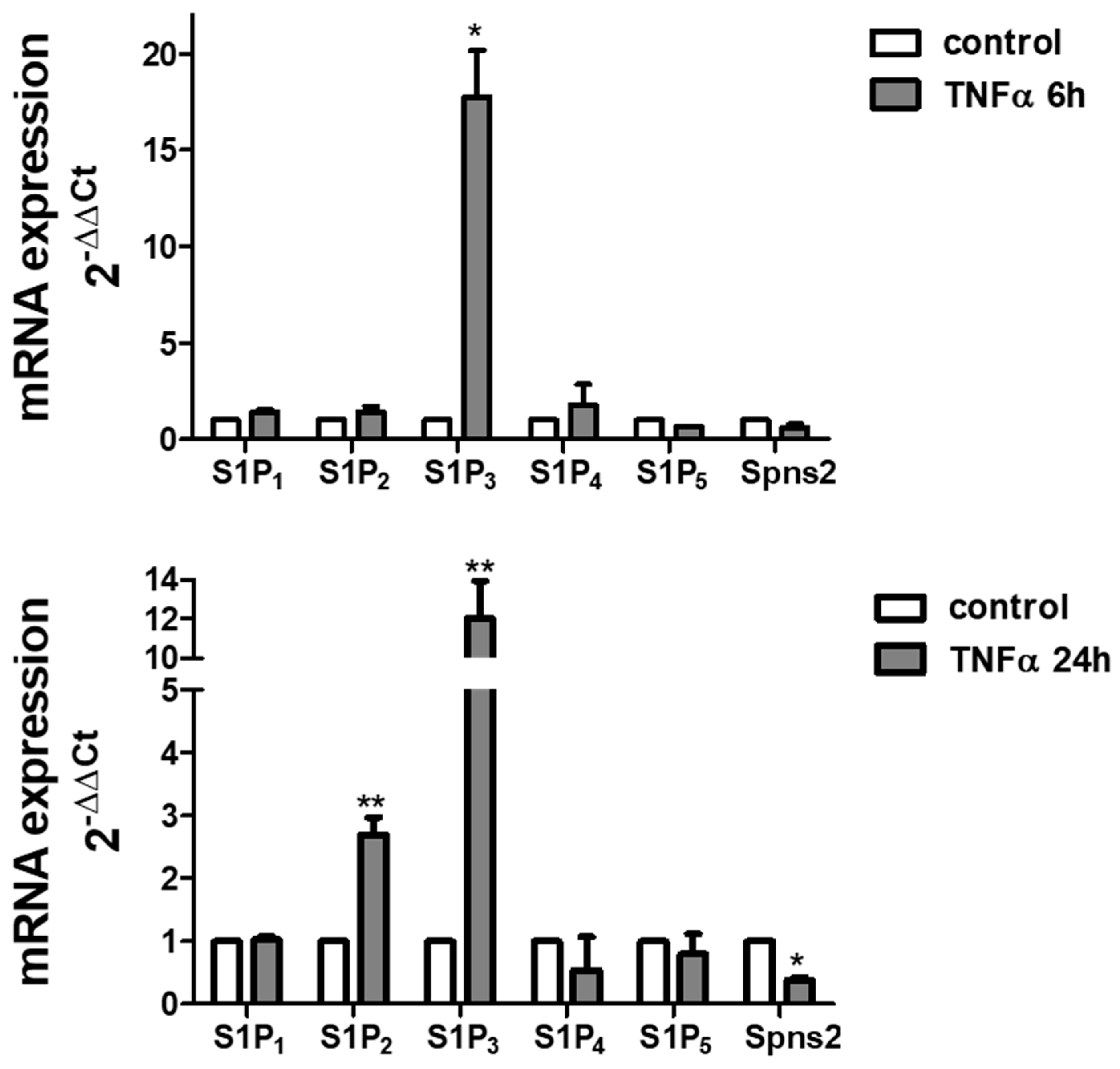
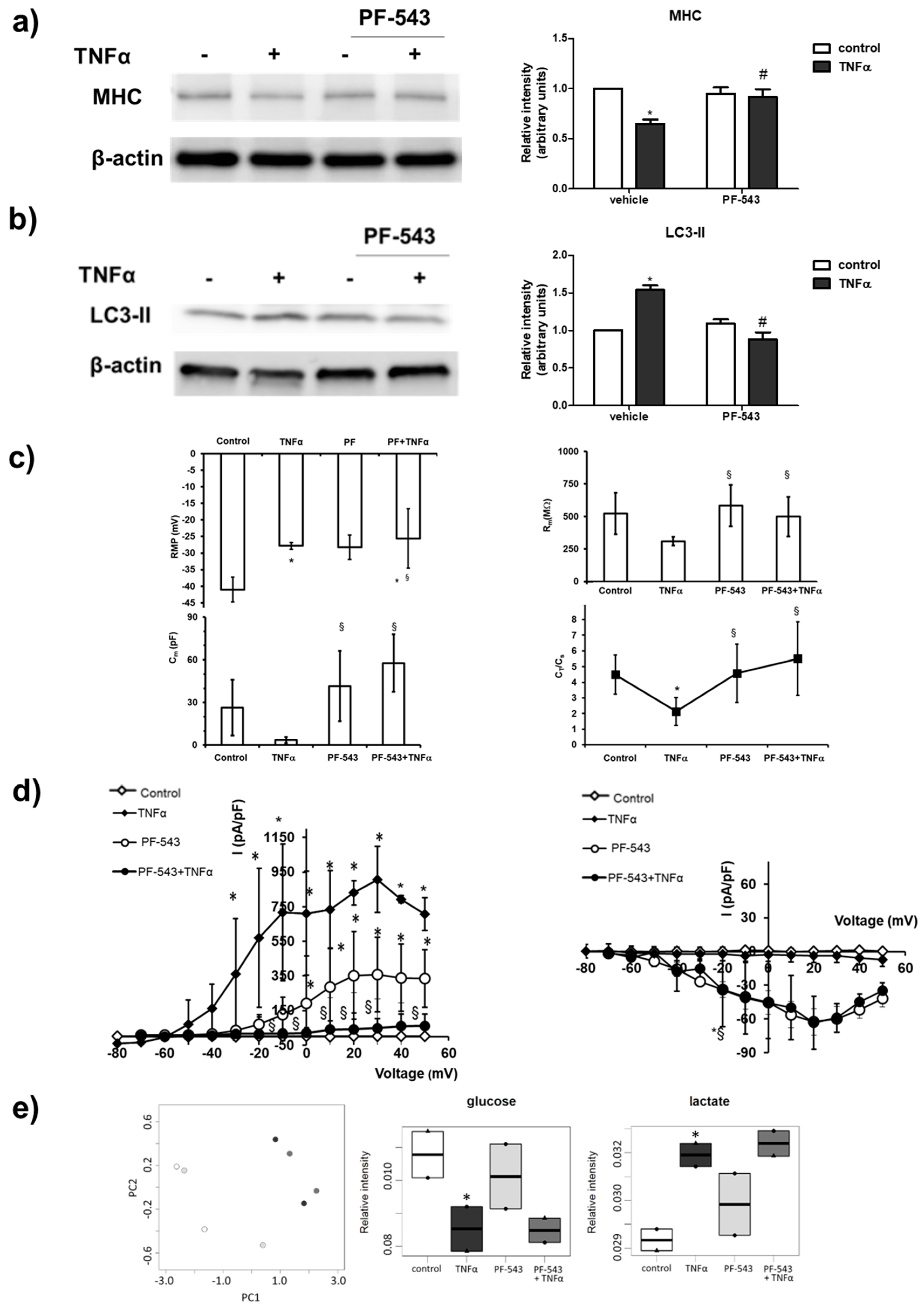
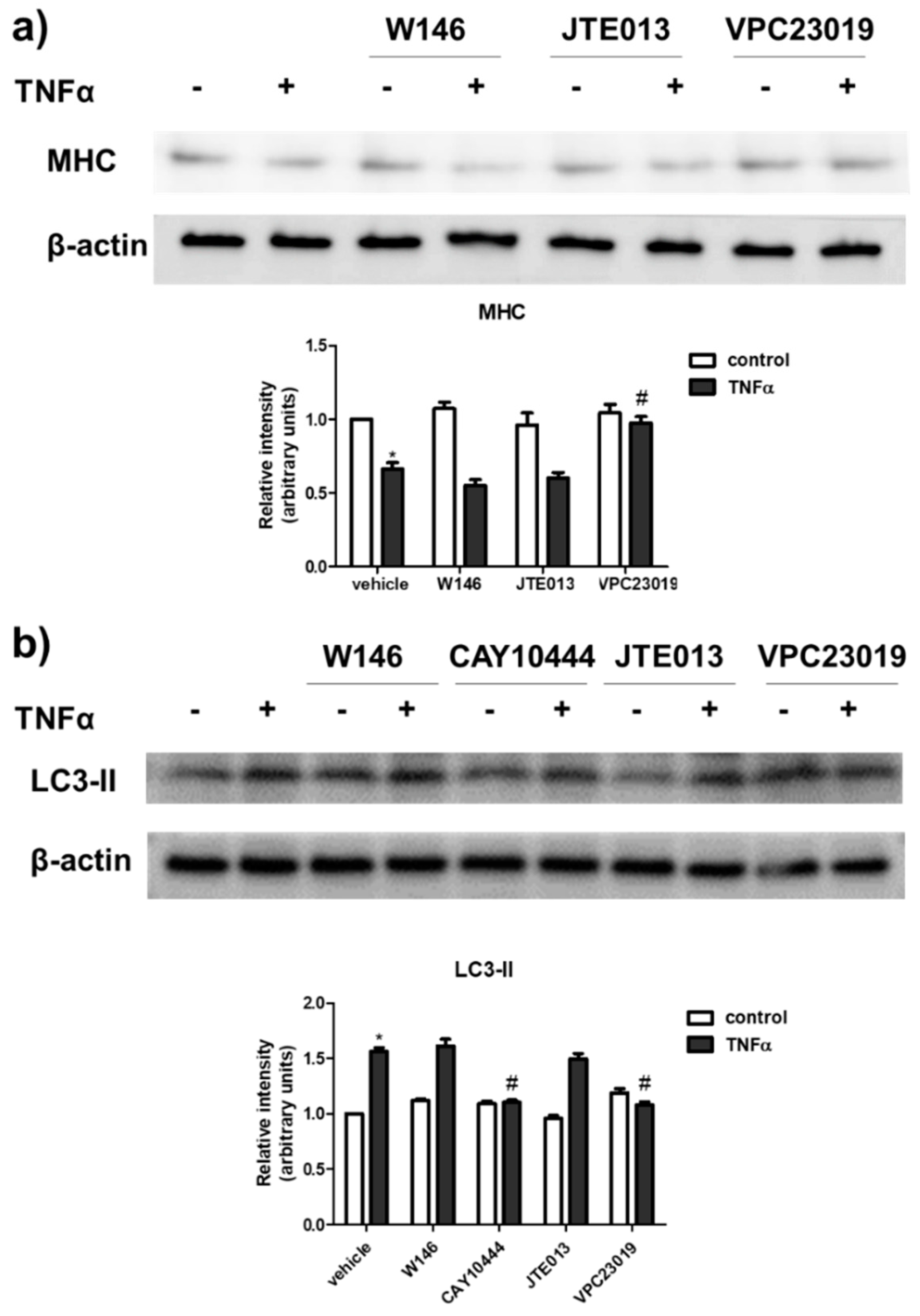
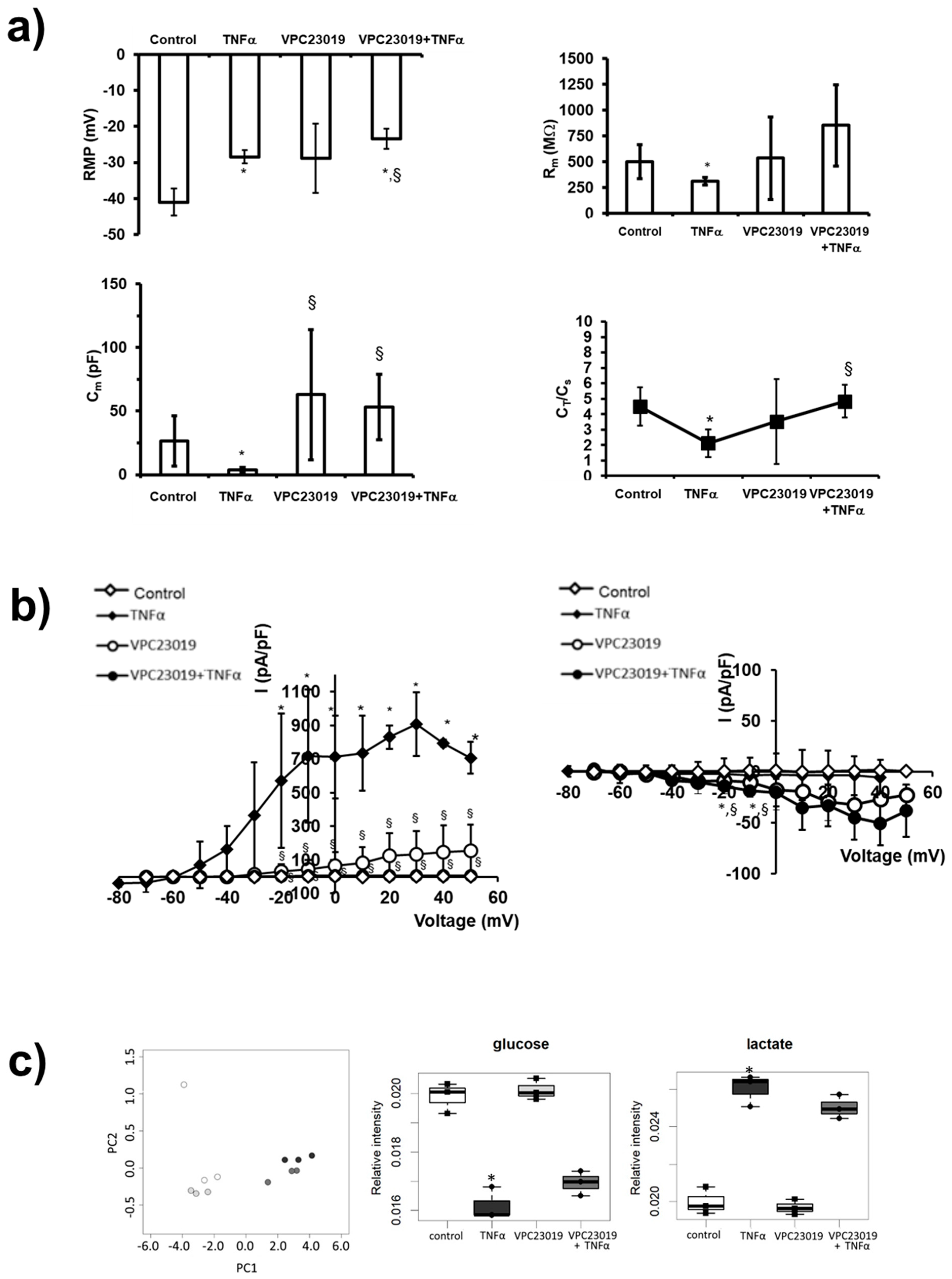
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Quantitative Real-Time Reverse Transcription PCR
4.5. NMR-Based Metabolomic Analyses
4.6. Electrophysiology
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S1P | sphingosine 1-phosphate |
| TNFα | tumor necrosis factor alpha |
| S1PR | sphingosine 1-phosphate receptors |
| SK | sphingosine kinase |
| SPL | S1P lyase |
| SPP | S1P phosphatases |
| LPP | lipid phosphate phosphatases |
| Spns2 | spinster homolog 2 |
| NMR | Nuclear magnetic resonance |
| RMP | resting membrane potential |
| Rm | membrane resistance |
| Cm | cell capacitance |
| PCA | principal component analysis |
References
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms Regulating Skeletal Muscle Growth and Atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, E.; Pichard, C.; Greenwood, C.E.; Kuo, G.C.; Cameron, R.G.; Kurian, R.; Kearns, J.P.; Allard, J.P.; Jeejeebhoy, K.N. Body Composition and Metabolic Rate in Rat during a Continuous Infusion of Cachectin. Am. J. Physiol. 1991, 260, E27–E36. [Google Scholar] [CrossRef]
- Buck, M.; Chojkier, M. Muscle Wasting and Dedifferentiation Induced by Oxidative Stress in a Murine Model of Cachexia Is Prevented by Inhibitors of Nitric Oxide Synthesis and Antioxidants. EMBO J. 1996, 15, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Frost, R.A.; Nairn, A.C.; MacLean, D.A.; Vary, T.C. TNF-Alpha Impairs Heart and Skeletal Muscle Protein Synthesis by Altering Translation Initiation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E336–E347. [Google Scholar] [CrossRef] [PubMed]
- Llovera, M.; López-Soriano, F.J.; Argilés, J.M. Chronic Tumour Necrosis Factor-Alpha Treatment Modifies Protein Turnover in Rat Tissues. Biochem. Mol. Biol. Int. 1993, 30, 29–36. [Google Scholar]
- Costelli, P.; Carbó, N.; Tessitore, L.; Bagby, G.J.; Lopez-Soriano, F.J.; Argilés, J.M.; Baccino, F.M. Tumor Necrosis Factor-Alpha Mediates Changes in Tissue Protein Turnover in a Rat Cancer Cachexia Model. J. Clin. Investig. 1993, 92, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Karlstad, M.D.; Choudhry, M.A.; Sayeed, M.M. Sepsis-Induced Myofibrillar Protein Catabolism in Rat Skeletal Muscle. Life Sci. 1994, 55, 1383–1391. [Google Scholar] [CrossRef]
