Safety of Surgery after Neoadjuvant Targeted Therapies in Non-Small Cell Lung Cancer: A Narrative Review
Abstract
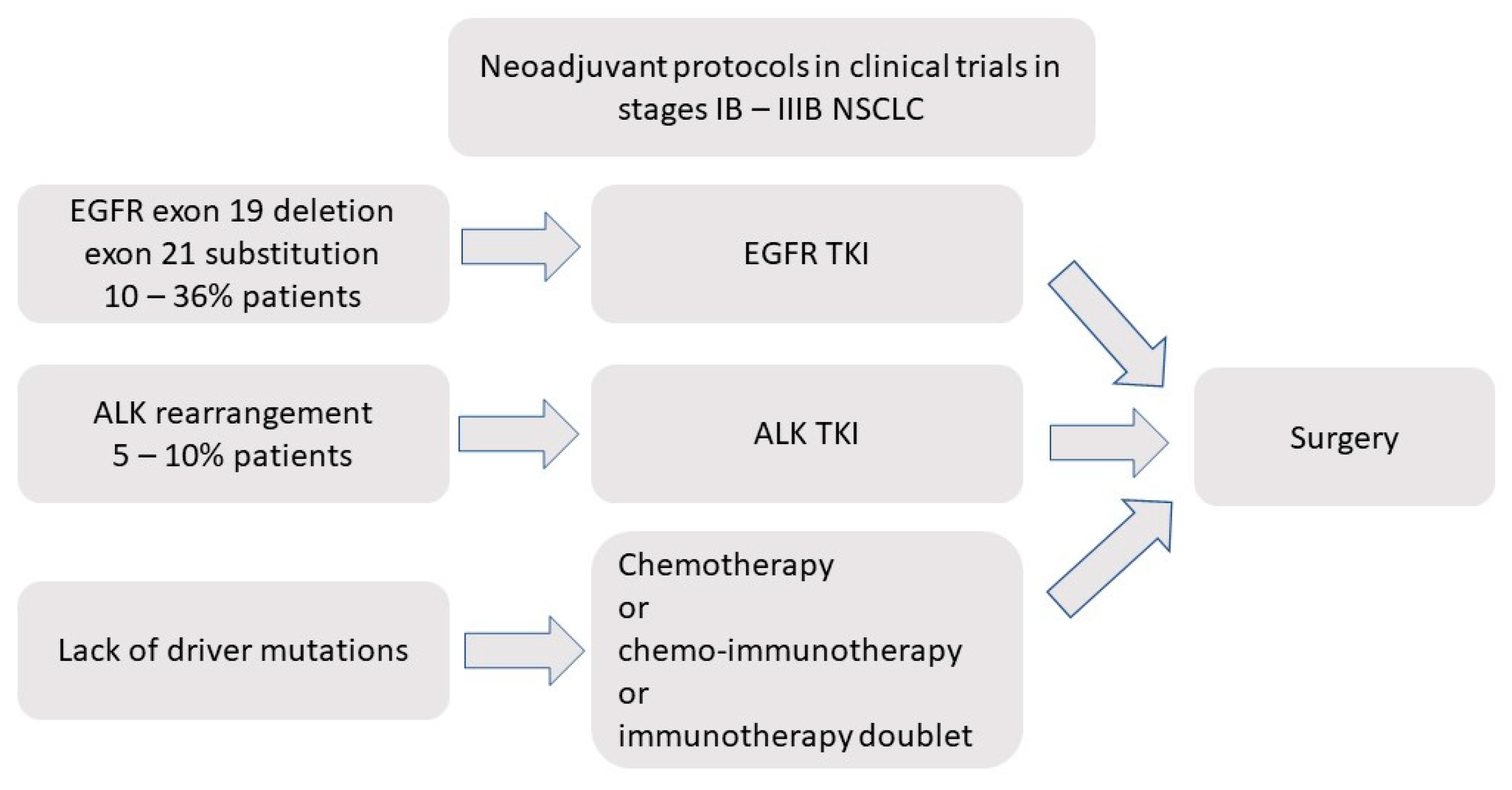
:1. Introduction
2. Typical Complications of EGFR-TKIs
2.1. Skin Toxicity
2.2. Gastrointestinal Toxicity
2.3. Pulmonary Toxicity
2.4. Cardiac Complications
2.5. Treatment-Related Mortality
3. Neoadjuvant EGFR-TKIs in Early NSCLC
4. Typical Complications in ALK Inhibitors
4.1. Gastrointestinal and Hepatic Complications
4.2. Pulmonary Complications
4.3. Cardiac Complications
4.4. Treatment-Related Mortality
5. Neoadjuvant Treatment with ALK Inhibitors in Early NSCLC
6. Final Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALK | anaplastic lymphoma kinase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CTCAE | Common Toxicity Criteria for Adverse Events |
| DFS | disease-free survival |
| EGFR | epidermal growth factor receptor |
| HR | hazard ratio |
| GI | gastrointestinal |
| ILD | interstitial lung disease |
| MPR | major pathological response |
| NR | not reported |
| NSCLC | non-smallcell lung cancer |
| OS | overall survival |
| PAL | persistent air leak |
| pCR | pathological complete response |
| PD-L1 | programmed cell death ligand-1 |
| SAE | serious adverse event |
| TKI | tyrosine kinase inhibitors |
References
- Horn, L.; Pao, W. EML4-ALK: Honing in on a New Target in Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4232–4235. [Google Scholar] [CrossRef]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and in Vivo Toxicology in Non-Human Primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Wang, D.; Chung, C.; Tian, D.; Rimner, A.; Huang, J.; Jones, D.R. A Systematic Review and Meta-Analysis of Stereotactic Body Radiation Therapy versus Surgery for Patients with Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2019, 157, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Lim, E.; Harris, G.; Patel, A.; Adachi, I.; Edmonds, L.; Song, F. Preoperative versus Postoperative Chemotherapy in Patients with Resectable Non-Small Cell Lung Cancer: Systematic Review and Indirect Comparison Meta-Analysis of Randomized Trials. J. Thorac. Oncol. 2009, 4, 1380–1388. [Google Scholar] [CrossRef]
- Burdett, S. Preoperative Chemotherapy for Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef] [Green Version]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Chmielewska, I.; Stencel, K.; Kalinka, E.; Ramlau, R.; Krawczyk, P. Neoadjuvant and Adjuvant Immunotherapy in Non-Small Cell Lung Cancer-Clinical Trials Experience. Cancers 2021, 13, 5048. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, D.; Ou, Q.; Liu, S.; Li, A.; Chen, Y.; Lin, D.; Gao, Q.; Zhou, H.; Liao, W.; et al. Association of Survival and Immune-Related Biomarkers with Immunotherapy in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis and Individual Patient-Level Analysis. JAMA Netw. Open 2019, 2, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calles, A.; Riess, J.W.; Brahmer, J.R. Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol Annu. Meet 2020, 40, 372–384. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, J.; Xiao, R.; Liu, Y.; Yu, F.; Cai, L.; Qiu, M.; He, F. EGFR Mutation Status in Non-Small Cell Lung Cancer Receiving PD-1/PD-L1 Inhibitors and Its Correlation with PD-L1 Expression: A Meta-Analysis. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef]
