Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison
Abstract
1. Introduction
2. Grapevine Structure and Metabolism
2.1. Compartmentation between Organs
2.2. Compartmentation between Tissues/Cell Types
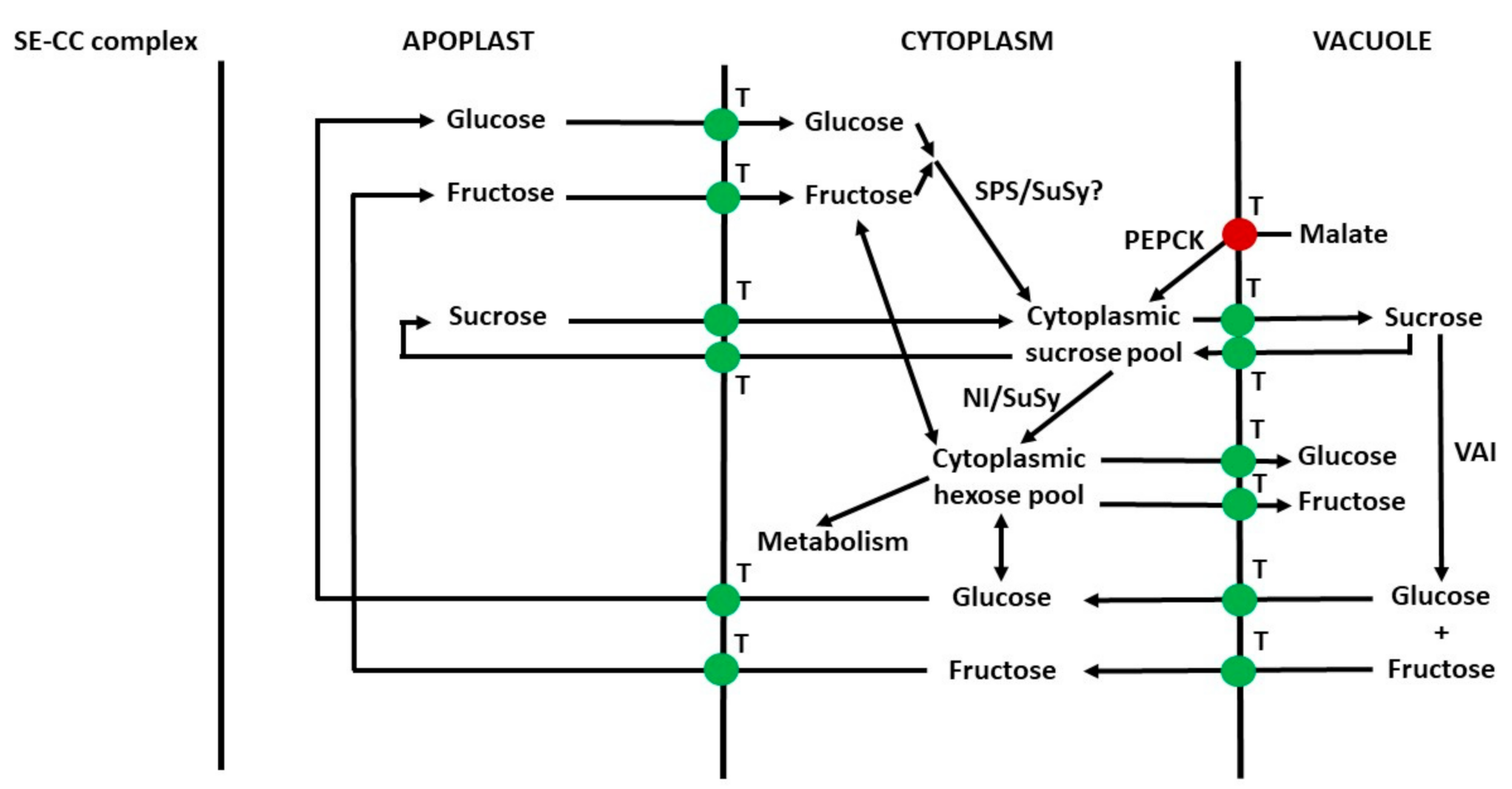
2.3. Compartmentation within Cells and between Apoplast and Symplast
2.4. Compartmentation of Sugars in Leaf Mesophyll Cells and Pericarp Parenchyma Cells
2.5. Distribution of Sugars between Symplast and Apoplast
3. Sucrose Metabolism Enzymes and Transporters
3.1. Entry of Sucrose into Metabolism
3.1.1. Sucrose Synthase
3.1.2. Neutral Invertase
3.1.3. Acid Invertase
3.2. Sugar Transporters
3.2.1. Sucrose Transporters
3.2.2. Hexose Transporters
3.2.3. SWEET Transporters
4. Pericarp Sugar Metabolism
4.1. Sucrose Synthase
4.2. Neutral Invertase
4.3. Acid Invertase
4.4. Sucrose Cycle
4.5. Sucrose:Hexose Ratio in Fruits before Ripening
4.6. Sucrose:Hexose Ratio in Fruits during Ripening
5. Leaf Sugar Metabolism
5.1. Photosynthesis
5.2. Sugar Content
5.3. Acid Invertase
6. Transport of Sugars within and between Organs
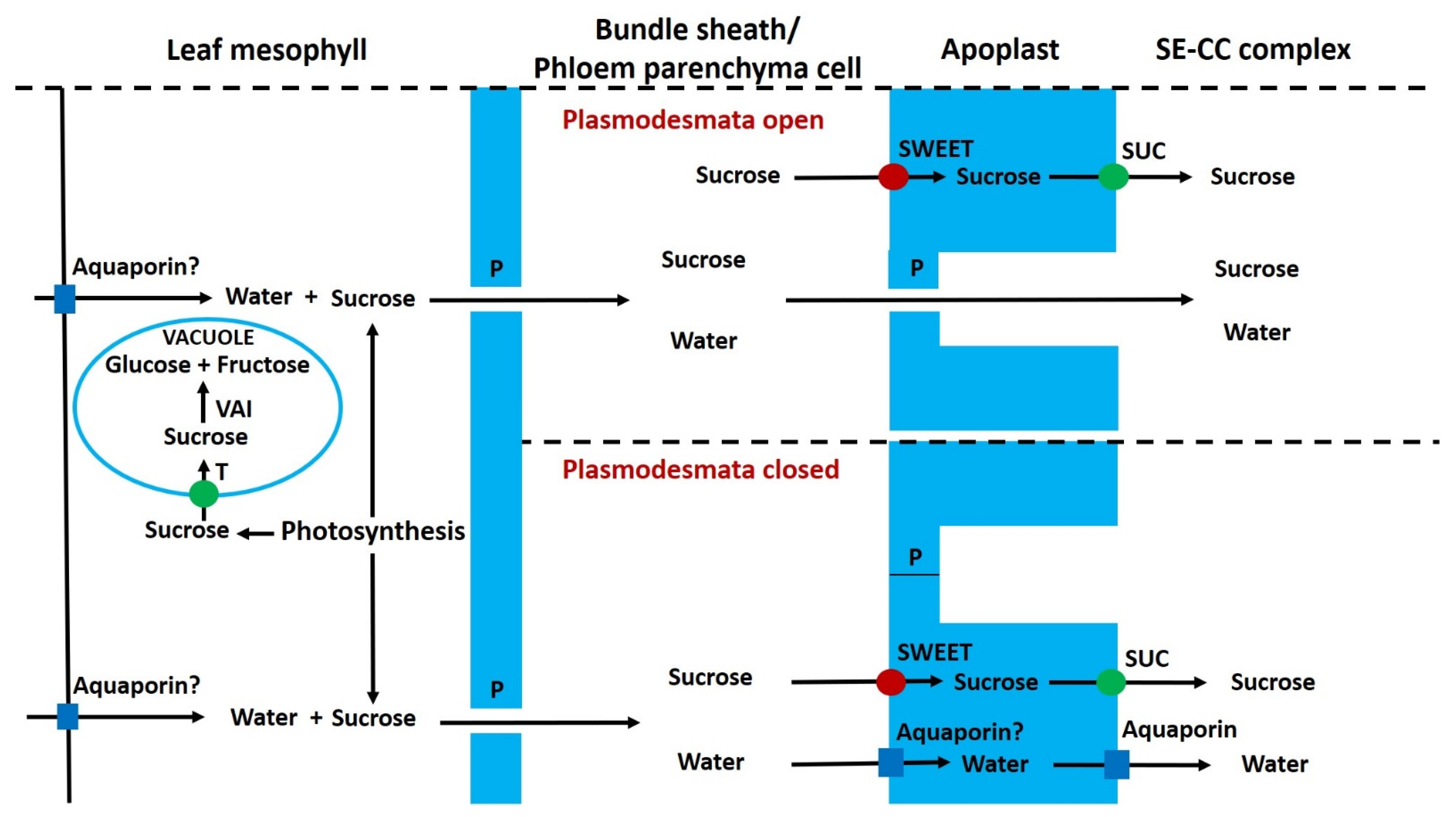
6.1. Pre-Phloem Transport and Phloem Loading of Sucrose in Leaves
6.2. Phloem and Xylem Sucrose Contents
6.3. Phloem and Xylem Flows into the Fruit
6.4. Post-Phloem Transport in the Seed
6.5. Phloem Unloading in the Pericarp
6.6. Symplastic Post-Phloem Transport in the Pericarp Before Ripening
6.7. Apoplastic Post-Phloem Transport in the Pericarp during Ripening
6.8. Phloem Unloading, Apoplastic Sugars and Invertase
6.9. Regulation of Apoplastic Sugar Concentration
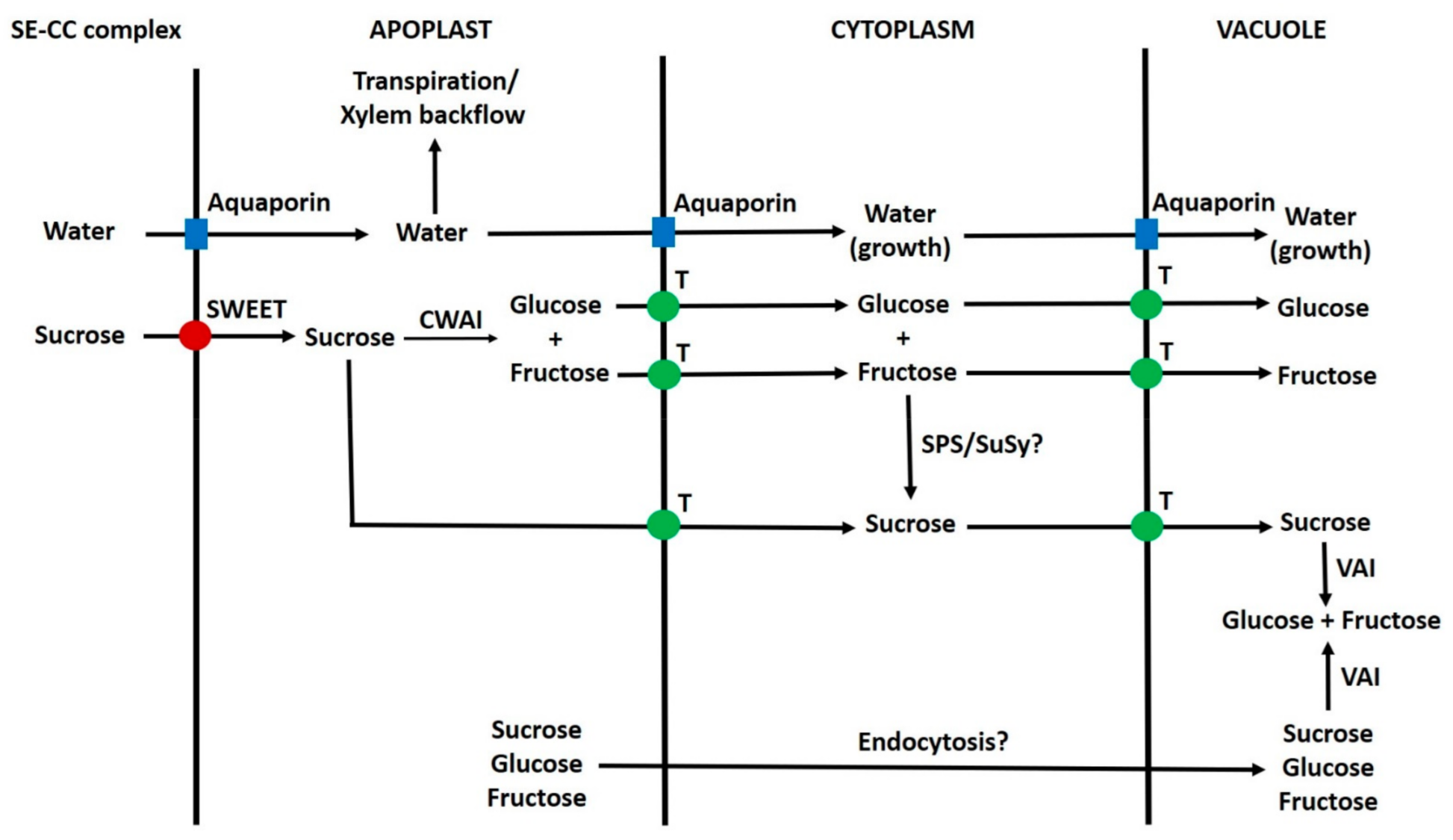
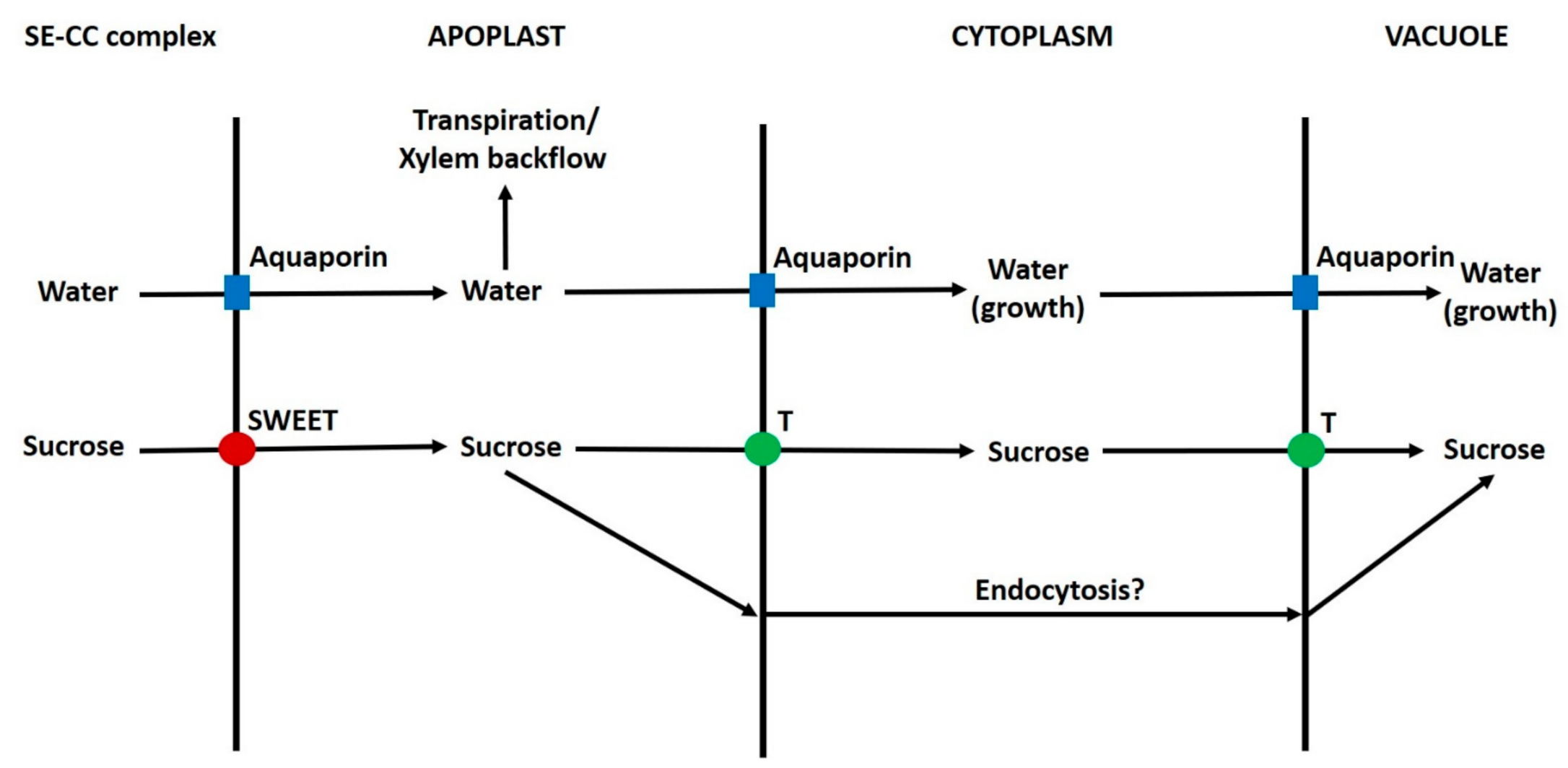
6.10. Apoplast to Vacuole Transport of Sugars in Parenchyma Cells
6.11. Summary of Transport of Sugars from the Phloem to Vacuoles of Sink Cells
7. Acid Invertase Osmoregulatory System
8. Conclusion and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, R.P.; Battistelli, A.; Bonghi, C.; Drincovich, M.F.; Falchi, R.; Lara, M.V.; Moscatello, S.; Vizzotto, G.; Famiani, F. Non-Structural Carbohydrate Metabolism in the Flesh of Stone Fruits of the Genus Prunus (Rosaceae)—A Review. Front. Plant Sci. 2020, 11, 549921. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An Update on Sugar Transport and Signalling in Grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Li, Y.-M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.-S. The Molecular Regulation of Carbon Sink Strength in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Chen, Z.-H.; Técsi, L.I.; Famiani, F.; Lea, P.J.; Leegood, R.C. Phosphoenolpyruvate Carboxykinase Plays a Role in Interactions of Carbon and Nitrogen Metabolism during Grape Seed Development. Planta 1999, 210, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Chen, Z.; Johnson, K.E.; Famiani, F.; Tecsi, L.; Leegood, R.C. Using Immunohistochemistry to Study Plant Metabolism: The Examples of Its Use in the Localization of Amino Acids in Plant Tissues, and of Phosphoenolpyruvate Carboxykinase and Its Possible Role in pH Regulation. J. Exp. Bot. 2001, 52, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Walker, R.P.; Técsi, L.; Chen, Z.; Proietti, P.; Leegood, R.C. An Immunohistochemical Study of the Compartmentation of Metabolism during the Development of Grape (Vitis vinifera L.) Berries. J. Exp. Bot. 2000, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E. Compartmentation in Plant Metabolism. J. Exp. Bot. 2007, 58, 35–47. [Google Scholar] [CrossRef]
- Swanson, C.A.; El-Shishiny, E.D.H. Translocation of Sugars in the Concord Grape. 12. Plant Physiol. 1958, 33, 33–37. [Google Scholar] [CrossRef]
- Ollat, N.; Carde, J.-P.; Gaudillère, J.-P.; Barrieu, F.; Diakou-Verdin, P.; Moing, A. Grape Berry Development: A Review. OENO One 2002, 36, 109–131. [Google Scholar] [CrossRef]
- Davies, C.; Boss, P.K.; Geros, H.; Lecourieux, F.; Delrot, S. Source/Sink Relationships and Molecular Biology of Sugar Accumulation in Grape Berries. In The Biochemistry of the Grape Berry; Bentham science: Sharjah, United Arab Emirates, 2012; 300p. [Google Scholar]
- Hunter, J.J.; Visser, J.H. Distribution of 14C-Photosynthetate in the Shoot of Vitis Vinifera L Cv Cabernet Sauvignon: Pt II. S. Afr. J. Enol. Vitic. 1988, 9, 10–15. [Google Scholar]
- Winkler, A.J.; Williams, W.O. Starch and Sugars of Vitis vinifera. Plant Physiol. 1945, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Ruffner, H.P.; Volschenk, C.G. Starch Concentrations in Grapevine Leaves, Berries and Roots and the Effect of Canopy Management. S. Afr. J. Enol. Vitic. 1995, 16, 35–40. [Google Scholar] [CrossRef]
- Bates, T.R.; Dunst, R.M.; Joy, P. Seasonal Dry Matter, Starch, and Nutrient Distribution in “Concord” Grapevine Roots. HortScience 2002, 37, 313–316. [Google Scholar] [CrossRef]
- Grimplet, J.; Deluc, L.G.; Tillett, R.L.; Wheatley, M.D.; Schlauch, K.A.; Cramer, G.R.; Cushman, J.C. Tissue-Specific mRNA Expression Profiling in Grape Berry Tissues. BMC Genom. 2007, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, Y.-L. Unraveling Mechanisms of Cell Expansion Linking Solute Transport, Metabolism, Plasmodesmtal Gating and Cell Wall Dynamics. Plant Signal. Behav. 2010, 5, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Ramos, M.S.; Nunes-Nesi, A.; Mutwil, M.; Giavalisco, P.; Steinhauser, D.; Schellenberg, M.; Willmitzer, L.; Persson, S.; Martinoia, E.; et al. Toward the Storage Metabolome: Profiling the Barley Vacuole. Plant Physiol. 2011, 157, 1469–1482. [Google Scholar] [CrossRef]
- Wada, H.; Castellarin, S.D.; Matthews, M.A.; Shackel, K.A.; Gambetta, G.A. Minimally Invasive, Pressure Probe Based Sampling Allows for in-situ Gene Expression Analyses in Plant Cells. BioRxiv 2019, 768978. [Google Scholar] [CrossRef]