- Li, Y.P.; Schwartz, R.J.; Waddell, I.D.; Holloway, B.R.; Reid, M.B. Skeletal Muscle Myocytes Undergo Protein Loss and Reactive Oxygen-Mediated NF-KappaB Activation in Response to Tumor Necrosis Factor Alpha. FASEB J. 1998, 12, 871–880. [Google Scholar] [CrossRef]
- Li, Y.P.; Atkins, C.M.; Sweatt, J.D.; Reid, M.B. Mitochondria Mediate Tumor Necrosis Factor-Alpha/NF-KappaB Signaling in Skeletal Muscle Myotubes. Antioxid. Redox Signal. 1999, 1, 97–104. [Google Scholar] [CrossRef]
- Tijerina, A.J. The Biochemical Basis of Metabolism in Cancer Cachexia. Dimens. Crit. Care Nurs. 2004, 23, 237–243. [Google Scholar] [CrossRef]
- Tisdale, M.J. Metabolic Abnormalities in Cachexia and Anorexia. Nutrition 2000, 16, 1013–1014. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling Pathways of the TNF Superfamily: A Double-Edged Sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Truong, T.-G.; Hannun, Y.A. Role for Neutral Sphingomyelinase-2 in Tumor Necrosis Factor Alpha-Stimulated Expression of Vascular Cell Adhesion Molecule-1 (VCAM) and Intercellular Adhesion Molecule-1 (ICAM) in Lung Epithelial Cells: P38 MAPK Is an Upstream Regulator of NSMase2. J. Biol. Chem. 2007, 282, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Cloessner, E.A.; Roddy, P.L.; Hannun, Y.A. Neutral Sphingomyelinase 2 (NSMase2) Is the Primary Neutral Sphingomyelinase Isoform Activated by Tumour Necrosis Factor-α in MCF-7 Cells. Biochem. J. 2011, 435, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Cartier, A.; Hla, T. Sphingosine 1-Phosphate: Lipid Signaling in Pathology and Therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An Update on the Biology of Sphingosine 1-Phosphate Receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Spiegel, S.; Maczis, M.A.; Maceyka, M.; Milstien, S. New Insights into Functions of the Sphingosine-1-Phosphate Transporter SPNS2. J. Lipid Res. 2019, 60, 484–489. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kawasaki-Nishi, S.; Otsuka, M.; Hisano, Y.; Yamaguchi, A.; Nishi, T. MFSD2B Is a Sphingosine 1-Phosphate Transporter in Erythroid Cells. Sci. Rep. 2018, 8, 4969. [Google Scholar] [CrossRef]
