YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development
Abstract
:1. Introduction
2. Results
2.1. Establishment of Trastuzumab/T-DM1-Dual-Resistant Cell Line
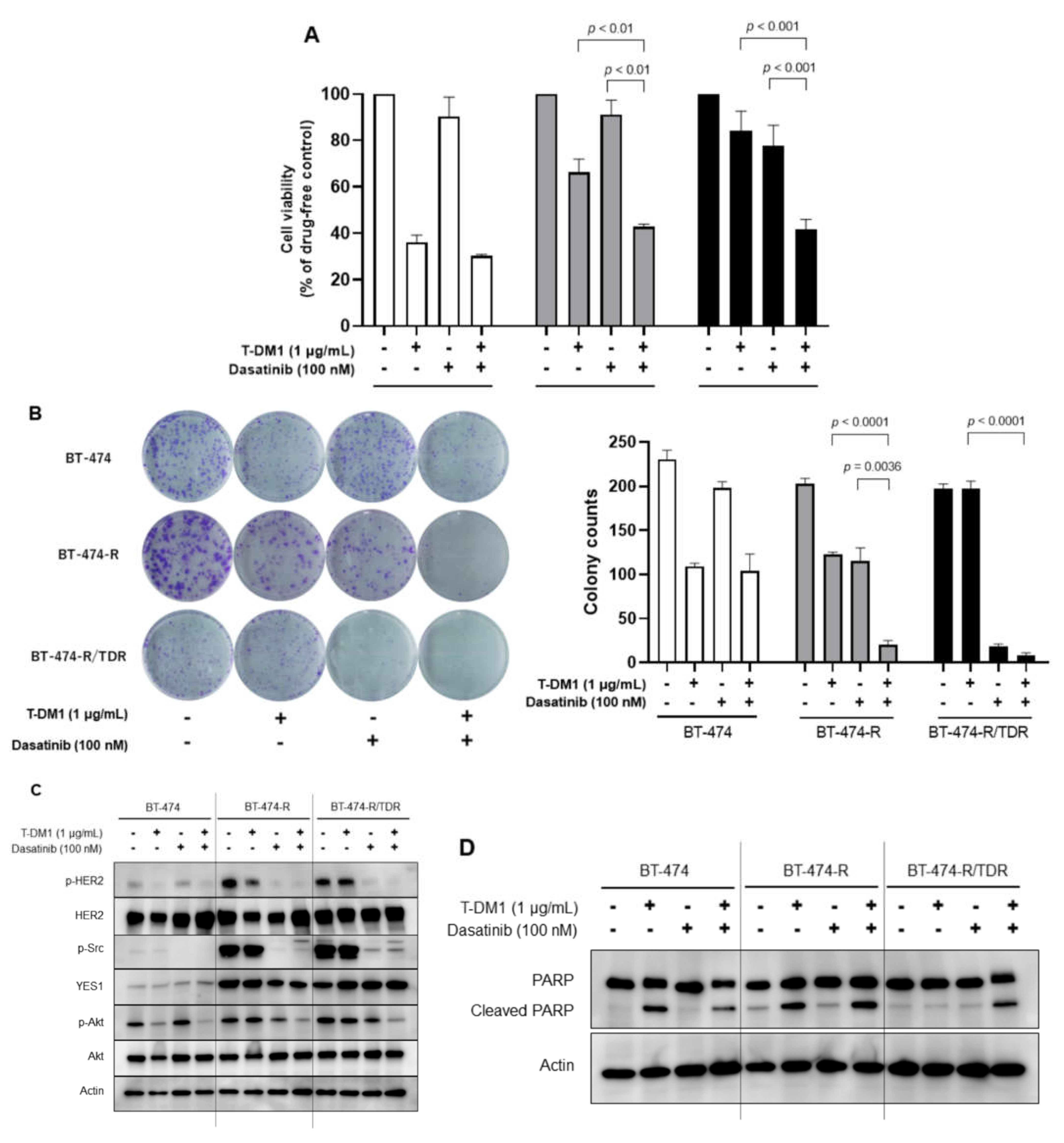
2.2. HER2-Related Signaling Status
2.3. YES1 Amplification and the Effects of the YES1 Knockdown in BT-474-R and BT-474-R/TDR
2.4. Effects of the Src Inhibitor Dasatinib in BT-474-R and BT-474-R/TDR
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drugs
4.2. Cell Viability Assay
4.3. Western Blotting
4.4. Copy Number Assay
4.5. siRNA Transfection
4.6. Colony Formation Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moasser, M.M. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene 2007, 26, 6577–6592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Breast Cancer Clinical Practice Guidelines. In Breast Cancer (Version 7, 2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 19 September 2021).