- Stencel, K.; Chmielewska, I.; Milanowski, J.; Ramlau, R. Non-Small-Cell Lung Cancer: New Rare Targets-New Targeted Therapies-State of The Art and Future Directions. Cancers 2021, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Ramlau, R.; Chorostowska-Wynimko, J.; Powrózek, T.; Lewandowska, M.A.; Limon, J.; Wasąg, B.; Pankowski, J.; Kozielski, J.; Kalinka-Warzocha, E.; et al. The Efficacy of EGFR Gene Mutation Testing in Various Samples from Non-Small Cell Lung Cancer Patients: A Multicenter Retrospective Study. J. Cancer Res. Clin. Oncol. 2015, 141, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenal, F.; Camps, C.; Majem, M.; Lopez-vivanco, G.; Isla, D.; Provencio, M.; Insa, A.; Massuti, B.; Gonzalez-larriba, J.L.; Paz-ares, L.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2019, 361, 958–967. [Google Scholar]
- Lindeman, N.I.; Cagle, P.T.; Beasley, M.B.; Chitale, D.A.; Dacic, S.; Giaccone, G.; Jenkins, R.B.; Kwiatkowski, D.J.; Saldivar, J.S.; Squire, J.; et al. Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Patho. J. Thorac. Oncol. 2013, 8, 823–859. [Google Scholar] [CrossRef] [Green Version]
- Letovanec, I.; Finn, S.; Zygoura, P.; Smyth, P.; Soltermann, A.; Bubendorf, L.; Speel, E.J.M.; Marchetti, A.; Nonaka, D.; Monkhorst, K.; et al. Evaluation of NGS and RT-PCR Methods for ALK Rearrangement in European NSCLC Patients: Results from the European Thoracic Oncology Platform Lungscape Project. J. Thorac. Oncol. 2018, 13, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackhall, F.H.; Peters, S.; Bubendorf, L.; Dafni, U.; Kerr, K.M.; Hager, H.; Soltermann, A.; O’Byrne, K.J.; Dooms, C.; Sejda, A.; et al. Prevalence and Clinical Outcomes for Patients with ALK-Positive Resected Stage I to III Adenocarcinoma: Results from the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014, 32, 2780–2787. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.C. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Han, J.; Choi, Y.-L. Real-World Analysis of the EGFR Mutation Test in Tissue and Plasma Samples from Non-Small Cell Lung Cancer. Diagnostics 2021, 11, 1695. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cress, W.D.; Muñoz-Antonia, T. Racial and Ethnic Differences in the Epidemiology and Genomics of Lung Cancer. Cancer Control. 2016, 23, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Płużański, A.; Piórek, A. Side Effects of Tyrosine Kinase Inhibitors—Management Guidelines. Oncol. Clin. Pract. 2016, 12, 113–118. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, H.; Weng, D.S.; Zhang, X.F.; Chen, C.L.; Zhou, Z.Q.; Tang, Y.; Xia, J.C. Pooled Safety Analyses of ALK-TKI Inhibitor in ALK-Positive NSCLC. BMC Cancer 2017, 17, 412. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.F.J.; McSherry, F.; Mayne, N.R.; Wang, X.; Berry, M.F.; Tong, B.; Harpole, D.H.; D’Amico, T.A.; Christensen, J.D.; Ready, N.E.; et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 105, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Stiles, B.M.; Sepesi, B.; Broderick, S.R.; Bott, M.J. Perioperative Considerations for Neoadjuvant Immunotherapy in Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2020, 160, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, A.W.; Marjanski, T.; Falcoz, P.-E.; Tsuboi, M.; Wu, Y.-L.; Sun, S.W.; Gitlitz, B.J. Important Surgical and Clinical Endpoints in Neoadjuvant Immunotherapy Trials in Resectable Non-Small Cell Lung Cancer. JTO Clin. Res. Rep. 2021, 2, 100221. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Swann, R.S.; Rusch, V.R.; Turrisi, A.T.; Shepherd, F.A.; Smith, C.; Chen, Y.; Robert, B.; Feins, R.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non–Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. J. Natl. Compr. Canc. Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef]
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.E.; OBrien, M.E.R.; Spigel, D.R.; Crinò, L.; Tsai, C.M.; Kim, J.H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib versus Placebo in Patients with Stage IB-IIIA Nonsmall-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Neal, J.W.; Chaft, J.E.; Azzoli, C.G.; Jänne, P.A.; Govindan, R.; Evans, T.L.; Costa, D.B.; Wakelee, H.A.; Heist, R.S.; et al. Select: A Phase II Trial of Adjuvant Erlotinib in Patients with Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 97–104. [Google Scholar] [CrossRef]
- Yue, D.; Xu, S.; Wang, Q.; Li, X.; Shen, Y.; Zhao, H.; Chen, C.; Mao, W.; Liu, W.; Liu, J.; et al. Erlotinib versus Vinorelbine plus Cisplatin as Adjuvant Therapy in Chinese Patients with Stage IIIA EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EVAN): A Randomised, Open-Label, Phase 2 Trial. Lancet Respir. Med. 2018, 6, 863–873. [Google Scholar] [CrossRef]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Shen, Y.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Xu, L.; et al. Gefitinib versus Vinorelbine plus Cisplatin as Adjuvant Treatment for Stage II–IIIA (N1–N2) EGFR-Mutant NSCLC (ADJUVANT/CTONG1104): A Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2018, 19, 139–148. [Google Scholar] [CrossRef]
- Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, W.; Bai, H.; Wang, H.; Shen, J.; Jing, B.; Shi, C.; et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2019, 24, 157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fu, F.; Hu, H.; Wang, S.; Li, Y.; Hu, H.; Chen, H. Gefitinib as Neoadjuvant Therapy for Resectable Stage II-IIIA Non–Small Cell Lung Cancer: A Phase II Study. J. Thorac. Cardiovasc. Surg. 2021, 161, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.Z.; Chen, K.N.; Chen, C.; Gu, C.D.; Wang, J.; Yang, X.N.; Mao, W.M.; Wang, Q.; Qiao, G.B.; Cheng, Y.; et al. Erlotinib versus Gemcitabine plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J. Clin. Oncol. 2019, 37, 2235–2245. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, X.; Yan, H.; Zhang, X.; Su, J.; Chen, Z.; Liao, R.; Nie, Q.; Dong, S.; Zhou, Q.; et al. Phase II Study of Biomarker-Guided Neoadjuvant Treatment Strategy for IIIA-N2 Non-Small Cell Lung Cancer Based on Epidermal Growth Factor Receptor Mutation Status. J. Hematol. Oncol. 