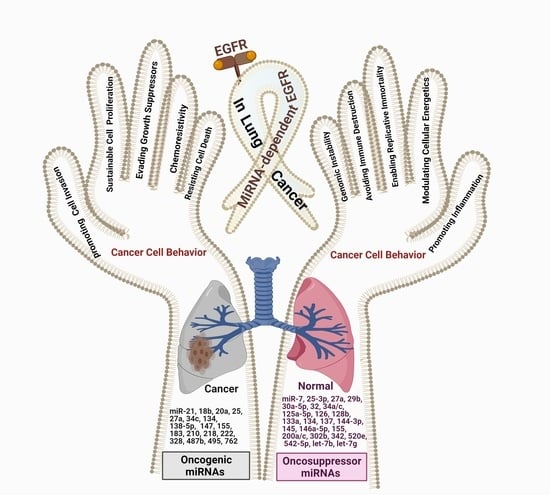
Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands
Abstract
1. Introduction
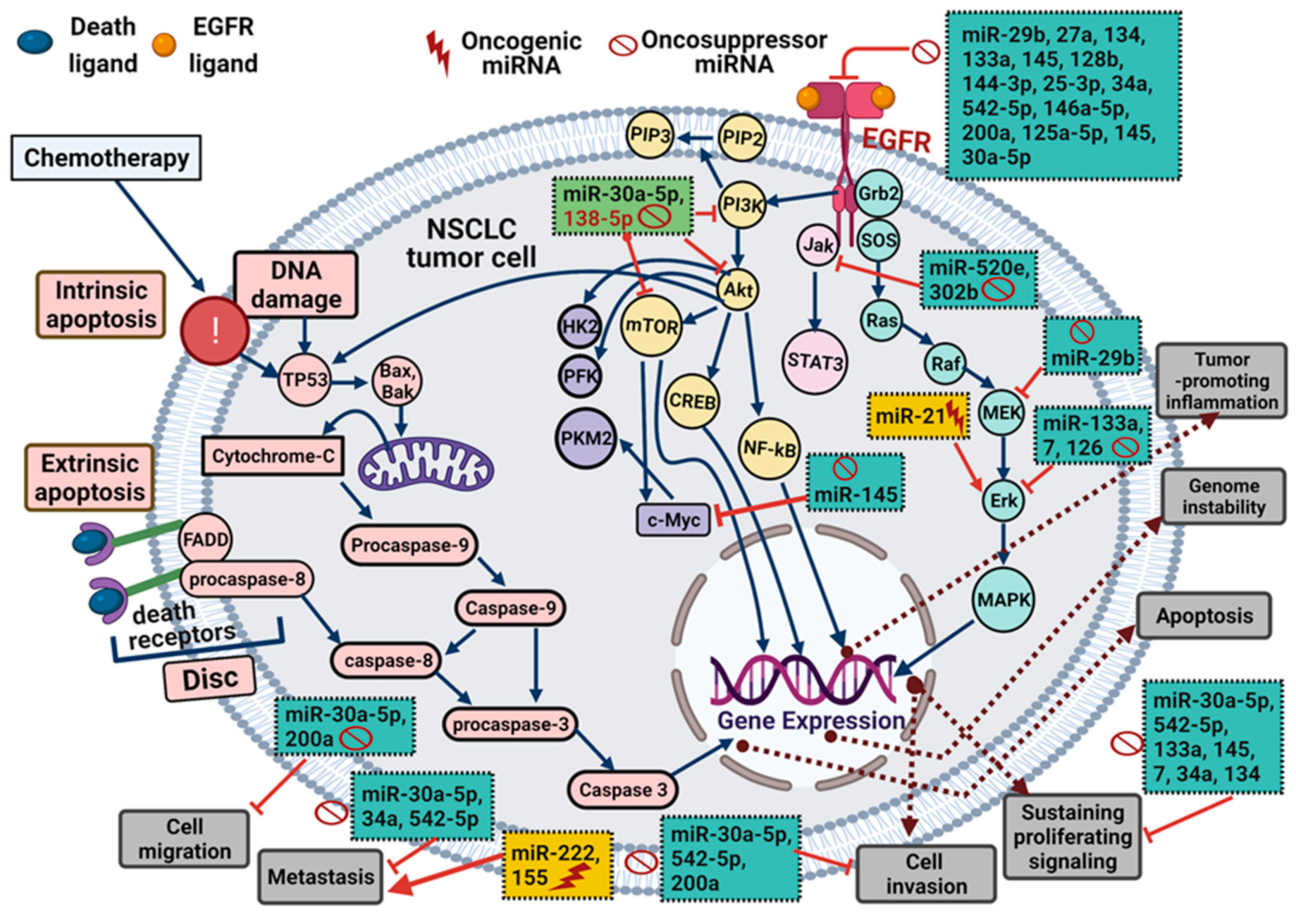
2. EGFR Overexpression/Hyperactivation and miRNA Expression Pattern: Effects on NSCLC Cell Behavior
2.1. Effect of miRNAs on EGFR Expression
2.2. Effect of EGFR Activation on miRNA Expression
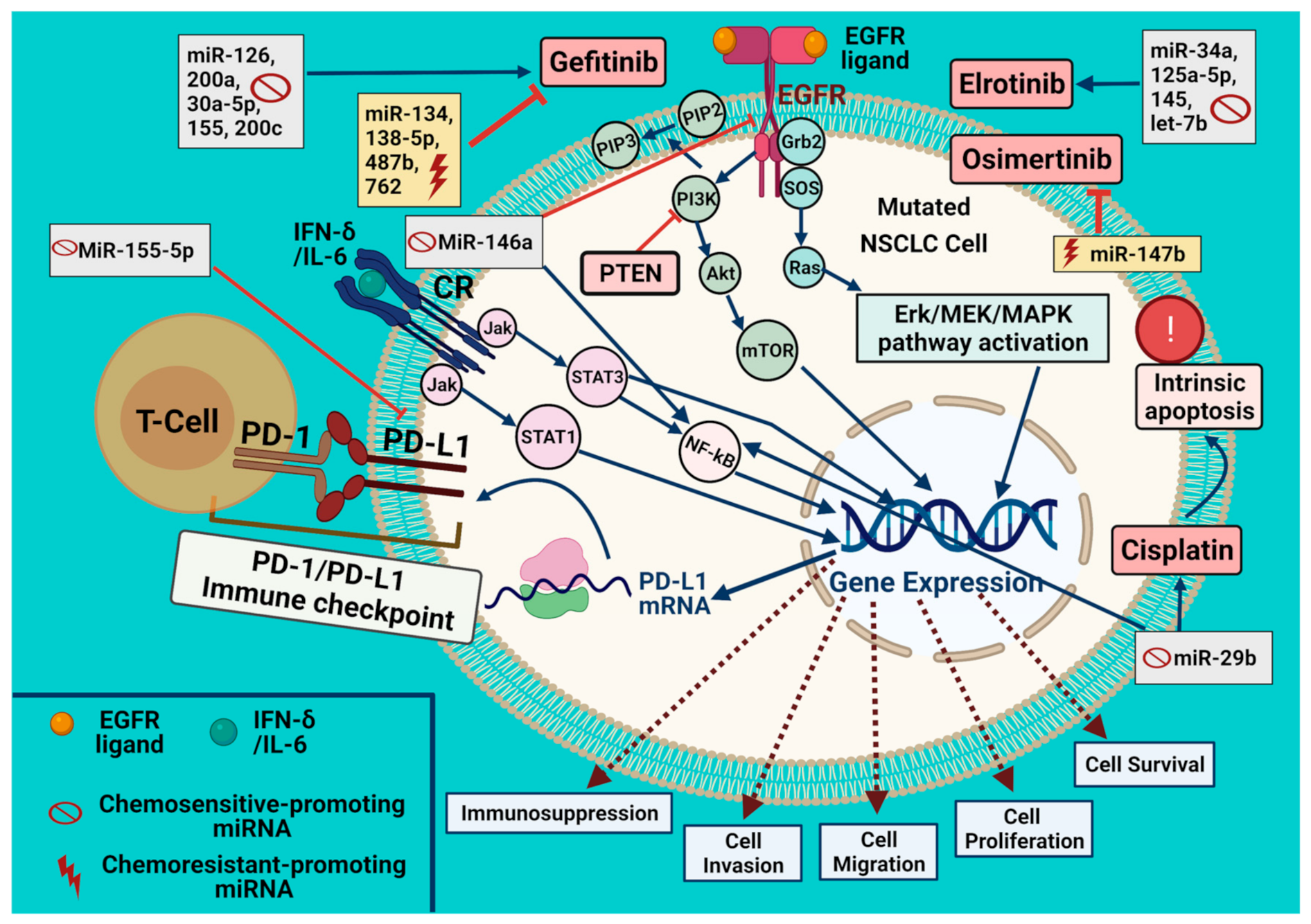
3. Specific miRNA Expression Patterns Affect the Behavior of EGFR-Mutated NSCLC
4. Effect of miRNAs on the Chemosensitivity to EGFR-TKIs
4.1. Erlotinib
4.2. Gefitinib
4.3. Other EGFR-TKI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ABR | ABR Activator of Rho GEF and GTPase |
| ADCY1 | Adenylate Cyclase 1 |
| ADCY6 | Adenylate Cyclase 6 |
| AKT | Protein kinase B |
| ALK | Anaplastic Lymphoma Kinase |
| AT1 | Angiotensin II type 1 |
| cAMP | cyclic adenosine -3’,5’-monophosphate |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CCNE1 | cyclin E |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CDKN1A | Cyclin-Dependent Kinase Inhibitor 1A |
| CTNNB1 | catenin beta 1 |
| CDR1as | Circular RNA CDR1as |
| c-MET | hepatocyte growth factor receptor |
| DUSP4 | Dual Specificity Phosphatase 4 |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| eIF4e | Eukaryotic translation initiation factor 4E |
| EMT | Epithelial-Mesenchymal Transition |
| ERF | Ets2 transcriptional repression factor |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| GABBR1 | Gamma-Aminobutyric Acid Type B Receptor Subunit 1 |
| GIPR | Gastric Inhibitory Polypeptide Receptor |
| GPR124 | G protein-coupled receptor124 |
| GRB2 | growth factor receptor-bound protein 2 |
| HIF-1α | Hypoxia Inducible Factor 1 Subunit Alpha |
| HuR | Human Antigen R |
| IC50 | Inhibit Cellular Proliferation by 50% |
| IGF-1R | Insulin-like Growth Factor Receptor-1 |
| IL-6 | Interleukin-6 |
| JAK1 | Janus kinase 1 |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| LADC | Lung Adenocarcinoma |
| 3LL | Lewis lung cancer |
| LSCC | Lung Squamous Carcinoma |
| MAGI2 | Membrane Associated Guanylate Kinase, WW And PDZ Domain Containing 2 |
| MAPK (MEK) | Mitogen-Activated Protein Kinase |
| mTOR | mammalian/mechanistic target of rapamycin. |
| MUC4 | Mucin 4 |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NSCLC | Non-Small Cell Lung Cancer |
| NRSF | Neuron-Restricted Silencing Factor |
| NUDT-1 | Nucleoside X-type Motif-1 |
| PDE4B | Phosphodiesterase 4b |
| PDE4C | Phosphodiesterase 4c |
| PDGFRa | platelet-derived growth factor receptor Alpha |
| PI3K | Phosphatidylinositol 3-kinase |
| PIK3CD | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Delta |
| PIK3R2 | phosphoinositide-3-kinase regulatory subunit 2 |
| PLCG1 | phospholipase C gamma 1 |
| PTEN | phosphatase tensin homologue |
| PTK2 | protein tyrosine kinase 2 |
| SCLC | Small Cell Lung Cancer |
| STAT | Signal Transducer and Activator of Transcription |
| RAAS | Renin-Angiotensin-Aldosterone System |
| Ras | Rat Sarcoma |
| Raf | Rapidly Accelerated Fibrosarcoma |
| RAF-1 | Rapidly Accelerated Fibrosarcoma homolog-1 |
| RHOA | Transforming protein RhoA |
| Ron | a protein tyrosine kinase related to c-MET |
| RTK | Receptor Tyrosine Kinase |
| TCA | Tricarboxylic Acid Cycle |
| TGFβ | Transforming Growth Factor Beta |
| TKIs | Tyrosine Kinase Inhibitors |
| TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 or A20 |
| TNM | Tumor, nodes, and metastasis |
| TRPM2 | Transient receptor potential cation channel, subfamily M, member 2 |
| VEGFA | Vascular endothelial growth factor-A |
| VHL | Von Hippel–Lindau disease |
| ZEB1 | zinc finger E-box binding homeobox 1 |
References
- Zhen, Q.; Liu, J.; Gao, L.; Liu, J.; Wang, R.; Chu, W.; Zhang, Y.; Tan, G.; Zhao, X.; Lv, B. MicroRNA-200a Targets EGFR and c-Met to Inhibit Migration, Invasion, and Gefitinib Resistance in Non-Small Cell Lung Cancer. Cytogenet. Genome Res. 2015, 146, 1–8. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Finn, S.P.; Cuffe, S.; O’Byrne, K.J.; Barr, M.P. The emerging role of microRNAs in resistance to lung cancer treatments. Cancer Treat. Rev. 2015, 41, 160–169. [Google Scholar] [CrossRef]
- Garinet, S.; Laurent-Puig, P.; Blons, H.; Oudart, J.-B. Current and Future Molecular Testing in NSCLC, What Can We Expect from New Sequencing Technologies? J. Clin. Med. 2018, 7, 144. [Google Scholar] [CrossRef]
- Wang, F.; Chan, L.W.C.; Law, H.K.W.; Cho, W.C.S.; Tang, P.; Yu, J.; Shyu, C.R.; Wong, S.C.C.; Yip, S.P.; Yung, B.Y.M. Exploring microRNA-mediated alteration of EGFR signaling pathway in non-small cell lung cancer using an mRNA: MiRNA regression model supported by target prediction databases. Genomics 2014, 104, 504–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Q.; Wang, S. MicroRNAs: A new key in lung cancer. Cancer Chemother. Pharmacol. 2014, 74, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Khedr, G.; Omar, A.; Bae, S.; Arafat, W.; Grant, S. Site of metastases as prognostic factors in unselected population of stage IV non-small cell lung cancer. Asian Pacific. J. Cancer Prev. 2018, 19, 1907–1910. [Google Scholar] [CrossRef]
- Wang, R.J.; Zheng, Y.H.; Wang, P.; Zhang, J.Z. Serum miR-125a-5p, miR-145 and miR-146a as diagnostic biomarkers in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 765–771. [Google Scholar]
- Souza, C.P.; Cinegaglia, N.C.; Felix, T.F.; Evangelista, A.F.; Oliveira, R.A.; Hasimoto, E.N.; Cataneo, D.C.; Cataneo, A.J.M.; Neto, C.S.; Viana, C.R.; et al. Deregulated microRNAs Are Associated with Patient Survival and Predicted to Target Genes That Modulate Lung Cancer Signaling Pathways. Cancers 2020, 12, 2711. [Google Scholar] [CrossRef]
- Song, F.; Xuan, Z.; Yang, X.; Ye, X.; Pan, Z.; Fang, Q. Identification of key microRNAs and hub genes in non-small-cell lung cancer using integrative bioinformatics and functional analyses. J. Cell. Biochem. 2020, 121, 2690–2703. [Google Scholar] [CrossRef] [PubMed]
- Kumarakulasinghe, N.B.; van Zanwijk, N.; Soo, R.A. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 2015, 20, 370–378. [Google Scholar] [CrossRef]
- Hsu, P.C.; Jablons, D.M.; Yang, C.T.; You, L. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int. J. Mol. Sci. 2019, 20, 3821. [Google Scholar] [CrossRef]
- Wang, K.; Park, J.O.; Zhang, M. Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and β-catenin. J. Gene Med. 2013, 15, 42–50. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.; Reid, T.R.; Cabrales, P.; Lybeck, M.; Oronsky, A.; Oronsky, N.; Carter, C.A. Navigating the “No Man’s Land” of TKI-Failed EGFR-Mutated Non–Small Cell Lung Cancer (NSCLC): A Review. Neoplasia 2018, 20, 92–98. [Google Scholar] [CrossRef]
- Yang, Z.; Tam, K.Y. Combination strategies using EGFR-TKi in NSCLC therapy: Learning from the gap between pre-clinical results and clinical outcomes. Int. J. Biol. Sci. 2018, 14, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, F.; Wang, L.; Wong, S.C.C.; Cho, W.C.S.; Chan, L.W.C. Associations of mRNA:microRNA for the shared downstream molecules of EGFR and alternative tyrosine kinase receptors in non-small cell lung cancer. Front. Genet. 2016, 7, 173. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.; Anido, U.; Lázaro, M.; Santomé, L.; Afonso, J.; Fernández, O.; Alegría, A.M.D.; Aparicio, L.A. Angiogenesis and Lung Cancer. Oncog. Inflamm. Parasit. Trop. Dis. Lung 2013. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2017, 12, 3–20. [Google Scholar] [CrossRef]
- Zhong, M.; Ma, X.; Sun, C.; Chen, L. MicroRNAs reduce tumor growth and contribute to enhance cytotoxicity induced by gefitinib in non-small cell lung cancer. Chem. Biol. Interact. 2010, 184, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, C.; Slack, F.J. Combinatorial action of MicroRNAs let-7 and miR-34 effectively synergizes with erlotinib to suppress non-small cell lung cancer cell proliferation. Cell Cycle 2015, 14, 2171–2180. [Google Scholar] [CrossRef]