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, S.; Ocaña, A.; Pandiella, A. Trastuzumab Emtansine: Mechanisms of Action and Resistance, Clinical Progress, and Beyond. Trends Cancer 2020, 6, 130–146. [Google Scholar] [CrossRef]
- Takeda, T.; Yamamoto, H.; Kanzaki, H.; Suzawa, K.; Yoshioka, T.; Tomida, S.; Cui, X.; Murali, R.; Namba, K.; Sato, H.; et al. Yes1 signaling mediates the resistance to Trastuzumab/Lap atinib in breast cancer. PLoS ONE 2017, 12, e0171356. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, H.; Mukhopadhya, N.K.; Cui, X.; Ramanujan, V.K.; Murali, R. Trastuzumab-Resistant Luminal B Breast Cancer Cells Show Basal-Like Cell Growth Features Through NF-κB-Activation. Monoclon. Antibodies Immunodiagn. Immunother. 2016, 35, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef] [Green Version]
- Irby, R.B.; Yeatman, T.J. Role of Src expression and activation in human cancer. Oncogene 2000, 19, 5636–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Huang, W.-C.; Li, P.; Guo, H.; Poh, S.-B.; Brady, S.W.; Xiong, Y.; Tseng, L.-M.; Li, S.-H.; Ding, Z.; et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011, 17, 461–469. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Gupta, R.C. Promising molecular targeted therapies in breast cancer. Indian J. Pharmacol. 2011, 43, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, F.M. Src Family Nonreceptor Tyrosine Kinases as Molecular Targets for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2007, 7, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Maa, M.C.; Leu, T.H.; McCarley, D.J.; Schatzman, R.C.; Parsons, S.J. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: Implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA 1995, 92, 6981–6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscardi, J.S.; Maa, M.-C.; Tice, D.A.; Cox, M.E.; Leu, T.-H.; Parsons, S.J. c-Src-mediated Phosphorylation of the Epidermal Growth Factor Receptor on Tyr845 and Tyr1101 Is Associated with Modulation of Receptor Function. J. Biol. Chem. 1999, 274, 8335–8343. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Jin, P.; Liu, J.; Wang, F.; Xi, S. HER2 and Src co-regulate proliferation, migration and transformation by downstream signaling pathways in arsenite-treated human uroepithelial cells. Metallomics 2018, 10, 1141–1159. [Google Scholar] [CrossRef]
- Takeda, T.; Yamamoto, H.; Suzawa, K.; Tomida, S.; Miyauchi, S.; Araki, K.; Nakata, K.; Miura, A.; Namba, K.; Shien, K.; et al. YES1 activation induces acquired resistance to neratinib in HER2 -amplified breast and lung cancers. Cancer Sci. 2020, 111, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E.; et al. Dasatinib in Imatinib-Resistant Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef] [Green Version]
- Seoane, S.; Montero, J.C.; Ocaña, A.; Pandiella, A. Effect of Multikinase Inhibitors on Caspase-Independent Cell Death and DNA Damage in HER2-Overexpressing Breast Cancer Cells. J. Natl. Cancer Inst. 2010, 102, 1432–1446. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Q.; Xu, P.; Fu, L.; Li, Y.; Fu, H.; Quan, H.; Lou, L. YES1 amplification confers trastuzumab–emtansine (T-DM1) resistance in HER2-positive cancer. Br. J. Cancer 2020, 123, 1000–1011. [Google Scholar] [CrossRef]