2015, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Gu, C.; Xie, H.; Zhao, S.; Xie, D.; Chen, C.; Jiang, G.; Dai, C.; Zhu, Y. Comprehensive Study of Neoadjuvant Targeted Therapy for Resectable Non-Small Cell Lung Cancer. Ann. Transl. Med. 2021, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Ma, Y.; Feng, Q.; Lu, F.; Chi, Y.; Wu, N.; Fang, J.; Yang, Y. Does Neoadjuvant Targeted Therapy Provide an Opportunity for Resectable EGFR-Mutant Lung Cancer: A Real-World Retrospective Study. J. Thorac. Dis. 2020, 12, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Chua, K.P.; Takano, A.; Alvarez, J.; Ong, B.H.; Koh, T.; Aung, Z.W.; Jain, A.; Lai, G.; Tan, W.L.; et al. P1.17-07 Neoadjuvant Gefitinib in Resectable Early Stage EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): A Window-of-Opportunity Study. J. Thorac. Oncol. 2019, 14, S609–S610. [Google Scholar] [CrossRef]
- Schaake, E.E.; Kappers, I.; Codrington, H.E.; Valdés Olmos, R.A.; Teertstra, H.J.; Van Pel, R.; Burgers, J.A.; Van Tinteren, H.; Klomp, H.M. Tumor Response and Toxicity of Neoadjuvant Erlotinib in Patients with Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 2731–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Liu, Y.; Zhou, B.; Wang, Z.; Liang, N.; Zhang, Y.; Dong, Z.; Li, J. Effects of Icotinib on Early-Stage Non-Small-Cell Lung Cancer as Neoadjuvant Treatment with Different Epidermal Growth Factor Receptor Phenotypes. Onco. Targets. Ther. 2016, 9, 1735–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J. P03.02 Neoadjuvant Osimertinib with/without Chemotherapy vs Chemotherapy for EGFR Mutated Resectable NSCLC: NeoADAURA. J. Thorac. Oncol. 2021, 16, S258. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, B.; Chen, Z.; Li, J.; Liang, H.; Chen, Y.; Zhu, F.; Li, C.; Xu, K.; Xiong, S.; et al. Toxicity Profile of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Patients with Lung Cancer: A Systematic Review and Network Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103305. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, Z.; Yin, Y.; Shen, C.; Chen, X.; Cai, Z.; Wang, J.; Chen, Z.; Yin, Y.; Zhang, B. Adverse Event Profiles of Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. Clin. Transl. Sci. 2021, 14, 919–933. [Google Scholar] [CrossRef]
- Ding, P.N.; Lord, S.J.; Gebski, V.; Links, M.; Bray, V.; Gralla, R.J.; Yang, J.C.H.; Lee, C.K. Risk of Treatment-Related Toxicities from EGFR Tyrosine Kinase Inhibitors: A Meta-Analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR-Mutated Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Mok, T.S.; Wu, Y.; Thongprasert, S.; Yang, C.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; Nishiwaki, Y.; et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Margaritopoulos, G.A.; Antoniou, K.M.; Wells, A.U. Comorbidities in Interstitial Lung Diseases. Eur. Respir. Rev. 2017, 26, 1–15. [Google Scholar] [CrossRef]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.