- Lea, P.J.; Chen, Z.-H.; Leegood, R.C.; Walker, R.P. Does Phosphoenolpyruvate Carboxykinase Have a Role in Both Amino Acid and Carbohydrate Metabolism? Amino Acids 2001, 20, 225–241. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Técsi, L.; Leegood, R.C.; Famiani, F. Phosphoenolpyruvate Carboxykinase and Gluconeogenesis in Grape Pericarp. Plant Physiol. Biochem. 2015, 97, 62–69. [Google Scholar] [CrossRef]
- Walker, R.P.; Benincasa, P.; Battistelli, A.; Moscatello, S.; Técsi, L.; Leegood, R.C.; Famiani, F. Gluconeogenesis and Nitrogen Metabolism in Maize. Plant Physiol. Biochem. 2018, 130, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Farré, E.M.; Tiessen, A.; Roessner, U.; Geigenberger, P.; Trethewey, R.N.; Willmitzer, L. Analysis of the Compartmentation of Glycolytic Intermediates, Nucleotides, Sugars, Organic Acids, Amino Acids, and Sugar Alcohols in Potato Tubers Using a Nonaqueous Fractionation Method. Plant Physiol. 2001, 127, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Nadwodnik, J.; Lohaus, G. Subcellular Concentrations of Sugar Alcohols and Sugars in Relation to Phloem Translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 2008, 227, 1079–1089. [Google Scholar] [CrossRef]
- Klie, S.; Krueger, S.; Krall, L.; Giavalisco, P.; Flügge, U.-I.; Willmitzer, L.; Steinhauser, D. Analysis of the Compartmentalized Metabolome—A Validation of the Non-Aqueous Fractionation Technique. Front. Plant Sci. 2011, 2, 55. [Google Scholar] [CrossRef]
- Ludewig, F.; Flügge, U.-I. Role of Metabolite Transporters in Source-Sink Carbon Allocation. Front. Plant Sci. 2013, 4, 231. [Google Scholar] [CrossRef]
- Kuang, L.; Chen, S.; Guo, Y.; Ma, H. Quantitative Proteome Analysis Reveals Changes in the Protein Landscape During Grape Berry Development with a Focus on Vacuolar Transport Proteins. Front. Plant Sci. 2019, 10, 641. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Chaves, M.M.; Barrieu, F. Water Relations of the Grape Berry and Aquaporins. In The Biochemistry of the Grape Berry; Bentham science: Sharjah, United Arab Emirates, 2012; 300p. [Google Scholar]
- Terrier, N.; Sauvage, F.-X.; Ageorges, A.; Romieu, C. Changes in Acidity and in Proton Transport at the Tonoplast of Grape Berries during Development. Planta 2001, 213, 20–28. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V.; Koroleva, O.A.; Batashev, D.R.; Knop, C.; Tomos, A.D.; Gamalei, Y.V.; Heldt, H.-W.; Lohaus, G. Phloem Loading in Two Scrophulariaceae Species. What Can Drive Symplastic Flow via Plasmodesmata? Plant Physiol. 2006, 140, 383–395. [Google Scholar] [CrossRef]
- Pollock, C.; Farrar, J.; Tomos, D.; Gallagher, J.; Lu, C.; Koroleva, O. Balancing Supply and Demand: The Spatial Regulation of Carbon Metabolism in Grass and Cereal Leaves. J. Exp. Bot. 2003, 54, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Skrivan, R.; Ruffner, H.P. Diurnal and Seasonal Physiological Changes in Leaves of Vitis vinifera L.: CO2 Assimilation Rates, Sugar Levels and Sucrolytic Enzyme Activity. Vitis 1994, 33, 189–195. [Google Scholar] [CrossRef]
- Duan, S.; Wu, Y.; Zhang, C.; Wang, L.; Song, S.; Ma, C.; Zhang, C.; Xu, W.; Bondada, B.; Wang, S. Differential Regulation of Enzyme Activities and Physio-Anatomical Aspects of Calcium Nutrition in Grapevine. Sci. Hortic. 2020, 272, 109423. [Google Scholar] [CrossRef]
- Kang, Y.; Outlaw, W.H., Jr.; Fiore, G.B.; Riddle, K.A. Guard Cell Apoplastic Photosynthate Accumulation Corresponds to a Phloem-Loading Mechanism. J. Exp. Bot. 2007, 58, 4061–4070. [Google Scholar] [CrossRef][Green Version]
- Sonnewald, U.; Hajirezaei, M.-R.; Kossmann, J.; Heyer, A.; Trethewey, R.N.; Willmitzer, L. Increased Potato Tuber Size Resulting from Apoplastic Expression of a Yeast Invertase. Nat. Biotechnol. 1997, 15, 794–797. [Google Scholar] [CrossRef]
- Lalonde, S.; Wipf, D.; Frommer, W.B. Transport Mechanisms for Organic Forms of Carbon and Nitrogen between Source and Sink. Annu. Rev. Plant Biol. 2004, 55, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit Ripening in Vitis vinifera: Apoplastic Solute Accumulation Accounts for Pre-Veraison Turgor Loss in Berries. Planta 2008, 227, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Matthews, M.A.; Shackel, K.A. Seasonal Pattern of Apoplastic Solute Accumulation and Loss of Cell Turgor during Ripening of Vitis vinifera Fruit under Field Conditions. J. Exp. Bot. 2009, 60, 1773–1781. [Google Scholar] [CrossRef]
- Knoche, M.; Grimm, E.; Schlegel, H.J. Mature Sweet Cherries Have Low Turgor. J. Am. Soc. Hortic. Sci. 2014, 139, 3–12. [Google Scholar] [CrossRef]
- Schumann, C.; Schlegel, H.J.; Grimm, E.; Knoche, M.; Lang, A. Water Potential and Its Components in Developing Sweet Cherry. J. Am. Soc. Hortic. Sci. 2014, 139, 349–355. [Google Scholar] [CrossRef]
- Damon, S.; Hewitt, J.; Nieder, M.; Bennett, A.B. Sink Metabolism in Tomato Fruit: II. Phloem Unloading and Sugar Uptake. Plant Physiol. 1988, 87, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.; Morrison, D.R.; Clearwater, M.J.; Ong, S.; Boldingh, H.L.; Minchin, P.E.H. Elucidating the Sugar Import Pathway into Developing Kiwifruit Berries (Actinidia deliciosa). N. Z. J. Crop. Hortic. Sci. 2013, 41, 189–206. [Google Scholar] [CrossRef][Green Version]
- Takayanagi, T.; Yokotsuka, K. Relationship Between Sucrose Accumulation and Sucrose-Metabolizing Enzymes in Developing Grapes. Am. J. Enol. Vitic. 1997, 48, 403. [Google Scholar]
- Baldicchi, A.; Farinelli, D.; Micheli, M.; Di Vaio, C.; Moscatello, S.; Battistelli, A.; Walker, R.P.; Famiani, F. Analysis of Seed Growth, Fruit Growth and Composition and Phospoenolpyruvate Carboxykinase (PEPCK) Occurrence in Apricot (Prunus armeniaca L.). Sci. Hortic. 2015, 186, 38–46. [Google Scholar] [CrossRef]