- Gasparini, P.; Cascione, L.; Landi, L.; Carasi, S.; Lovat, F.; Tibaldi, C.; Alì, G.; D’Incecco, A.; Minuti, G.; Chella, A.; et al. MicroRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 14924–14929. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef]
- Pak, M.G.; Lee, C.H.; Lee, W.J.; Shin, D.H.; Roh, M.S. Unique microRNAs in lung adenocarcinoma groups according to major TKI sensitive EGFR mutation status. Diagn. Pathol. 2015, 10, 99. [Google Scholar] [CrossRef]
- Giordano, M.; Boldrini, L.; Servadio, A.; Niccoli, C.; Melfi, F.; Lucchi, M.; Mussi, A.; Fontanini, G. Differential microRNA expression profiles between young and old lung adenocarcinoma patients. Am. J. Transl. Res. 2018, 10, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.P.; Drigo, S.A.; Carvalho, R.F.; Lapa, R.M.L.; Felix, T.F.; Patel, D.; Cheng, D.; Pintilie, M.; Liu, G.; Tsao, M.S. Circulating miR-16-5p, miR-92a-3p, and miR-451a in plasma from lung cancer patients: Potential application in early detection and a regulatory role in tumorigenesis pathways. Cancers 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Leonetti, A.; Assaraf, Y.G.; Veltsista, P.D.; El Hassouni, B.; Tiseo, M.; Giovannetti, E. MicroRNAs as a drug resistance mechanism to targeted therapies in EGFR-mutated NSCLC: Current implications and future directions. Drug Resist. Updat. 2019, 42, 1–11. [Google Scholar] [CrossRef]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Ishikawa, Y. MicroRNA In Lung Cancer: Novel Biomarkers and Potential Tools for Treatment. J. Clin. Med. 2016, 5, 36. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef]
- Gómez-Gómez, Y.; Organista-Nava, J.; Gariglio, P. Deregulation of the miRNAs Expression in Cervical Cancer: Human Papillomavirus Implications. Biomed. Res. Int. 2013, 2013, 15. [Google Scholar] [CrossRef]
- Zhao, X.M.; Liu, K.Q.; Zhu, G.; He, F.; Duval, B.; Richer, J.M.; Huang, D.S.; Jiang, C.J.; Hao, J.K.; Chen, L. Identifying cancer-related microRNAs based on gene expression data. Bioinformatics 2015, 31, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Jaiswal, B.S.; Stinson, J.; Janakiraman, V.; Bhatt, D.; Stern, H.M.; Yue, P.; Haverty, P.M.; Bourgon, R.; Zheng, J.; et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010, 466, 869–873. [Google Scholar] [CrossRef]
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587. [Google Scholar] [CrossRef]
- Qin, Q.; Wei, F.; Zhang, J.; Wang, X.; Li, B. miR-134 inhibits non-small cell lung cancer growth by targeting the epidermal growth factor receptor. J. Cell. Mol. Med. 2016, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Espinoza-Sánchez, N.A.; El-Damen, A.; Fahim, S.A.; Badawy, M.A.; Greve, B.; El-Shinawi, M.; Götte, M.; Ibrahim, S.A. Small extracellular vesicle-encapsulated miR-181b-5p, miR-222-3p and let-7a-5p: Next generation plasma biopsy-based diagnostic biomarkers for inflammatory breast cancer. PLoS ONE 2021, 16, e0250642. [Google Scholar] [CrossRef]
- Fahim, S.A.; Abdullah, M.S.; Espinoza-Sánchez, N.A.; Hassan, H.; Ibrahim, A.M.; Ahmed, S.H.; Shakir, G.; Badawy, M.A.; Zakhary, N.I.; Greve, B.; et al. Inflammatory Breast Carcinoma: Elevated microRNA miR-181b-5p and Reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p Expression as Potential Biomarkers with Diagnostic Value. Biomolecules 2020, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tian, Q.; Guan, L.; Niu, S.S. The Dual Role of miR-186 in Cancers: Oncomir Battling With Tumor Suppressor miRNA. Front. Oncol. 2020, 10, 233. [Google Scholar] [CrossRef]
- Ding, L.; Lan, Z.; Xiong, X.; Ao, H.; Feng, Y.; Gu, H.; Yu, M.; Cui, Q. The Dual Role of MicroRNAs in Colorectal Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2791. [Google Scholar] [CrossRef]
- Langsch, S.; Baumgartner, U.; Haemmig, S.; Schlup, O.; Schäfer, S.C.; Berezowska, S.; Rieger, G.; Dorn, P.; Tschan, M.P.; Vassella, E. miR-29b mediates NF-κkB signaling in KRAS-Induced non-small cell lung cancers. Cancer Res. 2016, 76, 4160–4169. [Google Scholar] [CrossRef]
- Song, G.; Zhou, Y.; Chen, R.; Li, Q.; Shan, B.; Duan, Y.; Wang, Y. Expression and Function Analysis of MicroRNA-29b in Xuanwei Lung Cancer. Clin. Lab. 2016, 62, 1739–1745. [Google Scholar] [CrossRef]
- Yu, D.H.; Ruan, X.-L.; Huang, J.-Y.; Liu, X.-P.; Ma, H.-L.; Chen, C.; Hu, W.-D.; Li, S. Analysis of the Interaction Network of Hub miRNAs-Hub Genes, Being Involved in Idiopathic Pulmonary Fibers and Its Emerging Role in Non-small Cell Lung Cancer. Front. Genet. 2020, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, M.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Carvalho, R.; Figueiras, A.; Santos, A.C.; Veiga, F. miR-29b and retinoic acid co-delivery: A promising tool to induce a synergistic antitumoral effect in non-small cell lung cancer cells. Drug Deliv. Transl. Res. 2020, 10, 1367–1380. [Google Scholar] [CrossRef]
- Wang, H.; Guan, X.; Tu, Y.; Zheng, S.; Long, J.; Li, S.; Qi, C.; Xie, X.; Zhang, H.; Zhang, Y. MicroRNA-29b attenuates non-small cell lung cancer metastasis by targeting matrix metalloproteinase 2 and PTEN. J. Exp. Clin. Cancer Res. 2015, 34, 59. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, S.I.; Tschan, M.P.; Federzoni, E.A.; Jaggi, R.; Fey, M.F.; Gugger, M.; Gautschi, O. MicroRNA-29b is involved in the Src-ID1 signaling pathway and is dysregulated in human lung adenocarcinoma. Oncogene 2012, 31, 4221–4232. [Google Scholar] [CrossRef] [PubMed]
- Grandis, J.R.; Sok, J.C. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol. Ther. 2004, 102, 37–46. [Google Scholar] [CrossRef]
- Tasdemir, S.; Taheri, S.; Akalin, H.; Kontas, O.; Onal, O.; Ozkul, Y. Increased EGFR mRNA expression levels in non-small cell lung cancer. Eurasian J. Med. 2019, 51, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cho, W.C.S. Genomic sequence analysis of EGFR regulation by microRNAs in lung cancer. Curr. Top. Med. Chem. 2012, 12, 920–926. [Google Scholar] [CrossRef]
- Mohan, A.; Ansari, A.; Masroor, M.; Saxena, A.; Pandey, R.M.; Upadhyay, A.; Luthra, K.; Khilnani, G.C.; Jain, D.; Kumar, R.; et al. Measurement of Serum EGFR mRNA Expression is a Reliable Predictor of Treatment Response and Survival Outcomes in Non- Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 3153–3163. [Google Scholar] [CrossRef]
- Masroor, M.; Mir, R.; Javid, J.; Prasant, Y.; Imtiyaz, A.; Mariyam, Z.; Mohan, A.; Ray, P.C.; Saxena, A. Cell Free EGFR mRNA Expression and Implications for Survival and Metastasis in Non-Small Cell Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2015, 16, 6445–6449. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Palmieri, D.; Laganá, A.; Garofalo, M.; Balatti, V.; Drusco, A.; Chiariello, M.; Nana-Sinkam, P.; Croce, C.M. Cross-talk between MET and EGFR in non-small cell lung cancer involves miR-27a and Sprouty2. Proc. Natl. Acad. Sci. USA 2013, 110, 8573–8578. [Google Scholar] [CrossRef]
- Zhang, Y.-W. The rationale for dual targeting MET and EGFR in non-small cell lung cancer. J. Cell Sci. Ther. 2013, 4, 4. [Google Scholar] [CrossRef]
- Peng, S.; Wang, R.; Zhang, X.; Ma, Y.; Zhong, L.; Li, K.; Nishiyama, A.; Arai, S.; Yano, S.; Wang, W. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol. Cancer 2019, 18, 165. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Zhang, F.; Sun, C.-C.; Li, S.-J.; Li, G.; Gong, F.-Y.; Bo, T.; He, J.; Hua, R.-X.; Hu, W.-D.; et al. miR-134: A Human Cancer Suppressor? Mol. Ther. Nucleic Acids 2017, 6, 140–149. [Google Scholar] [CrossRef]
- Azad, F.M.; Naeli, P.; Malakootian, M.; Baradaran, A.; Tavallaei, M.O.M.T.O.; Ghanei, M.; Mowla, S. Two lung development-related microRNAs, miR-134 and miR-187, are differentially expressed in lung tumors. Gene 2016, 577, 221–226. [Google Scholar] [CrossRef]