-P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS Clinical Guidelines on Fitness for Radical Therapy in Lung Cancer Patients (Surgery and Chemo-Radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic Evaluation of the Patient with Lung Cancer Being Considered for Resectional Surgery: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e166S–e190S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melosky, B.; Leighl, N.B.; Rothenstein, J.; Sangha, R.; Stewart, D.; Papp, K. Management of Egfr Tki-Induced Dermatologic Adverse Events. Curr. Oncol. 2015, 22, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, M.; Gregorc, V.; Belli, C.; Roca, E.; Lazzari, C.; Viganó, M.G.; Serafico, A.; Villa, E. Clinical Significance of Skin Toxicity Due to EGFR-Targeted Therapies. J. Oncol. 2009, 2009, 849051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuki, T. Skin Problems and EGFR-Tyrosine Kinase Inhibitor. Jpn. J. Clin. Oncol. 2016, 46, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcmann, M.; Sibilia, M. Mechanisms Underlying Skin Disorders Induced by EGFR Inhibitors. Mol. Cell Oncol. 2015, 2, e1004969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aw, D.C.W.; Tan, E.H.; Chin, T.M.; Lim, H.L.; Lee, H.Y.; Soo, R.A. Management of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Related Cutaneous and Gastrointestinal Toxicities. Asia Pac. J. Clin. Oncol. 2018, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.H.; Reguart, N.; Barinoff, J.; Köhler, J.; Uttenreuther-Fischer, M.; Stammberger, U.; O’Brien, D.; Wolf, J.; Cohen, E.E. Diarrhea Associated with Afatinib: An Oral ErbB Family Blocker. Expert. Rev. Anticancer Ther. 2013, 13, 729–736. [Google Scholar] [CrossRef]
- Hirsh, V.; Blais, N.; Burkes, R.; Verma, S.; Croitoru, K. Management of Diarrhea Induced by Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Curr. Oncol. 2014, 21, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Peerzada, M.M.; Spiro, T.P.; Daw, H.A. Pip Tyrosine Kinase Pneumonitis. Clin. Adv. Hematol. Oncol. 2011, 9, 824–836. [Google Scholar] [PubMed]
- Qi, W.X.; Sun, Y.J.; Shen, Z.; Yao, Y. Risk of Interstitial Lung Disease Associated with EGFR-TKIs in Advanced Non-Small-Cell Lung Cancer: A Meta-Analysis of 24 Phase III Clinical Trials. J. Chemother. 2015, 27, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, L. Tyrosine Kinase Inhibitors as Induction Therapy in Nonsmall-Cell Lung Cancer. Curr. Opin. Oncol. 2021, 33, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Gemma, A.; Kudoh, S.; Ando, M.; Ohe, Y.; Nakagawa, K.; Johkoh, T.; Yamazaki, N.; Arakawa, H.; Inoue, Y.; Ebina, M.; et al. Final Safety and Efficacy of Erlotinib in the Phase 4 POLARSTAR Surveillance Study of 10,708 Japanese Patients with Non-Small-Cell Lung Cancer. Cancer Sci. 2014, 105, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Zhang, G.; Zhang, X.; Lian, X. Pulmonary Toxicities of Gefitinib in Patients with Advanced Non-Small-Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e3008. [Google Scholar] [CrossRef]
- Shi, L.; Tang, J.; Tong, L.; Liu, Z. Risk of Interstitial Lung Disease with Gefitinib and Erlotinib in Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Clinical Trials. Lung Cancer 2014, 83, 231–239. [Google Scholar] [CrossRef]