- Otoguro, C.; Kaneko, K. Changes of Chemical Constituents in Small Mume Fruit during Growth and Maturaion. J. Jpn. Soc. Cold Pres. Food 1994, 20, 13–21. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Chen, Z.-H.; Leegood, R.C.; Famiani, F. Phosphoenolpyruvate Carboxykinase in Cherry (Prunus avium L.) Fruit during Development. J. Exp. Bot. 2011, 62, 5357–5365. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Sanada, T.; Yamaki, S. Seasonal Fluctuations of Some Enzymes Relating to Sucrose and Sorbitol Metabolism in Peach Fruit. J. Am. Soc. Hortic. Sci. 1990, 115, 278–281. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Frioni, T.; Palliotti, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Malate as Substrate for Catabolism and Gluconeogenesis during Ripening in the Pericarp of Different Grape Cultivars. Biol. Plant 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Donen, I. The Role of Sorbitol in the Carbon-Metabolism of the Kelsey Plum: Changes in Chemical Composition during Growth and Storage. Biochem. J. 1939, 33, 1611–1620. [Google Scholar] [CrossRef]
- Famiani, F.; Casulli, V.; Baldicchi, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Development and Metabolism of the Fruit and Seed of the Japanese Plum Ozark Premier (Rosaceae). J. Plant Physiol. 2012, 169, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Stommel, J.R. Enzymic Components of Sucrose Accumulation in the Wild Tomato Species Lycopersicon peruvianum. Plant Physiol. 1992, 99, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit Quality and Bioactive Compounds Relevant to Human Health of Sweet Cherry (Prunus avium L.) Cultivars Grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Farinelli, D.; Moscatello, S.; Battistelli, A.; Leegood, R.C.; Walker, R.P. The Contribution of Stored Malate and Citrate to the Substrate Requirements of Metabolism of Ripening Peach (Prunus persica L. Batsch) Flesh Is Negligible. Implications for the Occurrence of Phosphoenolpyruvate Carboxykinase and Gluconeogenesis. Plant Physiol. Biochem. 2016, 101, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, H.P.; Adler, S.; Rast, D.M. Soluble and Wall Associated Forms of Invertase in Vitis vinifera. Phytochemistry 1990, 29, 2083–2086. [Google Scholar] [CrossRef]
- Kliewer, W.M. Sugars and Organic Acids of Vitis vinifera. Plant Physiol. 1966, 41, 923–931. [Google Scholar] [CrossRef]
- Yamaki, S.; Ino, M. Alteration of Cellular Compartmentation and Membrane Permeability to Sugars in Immature and Mature Apple Fruit. J. Am. Soc. Hortic. Sci. 1992, 117, 951–954. [Google Scholar] [CrossRef]
- Ofosu-Anim, J.; Yamachi, S. Sugar Content and Compartmentation in Melon Fruit and the Restriction of Sugar Efflux from Flesh Tissue by ABA. J. Jpn. Soc. Hortic. Sci. 1994, 63, 685–692. [Google Scholar] [CrossRef][Green Version]
- Jiang, F.; Wang, Y.; Sun, H.; Yang, L.; Zhang, J.; Ma, L. Intracellular Compartmentation and Membrane Permeability to Sugars and Acids at Different Growth Stages of Peach. Sci. Hortic. 2013, 161, 210–215. [Google Scholar] [CrossRef]
- Ruan, Y.; Patrick, J.; Brady, C. The Composition of Apoplast Fluid Recovered from Intact Developing Tomato Fruit. Funct. Plant Biol. 1996, 23, 9–13. [Google Scholar] [CrossRef]
- Ofosu-Anim, J.; Yamachi, S. Sugar Content, Compartmentation, and Efflux in Strawberry Tissue. J. Am. Soc. Hortic. Sci. 1994, 119, 1024–1028. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Meinzer, F.C. Compartmentation of Solutes and Water in Developing Sugarcane Stalk Tissue. Plant Physiol. 1990, 93, 1147–1153. [Google Scholar] [CrossRef]
- Yelle, S.; Hewitt, J.D.; Robinson, N.L.; Damon, S.; Bennett, A.B. Sink Metabolism in Tomato Fruit: III. Analysis of Carbohydrate Assimilation in a Wild Species. Plant Physiol. 1988, 87, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Petreikov, M.; Carmi, N.; Shen, S.; Levin, I.; Granot, D.; Zamski, E.; Schaffer, A.A. Sucrose Uptake, Invertase Localization and Gene Expression in Developing Fruit of Lycopersicon esculentum and the Sucrose-Accumulating Lycopersicon hirsutum. Physiol. Plant. 2002, 115, 35–47. [Google Scholar] [CrossRef]
- Sarry, J.-E.; Sommerer, N.; Sauvage, F.-X.; Bergoin, A.; Rossignol, M.; Albagnac, G.; Romieu, C. Grape Berry Biochemistry Revisited upon Proteomic Analysis of the Mesocarp. Proteomics 2004, 4, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Goes da Silva, F.; Iandolino, A.; Al-Kayal, F.; Bohlmann, M.C.; Cushman, M.A.; Lim, H.; Ergul, A.; Figueroa, R.; Kabuloglu, E.K.; Osborne, C.; et al. Characterizing the Grape Transcriptome. Analysis of Expressed Sequence Tags from Multiple Vitis Species and Development of a Compendium of Gene Expression during Berry Development. Plant Physiol. 2005, 139, 574–597. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Glissant, D.; Grimplet, J.; Barrieu, F.; Abbal, P.; Couture, C.; Ageorges, A.; Atanassova, R.; Léon, C.; Renaudin, J.-P.; et al. Isogene Specific Oligo Arrays Reveal Multifaceted Changes in Gene Expression during Grape Berry (Vitis vinifera L.) Development. Planta 2005, 222, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and Metabolite Analyses of Cabernet Sauvignon Grape Berry Development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef]