- Mitra, P.; Oskeritzian, C.; Spiegel, S. The Role of ABC Transporters in Sphingosine-1-Phosphate Secretion. FASEB J. 2007, 21, A604. [Google Scholar] [CrossRef]
- Donati, C.; Cencetti, F.; Bruni, P. Sphingosine 1-Phosphate Axis: A New Leader Actor in Skeletal Muscle Biology. Front. Physiol. 2013, 4, 338. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Partridge, T.A.; Matsuda, R.; Zammit, P.S. Entry of Muscle Satellite Cells into the Cell Cycle Requires Sphingolipid Signaling. J. Cell Biol. 2006, 174, 245–253. [Google Scholar] [CrossRef]
- Nagata, Y.; Ohashi, K.; Wada, E.; Yuasa, Y.; Shiozuka, M.; Nonomura, Y.; Matsuda, R. Sphingosine-1-Phosphate Mediates Epidermal Growth Factor-Induced Muscle Satellite Cell Activation. Exp. Cell Res. 2014, 326, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Ieronimakis, N.; Pantoja, M.; Hays, A.L.; Dosey, T.L.; Qi, J.; Fischer, K.A.; Hoofnagle, A.N.; Sadilek, M.; Chamberlain, J.S.; Ruohola-Baker, H.; et al. Increased Sphingosine-1-Phosphate Improves Muscle Regeneration in Acutely Injured Mdx Mice. Skelet. Muscle 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-Phosphate Regulates Myogenic Differentiation: A Major Role for S1P2 Receptor. FASEB J. 2005, 19, 449–451. [Google Scholar] [CrossRef]
- Becciolini, L.; Meacci, E.; Donati, C.; Cencetti, F.; Rapizzi, E.; Bruni, P. Sphingosine 1-Phosphate Inhibits Cell Migration in C2C12 Myoblasts. Biochim. Biophys. Acta 2006, 1761, 43–51. [Google Scholar] [CrossRef]
- Nincheri, P.; Bernacchioni, C.; Cencetti, F.; Donati, C.; Bruni, P. Sphingosine Kinase-1/S1P1 Signalling Axis Negatively Regulates Mitogenic Response Elicited by PDGF in Mouse Myoblasts. Cell. Signal. 2010, 22, 1688–1699. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Cencetti, F.; Blescia, S.; Donati, C.; Bruni, P. Sphingosine Kinase/Sphingosine 1-Phosphate Axis: A New Player for Insulin-like Growth Factor-1-Induced Myoblast Differentiation. Skelet. Muscle 2012, 2, 15. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Germinario, E.; Esposito, A.; Megighian, A.; Midrio, M.; Ravara, B.; Damiani, E.; Libera, L.D.; Sabbadini, R.A.; Betto, R. Sphingosine 1-Phosphate Protects Mouse Extensor Digitorum Longus Skeletal Muscle during Fatigue. Am. J. Physiol. Cell Physiol. 2005, 288, C1367–C1373. [Google Scholar] [CrossRef]