- Akamatsu, H.; Inoue, A.; Mitsudomi, T.; Kobayashi, K.; Nakagawa, K.; Mori, K.; Nukiwa, T.; Nakanishi, Y.; Yamamoto, N. Interstitial Lung Disease Associated with Gefitinib in Japanese Patients with EGFR-Mutated Non-Small-Cell Lung Cancer: Combined Analysis of Two Phase III Trials (NEJ 002 and WJTOG 3405). Jpn J. Clin. Oncol. 2013, 43, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Uchel, T.; Karamian, G.; Loschner, A. Pulmonary Complications of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors in Patients with Non-Small Cell Lung Cancer. Cancer Treat. Res. Commun. 2021, 28, 100439. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT Diagnosis and Clinical Management of Drug-Related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper from the Fleischner Society. Radiology 2021, 298, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, M.; Hendriks, L.E.L.; Dinh, T.; Lalji, U.; Dingemans, A.-M.C. Current Perspective: Osimertinib-Induced QT Prolongation: New Drugs with New Side-Effects Need Careful Patient Monitoring. Eur. J. Cancer 2018, 91, 92–98. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant Chemotherapy and Nivolumab in Resectable Non-Small-Cell Lung Cancer (NADIM): An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Hao, J.; Hao, X.; Zhou, J.; Chen, N.; Liu, L.; Pu, Q. Chylothorax after Lung Cancer Surgery: A Key Factor Influencing Prognosis and Quality of Life. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Martucci, N.; Tracey, M.; Rocco, G. Postoperative Chylothorax. Thorac. Surg. Clin. 2015, 25, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, Y. Erlotinib as Neoadjuvant Treatment in Patients with IIIA-N2 Non-Small Cell Lung Cancer(NSCLC) With Activating Epidermal Growth Factor Receptor (EGFR) Mutation (NCT01217619, ESTERN). Ann. Oncol. 2012, 23, ix413. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W. Safety and Efficacy of Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitors in Non-small Cell Lung Cancer (Review). Oncol. Rep. 2021, 45, 13–28. [Google Scholar] [CrossRef]
- Kassem, L.; Shohdy, K.S.; Lasheen, S.; Abdel-Rahman, O.; Ali, A.; Abdel-Malek, R.R. Safety Issues with the ALK Inhibitors in the Treatment of NSCLC: A Systematic Review. Crit. Rev. Oncol. Hematol. 2019, 134, 56–64. [Google Scholar] [CrossRef]
- Hou, H.; Sun, D.; Liu, K.; Jiang, M.; Liu, D.; Zhu, J.; Zhou, N.; Cong, J.; Zhang, X. The Safety and Serious Adverse Events of Approved ALK Inhibitors in Malignancies: A Meta-Analysis. Cancer Manag. Res. 2019, 11, 4109–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gristina, V.; Mantia, M.L.; Iacono, F.; Galvano, A.; Russo, A.; Bazan, V. The Emerging Therapeutic Landscape of Alk Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 474. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Bazhenova, L.A.; Langer, C.J.; Salgia, R.; Gold, K.A.; Rosell, R.; Shaw, A.T.; Weiss, G.J.; Tugnait, M.; Narasimhan, N.I.; et al. Activity and Safety of Brigatinib in ALK-Rearranged Non-Small-Cell Lung Cancer and Other Malignancies: A Single-Arm, Open-Label, Phase 1/2 Trial. Lancet Oncol. 2016, 17, 1683–1696. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, M.; Sun, Y.; Cheng, Z.; Zhang, Z.; Hou, S.; Wang, X.; Liu, J. Incidence and Risk of Hepatic Toxicities Associated with Anaplastic Lymphoma Kinase Inhibitors in the Treatment of Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2018, 9, 9480–9488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, K.Y.; Scranton, J.R.; Cadogan, M.A.; Tassell, V.; Nadanaciva, S.; Wilner, K.D.; Stollenwerk, N.S. Interstitial Lung Disease Associated With Crizotinib in Patients With Advanced Non–Small Cell Lung Cancer: Independent Review of Four PROFILE Trials. Clin. Lung Cancer 2017, 18, 472–479. [Google Scholar] [CrossRef]
- Pellegrino, B.; Facchinetti, F.; Bordi, P.; Silva, M.; Gnetti, L.; Tiseo, M. Lung Toxicity in Non-Small-Cell Lung Cancer Patients Exposed to ALK Inhibitors: Report of a Peculiar Case and Systematic Review of the Literature. Clin. Lung Cancer 2018, 19, e151–e161. [Google Scholar] [CrossRef]
- Zaborowska-Szmit, M.; Krzakowski, M.; Kowalski, D.M.; Szmit, S. Cardiovascular Complications of Systemic Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, Z.; Yan, H.; He, Q.; Yang, X.; Luo, P. A Comprehensive Review of Clinical Cardiotoxicity Incidence of FDA-Approved Small-Molecule Kinase Inhibitors. Front. Pharmacol. 2020, 11, 891. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus Crizotinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, J.; Li, C.; Jiang, L.; Lin, H.; Lu, P.; Luo, Q.; Yang, G. Perioperative Crizotinib in a Patient with Stage IIIB ALK-Positive Non-Small Cell Lung Cancer: A Case Report. Ann. Transl. Med. 2020, 8, 770. [Google Scholar] [CrossRef]
- Xie, X.H.; Zhan, Z.J.; Qin, Y.Y.; Jiang, J.H.; Yin, W.Q.; Zheng, R.H.; Li, S.Y.; Zhou, C.Z. Case Report: Neoadjuvant and Adjuvant Crizotinib Targeted Therapy in Stage IIIA-N2 ALK-Positive Non-Small-Cell Lung Cancer. Front. Oncol. 2021, 11, 785. [Google Scholar] [CrossRef]
- Kilickap, S.; Onder, S.; Dizdar, O.; Erman, M.; Uner, A. Short-Time Use of Crizotinib as Neoadjuvant in ALK-Positive Non-Small Cell Lung Carcinoma Can Be a Chance for Resectability. Cancer Chemother. Pharmacol. 2019, 83, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.L.; Nie, Q.; Dong, S.; Shao, Y.; Yang, X.N.; Wu, Y.L.; Yang, Y.; Zhong, W.Z. Neoadjuvant Crizotinib in Resectable Locally Advanced Non–Small Cell Lung Cancer with ALK Rearrangement. J. Thorac. Oncol. 2019, 14, 726–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanishi, N.; Yoneda, K.; Taira, A.; Ichiki, Y.; Sato, N.; Hisaoka, M.; Tanaka, F. Major Pathologic Response to Alectinib in ALK-Rearranged Adenocarcinoma of the Lung. Surg. Case Rep. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Chaft, J.; Nicholas, A.; Patterson, A.; Waqar, S.; Toloza, E.; Haura, E.; Raz, D.; Reckamp, K.; Merritt, R.; et al. PS01.05 Surgical and Clinical Outcomes With Neoadjuvant Atezolizumab in Resectable Stage IB–IIIB NSCLC: LCMC3 Trial Primary Analysis. J. Thorac. Oncol. 2021, 16, S59–S61. [Google Scholar] [CrossRef]
- Gavralidis, A.; Justin, F. Immunotherapy in EGFR-Mutant and ALK-Positive Lung Cancer. Cancer J. 2020, 26, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | EGFR-TKI in Study Group | Number of Patients in Study Group | Delay of Surgery | EGFR-TKI Complications | Intraoperative Complications in EGFR-TKI Group | R0 Resection Rate in EGFR-TKI Group | Postoperative Complications in EGFR-TKI Group |