- Li, Y.-L.; Liu, X.-M.; Zhang, C.-Y.; Zhou, J.-B.; Shao, Y.; Liang, C.; Wang, H.-M.; Hua, Z.-Y.; Lu, S.-D.; Ma, Z.-L. MicroRNA-34a/EGFR axis plays pivotal roles in lung tumorigenesis. Oncogenesis 2017, 6, e37. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, E.J.; Lee, S.; Tan, X.; Liu, X.; Park, S.; Kang, K.; Yoon, J.-S.; Ko, Y.H.; Kurie, J.M.; et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, X.; Tang, D.; Wang, J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS ONE 2014, 9, e90022. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Navarro, A.; Viñolas, N.; Marrades, R.M.; Diaz, T.; Gel, B.; Quera, A.; Bandres, E.; Garcia-Foncillas, J.; Ramirez, J.; et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis 2009, 30, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Sun, X.X.; Wu, H.R.; Xu, G.W.; Wang, G.X.; Sun, X.H.; Xu, M.Q.; Xie, M.R. Mechanism research of miR-34a regulates Axl in non-small-cell lung cancer with gefitinib-acquired resistance. Thorac. Cancer 2020, 11, 156–165. [Google Scholar] [CrossRef]
- He, X.; Yang, A.; McDonald, D.G.; Riemer, E.C.; Vanek, K.N.; Schulte, B.A.; Wang, G.Y. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget 2017, 8, 69797–69807. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lu, P.; Sun, G.; Yang, L.; Wang, Z.; Wang, Z. miR-34a sensitizes lung cancer cells to cisplatin via p53/miR-34a/MYCN axis. Biochem. Biophys. Res. Commun. 2017, 482, 22–27. [Google Scholar] [CrossRef]
- Guo, N.; Zhao, Y.; Zhang, W.; Li, S.; Li, S.; Yu, J. MicroRNA-133a downregulated EGFR expression in human non-small cell lung cancer cells via AKT/ERK signaling. Oncol. Lett. 2018, 16, 6045–6050. [Google Scholar] [CrossRef]
- Lan, D.; Zhang, X.; He, R.; Tang, R.; Li, P.; He, Q.; Chen, G. MiR-133a is downregulated in non-small cell lung cancer: A study of clinical significance. Eur. J. Med. Res. 2015, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Chen, H.; Mo, Y.; Ye, H.; Luo, Y.; Guo, K.; Mai, Z.; Zhang, Y.; Chen, B.; et al. Down-regulation of miR-133a as a poor prognosticator in non-small cell lung cancer. Gene 2016, 591, 333–337. [Google Scholar] [CrossRef]
- He, R.Q.; Li, X.J.; Liang, L.; Xie, Y.; Luo, D.Z.; Ma, J.; Peng, Z.G.; Hu, X.H.; Chen, G. The suppressive role of miR-542-5p in NSCLC: The evidence from clinical data and in vivo validation using a chick chorioallantoic membrane model. BMC Cancer 2017, 17, 655. [Google Scholar] [CrossRef] [PubMed]
- Iacona, J.R.; Monteleone, N.J.; Lemenze, A.D.; Cornett, A.L.; Lutz, C.S. Transcriptomic studies provide insights into the tumor suppressive role of miR-146a-5p in non-small cell lung cancer (NSCLC) cells. RNA Biol. 2019, 16, 1721–1732. [Google Scholar] [CrossRef]
- Zhao, Q.; Cao, J.; Wu, Y.C.; Liu, X.; Han, J.; Huang, X.C.; Jiang, L.H.; Hou, X.X.; Mao, W.M.; Ling, Z.Q. Circulating miRNAs is a potential marker for gefitinib sensitivity and correlation with EGFR mutational status in human lung cancers. Am. J. Cancer Res. 2015, 5, 1692–1705. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Y.; Li, W.; Qin, H.; Lei, Z.; Shen, D.; Gu, D.; Huang, J.A.; Liu, Z. CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol. Cancer 2017, 16, 34. [Google Scholar] [CrossRef]
- Amri, J.; Molaee, N.; Baazm, M.; Karami, H. Targeting epidermal growth factor receptor by MiRNA-145 inhibits cell growth and sensitizes NSCLC cells to erlotinib. Asian Pacific. J. Cancer Prev. 2019, 20, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Amri, J.; Molaee, N.; Karami, H. Up-regulation of MiRNA-125a-5p inhibits cell proliferation and increases EGFR-TKI induced apoptosis in lung cancer cells. Asian Pac. J. Cancer Prev. 2019, 20, 3361–3367. [Google Scholar] [CrossRef]
- Meng, F.; Wang, F.; Wang, L.; Wong, S.C.C.; Cho, W.C.S.; Chan, L.W.C. MiR-30a-5p overexpression may overcome EGFR-inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front. Genet. 2016, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Liu, Y.N.; Wu, S.G.; Hsu, C.L.; Chang, T.H.; Tsai, M.F.; Lin, Y.T.; Shih, J.Y. MiR-200c-3p suppression is associated with development of acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in EGFR mutant non-small cell lung cancer via a mediating epithelial-to-mesenchymal transition (EMT) process. Cancer Biomark. 2020, 28, 351–363. [Google Scholar] [CrossRef]
- Ge, P.; Cao, L.; Chen, X.; Jing, R.; Yue, W. MiR-762 activation confers acquired resistance to gefitinib in non-small cell lung cancer. BMC Cancer 2019, 19, 1203. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Li, W.; Ping, W.; Deng, Y.; Fu, X. MiR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem. Biophys. Res. Commun. 2014, 446, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Wells, J.M.; Chow, K.H.; Huang, H.; Yuan, M.; Saxena, T.; Melnick, M.A.; Politi, K.; Asara, J.M.; Costa, D.B.; et al. miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nat. Metab. 2019, 1, 460–474. [Google Scholar] [CrossRef]
- Li, B.; Ren, S.; Li, X.; Wang, Y.; Garfield, D.; Zhou, S.; Chen, X.; Su, C.; Chen, M.; Kuang, P.; et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer 2014, 83, 146–153. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Wang, S.; Su, X.; Bai, H.; Zhao, J.; Duan, J.; An, T.; Zhuo, M.; Wang, Z.; Wu, M.; Li, Z.; et al. Identification of plasma microRNA profiles for primary resistance to EGFR-TKIs in advanced non-small cell lung cancer (NSCLC) patients with EGFR activating mutation. J. Hematol. Oncol. 2015, 8, 127. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Sun, G.; Xiong, S. Restoration of miR-7 expression suppresses the growth of Lewis lung cancer cells by modulating epidermal growth factor receptor signaling. Oncol. Rep. 2014, 32, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Lin, H.H.; Lien, Y.C.; Wang, Y.H.; Hong, C.F.; Kao, Y.R.; Lin, S.C.; Chang, Y.C.; Lin, S.Y.; Chen, S.J.; et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res. 2010, 70, 8822–8831. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Gao, F.H.; Shi, J.; Yuan, H.H.; Jiang, B. EGFR-ERK signaling pathway down-regulates miRNA-145 in lung cancer cells. Zhonghua Zhong Liu Za Zhi 2013, 35, 187–192. [Google Scholar] [CrossRef]
- Tao, S.; Ju, X.; Zhou, H.; Zeng, Q. Circulating microRNA-145 as a diagnostic biomarker for non-small-cell lung cancer: A systemic review and meta-analysis. Int. J. Biol. Markers 2020, 35, 51–60. [Google Scholar] [CrossRef]
- Chen, G.; Umelo, I.A.; Lv, S.; Teugels, E.; Fostier, K.; Kronenberger, P.; Dewaele, A.; Sadones, J.; Geers, C.; De Grève, J. miR-146a Inhibits Cell Growth, Cell Migration and Induces Apoptosis in Non-Small Cell Lung Cancer Cells. PLoS ONE 2013, 8, e60317. [Google Scholar] [CrossRef]
- Zhong, S.; Golpon, H.; Zardo, P.; Borlak, J. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl. Res. 2021, 230, 164–196. [Google Scholar] [CrossRef]
- Wani, J.; Majid, S.; Khan, A.; Arafah, A.; Ahmad, A.; Jan, B.; Shah, N.; Kazi, M.; Rehman, M. Clinico-Pathological Importance of miR-146a in Lung Cancer. Diagnostics 2021, 11, 274. [Google Scholar] [CrossRef]