- Zanin, M.; Germinario, E.; Dalla Libera, L.; Sandonà, D.; Sabbadini, R.A.; Betto, R.; Danieli-Betto, D. Trophic Action of Sphingosine 1-Phosphate in Denervated Rat Soleus Muscle. Am. J. Physiol. Cell Physiol. 2008, 294, C36–C46. [Google Scholar] [CrossRef]
- Donati, C.; Nincheri, P.; Cencetti, F.; Rapizzi, E.; Farnararo, M.; Bruni, P. Tumor Necrosis Factor-Alpha Exerts pro-Myogenic Action in C2C12 Myoblasts via Sphingosine Kinase/S1P2 Signaling. FEBS Lett. 2007, 581, 4384–4388. [Google Scholar] [CrossRef] [PubMed]
- Langen, R.C.J.; Van Der Velden, J.L.J.; Schols, A.M.W.J.; Kelders, M.C.J.M.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W. Tumor Necrosis Factor-Alpha Inhibits Myogenic Differentiation through MyoD Protein Destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef] [PubMed]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, D.; Vidal, H.; et al. TNF-α- and Tumor-Induced Skeletal Muscle Atrophy Involves Sphingolipid Metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Carraro, U.; Kern, H.; Pond, A.; Adami, N.; Biral, D.; Vindigni, V.; Boncompagni, S.; Pietrangelo, T.; Bosco, G.; et al. A Subpopulation of Rat Muscle Fibers Maintains an Assessable Excitation-Contraction Coupling Mechanism After Long-Standing Denervation Despite Lost Contractility. J. Neuropathol. Exp. Neurol. 2009, 68, 1256–1268. [Google Scholar] [CrossRef]
- Pitson, S.M.; Xia, P.; Leclercq, T.M.; Moretti, P.A.B.; Zebol, J.R.; Lynn, H.E.; Wattenberg, B.W.; Vadas, M.A. Phosphorylation-Dependent Translocation of Sphingosine Kinase to the Plasma Membrane Drives Its Oncogenic Signalling. J. Exp. Med. 2005, 201, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Bellamy, A.; Milstien, S.; Kordula, T.; Spiegel, S. Sphingosine Kinase Type 2 Activation by ERK-Mediated Phosphorylation. J. Biol. Chem. 2007, 282, 12058–12065. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and Molecular Mechanisms of Muscle Atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Bossola, M.; Muscaritoli, M.; Costelli, P.; Grieco, G.; Bonelli, G.; Pacelli, F.; Rossi Fanelli, F.; Doglietto, G.B.; Baccino, F.M. Increased Muscle Proteasome Activity Correlates with Disease Severity in Gastric Cancer Patients. Ann. Surg. 2003, 237, 384–389. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Masiero, E.; Sandri, M. Autophagy Inhibition Induces Atrophy and Myopathy in Adult Skeletal Muscles. Autophagy 2010, 6, 307–309. [Google Scholar] [CrossRef]