- Redin, E.; Garmendia, I.; Lozano, T.; Serrano, D.; Senent, Y.; Redrado, M.; Villalba, M.; De Andrea, C.E.; Exposito, F.; Ajona, D.; et al. SRC family kinase (SFK) inhibitor dasatinib improves the antitumor activity of anti-PD-1 in NSCLC models by inhibiting Treg cell conversion and proliferation. J. Immunother. Cancer 2021, 9, e001496. [Google Scholar] [CrossRef]
- Paul, D.; Vukelja, S.J.; Holmes, F.A.; Blum, J.L.; McIntyre, K.J.; Lindquist, D.L.; Osborne, C.R.; Sanchez, I.J.; Goldschmidt, J.H.; Wang, Y.; et al. Randomized phase-II evaluation of letrozole plus dasatinib in hormone receptor positive metastatic breast cancer patients. NPJ Breast Cancer 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Ocana, A.; Gil-Martin, M.; Antolín, S.; Atienza, M.; Montaño, Á.; Ribelles, N.; Urruticoechea, A.; Falcón, A.; Pernas, S.; Orlando, J.; et al. Efficacy and safety of dasatinib with trastuzumab and paclitaxel in first line HER2-positive metastatic breast cancer: Results from the phase II GEICAM/2010-04 study. Breast Cancer Res. Treat. 2019, 174, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor Cells Chronically Treated with a Trastuzumab–Maytansinoid Antibody–Drug Conjugate Develop Varied Resistance Mechanisms but Respond to Alternate Treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Guo, J.; Shen, B.-Q.; Yadav, D.B.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol. Cancer Ther. 2018, 17, 1441–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Shu, S.; Osada, M.; Fujimura, T.; Yoshiura, S.; Harada, N.; Yoshimura, Y. Combination efficacy of pertuzumab and trastuzumab for trastuzumab emtansine-resistant cells exhibiting attenuated lysosomal trafficking or efflux pumps upregulation. Cancer Chemother. Pharmacol. 2020, 86, 641–654. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2 + breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Jacot, W.; Pons, E.; Frenel, J.-S.; Guiu, S.; Levy, C.; Heudel, P.E.; Bachelot, T.; D’Hondt, V.; Darlix, A.; Firmin, N.; et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 2016, 157, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu, H.; Olsson, M.; Zhivotovsky, B. Death through a tragedy: Mitotic catastrophe. Cell Death Differ. 2008, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujihara, M.; Shien, T.; Shien, K.; Suzawa, K.; Takeda, T.; Zhu, Y.; Mamori, T.; Otani, Y.; Yoshioka, R.; Uno, M.; et al. YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development. Int. J. Mol. Sci. 2021, 22, 12809. https://doi.org/10.3390/ijms222312809
Fujihara M, Shien T, Shien K, Suzawa K, Takeda T, Zhu Y, Mamori T, Otani Y, Yoshioka R, Uno M, et al. YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development. International Journal of Molecular Sciences. 2021; 22(23):12809. https://doi.org/10.3390/ijms222312809
Chicago/Turabian StyleFujihara, Miwa, Tadahiko Shien, Kazuhiko Shien, Ken Suzawa, Tatsuaki Takeda, Yidan Zhu, Tomoka Mamori, Yusuke Otani, Ryo Yoshioka, Maya Uno, and et al. 2021. "YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development" International Journal of Molecular Sciences 22, no. 23: 12809. https://doi.org/10.3390/ijms222312809
APA StyleFujihara, M., Shien, T., Shien, K., Suzawa, K., Takeda, T., Zhu, Y., Mamori, T., Otani, Y., Yoshioka, R., Uno, M., Suzuki, Y., Abe, Y., Hatono, M., Tsukioki, T., Takahashi, Y., Kochi, M., Iwamoto, T., Taira, N., Doihara, H., & Toyooka, S. (2021). YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development. International Journal of Molecular Sciences, 22(23), 12809. https://doi.org/10.3390/ijms222312809