- Negri, A.S.; Prinsi, B.; Rossoni, M.; Failla, O.; Scienza, A.; Cocucci, M.; Espen, L. Proteome Changes in the Skin of the Grape Cultivar Barbera among Different Stages of Ripening. BMC Genom. 2008, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esteso, M.J.; Sellés-Marchart, S.; Lijavetzky, D.; Pedreño, M.A.; Bru-Martínez, R. A DIGE-Based Quantitative Proteomic Analysis of Grape Berry Flesh Development and Ripening Reveals Key Events in Sugar and Organic Acid Metabolism. J. Exp. Bot. 2011, 62, 2521–2569. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esteso, M.J.; Vilella-Antón, M.T.; Pedreño, M.Á.; Valero, M.L.; Bru-Martínez, R. ITRAQ-Based Protein Profiling Provides Insights into the Central Metabolism Changes Driving Grape Berry Development and Ripening. BMC Plant Biol. 2013, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.W.; Léon, C.; Feil, R.; Lunn, J.E.; Delrot, S.; Gomès, E. Metabolic Profiling Reveals Coordinated Switches in Primary Carbohydrate Metabolism in Grape Berry (Vitis vinifera L.), a Non-Climacteric Fleshy Fruit. J. Exp. Bot. 2013, 64, 1345–1355. [Google Scholar] [CrossRef]
- Lecourieux, D.; Kappel, C.; Claverol, S.; Pieri, P.; Feil, R.; Lunn, J.E.; Bonneu, M.; Wang, L.; Gomès, E.; Delrot, S.; et al. Proteomic and Metabolomic Profiling Underlines the Stage- and Time-Dependent Effects of High Temperature on Grape Berry Metabolism. J. Integr. Plant Biol. 2020, 62, 1132–1158. [Google Scholar] [CrossRef] [PubMed]
- Beckles, D.M.; Hong, N.; Stamova, L.; Luengwilai, K. Biochemical Factors Contributing to Tomato Fruit Sugar Content: A Review. Fruits 2012, 67, 49–64. [Google Scholar] [CrossRef]
- Afoufa-Bastien, D.; Medici, A.; Jeauffre, J.; Coutos-Thévenot, P.; Lemoine, R.; Atanassova, R.; Laloi, M. The Vitis Vinifera Sugar Transporter Gene Family: Phylogenetic Overview and Macroarray Expression Profiling. BMC Plant Biol. 2010, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Davies, C.; Dry, I.B. Isolation, Functional Characterization, and Expression Analysis of Grapevine (Vitis vinifera L.) Hexose Transporters: Differential Roles in Sink and Source Tissues. J. Exp. Bot. 2007, 58, 1985–1997. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 Mediates Sugar Accumulation in Grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Wu, W.; Li, X.; Zhang, C.; Fang, J. Enzyme Activities and Gene Expression of Starch Metabolism Provide Insights into Grape Berry Development. Hortic. Res. 2017, 4, 17018. [Google Scholar] [CrossRef][Green Version]
- Ren, R.; Yue, X.; Li, J.; Xie, S.; Guo, S.; Zhang, Z. Coexpression of Sucrose Synthase and the SWEET Transporter, Which Are Associated with Sugar Hydrolysis and Transport, Respectively, Increases the Hexose Content in Vitis vinifera L. Grape Berries. Front. Plant Sci. 2020, 11, 321. [Google Scholar] [CrossRef]
- Nonis, A.; Ruperti, B.; Pierasco, A.; Canaguier, A.; Adam-Blondon, A.-F.; Di Gaspero, G.; Vizzotto, G. Neutral Invertases in Grapevine and Comparative Analysis with Arabidopsis, Poplar and Rice. Planta 2008, 229, 129. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and Celluloses Show Specific Dynamics in the Internal and External Surfaces of Grape Berry Skin During Ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef] [PubMed]
- Karlson, P. Regulation in Metabolism; Newsholme, E.A., Start, C., Eds.; John Wiley and Sons, Ltd.: New York, NY, USA; London, UK, 1974; Volume 2. [Google Scholar]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Stitt, M.; Huber, S.; Kerr, P. Control of Photosynthetic Sucrose Formation. In Photosynthesis; Hatch, M.D., Boardman, N.K., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 327–409. ISBN 978-0-12-675410-0. [Google Scholar]
- Huber, S.C.; Huber, J.L. Role and Regulation of Sucrose-Phosphate Synthase in Higher Plants. Annu. Rev. Plant Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef]
- Sturm, A. Invertases. Primary Structures, Functions, and Roles in Plant Development and Sucrose Partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Winter, H.; Huber, S.C. Regulation of Sucrose Metabolism in Higher Plants: Localization and Regulation of Activity of Key Enzymes. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Leegood, R.C.; Walker, R.P. Regulation and Roles of Phosphoenolpyruvate Carboxykinase in Plants. Arch. Biochem. Biophys. 2003, 414, 204–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. Metabolons, Enzyme–Enzyme Assemblies That Mediate Substrate Channeling, and Their Roles in Plant Metabolism. Plant Commun. 2021, 2, 100081. [Google Scholar] [CrossRef]
- Diakou, P.; Svanella, L.; Raymond, P.; Gaudillère, J.-P.; Moing, A. Phosphenolpyruvate Carbosxylase during Grape Berry Development: Protein Level, Enzyme Activity and Regulation. Funct. Plant Biol. 2000, 27, 221–229. [Google Scholar] [CrossRef]
- Walker, R.P.; Paoletti, A.; Leegood, R.C.; Famiani, F. Phosphorylation of Phosphoenolpyruvate Carboxykinase (PEPCK) and Phosphoenolpyruvate Carboxylase (PEPC) in the Flesh of Fruits. Plant Physiol. Biochem. 2016, 108, 323–327. [Google Scholar] [CrossRef]
- Hawker, J.S. Changes in the Activities of Enzymes Concerned with Sugar Metabolism during the Development of Grape Berries. Phytochemistry 1969, 8, 9–17. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Wu, B.; Liu, H.; Guan, L.; Fan, P.; Li, S. Carbohydrate Metabolism in Grape Cultivars That Differ in Sucrose Accumulation. Vitis 2015, 50, 51–57. [Google Scholar]
- Moriguchi, T.; Abe, K.; Sanada, T.; Yamaki, S. Levels and Role of Sucrose Synthase, Sucrose-Phosphate Synthase, and Acid Invertase in Sucrose Accumulation in Fruit of Asian Pear. J. Am. Soc. Hortic. Sci. 1992, 117, 274–278. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Pueppke, S.G. Cherry Fruit Invertase: Partial Purification, Characterization and Activity during Fruit Development. J. Plant Physiol. 1990, 135, 662–666. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-Metabolizing Enzymes in Transport Tissues and Adjacent Sink Structures in Developing Citrus Fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef]
