Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole
Abstract
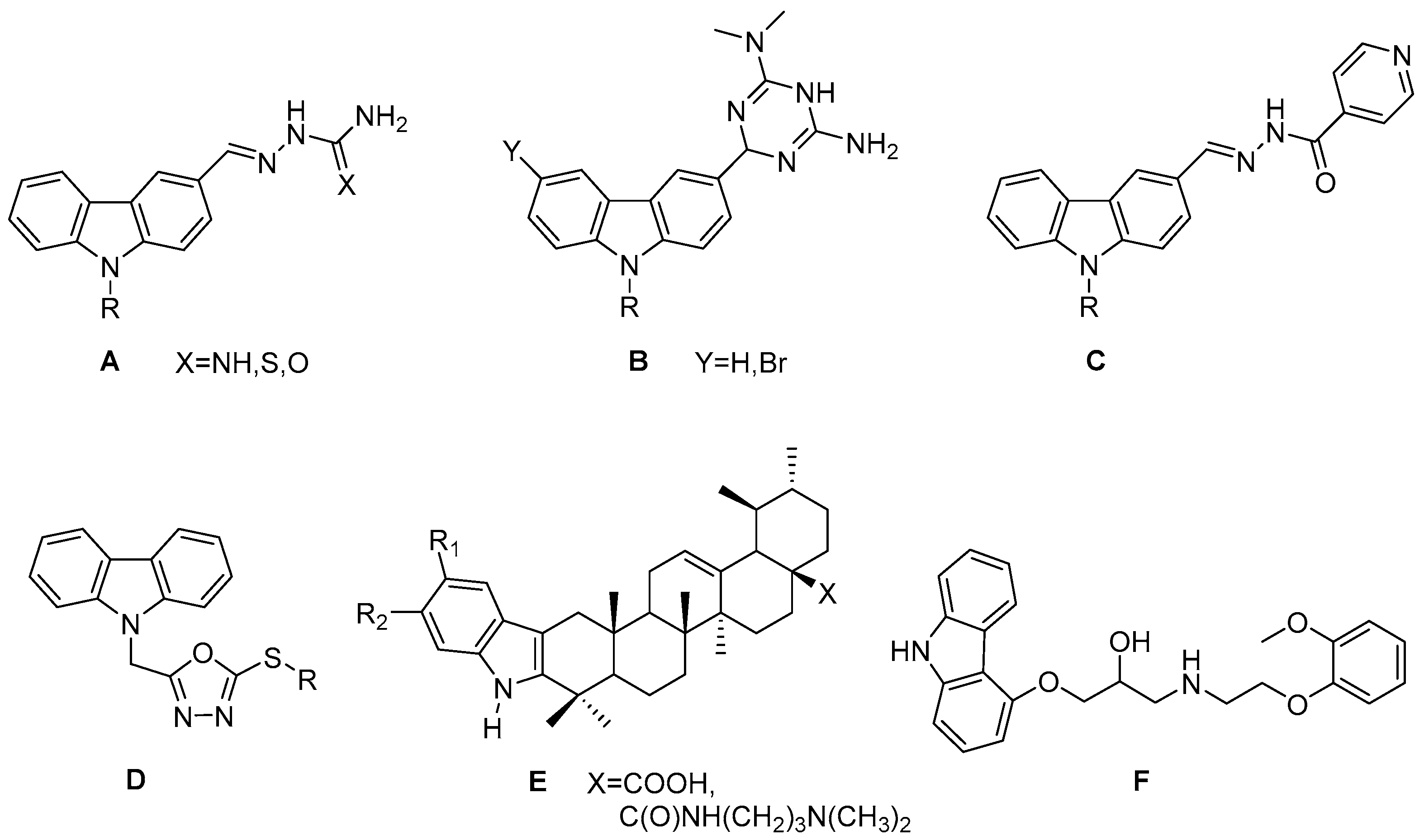
:1. Introduction
2. Results
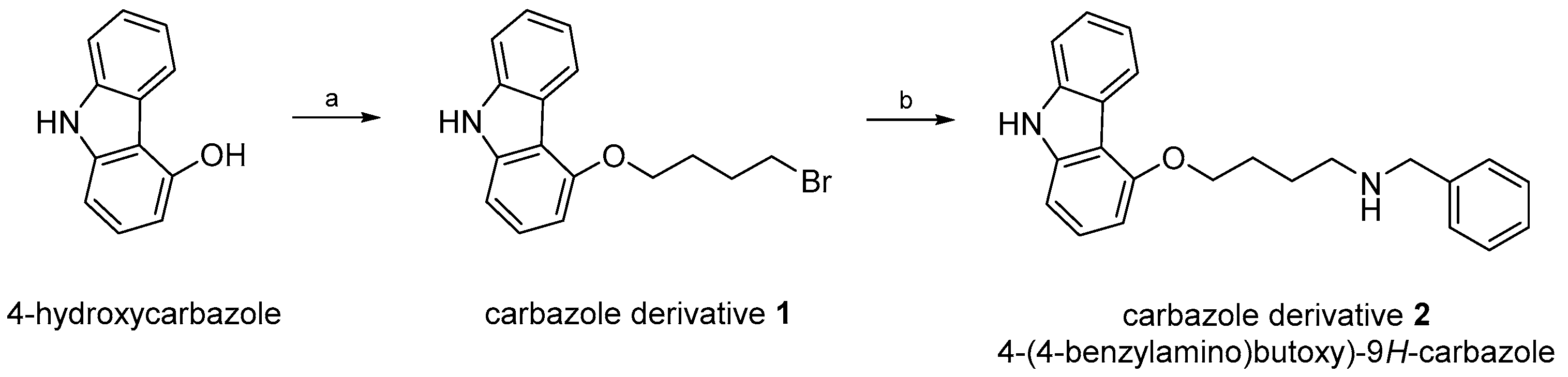
2.1. Synthesis and Characterisation of 4-(4-(Benzylamino)butoxy)-9H-carbazole
2.2. Antimicrobial Properties of Carbazole Derivative 2
2.3. Cytotoxicity and Haemolytic Activity of Carbazole Derivative 2
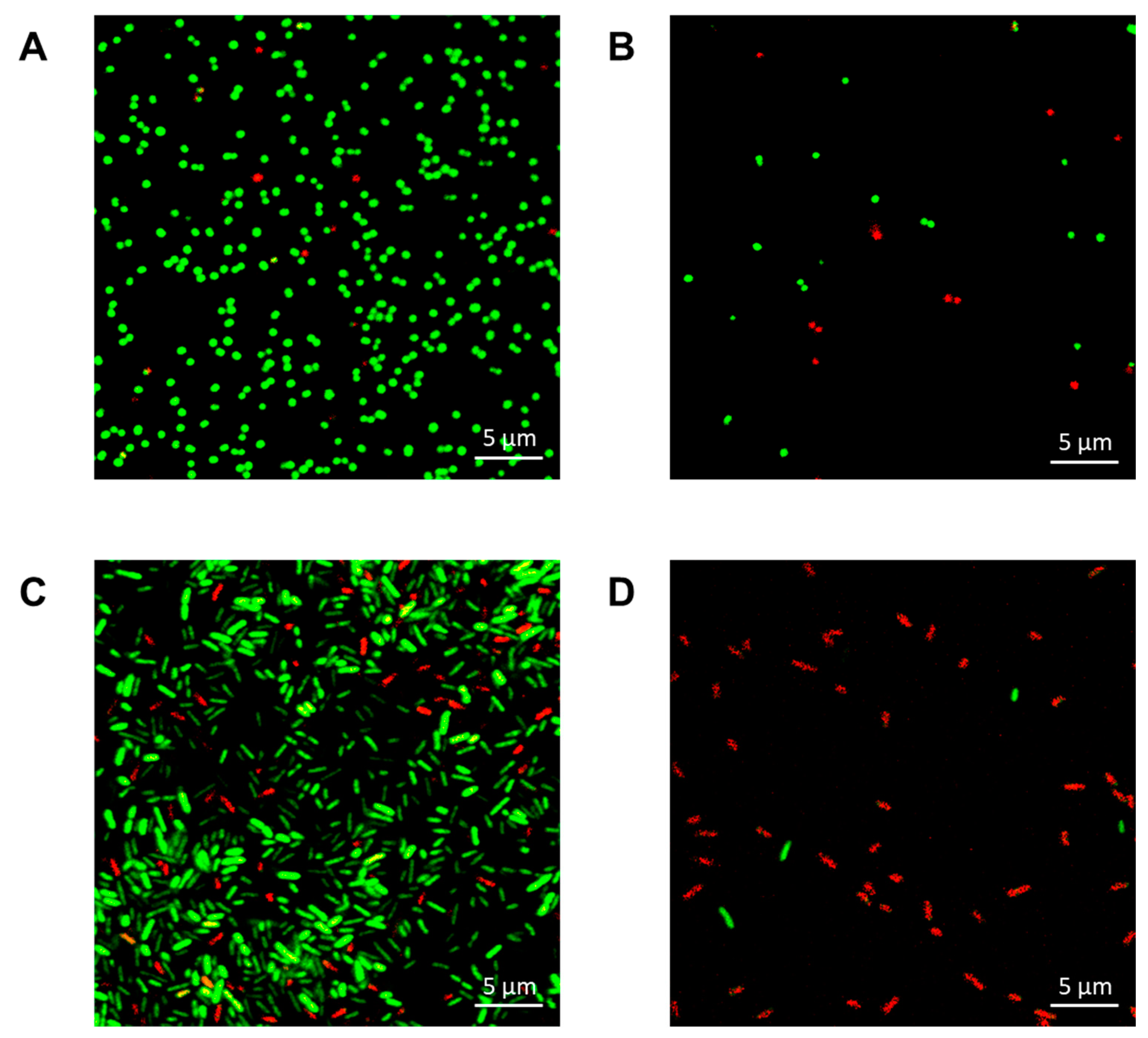
2.4. The Mechanism of Carbazole Derivative 2 Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of 4-(4-(Benzylamino)butoxy)-9H-carbazole 2
4.2.2. Examination of Antimicrobial Activity
4.2.3. Assessment of the Haemolytic Activity of Carbazole Derivative 2
4.2.4. Determination of the Cytotoxic Activity of Carbazole Derivative 2
4.2.5. Confocal Analysis of Bacterial Cells Viability
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roope, L.