- Paolini, A.; Omairi, S.; Mitchell, R.; Vaughan, D.; Matsakas, A.; Vaiyapuri, S.; Ricketts, T.; Rubinsztein, D.C.; Patel, K. Attenuation of Autophagy Impacts on Muscle Fibre Development, Starvation Induced Stress and Fibre Regeneration Following Acute Injury. Sci. Rep. 2018, 8, 9062. [Google Scholar] [CrossRef] [PubMed]
- Calise, S.; Blescia, S.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Bruni, P. Sphingosine 1-Phosphate Stimulates Proliferation and Migration of Satellite Cells: Role of S1P Receptors. Biochim. Biophys. Acta 2012, 1823, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, A.A.; Casper, E.S.; Gabrilove, J.L.; Templeton, M.A.; Sherwin, S.A.; Oettgen, H.F. Phase I Trial of Intramuscularly Administered Tumor Necrosis Factor in Patients with Advanced Cancer. J. Clin. Oncol. 1989, 7, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Weinman, S.; Boldogh, I.; Walker, R.K.; Brasier, A.R. Tumor Necrosis Factor-Alpha-Inducible IkappaBalpha Proteolysis Mediated by Cytosolic m-Calpain. A Mechanism Parallel to the Ubiquitin-Proteasome Pathway for Nuclear Factor-Kappab Activation. J. Biol. Chem. 1999, 274, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Takekura, H.; Fujinami, N.; Nishizawa, T.; Ogasawara, H.; Kasuga, N. Eccentric Exercise-Induced Morphological Changes in the Membrane Systems Involved in Excitation–Contraction Coupling in Rat Skeletal Muscle. J. Physiol. 2001, 533, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.; Benoit, H.; Freyssenet, D. Cellular and Molecular Events Controlling Skeletal Muscle Mass in Response to Altered Use. Pflügers Arch. 2008, 456, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β Mediates Muscle Weakness Associated with Bone Metastases in Mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Zentella, A.; Manogue, K.; Cerami, A. Cachectin/TNF-Mediated Lactate Production in Cultured Myocytes Is Linked to Activation of a Futile Substrate Cycle. Cytokine 1993, 5, 436–447. [Google Scholar] [CrossRef]
- Alvarez, B.; Quinn, L.S.; Busquets, S.; Quiles, M.T.; Lopez-Soriano, F.J.; Argiles, J.M. Tumor Necrosis Factor-Alpha Exerts Interleukin-6-Dependent and -Independent Effects on Cultured Skeletal Muscle Cells. Biochim. Biophys. Acta 2002, 1542, 66–72. [Google Scholar] [CrossRef]