- Li, L.; Wang, D. MicroRNA-128-b regulates epidermal growth factor receptor expression in non-small cell lung cancer. Mol. Med. Rep. 2019, 20, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, J.; Fang, L.; Huang, Y.; Li, R.; Xu, X.; Hu, Z.; Zhang, L.; Yang, Y.; Zhu, X.; et al. Simultaneous overactivation of Wnt/β-catenin and TGFβ signalling by miR-128-3p confers chemoresistance-associated metastasis in NSCLC. Nat. Commun. 2017, 8, 15870. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Li, S.; Lv, B.; Shi, J.; Yan, H.; Zhang, H.; He, Y. Long Non-coding RNA MIAT Mediates Non-small Cell Lung Cancer Development Through Regulating the miR-128-3p/PELI3 Axis. Biochem. Genet. 2020, 58, 867–882. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, X.; Zhou, W.; Wu, Y.; Yang, Y. MiR-128 reverses the gefitinib resistance of the lung cancer stem cells by inhibiting the c-met/PI3K/AKT pathway. Oncotarget 2016, 7, 73188–73199. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.; Park, H.; Chandimali, N.; Huynh, D.L.; Zhang, J.J.; Ghosh, M.; Gera, M.; Kim, N.; Bak, Y.; Yoon, D.Y.; et al. MicroRNA-128 suppresses paclitaxel-resistant lung cancer by inhibiting MUC1-C and BMI-1 in cancer stem cells. Oncotarget 2017, 8, 110540–110551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-G.; Men, W.-F.; Tang, J. MicroRNA-7 enhances cytotoxicity induced by gefitinib in non-small cell lung cancer via inhibiting the EGFR and IGF1R signalling pathways. Contemp. Oncol. 2015, 19, 201–206. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, D.; Wei, Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco. Targets. Ther. 2018, 11, 3979–3987. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, C.H.; Zhou, Y.; Liao, Z.Y.; Zhu, S.F.; Hu, Y.; Chen, C.; Luo, J.M.; Wen, Z.K.; Xu, L. TLR9 signaling repressed tumor suppressor miR-7 expression through up-regulation of HuR in human lung cancer cells. Cancer Cell Int. 2013, 13, 90. [Google Scholar] [CrossRef]
- Xiao, H. MiR-7-5p suppresses tumor metastasis of non-small cell lung cancer by targeting NOVA2. Cell. Mol. Biol. Lett. 2019, 24, 1V. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, C.; Zhao, J.; Wang, H.R.; Guo, M.; Zhou, Y.; Luo, J.; Zhang, J.; Xu, L. Targeted Expression of miR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway. Mol. Ther. Nucleic Acids 2017, 6, 183–197. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, Z.; Yu, L.; Che, J.; Zhang, J.; Yang, J.; Dong, Y. MicroRNA-144-3p suppressed TGF-β1-induced lung cancer cell invasion and adhesion by regulating the Src-Akt-Erk pathway. Cell Biol. Int. 2019, 44, 51–61. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Guo, Y.-N.; Shi, K.; Huang, H.-M.; Huang, S.-P.; Xu, W.-Q.; Li, Z.-Y.; Wei, K.-L.; Gan, T.-Q.; Chen, G. Down-regulation of microRNA-144-3p and its clinical value in non-small cell lung cancer: A comprehensive analysis based on microarray, miRNA-sequencing, and quantitative real-time PCR data. Respir. Res. 2019, 20, 48. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Luo, H.; Zhang, J.; Wang, J.; Guo, H. Identification of the differential expression of genes and upstream microRNAs in small cell lung cancer compared with normal lung based on bioinformatics analysis. Medicine 2020, 99, e19086. [Google Scholar] [CrossRef]
- Kumar, S.; Sharawat, S.K.; Ali, A.; Gaur, V.; Malik, P.; Kumar, S.; Mohan, A.; Guleria, R. Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients. Curr. Probl. Cancer 2020, 44, 100540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, M.; Liu, Z.; Liu, B. Clinical significance of serum miR-25 in non-small-cell lung cancer. Br. J. Biomed. Sci. 2019, 76, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, X. miR-25 promotes invasion of human non-small cell lung cancer via CDH1. Bioengineered 2019, 10, 271–281. [Google Scholar] [CrossRef]
- Wu, T.; Hu, H.; Zhang, T.; Jiang, L.; Li, X.; Liu, S.; Zheng, C.; Yan, G.; Chen, W.; Ning, Y.; et al. miR-25 Promotes Cell Proliferation, Migration, and Invasion of Non-Small-Cell Lung Cancer by Targeting the LATS2/YAP Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9719723. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, W.; Kong, D.; Li, X.; Lu, H.; Liu, S.; Wang, J.; Du, L.; Kong, Q.; Huang, X.; et al. miR-25 targets the modulator of apoptosis 1 gene in lung cancer. Carcinogenesis 2015, 36, 925–935. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Cui, J.Y.; Yuan, J.; Wang, X. MiR-451a suppressed cell migration and invasion in non-small cell lung cancer through targeting ATF2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5554–5561. [Google Scholar] [CrossRef]
- Yin, P.; Peng, R.; Peng, H.; Yao, L.; Sun, Y.; Wen, L.; Wu, T.; Zhou, J.; Zhang, Z. MiR-451 suppresses cell proliferation and metastasis in A549 lung cancer cells. Mol. Biotechnol. 2015, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Li, L.H.; Tang, J.B.; Sheng, Y.L. Mir-451 inhibits proliferation and migration of non-small cell lung cancer cells via targeting LKB1/AMPK. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 274–280. [Google Scholar] [CrossRef]
- Nie, R.; Niu, W.; Tang, T.; Zhang, J.; Zhang, X. Integrating microRNA expression, miRNA-mRNA regulation network and signal pathway: A novel strategy for lung cancer biomarker discovery. PeerJ 2021, 9, e12369. [Google Scholar] [CrossRef]
- Monteleone, N.J.; Lutz, C.S. miR-708-5p enhances erlotinib/paclitaxel efficacy and overcomes chemoresistance in lung cancer cells. Oncotarget 2020, 11, 4699–4721. [Google Scholar] [CrossRef]
- Alcantara, K.M.M.; Garcia, R.L. MicroRNA-92a promotes cell proliferation, migration and survival by directly targeting the tumor suppressor gene NF2 in colorectal and lung cancer cells. Oncol. Rep. 2019, 41, 2103–2116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Du, Y.; Mi, Y.; Wang, L. The HNF1A-AS1/miR-92a-3p axis affects the radiosensitivity of non-small cell lung cancer by competitively regulating the JNK pathway. Cell Biol. Toxicol. 2021, 37, 715–729. [Google Scholar] [CrossRef]
- Wei, J.; Jia, A.; Ma, L.; Wang, Y.; Qiu, L.; Xiao, B. MicroRNA-16 inhibits the proliferation and metastasis of human lung cancer cells by modulating the expression of YAP1. JBUON 2020, 25, 862–868. [Google Scholar] [PubMed]
- Chen, T.M.; Xiao, Q.; Wang, X.J.; Wang, Z.Q.; Hu, J.W.; Zhang, Z.; Gong, Z.N.; Chen, S.L. miR-16 regulates proliferation and invasion of lung cancer cells via the ERK/MAPK signaling pathway by targeted inhibition of MAPK kinase 1 (MEK1). J. Int. Med. Res. 2019, 47, 5194–5204. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Feng, Y.; Shi, K.; Zhang, H.; Qian, R. Long non-coding RNA TRPM2-AS sponges microRNA-138-5p to activate epidermal growth factor receptor and PI3K/AKT signaling in non-small cell lung cancer. Ann. Transl. Med. 2020, 8, 1313. [Google Scholar] [CrossRef]
- He, Q.; Fang, Y.; Lu, F.; Pan, J.; Wang, L.; Gong, W.; Fei, F.; Cui, J.; Zhong, J.; Hu, R.; et al. Analysis of differential expression profile of miRNA in peripheral blood of patients with lung cancer. J. Clin. Lab. Anal. 2019, 33, e23003. [Google Scholar] [CrossRef]
- He, Y.; Ren, S.; Wang, Y.; Li, X.; Zhou, C.; Hirsch, F.R. Serum microRNAs improving the diagnostic accuracy in lung cancer presenting with pulmonary nodules. J. Thorac. Dis. 2018, 10, 5080–5085. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Li, P.; Song, P.; Li, Y.; Zhou, S.; Su, Q.; Li, X.; Yu, Y.; Li, P.; Feng, M.; et al. MicroRNA-138-5p Suppresses Non-small Cell Lung Cancer Cells by Targeting PD-L1/PD-1 to Regulate Tumor Microenvironment. Front. Cell Dev. Biol. 2020, 8, 540. [Google Scholar] [CrossRef]
- Yamaguchi, G.; Takanashi, M.; Tanaka, M.; Fujita, K.; Ohira, T.; Kuroda, M.; Ikeda, N. Isolation of miRNAs that target EGFR mRNA in human lung cancer. Biochem. Biophys. Res. Commun. 2012, 420, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Joerger, M.; Baty, F.; Früh, M.; Droege, C.; Stahel, R.; Betticher, D.; von Moos, R.; Ochsenbein, A.; Pless, M.; Gautschi, O.; et al. Circulating microRNA profiling in patients with advanced non-squamous NSCLC receiving bevacizumab/erlotinib followed by platinum-based chemotherapy at progression (SAKK 19/05). Lung Cancer 2014, 85, 306–313. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Zheng, M.; Ge, J.; Li, J.; Yu, P. MiR-542-3p exerts tumor suppressive functions in non-small cell lung cancer cells by upregulating FTSJ2. Life Sci. 2017, 188, 87–95. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Jiang, C.; Li, W.; Chen, Y.; Ying, G. Multiple MicroRNAs synergistically promote tolerance to epidermal growth factor receptor-targeted drugs in smoked lung cancer therapies. J. Cancer Res. Ther. 2019, 15, 876–881. [Google Scholar] [CrossRef]