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Cima-Cabal, M.D.; Duarte, A.C.; Rodriguez, A.; García, P.; García-Suárez, M. Developing Diagnostic and Therapeutic Approaches to Bacterial Infections for a New Era: Implications of Globalization. Antibiotics 2020, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Głuszyńska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.J.; Li, M.Y.; Jin, X.J.; Zheng, C.J.; Piao, H.R. Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Ansari, M.F.; Zhang, S.L.; Zhou, C.H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pestic. Biochem. Physiol. 2021, 175, 104849. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.; Miao, T.; Zhang, K. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic. Acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Bernat, P.; Felczak, A.; Różalska, S.; Lisowska, K. Antibacterial activity of high concentrations of carvedilol against Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents. 2018, 51, 458–467. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Kirkwood, N.K.; Kenyon, E.J.; Huckvale, R.; Cantillon, D.M.; Waddell, S.J.; Ward, S.E.; Richardson, G.P.; Kros, C.J.; Derudas, M. Design, Synthesis, and Biological Evaluation of a New Series of Carvedilol Derivatives That Protect Sensory Hair Cells from Aminoglycoside-Induced Damage by Blocking the Mechanoelectrical Transducer Channel. J. Med. Chem 2019, 62, 5312–5329. [Google Scholar] [CrossRef] [PubMed]
- Stanek, M.; Picard, L.P.; Schmidt, M.F.; Kaindl, J.M.; Hübner, H.; Bouvier, M.; Weikert, D.; Gmeiner, P. Hybridization of β-Adrenergic Agonists and Antagonists Confers G Protein Bias. J. Med. Chem. 2019, 62, 5111–5131. [Google Scholar] [CrossRef]
- Zhang, F.F.; Gan, L.L.; Zhou, C.H. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 1881–1884. [Google Scholar] [CrossRef]
- Jasass, R.S.; Alshehrei, F.; Farghaly, T.A. Microwave-Assisted Synthesis of Antimicrobial Agents Containing Carbazole and Thiazole Moieties. J. Heterocyclic Chem. 2018, 55, 2099–2106. [Google Scholar] [CrossRef]