- Ninomiya, S.; Nakamura, N.; Nakamura, H.; Mizutani, T.; Kaneda, Y.; Yamaguchi, K.; Matsumoto, T.; Kitagawa, J.; Kanemura, N.; Shiraki, M.; et al. Low Levels of Serum Tryptophan Underlie Skeletal Muscle Atrophy. Nutrients 2020, 12, 978. [Google Scholar] [CrossRef]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased Plasma Proline Concentrations Are Associated with Sarcopenia in the Elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-α and Cancer Cachexia: Molecular Insights and Clinical Implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Li, Y.P.; Schwartz, R.J. TNF-Alpha Regulates Early Differentiation of C2C12 Myoblasts in an Autocrine Fashion. FASEB J. 2001, 15, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, L.; Gamble, J.R.; Vadas, M.A. Activation of Sphingosine Kinase by Tumor Necrosis Factor-Alpha Inhibits Apoptosis in Human Endothelial Cells. J. Biol. Chem. 1999, 274, 34499–34505. [Google Scholar] [CrossRef] [PubMed]
- Radeff-Huang, J.; Seasholtz, T.M.; Chang, J.W.; Smith, J.M.; Walsh, C.T.; Brown, J.H. Tumor Necrosis Factor-Alpha-Stimulated Cell Proliferation Is Mediated through Sphingosine Kinase-Dependent Akt Activation and Cyclin D Expression. J. Biol. Chem. 2006, 282, 863–870. [Google Scholar] [CrossRef]
- Pettus, B.J.; Bielawski, J.; Porcelli, A.M.; Reames, D.L.; Johnson, K.R.; Morrow, J.; Chalfant, C.E.; Obeid, L.M.; Hannun, Y.A. The Sphingosine Kinase 1/Sphingosine-1-Phosphate Pathway Mediates COX-2 Induction and PGE2 Production in Response to TNF-Alpha. FASEB J. 2003, 17, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, F.; Bernacchioni, C.; Nincheri, P.; Donati, C.; Bruni, P. Transforming Growth Factor-Beta1 Induces Transdifferentiation of Myoblasts into Myofibroblasts via up-Regulation of Sphingosine Kinase-1/S1P3 Axis. Mol. Biol. Cell 2010, 21, 1111–1124. [Google Scholar] [CrossRef]
- Meacci, E.; Nuti, F.; Donati, C.; Cencetti, F.; Farnararo, M.; Bruni, P. Sphingosine Kinase Activity Is Required for Myogenic Differentiation of C2C12 Myoblasts. J. Cell. Physiol. 2008, 214, 210–220. [Google Scholar] [CrossRef]