- Cho, W.C.S.; Chow, A.S.C.; Au, J.S.K. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur. J. Cancer 2009, 45, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Seike, M.; Okano, T.; Matsuda, K.; Miyanaga, A.; Mizutani, H.; Noro, R.; Minegishi, Y.; Kubota, K.; Gemma, A. MiR-134/487b/655 cluster regulates TGF-β-induced epithelial- mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in Lung Adenocarcinoma Cells. Mol. Cancer Ther. 2014, 13, 444–453. [Google Scholar] [CrossRef]
- Mou, K.; Gu, W.; Gu, C.; Zhang, J.; Qwang, W.; Ren, G.; Tian, J. Relationship between miR-7 expression and treatment outcomes with gefitinib in non-small cell lung cancer. Oncol. Lett. 2016, 12, 4613–4617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, Q.; Mao, Z.-D.; Shi, Y.-J.; Chen, Y.; Sun, Y.; Zhang, Q.; Song, L.; Peng, L.-P. MicroRNA-7 inhibits cell proliferation, migration and invasion in human non-small cell lung cancer cells by targeting FAK through ERK/MAPK signaling pathway. Oncotarget 2016, 7, 77468–77481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, K.; Liao, Z.; Li, Y.; Yang, H.; Chen, C.; Zhou, Y.; Tao, Y.; Guo, M.; Ren, T.; et al. Promoter mutation of tumor suppressor microRNA-7 is associated with poor prognosis of lung cancer. Mol. Clin. Oncol. 2015, 3, 1329–1336. [Google Scholar] [CrossRef]
- Bagheri, A.; Khorshid, H.R.K.; Tavallaie, M.; Mowla, S.J.; Sherafatian, M.; Rashidi, M.; Zargari, M.; Boroujeni, M.E.; Hosseini, S.M. A panel of noncoding RNAs in non-small-cell lung cancer. J. Cell. Biochem. 2018, 120, 8280–8290. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weng, Q.; Shi, Y.; Mao, W.; Zhao, Z.; Wu, R.; Ren, J.; Fang, S.; Lu, C.; Du, Y.; et al. MicroRNA-155-5p suppresses PD-L1 expression in lung adenocarcinoma. FEBS Open Bio 2020, 10, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef]
- Hejleh, T.A.; Furqan, M.; Ballas, Z.; Clamon, G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Kiakou, M.; Tsapakidis, K.; Tolia, M.; Aravantinou-Fatorou, E.; Baxevanos, P.; Kyrgias, G.; Theocharis, S. PD-1 and PD-L1 as immunotherapy targets and biomarkers in non-small cell lung cancer. JBUON 2019, 24, 883–888. [Google Scholar]
- Akbay, E.A.; Koyama, S.; Carretero, J.; Altabef, A.; Tchaicha, J.H.; Christensen, C.L.; Mikse, O.R.; Cherniack, A.D.; Beauchamp, E.M.; Pugh, T.J.; et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013, 3, 1355–1363. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Liu, T.; Du, W.; Zhu, J.; Liu, Z.; Huang, J.-A. The canonical TGF-β/Smad signalling pathway is involved in PD-L1-induced primary resistance to EGFR-TKIs in EGFR-mutant non-small-cell lung cancer. Respir. Res. 2019, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Tanaka, T.; Kaneda, H.; Togashi, Y.; Sakai, K.; Hayashi, K.; Tomida, S.; Chiba, Y.; Yonesaka, K.; et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fang, W.; Zhan, J.; Hong, S.; Tang, Y.; Kang, S.; Zhang, Y.; He, X.; Zhou, T.; Qin, T.; et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J. Thorac. Oncol. 2015, 10, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, J.; Wang, Y.; Huang, L.; Sun, L.; Zhao, J.; Zhang, S.; Jing, W.; Ma, J.; Han, C. Concurrent Genetic Alterations and Other Biomarkers Predict Treatment Efficacy of EGFR-TKIs in EGFR-Mutant Non-Small Cell Lung Cancer: A Review. Front. Oncol. 2020, 10, 610923. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.; Trivedi, M.; Shah, P.; Ouyang, Q.; Oka, A.; Gandham, S.; Amiji, M.M. Combination wt-p53 and MicroRNA-125b transfection in a genetically engineered lung cancer model using dual CD44/EGFR-targeting nanoparticles. Mol. Ther. 2016, 24, 759–769. [Google Scholar] [CrossRef]
- Cornett, A.L.; Lutz, C.S. Regulation of COX-2 expression by miR-146a in lung cancer cells. RNA 2014, 20, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.H.; Pasha, H.F.; Gad, D.M.; Toam, M.M. miR-146a and miR-196a-2 genes polymorphisms and its circulating levels in lung cancer patients. J. Biochem. 2019, 220, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Lin, Z.; Cai, L. Association between miRNA-146a polymorphism and lung cancer susceptibility: A meta-analysis involving 6506 cases and 6576 controls. Gene 2020, 757, 144940. [Google Scholar] [CrossRef]
- Lopes, G.L.; Vattimo, E.; Junior, G.D.C. Identifying activating mutations in the EGFR gene: Prognostic and therapeutic implications in non-small cell lung cancer. J. Bras. Pneumol. 2015, 41, 365–375. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Pancewicz-Wojtkiewicz, J. Epidermal growth factor receptor and notch signaling in non-small-cell lung cancer. Cancer Med. 2016, 5, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Chung, J.-Y.; Ylaya, K.; Conway, C.; Takikita, M.; Fukuoka, J.; Doki, Y.; Hanaoka, J.; Hewitt, S.M. Profiling of phospho-AKT, phospho-mTOR, phospho-MAPK and EGFR in non-small cell lung cancer. J. Histochem. Cytochem. 2014, 62, 335–346. [Google Scholar] [CrossRef]
- Wannesson, L.; Viteri, S.; Costa, C.; Karachaliou, N.; Molina-Vila, M.; Rosell, R. Signaling pathways modulating dependence of lung cancer on mutant epidermal growth factor receptor and mechanisms of intrinsic and acquired resistance to tyrosine kinase inhibitors. Curr. Pharm. Des. 2014, 20, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Zhang, G.; Haura, E.B. Targeting epidermal growth factor receptor: Central signaling kinase in lung cancer. Biochem. Pharmacol. 2010, 80, 613–623. [Google Scholar] [CrossRef]
- Pathak, A.; Rajappa, S.; Gore, A. Oncogenic drivers in nonsmall cell lung cancer and resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Indian J. Cancer 2017, 54, S1–S8. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Barni, S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated nonsmall-cell lung cancer: A meta-analysis of 13 randomized trials. Clin. Lung Cancer 2012, 13, 107–114. [Google Scholar] [CrossRef]
- Sgambato, A.; Casaluce, F.; Maione, P.; Rossi, A.; Rossi, E.; Napolitano, A.; Palazzolo, G.; Bareschino, A.M.; Schettino, C.; Sacco, C.P.; et al. The Role of EGFR Tyrosine Kinase Inhibitors in the First-Line Treatment of Advanced Non Small Cell Lung Cancer Patients Harboring EGFR Mutation. Curr. Med. Chem. 2012, 19, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Subat, S.; Inamura, K.; Ninomiya, H.; Nagano, H.; Okumura, S.; Ishikawa, Y. Unique MicroRNA and mRNA Interactions in EGFR-Mutated Lung Adenocarcinoma. J. Clin. Med. 2018, 7, 419. [Google Scholar] [CrossRef]
- Seike, M.; Goto, A.; Okano, T.; Bowman, E.D.; Schetter, A.J.; Horikawa, I.; Mathe, E.A.; Jen, J.; Yang, P.; Sugimura, H.; et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc. Natl. Acad. Sci. USA 2009, 106, 12085–12090. [Google Scholar] [CrossRef]
- Bica-Pop, C.; Cojocneanu-Petric, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. 2018, 75, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, J.; Liu, L.; Shen, H.; Zeng, H.; Shu, Y. A systematic-analysis of predicted miR-21 targets identifies a signature for lung cancer. Biomed. Pharmacother. 2012, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, J.; Tao, Y.; Guo, M.; Ya, Z.; Chen, C.; Qin, N.; Zheng, J.; Luo, J.; Xu, L. MicroRNA-21: A promising biomarker for the prognosis and diagnosis of non-small cell lung cancer. Oncol. Lett. 2018, 16, 2777–2782. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef]
- Yang, J.-S.; Li, B.-J.; Lu, H.-W.; Chen, Y.; Lu, C.; Zhu, R.-X.; Liu, S.-H.; Yi, Q.-T.; Li, J.; Song, C.-H. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015, 36, 3035–3042. [Google Scholar] [CrossRef]