- Moscatello, S.; Famiani, F.; Proietti, S.; Farinelli, D.; Battistelli, A. Sucrose Synthase Dominates Carbohydrate Metabolism and Relative Growth Rate in Growing Kiwifruit (Actinidia deliciosa, Cv Hayward). Sci. Hortic. 2011, 128, 197–205. [Google Scholar] [CrossRef]
- Vizzotto, G.; Pinton, R.; Varanini, Z.; Costa, G. Sucrose Accumulation in Developing Peach Fruit. Physiol. Plant. 1996, 96, 225–230. [Google Scholar] [CrossRef]
- Hubbard, N.L.; Pharr, D.M.; Huber, S.C. Sucrose Phosphate Synthase and Other Sucrose Metabolizing Enzymes in Fruits of Various Species. Physiol. Plant. 1991, 82, 191–196. [Google Scholar] [CrossRef]
- Yelle, S.; Chetelat, R.T.; Dorais, M.; DeVerna, J.W.; Bennett, A.B. Sink Metabolism in Tomato Fruit: IV. Genetic and Biochemical Analysis of Sucrose Accumulation. Plant Physiol. 1991, 95, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Desnoues, E.; Gibon, Y.; Baldazzi, V.; Signoret, V.; Génard, M.; Quilot-Turion, B. Profiling Sugar Metabolism during Fruit Development in a Peach Progeny with Different Fructose-to-Glucose Ratios. BMC Plant Biol. 2014, 14, 336. [Google Scholar] [CrossRef]
- Davies, C.; Robinson, S.P. Sugar Accumulation in Grape Berries (Cloning of Two Putative Vacuolar Invertase cDNAs and Their Expression in Grapevine Tissues). Plant Physiol. 1996, 111, 275–283. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, M.; Li, X.; Jiu, S.; Wang, C.; Fang, J. Genome-Wide Analysis of the Sucrose Synthase Gene Family in Grape (Vitis vinifera): Structure, Evolution, and Expression Profiles. Genes 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, C.P.P.; Ap Rees, T. Invertase Activity during the Development of Carrot Roots. Phytochemistry 1970, 9, 239–247. [Google Scholar] [CrossRef]
- Nonis, A.; Ruperti, B.; Falchi, R.; Casatta, E.; Thamasebi Enferadi, S.; Vizzotto, G. Differential Expression and Regulation of a Neutral Invertase Encoding Gene from Peach (Prunus Persica): Evidence for a Role in Fruit Development. Physiol. Plant. 2007, 129, 436–446. [Google Scholar] [CrossRef]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal Growth of Arabidopsis Requires Cytosolic Invertase but Not Sucrose Synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Kossmann, J.; Botha, F.C.; Groenewald, J.-H. Reduced Neutral Invertase Activity in the Culm Tissues of Transgenic Sugarcane Plants Results in a Decrease in Respiration and Sucrose Cycling and an Increase in the Sucrose to Hexose Ratio. Funct. Plant Biol. 2010, 37, 22–31. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Ding, S.; Zhang, Q.; Chen, Y.; Zhang, J. Molecular Cloning, Structure, Phylogeny and Expression Analysis of the Invertase Gene Family in Sugarcane. BMC Plant Biol. 2017, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, K.B.; Gabler, A.; Simon, U.K.; Luschin-Ebengreuth, N.; Hatz, M.; Berger, S.; Muhammad, N.; Zeier, J.; Sinha, A.K.; Roitsch, T. Post-Translational Derepression of Invertase Activity in Source Leaves via Down-Regulation of Invertase Inhibitor Expression Is Part of the Plant Defense Response. Mol. Plant 2010, 3, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.-L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Giaquinta, R. Evidence for Phloem Loading from the Apoplast: Chemical Modification of Membrane Sulfhydryl Groups. Plant Physiol. 1976, 57, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G.; Matile, P. Solute Accumulation by Grape Pericarp Cells. I. Sugar Uptake by Skin, Segments. Biochem. Physiol. Pflanz. 1980, 175, 369–381. [Google Scholar] [CrossRef]
- Wyse, R. Sucrose Uptake by Sugar Beet Tap Root Tissue. Plant Physiol. 1979, 64, 837–841. [Google Scholar] [CrossRef]
- Etxeberria, E.; Pozueta-Romero, J.; Gonzalez, P. In and out of the Plant Storage Vacuole. Plant Sci. 2012, 190, 52–61. [Google Scholar] [CrossRef]
- Brown, S.C.; Coombe, B.G. Solute Accumulation by Grape Pericarp Cells II. Studies with Protoplasts and Isolated Vacuoles. Biochem. Physiol. Pflanz. 1984, 179, 157–171. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Feng, J.; Zeng, L.; Wang, Z.; Chen, S. Grape Berry Plasma Membrane Proteome Analysis and Its Differential Expression during Ripening. J. Exp. Bot. 2008, 59, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Reinders, A.; Schulze, W.; Kühn, C.; Barker, L.; Schulz, A.; Ward, J.M.; Frommer, W.B. Protein–Protein Interactions between Sucrose Transporters of Different Affinities Colocalized in the Same Enucleate Sieve Element. Plant Cell 2002, 14, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yin, S.; Ma, Y.; Song, M.; Song, Y.; Mu, S.; Li, Y.; Liu, X.; Ren, Y.; Gao, C.; et al. Carbon Export from Leaves Is Controlled via Ubiquitination and Phosphorylation of Sucrose Transporter SUC2. Proc. Natl. Acad. Sci. USA 2020, 117, 6223. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, J.; Tu, W.; Deng, Z.; Dong, W.; Gao, H.; Xu, J.; Zhang, N.; Yin, L.; Meng, Q.; et al. Expression of Sucrose Transporters from Vitis Vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2624. [Google Scholar] [CrossRef]
- Vignault, C.; Vachaud, M.; Cakir, B.; Glissant, D.; Dédaldéchamp, F.; Büttner, M.; Atanassova, R.; Fleurat-Lessard, P.; Lemoine, R.; Delrot, S. VvHT1 Encodes a Monosaccharide Transporter Expressed in the Conducting Complex of the Grape Berry Phloem. J. Exp. Bot. 2005, 56, 1409–1418. [Google Scholar] [CrossRef]
- Cakir, B.; Giachino, R.R.A. VvTMT2 Encodes a Putative Tonoplast Monosaccharide Transporter Expressed during Grape Berry (Vitis vinifera Cv. Sultanine) Ripening. Plant Omics 2012, 5, 576–583. [Google Scholar]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kühn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA Interference of LIN5 in Tomato Confirms Its Role in Controlling Brix Content, Uncovers the Influence of Sugars on the Levels of Fruit Hormones, and Demonstrates the Importance of Sucrose Cleavage for Normal Fruit Development and Fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef]