- Pierucci, F.; Frati, A.; Battistini, C.; Matteini, F.; Iachini, M.C.; Vestri, A.; Penna, F.; Costelli, P.; Meacci, E. Involvement of Released Sphingosine 1-Phosphate/Sphingosine 1-Phosphate Receptor Axis in Skeletal Muscle Atrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3598–3614. [Google Scholar] [CrossRef]
- Pfitzenmaier, J.; Vessella, R.; Higano, C.S.; Noteboom, J.L.; Wallace, D.; Corey, E. Elevation of Cytokine Levels in Cachectic Patients with Prostate Carcinoma. Cancer 2003, 97, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Bernacchioni, C.; Ghini, V.; Cencetti, F.; Japtok, L.; Donati, C.; Bruni, P.; Turano, P. NMR Metabolomics Highlights Sphingosine Kinase-1 as a New Molecular Switch in the Orchestration of Aberrant Metabolic Phenotype in Cancer Cells. Mol. Oncol. 2017, 11, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Bernacchioni, C.; Cencetti, F.; Ouro, A.; Bruno, M.; Gomez-Muñoz, A.; Donati, C.; Bruni, P. Lysophosphatidic Acid Signaling Axis Mediates Ceramide 1-Phosphate-Induced Proliferation of C2C12 Myoblasts. Int. J. Mol. Sci. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Blankenbach, K.V.; Thomas, D.; Meyer zu Heringdorf, D.; Bruni, P. Bradykinin Mediates Myogenic Differentiation in Murine Myoblasts through the Involvement of SK1/Spns2/S1P 2 Axis. Cell. Signal. 2018, 45, 110–121. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Bruno, M.; Squecco, R.; Idrizaj, E.; Berbeglia, M.; Bruni, P.; Donati, C. Sphingosine 1-Phosphate-Mediated Activation of Ezrin-Radixin-Moesin Proteins Contributes to Cytoskeletal Remodeling and Changes of Membrane Properties in Epithelial Otic Vesicle Progenitors. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 554–565. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Takis, P.G.; Ghini, V.; Tenori, L.; Turano, P.; Luchinat, C. Uniqueness of the NMR Approach to Metabolomics. TrAC Trends Anal. Chem. 2018, 120, 115300. [Google Scholar] [CrossRef]
- Ghini, V.; Quaglio, D.; Luchinat, C.; Turano, P. NMR for Sample Quality Assessment in Metabolomics. New Biotechnol. 2019, 52, 25–34. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Quaglio, D.; Monaco, L.; Lauro, C.; Ghirga, F.; Ingallina, C.; De Martino, M.; Fucile, S.; Porzia, A.; Di Castro, M.A.; et al. 1H-NMR Metabolomics Reveals the Glabrescione B Exacerbation of Glycolytic Metabolism beside the Cell Growth Inhibitory Effect in Glioma. Cell Commun. Signal. 2019, 17, 108. [Google Scholar] [CrossRef]