- Szpechcinski, A.; Florczuk, M.; Duk, K.; Zdral, A.; Rudzinski, S.; Bryl, M.; Czyzewicz, G.; Rudzinski, P.; Kupis, W.; Wojda, E.; et al. The expression of circulating miR-504 in plasma is associated with EGFR mutation status in non-small-cell lung carcinoma patients. Cell. Mol. Life Sci. 2019, 76, 3641–3656. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.J.; Park, H.Y.; Kim, A.; Shin, D.H.; Lee, C.H. Differential MicroRNA expression between EGFR T790M and L858R mutated lung cancer. J. Pathol. Transl. Med. 2018, 52, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bjaanæs, M.M.; Halvorsen, A.R.; Solberg, S.; Jørgensen, L.; Dragani, T.A.; Galvan, A.; Colombo, F.; Anderlini, M.; Pastorino, U.; Kure, E.; et al. Unique microRNA-profiles in EGFR-mutated lung adenocarcinomas. Int. J. Cancer 2014, 135, 1812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Ding, H.; Wang, W.; Liao, Z.; Fu, Z.; Hong, Y.; Zhou, Y.; Zhang, C.-Y.; Chen, X. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget 2016, 7, 28075–28085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, L.; Hu, Q.; Xia, J.; Sun, J.; Wang, X.; Xiong, H.; Gurbani, D.; Li, L.; Liu, Y.; et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol. Cancer 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Dacic, S.; Kelly, L.; Shuai, Y.; Nikiforova, M.N. MiRNA expression profiling of lung adenocarcinomas: Correlation with mutational status. Mod. Pathol. 2010, 23, 1577–1582. [Google Scholar] [CrossRef]
- Wischmann, F.; Götte, M.; Rezai, M.; Eich, H.T.; Greve, B. EGFR mediated Inhibition of miR-218 Maturation as cellular Radiation Response. In Proceedings of the Conference: 21st Annual Meeting of the German-Society-for-Radiation-Oncology, Hamburg, Germany, 25–28 June 2015; Springer: Berlin/Heidelberg, Germany, 2015; Volume 191. [Google Scholar]
- Ma, W.; Kang, Y.; Ning, L.; Tan, J.; Wang, H.; Ying, Y. Identification of microRNAs involved in gefitinib resistance of non-small-cell lung cancer through the insulin-like growth factor receptor 1 signaling pathway. Exp. Ther. Med. 2017, 14, 2853–2862. [Google Scholar] [CrossRef]
- Zhao, J.; Kelnar, K.; Bader, A.G. In-depth analysis shows synergy between erlotinib and miR-34a. PLoS ONE 2014, 9, e89105. [Google Scholar] [CrossRef]
- Hisakane, K.; Seike, M.; Sugano, T.; Yoshikawa, A.; Matsuda, K.; Takano, N.; Takahashi, S.; Noro, R.; Gemma, A. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial-mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac. Cancer 2021, 12, 1690–1698. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, C.; Wang, Y.; Yang, Z.; Li, C.; Fang, W.; Jin, Y.; Hou, K.; Cheng, Y.; Qi, J.; et al. LncRNA APCDD1L-AS1 induces icotinib resistance by inhibition of EGFR autophagic degradation via the miR-1322/miR-1972/miR-324-3p-SIRT5 axis in lung adenocarcinoma. Biomark. Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Tsai, M.-F.; Wu, S.-G.; Chang, T.-H.; Tsai, T.-H.; Gow, C.-H.; Wang, H.-Y.; Shih, J.-Y. miR-146b-5p Enhances the Sensitivity of NSCLC to EGFR Tyrosine Kinase Inhibitors by Regulating the IRAK1/NF-κB Pathway. Mol. Ther. Nucleic Acids 2020, 22, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Lin, J.; Lin, N.; Zou, C.; Kurata, J.; Lin, R.; He, Z.; Su, Y. Down-regulation of miR-214 reverses erlotinib resistance in non-small-cell lung cancer through up-regulating LHX6 expression. Sci. Rep. 2017, 7, 781. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, A.; Cafarotti, S.; Indini, A.; Galli, A.; Russo, A.; Cesario, A.; Lococo, F.M.; Russo, P.; Mainini, A.F.; Bonifati, L.G.; et al. Egfr-targeted therapy for non-small cell lun cancer: Focus on EGFR oncogenic mutation. Int. J. Med. Sci. 2013, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Shimura, E.; Nagasawa, A.; Aiuchi, T.; Suda, Y.; Hamada, Y.; Ikegami, D.; Iwasawa, C.; Arakawa, K.; Igarashi, K.; et al. Chronic treatment of non-small-cell lung cancer cells with gefitinib leads to an epigenetic loss of epithelial properties associated with reductions in microRNA-155 and -200c. PLoS ONE 2017, 12, e0172115. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, L.; Niu, X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Ye, R.; Tang, R.; Gan, S.; Li, R.; Cheng, Y.; Guo, L.; Zeng, C.; Sun, Y. New insights into long non-coding RNAs in non-small cell lung cancer. Biomed. Pharmacother. 2020, 131, 110775. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, X.; Gao, W.; Zhang, Z.; Liu, P.; Wang, R.; Huang, P.; Yin, Y.; Shu, Y. MicroRNA-133b inhibits the growth of non-small-cell lung cancer by targeting the epidermal growth factor receptor. FEBS J. 2012, 279, 3800–3812. [Google Scholar] [CrossRef]
- Kawana, S.; Saito, R.; Miki, Y.; Kimura, Y.; Abe, J.; Sato, I.; Endo, M.; Sugawara, S.; Sasano, H. Suppression of tumor immune microenvironment via microRNA-1 after epidermal growth factor receptor-tyrosine kinase inhibitor resistance acquirement in lung adenocarcinoma. Cancer Med. 2021, 10, 718–727. [Google Scholar] [CrossRef]
- Marin, I.; Ofek, E.; Bar, J.; Prisant, N.; Perelman, M.; Avivi, C.; Lavy-Shahaf, G.; Onn, A.; Katz, R.; Barshack, I. MiR-21, EGFR and PTEN in non-small cell lung cancer: An in situ hybridisation and immunohistochemistry study. J. Clin. Pathol. 2020, 73, 636–641. [Google Scholar] [CrossRef]
- Sun, W.; Yuan, X.; Tian, Y.; Wu, H.; Xu, H.; Hu, G.; Wu, K. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J. Hematol. Oncol. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Bach, D.-H.; Kim, D.; Bae, S.Y.; Kim, W.K.; Hong, J.-Y.; Lee, H.-J.; Rajasekaran, N.; Kwon, S.; Fan, Y.; Luu, T.-T.; et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. Mol. Ther. Nucleic Acids 2018, 11, 455–467. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, P.; Mi, Y.; Wang, W.; Pan, X.; Wu, X.; He, Q.; Liu, H.; Tang, W.; An, H. Plasma MiRNA alterations between NSCLC patients harboring Del19 and L858R EGFR mutations. Oncotarget 2016, 7, 54965–54972. [Google Scholar] [CrossRef]
- Hanafi, A.R.; Jayusman, A.M.; Alfasunu, S.; Sadewa, A.H.; Pramono, D.; Heriyanto, D.S.; Haryana, S.M. Serum MiRNA as predictive and prognosis biomarker in advanced stage non-small cell lung cancer in Indonesia. Chin. J. Lung Cancer 2020, 23, 321–332. [Google Scholar] [CrossRef]
- Remon, J.; Alvarez-Berdugo, D.; Majem, M.; Moran, T.; Reguart, N.; Lianes, P. miRNA-197 and miRNA-184 are associated with brain metastasis in EGFR-mutant lung cancers. Clin. Transl. Oncol. 2016, 18, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gober, M.K.; Collard, J.P.; Thompson, K.; Black, E.P. A microRNA signature of response to erlotinib is descriptive of TGFβ behaviour in NSCLC. Sci. Rep. 2017, 7, 4202. [Google Scholar] [CrossRef]
- Bryant, J.L.; Britson, J.; Balko, J.M.; Willian, M.; Timmons, R.; Frolov, A.; Black, E.P. A microRNA gene expression signature predicts response to erlotinib in epithelial cancer cell lines and targets EMT. Br. J. Cancer 2012, 106, 148–156. [Google Scholar] [CrossRef]
- Makris, D.; Scherpereel, A.; Copin, M.C.; Colin, G.; Brun, L.; Lafitte, J.J.; Marquette, C.H. Fatal interstitial lung disease associated with oral erlotinib therapy for lung cancer. BMC Cancer 2007, 7, 1–4. [Google Scholar] [CrossRef]
- Ricciuti, B.; Mecca, C.; Cenci, M.; Leonardi, G.C.; Perrone, L.; Mencaroni, C.; Crino, L.; Grignani, F.; Baglivo, S.; Chiari, R.; et al. MiRNAs and resistance to EGFR—TKIs in EGFR-mutant non-small cell lung cancer: Beyond “traditional mechanisms” of resistance. Ecancermedicalscience 2015, 9, 569. [Google Scholar] [CrossRef]
- Bisagni, A.; Pagano, M.; Maramotti, S.; Zanelli, F.; Bonacini, M.; Tagliavini, E.; Braglia, L.; Paci, M.; Mozzarelli, A.; Croci, S. Higher expression of miR-133b is associated with better efficacy of erlotinib as the second or third line in non-small cell lung cancer patients. PLoS ONE 2018, 13, e0196350. [Google Scholar] [CrossRef] [PubMed]
- Haque, I.; Kawsar, H.I.; Motes, H.; Sharma, M.; Banerjee, S.; Banerjee, S.K.; Godwin, A.K.; Huang, C.H. Downregulation of miR-506-3p Facilitates EGFR-TKI Resistance through Induction of Sonic Hedgehog Signaling in Non-Small-Cell Lung Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Chen, J.; Cui, J.-D.; Guo, X.-T.; Cao, X.; Li, Q. Increased expression of miR-641 contributes to erlotinib resistance in non-small-cell lung cancer cells by targeting NF1. Cancer Med. 2018, 7, 1394–1403. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, R.; Chen, J.; Ning, D. CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes to cisplatin resistance in non-small cell lung cancer (NSCLC). Cancer Cell Int. 2020, 20, 289. [Google Scholar] [CrossRef]