- Ko, H.-Y.; Ho, L.-H.; Neuhaus, H.E.; Guo, W.-J. SWEET15 Exports Sucrose from Phloem and Seed Coat in Tomato to Supply Carbon for Fruit and Seed Development. BioRxiv 2020. [Google Scholar] [CrossRef]
- Breia, R.; Conde, A.; Pimentel, D.; Conde, C.; Fortes, A.M.; Granell, A.; Gerós, H. VvSWEET7 Is a Mono- and Disaccharide Transporter Up-Regulated in Response to Botrytis cinerea Infection in Grape Berries. Front. Plant Sci. 2019, 10, 1753. [Google Scholar] [CrossRef]
- Thudichum, J.L.W.; Dupré, A. A Treatise on the Origin, Nature, and Varieties of Wine: Being a Complete Manual of Viticulture and Oenology; Macmillan: London, UK, 1872. [Google Scholar]
- Gore, H.C. The Occurence of Sucrose in Grapes of American Origin. Ind. Eng. Chem. 1916, 8, 333–334. [Google Scholar] [CrossRef]
- Amerine, M.A.; Thoukis, G. The Glucose-Fructose Ratio of California Grapes. Vitis 1958, 1, 224–229. [Google Scholar]
- Brandt, M.; Scheidweiler, M.; Rauhut, D.; Patz, C.-D.; Will, F.; Zorn, H.; Stoll, M. The Influence of Temperature and Solar Radiation on Phenols in Berry Skin and Maturity Parameters of Vitis vinifera L. Cv. Riesling. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines, 3rd ed.; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-816365-8. [Google Scholar]
- Hajirezaei, M.; Takahata, Y.; Trethewey, R.N.; Willmitzer, L.; Sonnewald, U. Impact of Elevated Cytosolic and Apoplastic Invertase Activity on Carbon Metabolism during Potato Tuber Development. J. Exp. Bot. 2000, 51, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Paoletti, A.; Proietti, P.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The Occurrence of Phosphoenolpyruvate Carboxykinase (PEPCK) in the Pericarp of Different Grapevine Genotypes and in Grape Leaves and Developing Seeds. J. Hort. Sci. Biotechnol. 2018, 93, 456–465. [Google Scholar] [CrossRef]
- Reshef, N.; Fait, A.; Agam, N. Grape Berry Position Affects the Diurnal Dynamics of Its Metabolic Profile. Plant Cell Environ. 2019, 42, 1897–1912. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Farinelli, D.; Palliotti, A.; Moscatello, S.; Battistelli, A.; Walker, R.P. Is Stored Malate the Quantitatively Most Important Substrate Utilised by Respiration and Ethanolic Fermentation in Grape Berry Pericarp during Ripening? Plant Physiol. Biochem. 2014, 76, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Shahood, R.; Torregrosa, L.; Savoi, S.; Romieu, C. First Quantitative Assessment of Growth, Sugar Accumulation and Malate Breakdown in a Single Ripening Berry. OENO One 2020, 54, 1077–1092. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Kliewer, W.M. Phosphoenolpyruvate Carboxykinase Activity in Grape Berries. Plant Physiol. 1975, 56, 67–71. [Google Scholar] [CrossRef]
- Famiani, F.; Moscatello, S.; Ferradini, N.; Gardi, T.; Battistelli, A.; Walker, R.P. Occurrence of a Number of Enzymes Involved in Either Gluconeogenesis or Other Processes in the Pericarp of Three Cultivars of Grape (Vitis vinifera L.) during Development. Plant Physiol. Biochem. 2014, 84, 261–270. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The Organic Acids That Are Accumulated in the Flesh of Fruits: Occurrence, Metabolism and Factors Affecting Their Contents—A Review. Rev. Chapingo. Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic Acids in Fruits. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 371–430. ISBN 978-1-119-43107-7. [Google Scholar]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in Grape Berries: The Role of Seed Respiration and Lenticels on the Berry Pedicel and the Possible Link to Cell Death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef]
- Boeckx, J.; Pols, S.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Regulation of the Central Carbon Metabolism in Apple Fruit Exposed to Postharvest Low-Oxygen Stress. Front. Plant Sci. 2019, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Beauvoit, B.P.; Biais, B.; Chabane, M.; Allwood, J.W.; Deborde, C.; Maucourt, M.; Goodacre, R.; Cabasson, C.; Moing, A.; et al. Central Metabolism Is Tuned to the Availability of Oxygen in Developing Melon Fruit. Front. Plant Sci. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the Crucial Role of Sucrose Synthase for Sink Strength Using Transgenic Potato Plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef]
- Nolte, K.D.; Koch, K.E. Companion-Cell Specific Localization of Sucrose Synthase in Zones of Phloem Loading and Unloading. Plant Physiol. 1993, 101, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.T.; Schurr, U.; Pfister, M.; Geigenberger, P. Phloem Metabolism and Function Have to Cope with Low Internal Oxygen. Plant Physiol. 2003, 131, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Giaquinta, R.T. Sucrose Translocation and Storage in the Sugar Beet. Plant Physiol. 1979, 63, 828–832. [Google Scholar] [CrossRef]
- Lemoine, R.; Daie, J.; Wyse, R. Evidence for the Presence of a Sucrose Carrier in Immature Sugar Beet Tap Roots. Plant Physiol. 1988, 86, 575–580. [Google Scholar] [CrossRef]
- Geigenberger, P.; Stitt, M. Sucrose Synthase Catalyses a Readily Reversible Reaction in Vivo in Developing Potato Tubers and Other Plant Tissues. Planta 1993, 189, 329–339. [Google Scholar] [CrossRef]
- Nguyen-Quoc, B.; Foyer, C.H. A Role for ‘Futile Cycles’ Involving Invertase and Sucrose Synthase in Sucrose Metabolism of Tomato Fruit. J. Exp. Bot. 2001, 52, 881–889. [Google Scholar] [CrossRef]
- Yamaki, S. Metabolism and Accumulation of Sugars Translocated to Fruit and Their Regulation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef]