- Perrin, E.; Ghini, V.; Giovannini, M.; Di Patti, F.; Cardazzo, B.; Carraro, L.; Fagorzi, C.; Turano, P.; Fani, R.; Fondi, M. Diauxie and Co-Utilization of Carbon Sources Can Coexist during Bacterial Growth in Nutritionally Complex Environments. Nat. Commun. 2020, 11, 3135. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Chellini, F.; Idrizaj, E.; Tani, A.; Garella, R.; Pancani, S.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Sassoli, C. Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-Β1-Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells 2020, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Rojas, E.; Suarez-Isla, B.A. Fast Charge Movements in Skeletal Muscle Fibres from Rana Temporaria. J. Physiol. 1982, 324, 319–345. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Idrizaj, E.; Morelli, A.; Gallina, P.; Vannelli, G.B.; Francini, F. An Electrophysiological Study on the Effects of BDNF and FGF2 on Voltage Dependent Ca(2+) Currents in Developing Human Striatal Primordium. Mol. Cell. Neurosci. 2016, 75, 50–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernacchioni, C.; Ghini, V.; Squecco, R.; Idrizaj, E.; Garella, R.; Puliti, E.; Cencetti, F.; Bruni, P.; Donati, C. Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes. Int. J. Mol. Sci. 2021, 22, 1280. https://doi.org/10.3390/ijms22031280
Bernacchioni C, Ghini V, Squecco R, Idrizaj E, Garella R, Puliti E, Cencetti F, Bruni P, Donati C. Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes. International Journal of Molecular Sciences. 2021; 22(3):1280. https://doi.org/10.3390/ijms22031280
Chicago/Turabian StyleBernacchioni, Caterina, Veronica Ghini, Roberta Squecco, Eglantina Idrizaj, Rachele Garella, Elisa Puliti, Francesca Cencetti, Paola Bruni, and Chiara Donati. 2021. "Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes" International Journal of Molecular Sciences 22, no. 3: 1280. https://doi.org/10.3390/ijms22031280
APA StyleBernacchioni, C., Ghini, V., Squecco, R., Idrizaj, E., Garella, R., Puliti, E., Cencetti, F., Bruni, P., & Donati, C. (2021). Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes. International Journal of Molecular Sciences, 22(3), 1280. https://doi.org/10.3390/ijms22031280