- Gu, W.; Qiao, C.; Wang, S.F.; Hao, Y.; Miao, T.T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2014, 24, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Nowak, M.; Piwoński, I.; Lisowska, K. The Synergy of Ciprofloxacin and Carvedilol against Staphylococcus aureus-Prospects of a New Treatment Strategy? Molecules 2019, 24, 4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the Protocol for the LIVE/DEAD® BacLightTM Bacterial Viability Kit for Rapid Determination of Bacterial Load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]

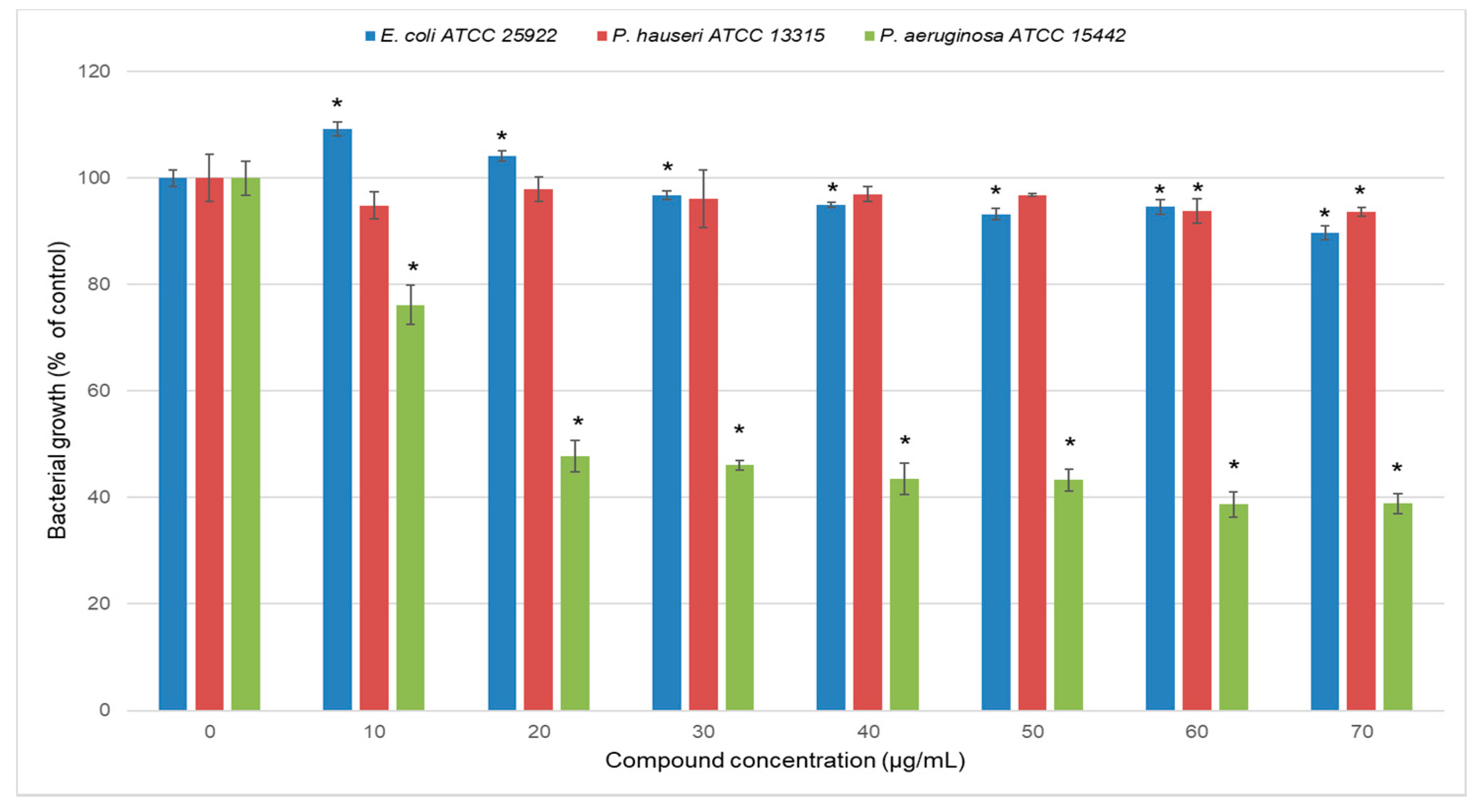
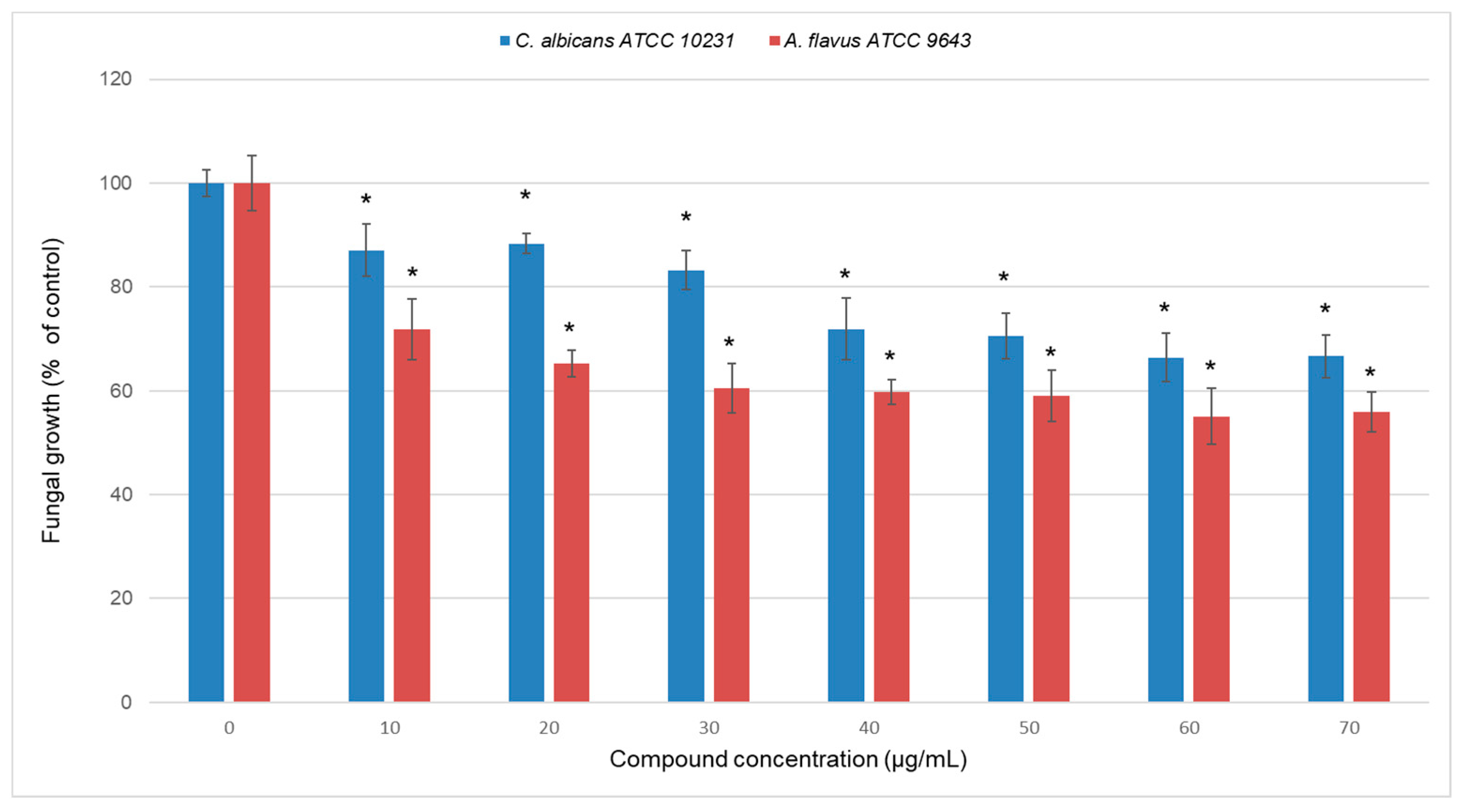


| STRAIN | MIC | MBC/MFC |
|---|---|---|
| Gram-positive bacteria | ||
| S. aureus ATCC 29213 | 30 | >70 |
| S. aureus ATCC 25923 | 40 | >70 |
| S. aureus ATCC 6358 | 30 | >70 |
| S. aureus ATCC 700699 | 40 | 70 |
| S. aureus ATCC 43300 | 40 | >70 |
| S. epidermidis ATCC 12228 | 50 | 70 |
| S. pyogenes ATCC 19615 | 40 | 60 |
| Gram-negative bacteria | ||
| E. coli ATCC 25922 | >70 | >70 |
| P. hauseri ATCC 13315 | >70 | >70 |
| P. aeruginosa ATCC 15442 | >70 | >70 |
| Fungi | ||
| C. albicans ATCC 10231 | >70 | >70 |
| A. flavus ATCC 9643 | >70 | >70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzka, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole. Int. J. Mol. Sci. 2021, 22, 12796. https://doi.org/10.3390/ijms222312796
Zawadzka K, Felczak A, Głowacka IE, Piotrowska DG, Lisowska K. Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole. International Journal of Molecular Sciences. 2021; 22(23):12796. https://doi.org/10.3390/ijms222312796
Chicago/Turabian StyleZawadzka, Katarzyna, Aleksandra Felczak, Iwona E. Głowacka, Dorota G. Piotrowska, and Katarzyna Lisowska. 2021. "Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole" International Journal of Molecular Sciences 22, no. 23: 12796. https://doi.org/10.3390/ijms222312796
APA StyleZawadzka, K., Felczak, A., Głowacka, I. E., Piotrowska, D. G., & Lisowska, K. (2021). Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)butoxy)-9H-carbazole. International Journal of Molecular Sciences, 22(23), 12796. https://doi.org/10.3390/ijms222312796