- Zhao, J.; Guerrero, A.; Kelnar, K.; Peltier, H.J.; Bader, A.G. Synergy between next generation EGFR tyrosine kinase inhibitors and miR-34a in the inhibition of non-small cell lung cancer. Lung Cancer 2017, 108, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lim, L.P.; De Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; Ceppi, P.; Kumarswamy, R.; Scagliotti, G.V.; Papotti, M.; Allgayer, H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 2011, 30, 2888–2899. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Sci 2007, 316, 1039. [Google Scholar] [CrossRef]
- Giaccone, G. The Role of Gefitinib in Lung Cancer Treatment. Clin. Cancer Res. 2004, 10, 4233s–4237s. [Google Scholar] [CrossRef] [PubMed]
- Kita, K.; Fukuda, K.; Takahashi, H.; Tanimoto, A.; Nishiyama, A.; Arai, S.; Takeuchi, S.; Yamashita, K.; Ohtsubo, K.; Otani, S.; et al. Patient-derived xenograft models of non-small cell lung cancer for evaluating targeted drug sensitivity and resistance. Cancer Sci. 2019, 110, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Chen, J.; Li, Y.; Tang, X.; Wang, J.; Xu, W.; Song, J.; Li, Y.; Tao, H.; Chen, Q. miR-1-3p and miR-206 sensitizes HGF-induced gefitinib-resistant human lung cancer cells through inhibition of c-Met signalling and EMT. J. Cell. Mol. Med. 2018, 22, 3526–3536. [Google Scholar] [CrossRef]
- Sun, C.; Gao, W.; Liu, J.; Cheng, H.; Hao, J. FGL1 regulates acquired resistance to Gefitinib by inhibiting apoptosis in non-small cell lung cancer. Respir. Res. 2020, 21, 210. [Google Scholar] [CrossRef]
- Yu, G.; Zhong, N.; Chen, G.; Huang, B.; Wu, S. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumour Biol. 2014, 35, 10341–10349. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, M.; Rodriguez, J.A.; Gallegos-Ruiz, M.; Kruyt, F.; Giaccone, G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int. J. Cancer 2006, 118, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yang, F.; Qin, Z.; Jing, X.; Shu, Y.; Shen, H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: A systematic review with meta-analysis. BMC Cancer 2019, 19, 1103. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Eklo, K.; Berg, T.; Sorbye, S.W.; Lonvik, K.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Stenvold, H.; Bremnes, R.M.; et al. Prognostic impact of MiR-155 in non-small cell lung cancer evaluated by in situ hybridization. J. Transl. Med. 2011, 9, 6. [Google Scholar] [CrossRef]
- Xie, K.; Ma, H.; Liang, C.; Wang, C.; Qin, N.; Shen, W.; Gu, Y.; Yan, C.; Zhang, K.; Dai, N.; et al. A functional variant in miR-155 regulation region contributes to lung cancer risk and survival. Oncotarget 2015, 6, 42781–42792. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bao, T.; Li, Z.; Ji, G.; Zhang, L. Function of miR-200a in proliferation and apoptosis of non-small cell lung cancer cells. Oncol. Lett. 2020, 20, 1256–1262. [Google Scholar] [CrossRef]
- Liu, C.; Hu, W.; Li, L.-L.; Wang, Y.-X.; Zhou, Q.; Zhang, F.; Song-Yang, Y.-Y.; Zhu, W.; Sun, C.-C.; Li, D.-J. Roles of miR-200 family members in lung cancer: More than tumor suppressors. Future Oncol. 2018, 14, 2875–2886. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Song, W.W.; Wang, Y.; Zhao, W.; Wu, J.; Xu, W. MicroRNA-200 families and prognostic value in various carcinomas: A systematic review and meta-analysis. Aging Med. Milt. 2018, 1, 39–45. [Google Scholar] [CrossRef]
- Wang, S.-K.; Lin, K.; Xu, T.; He, B.-S.; Pan, Y.-Q.; Sun, H.-L.; Peng, H.-X.; Hu, X.-X. MicroRNA expression profiles predict progression and clinical outcome in lung adenocarcinoma. Onco. Targets. Ther. 2016, 9, 5679–5692. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, S.; Zhao, J.; Zhou, Y.; Xu, L. MicroRNA-126: A new and promising player in lung cancer. Oncol. Lett. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Zhou, W.; Nie, J.; Zhang, D. Differential expression of miR-126-5p in lung adenocarcinoma and the possible mechanism. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 1186–1190. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, Y.; Lu, J.; Xu, H.; Lei, L.; Chen, C.; Zhao, J.; Xu, L. The prognostic value of miR-126 expression in non-small-cell lung cancer: A meta-analysis. Cancer Cell Int. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ning, Z.; Jun, D.; Lu, Z.; Xin-Ting, F.; Yu, W.; Jing-Kang, H. MicroRNA-138 targets SOX4 to regulate the proliferation and metastasis of human lung cancer cells. JBUON 2020, 25, 835–841. [Google Scholar]
- Tang, X.; Jiang, J.; Zhu, J.; He, N.; Tan, J. HOXA4-regulated miR-138 suppresses proliferation and gefitinib resistance in non-small cell lung cancer. Mol. Genet. Genomics 2019, 294, 85–93. [Google Scholar] [CrossRef]
- Han, Z.; Zhou, X.; Li, S.; Qin, Y.; Chen, Y.; Liu, H. Inhibition of miR-23a increases the sensitivity of lung cancer stem cells to erlotinib through PTEN/PI3K/Akt pathway. Oncol. Rep. 2017, 38, 3064–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Wang, Y.-H.; Xia, H.-P.; Zhou, S.-W.; Schmid-Bindert, G.; Zhou, C.-C. MicroRNA-214 regulates the acquired resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant cell lines. Asian Pac. J. Cancer Prev. 2012, 13, 255–260. [Google Scholar] [CrossRef]
- Yin, J.; Hu, W.; Pan, L.; Fu, W.; Dai, L.; Jiang, Z.; Zhang, F.; Zhao, J. let-7 and miR-17 promote self-renewal and drive gefitinib resistance in non-small cell lung cancer. Oncol. Rep. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- Wang, F.; Meng, F.; Wong, S.C.C.; Cho, W.C.; Yang, S.; Chan, L.W. Combination therapy of gefitinib and miR-30a-5p may overcome acquired drug resistance through regulating the PI3K/AKT pathway in non-small cell lung cancer. Ther. Adv. Respir. Dis. 2020, 14, 1753466620915156. [Google Scholar] [CrossRef]
- Wang, H.; Kanmangne, D.; Li, R.; Qian, Z.; Xia, X.; Wang, X.; Wang, T. miR-30a-3p suppresses the proliferation and migration of lung adenocarcinoma cells by downregulating CNPY2. Oncol. Rep. 2020, 43, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Rao, Z.; Chen, C. miR-30a suppresses lung cancer progression by targeting SIRT1. Oncotarget 2017, 9, 4924–4934. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, S.; Ma, Y.; Fan, Z.; Yan, Z.; Li, W.; Song, Q.; You, W.; Lyu, Z.; Song, Y.; et al. miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression. J. Mol. Med. 2017, 95, 861–871. [Google Scholar] [CrossRef]
- Luan, N.; Wang, Y.; Liu, X. Absent expression of miR-30a promotes the growth of lung cancer cells by targeting MEF2D. Oncol. Lett. 2018, 16, 1173–1179. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; He, J. MiR-133a-3p attenuates resistance of non-small cell lung cancer cells to gefitinib by targeting SPAG5. J. Clin. Lab. Anal. 2021, 35, e23853. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lv, D.; Wang, C.; Li, L.; Zhao, Q.; Chen, H.; Xu, L. Epigenetic silencing of miR-483-3p promotes acquired gefitinib resistance and EMT in EGFR-mutant NSCLC by targeting integrin β3. Oncogene 2018, 37, 4300–4312. [Google Scholar] [CrossRef]
- Zhu, J.; Qi, Y.; Wu, J.; Shi, M.; Feng, J.; Chen, L. Evaluation of plasma microRNA levels to predict insensitivity of patients with advanced lung adenocarcinomas to pemetrexed and platinum. Oncol. Lett. 2016, 12, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sun, L.; Liu, T.; Zhu, J.; Zeng, Y.; Zhang, Y.; Wang, X.; Liu, Z.; Huang, J.-A. The miR-625-3p/AXL axis induces non-T790M acquired resistance to EGFR-TKI via activation of the TGF-β/Smad pathway and EMT in EGFR-mutant non-small cell lung cancer. Oncol. Rep. 2020, 44, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Q.; Zhang, M.; Su, W.; Wang, Z.; Li, Y.; Zhang, J.; Beer, D.G.; Yang, S.; Chen, G. Serum microRNA Signature Is Capable of Early Diagnosis for Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2019, 15, 1712–1722. [Google Scholar] [CrossRef]