|---|---|---|---|---|---|---|---|
| Schaake et al., 2012 [44] | Erlotinib | 60 | 24 days | Rash 61% Diarrhea 35% Pneumonitis 5% | 0% | 7% of patients found out to be unresectable | Pneumonia 2% PAL 2% Blood transfusion 3% |
| Han et al., 2012 [75] | Erlotinib | 7 | 56 days | NR | 0% | 20% of resected patients | NR |
| Zhong et al., 2015 [40] | Erlotinib | 24 | 6 weeks of treatment | Rash 100% Diarrhea 42% | 0% | 50% | 0% |
| Tan et al., 2019 [43] | Gefitinib | 14 | At least 4 weeks of treatment | AST/ALT elevation 8% | NR | NR | NR |
| W.Z. Zhong et al., 2019 [39] | Erlotinib | 37 | 6 weeks of treatment | Rash 76% Diarrhea 68% Deterioration of pulmonary function precluding surgery 3% | 0% | 73% | Arrhytmia 6% Lung infection 6% Poor wound healing 6% PAL 3% Pneumothorax 3% |
| Xiong et al., 2019 [37] | Erlotinib | 19 | 56 days | Rash 26% 13% were not operated due to SAE | NR | 68% of resected patients | NR |
| Lv et al., 2020 [42] | Different agents | 43 | 8 weeks of treatment | NR | 0% | 95% | Chylothorax 7% Atelectasis 5% Arrhytmia 2% |
| Y. Zhang et al., 2021 [38] | Gefitinib | 35 | 61 days | Skin toxicity 69% GI symptoms 49% | 0% | 12% of patients found out to be stage IV at surgery | Chylothorax 12% |
| Bao et al., 2021 [41] | Different agents | 42 | NR | NR | NR | NR | NR |
| Study | ALK Inhibitor in Study Group | Number of Patients | Delay of Surgery | ALK Inhibitor Complications | Intraoperative Complications | Postoperative Complications |
|---|---|---|---|---|---|---|
| Tian et al., 2020 [89] | Crizotinib | 1 | 12 weeks | Grade 1 hepatic damageMild edema | None | None |
| Kilickap et al., 2019 [90] | Crizotinib | 1 | 6 weeks | NR | NR | NR |
| Xie et al., 2021 [91] | Gemcitabine, cisplatine, crizotinib | 1 | 2 months | None | NR | NR |
| C. Zhang et al., 2019 [92] | Crizotinib | 11 | Median 41 days | Grade 4 hepatitis 9% | None | Pneumonia 9% Dyspnoe 9% |
| Imanishi et al., 2018 [93] | Alectinib | 1 | 3 months | None | None | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjanski, T.; Dziedzic, R.; Kowalczyk, A.; Rzyman, W. Safety of Surgery after Neoadjuvant Targeted Therapies in Non-Small Cell Lung Cancer: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 12244. https://doi.org/10.3390/ijms222212244
Marjanski T, Dziedzic R, Kowalczyk A, Rzyman W. Safety of Surgery after Neoadjuvant Targeted Therapies in Non-Small Cell Lung Cancer: A Narrative Review. International Journal of Molecular Sciences. 2021; 22(22):12244. https://doi.org/10.3390/ijms222212244
Chicago/Turabian StyleMarjanski, Tomasz, Robert Dziedzic, Anna Kowalczyk, and Witold Rzyman. 2021. "Safety of Surgery after Neoadjuvant Targeted Therapies in Non-Small Cell Lung Cancer: A Narrative Review" International Journal of Molecular Sciences 22, no. 22: 12244. https://doi.org/10.3390/ijms222212244
APA StyleMarjanski, T., Dziedzic, R., Kowalczyk, A., & Rzyman, W. (2021). Safety of Surgery after Neoadjuvant Targeted Therapies in Non-Small Cell Lung Cancer: A Narrative Review. International Journal of Molecular Sciences, 22(22), 12244. https://doi.org/10.3390/ijms222212244