- Burger, Y.; Schaffer, A.A. The Contribution of Sucrose Metabolism Enzymes to Sucrose Accumulation in Cucumis melo. J. Am. Soc. Hortic. Sci. 2007, 132, 704–712. [Google Scholar] [CrossRef]
- Walker, R.P.; Winters, A.L.; Pollock, C.J. Purification and Characterization of Invertases from Leaves of Lolium temulentum. New Phytol. 1997, 135, 259–266. [Google Scholar] [CrossRef]
- Dreier, L.P.; Hunter, J.J.; Ruffner, H.P. Invertase Activity, Grape Berry Development and Cell Compartmentation. Plant Physiol Biochem. 1998, 36, 865–872. [Google Scholar] [CrossRef]
- Porntaveewat, W.; Takayanagi, T.; Yokotsuka, K. Purification and Properties of Invertase from Muscat Bailey A Grapes. J. Ferment. Bioeng. 1994, 78, 288–292. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Huerlimann, M.; Skrivan, R. Soluble Invertase from Grape Berries: Purification, Deglycosylation and Antibody Specifity. Plant Physiol. Biochem. 1995, 33, 25–31. [Google Scholar]
- Pan, Q.-H.; Li, M.-J.; Peng, C.-C.; Zhang, N.; Zou, X.; Zou, K.-Q.; Wang, X.-L.; Yu, X.-C.; Wang, X.-F.; Zhang, D.-P. Abscisic Acid Activates Acid Invertases in Developing Grape Berry. Physiol. Plant. 2005, 125, 157–170. [Google Scholar] [CrossRef]
- Jégou, S.; Conreux, A.; Villaume, S.; Hovasse, A.; Schaeffer, C.; Cilindre, C.; Van Dorsselaer, A.; Jeandet, P. One Step Purification of the Grape Vacuolar Invertase. Anal. Chim. Acta 2009, 638, 75–78. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, X.-L.; Wang, X.-F.; Xia, G.-H.; Pan, Q.-H.; Fan, R.-C.; Wu, F.-Q.; Yu, X.-C.; Zhang, D.-P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Laurière, C.; Laurière, M.; Sturm, A.; Faye, L.; Chrispeels, M.J. Characterization of β-Fructosidase: An Extracellular Glycoprotein of Carrot Cells. Biochimie 1988, 70, 1483–1491. [Google Scholar] [CrossRef]
- Unger, C.; Hofsteenge, J.; Sturm, A. Purification and Characterization of a Soluble β-Fructofuranosidase from Daucus carota. Eur. J. Biochem. 1992, 204, 915–921. [Google Scholar] [CrossRef]
- Cheng, W.H.; Taliercio, E.W.; Chourey, P.S. The Miniature Seed Locus of Maize Encodes a Cell Wall Invertase Required for Normal Development of Endosperm and Maternal Cells in the Pedicel. Plant Cell 1996, 8, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Q.; Lüscher, M.; Sturm, A. Antisense Repression of Vacuolar and Cell Wall Invertase in Transgenic Carrot Alters Early Plant Development and Sucrose Partitioning. Plant Cell 1999, 11, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.P.; Lu, Y.M.; Wang, Y.Z.; Duan, C.Q.; Yan, H.Y. Acid Invertase Is Predominantly Localized to Cell Walls of Both the Practically Symplasmically Isolated Sieve Element/Companion Cell Complex and Parenchyma Cells in Developing Apple Fruits. Plant Cell Environ. 2001, 24, 691–702. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression Patterns of Genes Involved in Sugar Metabolism and Accumulation during Apple Fruit Development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Feng, F.; Zhang, S.; Ma, F.; Cheng, L. Proteomic Analysis Reveals Dynamic Regulation of Fruit Development and Sugar and Acid Accumulation in Apple. J. Exp. Bot. 2016, 67, 5145–5157. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, P.; Ma, F.; Dandekar, A.M.; Cheng, L. Sugar Metabolism and Accumulation in the Fruit of Transgenic Apple Trees with Decreased Sorbitol Synthesis. Hortic. Res. 2018, 5, 60. [Google Scholar] [CrossRef]
- Hardy, P.J. Metabolism of Sugars and Organic Acids in Immature Grape Berries. Plant Physiol. 1968, 43, 224–228. [Google Scholar] [CrossRef]
- Wang, J.; Nayak, S.; Koch, K.; Ming, R. Carbon Partitioning in Sugarcane (Saccharum species). Front. Plant Sci. 2013, 4, 201. [Google Scholar] [CrossRef]
- Sacher, J.A.; Hatch, M.D.; Glasziou, K.T. Sugar Accumulation Cycle in Sugar Cane. III. Physical and Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963, 38, 348–354. [Google Scholar] [CrossRef]
- Dancer, J.; Hatzfeld, W.-D.; Stitt, M. Cytosolic Cycles Regulate the Turnover of Sucrose in Heterotrophic Cell-Suspension Cultures of Chenopodium rubrum L. Planta 1990, 182, 223–231. [Google Scholar] [CrossRef]
- Geigenberger, P.; Reimholz, R.; Geiger, M.; Merlo, L.; Canale, V.; Stitt, M. Regulation of Sucrose and Starch Metabolism in Potato Tubers in Response to Short-Term Water Deficit. Planta 1997, 201, 502–518. [Google Scholar] [CrossRef]
- Fernie, A.R.; Willmitzer, L.; Trethewey, R.N. Sucrose to Starch: A Transition in Molecular Plant Physiology. Trends Plant Sci. 2002, 7, 35–41. [Google Scholar] [CrossRef]
- Giaquinta, R.T.; Lin, W.; Sadler, N.L.; Franceschi, V.R. Pathway of Phloem Unloading of Sucrose in Corn Roots. Plant Physiol. 1983, 72, 362–367. [Google Scholar] [CrossRef]
- Pritchard, J.; Tomos, A.D.; Farrar, J.F.; Minchin, P.E.H.; Gould, N.; Paul, M.J.; MacRae, E.A.; Ferrieri, R.A.; Gray, D.W.; Thorpe, M.R. Turgor, Solute Import and Growth in Maize Roots Treated with Galactose. Funct. Plant Biol. 2004, 31, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.-R.; Lian, H.; Ni, D.-A.; He, Y.; Chen, X.-Y.; Ruan, Y.-L. Evidence That High Activity of Vacuolar Invertase Is Required for Cotton Fiber and Arabidopsis Root Elongation through Osmotic Dependent and Independent Pathways, Respectively. Plant Physiol. 2010, 154, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the Grape (Vitis vinifera L.) Berry: Transport and Function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Flütsch, S.; Wang, Y.; Takemiya, A.; Vialet-Chabrand, S.R.M.; Klejchová, M.; Nigro, A.; Hills, A.; Lawson, T.; Blatt, M.R.; Santelia, D. Guard Cell Starch Degradation Yields Glucose for Rapid Stomatal Opening in Arabidopsis. Plant Cell 2020, 32, 2325–2344. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Drincovich, M.F.; Lara, M.V.; Vizzotto, G.; Walker, R.P.; Famiani, F.; Bonghi, C. Metabolism of Stone Fruits: Reciprocal Contribution Between Primary Metabolism and Cell Wall. Front. Plant. Sci. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Shackel, K.A.; Matthews, M.A.; Morrison, J.C. Dynamic Relation between Expansion and Cellular Turgor in Growing Grape (Vitis vinifera L.) Leaves. Plant Physiol. 1987, 84, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.; Shackel, K.A.; Matthews, M.A. Mesocarp Cell Turgor in Vitis vinifera L. Berries throughout Development and Its Relation to Firmness, Growth, and the Onset of Ripening. Planta 2008, 228, 1067. [Google Scholar] [CrossRef]