- Raponi, M.; Dossey, L.; Jatkoe, T.; Wu, X.; Chen, G.; Fan, H.; Beer, D.G. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009, 69, 5776–5783. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, N.H.H.; Schetter, A.J.; Welsh, J.A.; Yoneda, M.; Bowman, E.D.; Harris, C.C. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int. J. Cancer 2012, 130, 1378–1386. [Google Scholar] [CrossRef]
- Cinegaglia, N.C.; Andrade, S.C.S.; Tokar, T.; Pinheiro, M.; Severino, F.E.; Oliveira, R.A.; Hasimoto, E.N.; Cataneo, D.C.; Cataneo, A.J.M.; Defaveri, J.; et al. Integrative transcriptome analysis identifies deregulated microRNA-transcription factor networks in lung adenocarcinoma. Oncotarget 2016, 7, 28920–28934. [Google Scholar] [CrossRef]
- Fan, J.; Xu, G.; Chang, Z.; Zhu, L.; Yao, J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin. Sci. 2020, 134, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yan, Y.; Yang, Y.; Hong, X.; Wang, M.; Yang, Z.; Liu, B.; Ye, L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020, 73, 109675. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Zhang, Y.; Ding, Z.; Zheng, Y.; Chen, S.; Wan, Y. Integrative analysis of mRNA and miRNA expression profiles reveals seven potential diagnostic biomarkers for non-small cell lung cancer. Oncol. Rep. 2020, 43, 99–112. [Google Scholar] [CrossRef]
- Xie, S.; Liu, G.; Huang, J.; Hu, H.B.; Jiang, W. miR-210 promotes lung adenocarcinoma proliferation, migration, and invasion by targeting lysyl oxidase-like 4. J. Cell. Physiol. 2019, 234, 14050–14057. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Fei, X.; Liu, S.; Liao, J.; Li, Y. LncRNA LOXL1-AS1 promotes invasion and proliferation of non-small-cell lung cancer through targeting miR-324-3p. Am. J. Transl. Res. 2019, 11, 6403. [Google Scholar] [PubMed]
- Lin, M.H.; Chen, Y.Z.; Lee, M.Y.; Weng, K.P.; Chang, H.T.; Yu, S.Y.; Dong, B.J.; Kuo, F.R.; Hung, L.T.; Liu, L.F.; et al. Comprehensive identification of microRNA arm selection preference in lung cancer: miR-324-5p and -3p serve oncogenic functions in lung cancer. Oncol. Lett. 2018, 15, 9818–9826. [Google Scholar] [CrossRef]
| Type of miRNA | Proposed Mechanism of Action in Lung Cancer | Preclinical and Clinical Studies | Methodology | Reference |
|---|---|---|---|---|
| Oncosuppressor miRNAs | ||||
| miR-27a |
| A549, H1299, and CALU-1 NSCLC cell lines | qRT-PCR Western blot Luciferase reporter assay | [52] |
| miR-133a |
| Human NSCLC tissues and adjacent normal lung tissue H358 human NSCLC cell line transfected with miR-133a mimics | qRT-PCR Immunohistochemistry (IHC) | [64,65,66] |
| miR-25-3p |
| miRNA regression model supported by target prediction databases | Multiple linear regression based on expression levels | [8] |
| miR-128b |
| Human NSCLC and adjacent normal lung tissue samples | qRT-PCR Semi-quantitative RT-PCR IHC analysis | [49] |
| miR-134 |
| NSCLC cell lines (A549, H1299, H520, and H1975) | qRT-PCR MTT assay Flow cytometry Luciferase reporter assay RNAi and rescue experiments A549 xenograft in nude mice | [36] |
| miR-34a |
| Human NSCLC and adjacent normal lung tissue samples A549 (EGFR-wild type), SPC-A1 and HCC827 (EGFR-mutated) cell lines lung carcinoma xenograft mouse model | qRT-PCR Cell proliferation assay Cell transwell assay Luciferase reporter assay Western blot analysis xenograft assay IHC analysis | [20,57] |
| miR-542-5p |
| Human NSCLC and adjacent normal lung tissue samples | qRT-PCR | [67] |
| miR-146a-5p |
| A549 cells | RNA-Seq Gene ontology analysis qRT-PCR | [68] |
| miR-183 miR-210 miR-34c |
| Human mutated LADC and adjacent normal lung tissue samples | Microarray analysis qRT-PCR | [25] |
| miR-125b |
| Human NSCLC and adjacent normal lung tissue samples and plasma gefitinib-sensitive PC9, and gefitinib-resistant A549 and H1299 human lung ADC cells | MicroRNA array Genotyping of EGFR mutational status | [69] |
| miR-30a-5p |
| Human NSCLC and adjacent normal lung tissue samples lung carcinoma xenograft mouse model | CCK-8 and clonogenic assays Wound healing, migration and invasion assays | [70] |
| miR-145 |
| NSCLC cell line A549 | qRT-PCR Trypan blue and MTT assays ELISA cell death assay Combination effect analysis | [71] |
| miR-200a |
| lung cancer cell lines H3255 (L858R EGFR allele), H1975 (L858R/T790M mutations in EGFR), and HCC827 | qRT-PCR Luciferase assays Western Blot Wound-Healing Assay Cell Invasion Assay MTS assay and BrdU incorporation assay | [1] |
| miR-125a-5p |
| A549 lung cancer cells | qRT-PCR Trypan blue assays MTT assay Combination index (Chou-Talalay) ELISA cell death assay kit | [72] |
| miR-30a-5p |
| Gefitinib-resistant NSCLC cell lines, H460 and H1975 | Western Blot Annexin V-FITC Apoptosis Detection Kit CytoSelect™ Cell Invasion Assay Kit Wound healing assay | [15,73] |
| let-7b |
| NSCLC cells bearing clinically relevant mutations in KRAS and TP53 (H358, H23, H441, Calu-6) or NRAS and TP53 (H1299) | qRT-PCR Sulforhodamine B (SRB) assays | [20,49] |
| miR-200c-3p |
| EGFR-mutant NSCLC and adjacent normal lung tissue EGFR TKI-sensitive cell lines- PC9 and HCC287, and gefitinib-resistant PC9/gef cells that harbor a deletion in exon 19 of EGFR | MicroRNA array qRT-PCR Western Blot Migration assay Cytotoxicity and apoptosis assays | [74] |
| miR-762 |
| NSCLC Cell lines with EGFR mutations PC-9 (E746-A750 del) NCI-H820 (E746-E749 del) NCI-H1650 (E746-A750 del) NCI-H1975 (L858R) A549, NCI-H2170, NCI-H1993, NCI-H2126, NCI-H1299, NCI-H1648, NCI-H1703 and NCI-H2347 (WT) lung carcinoma xenograft mouse model | qRT-PCR Cytotoxicity In vivo chemosensitivity Luciferase reporter assay | [75] |
| miR-126 |
| NCI-H460 (H460) and A549 cells lung carcinoma xenograft mouse model | qRT-PCR Growth inhibition assay Western Blot | [19] |
| miR-138-5p |
| Human NSCLC and adjacent normal lung tissue samples NSCLC cell lines PC9 and H1975 | qRT-PCR Luciferase assays Western Blot IHC | [76] |
| Oncogenic miRNAs | ||||
| miR-147b |
| EGFR-wild type cell lines H358, H460, A549, H1299, and H69 (ATCC) EGFR-mutant cell lines H1650, H1975, HCC827, HCC827GR, PC9, PC9ER, and H3255 Patient-derived Xenograft Tumor Specimens | MicroRNA array Colony Formation Assay High-Throughput Sequencing Western Blot H&E Staining and Immunofluorescence. Targeted Mass Spectrometry. | [77] |
| miR-21 |
|
| Gene expression data (target scan database) qRT-PCR | [78,79,80] |
| Oncogenic/Oncosuppressor miRNAs | ||||
| miR-29b |
| mutated KRASG12V Cells | Microarray analysis Mechanistic investigations | [41] |
| miR-7 |
| EGFR-silenced cells EGFR mutant (L858R) CL1-5 cells. | MiRNA microarray analysis qRT-PCR | [81,82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanein, S.S.; Ibrahim, S.A.; Abdel-Mawgood, A.L. Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands. Int. J. Mol. Sci. 2021, 22, 12496. https://doi.org/10.3390/ijms222212496
Hassanein SS, Ibrahim SA, Abdel-Mawgood AL. Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands. International Journal of Molecular Sciences. 2021; 22(22):12496. https://doi.org/10.3390/ijms222212496
Chicago/Turabian StyleHassanein, Sarah Sayed, Sherif Abdelaziz Ibrahim, and Ahmed Lotfy Abdel-Mawgood. 2021. "Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands" International Journal of Molecular Sciences 22, no. 22: 12496. https://doi.org/10.3390/ijms222212496
APA StyleHassanein, S. S., Ibrahim, S. A., & Abdel-Mawgood, A. L. (2021). Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands. International Journal of Molecular Sciences, 22(22), 12496. https://doi.org/10.3390/ijms222212496