- Klann, E.M.; Hall, B.; Bennett, A.B. Antisense Acid Invertase (TIV1) Gene Alters Soluble Sugar Composition and Size in Transgenic Tomato Fruit. Plant Physiol. 1996, 112, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Vinson, A.E. The Function of Invertase in the Formation of Cane and Invert Sugar Dates. Bot. Gaz. 1907, 43, 393–407. [Google Scholar] [CrossRef]
- Hubbard, N.L.; Huber, S.C.; Pharr, D.M. Sucrose Phosphate Synthase and Acid Invertase as Determinants of Sucrose Concentration in Developing Muskmelon (Cucumis melo L.) Fruits. Plant Physiol. 1989, 91, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Paczek, V.; Dubois, F.; Sangwan, R.; Morot-Gaudry, J.-F.; Roubelakis-Angelakis, K.A.; Hirel, B. Cellular and Subcellular Localisation of Glutamine Synthetase and Glutamate Dehydrogenase in Grapes Gives New Insights on the Regulation of Carbon and Nitrogen Metabolism. Planta 2002, 216, 245–254. [Google Scholar] [CrossRef]
- Hale, C.R.; Weaver, R. The Effect of Developmental Stage on Direction of Translocation of Photosynthate in Vitis vinifera. Hildgardia 1962, 33, 89–131. [Google Scholar] [CrossRef]
- Merry, A.M.; Evans, K.J.; Corkrey, R.; Wilson, S.J. Coincidence of Maximum Severity of Powdery Mildew on Grape Leaves and the Carbohydrate Sink-to-Source Transition. Plant Pathol. 2013, 62, 842–850. [Google Scholar] [CrossRef]
- Moser, C.; Pindo, M.; Bertamini, M.; Segala, C.; Nedunchezhian, N.; Fontana, P.; Velasco, R.; Blanzieri, E. Gene Expression Profiling during Grape Leaf Development and Senescence by High Density Filters. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 August 2005; pp. 441–446. [Google Scholar]
- Ruffner, H.P.; Brem, S.; Rast, D.M. Pathway of Photosynthetic Malate Formation in Vitis vinifera, a C3 Plant. Plant Physiol. 1983, 73, 582–585. [Google Scholar] [CrossRef]
- Dayer, S.; Prieto, J.A.; Galat, E.; Peña, J.P. Leaf Carbohydrate Metabolism in Malbec Grapevines: Combined Effects of Regulated Deficit Irrigation and Crop Load. Aust. J. Grape Wine Res. 2016, 22, 115–123. [Google Scholar] [CrossRef]
- Quereix, A.; Dewar, R.C.; Gaudillere, J.; Dayau, S.; Valancogne, C. Sink Feedback Regulation of Photosynthesis in Vines: Measurements and a Model. J. Exp. Bot. 2001, 52, 2313–2322. [Google Scholar] [CrossRef]
- Chaumont, M.; Morot-Gaudry, J.-F.; Foyer, C.H. Seasonal and Diurnal Changes in Photosynthesis and Carbon Partitioning in Vitis vinifera Leaves in Vines with and Without Fruit. J. Exp. Bot. 1994, 45, 1235–1243. [Google Scholar] [CrossRef]
- Chen, L.-S.; Cheng, L. Carbon Assimilation and Carbohydrate Metabolism of ‘Concord’ Grape (Vitis labrusca L.) Leaves in Response to Nitrogen Supply. J. Am. Soc. Hortic. Sci. 2003, 128, 754–760. [Google Scholar] [CrossRef]
- Patakas, A.; Nikolaou, N.; Zioziou, E.; Radoglou, K.; Noitsakis, B. The Role of Organic Solute and Ion Accumulation in Osmotic Adjustment in Drought-Stressed Grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Gerhardt, R.; Stitt, M.; Heldt, H.W. Subcellular Metabolite Levels in Spinach Leaves: Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987, 83, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and Primary Photosynthetic Metabolism–More than the Icing on the Cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Neves, A.; Breia, R.; Pimentel, D.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Cunha, A.; Gerós, H.; Moutinho-Pereira, J. Kaolin Particle Film Application Stimulates Photoassimilate Synthesis and Modifies the Primary Metabolome of Grape Leaves. J. Plant Physiol. 2018, 223, 47–56. [Google Scholar] [CrossRef]
- Kingston-Smith, A.H.; Walker, R.P.; Pollock, C.J. Invertase in Leaves: Conundrum or Control Point? J. Exp. Bot. 1999, 50, 735–743. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The Physiology of Drought Stress in Grapevine: Towards an Integrative Definition of Drought Tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Dayer, S.; Murcia, G.; Prieto, J.A.; Durán, M.; Martínez, L.; Píccoli, P.; Perez Peña, J. Non-Structural Carbohydrates and Sugar Export in Grapevine Leaves Exposed to Different Light Regimes. Physiol. Plant. 2021, 171, 728–738. [Google Scholar] [CrossRef]
- Chen, L.-S.; Smith, B.R.; Cheng, L. CO2 Assimilation, Photosynthetic Enzymes, and Carbohydrates of ‘Concord’ Grape Leaves in Response to Iron Supply. J. Am. Soc. Hortic. Sci. 2004, 129, 738–744. [Google Scholar] [CrossRef]
- Roper, T.R.; Williams, L.E. Net CO2 Assimilation and Carbohydrate Partitioning of Grapevine Leaves in Response to Trunk Girdling and Gibberellic Acid Application. Plant Physiol. 1989, 89, 1136–1140. [Google Scholar] [CrossRef]
- Hendrix, D.L.; Huber, S.C. Diurnal Fluctuations in Cotton Leaf Carbon Export, Carbohydrate Content, and Sucrose Synthesizing Enzymes. Plant Physiol. 1986, 81, 584–586. [Google Scholar] [CrossRef]
- Du, Y.-C.; Nose, A.; Kondo, A.; Wasano, K. Diurnal Changes in Photosynthesis in Sugarcane Leaves: II. Enzyme Activities and Metabolite Levels Relating to Sucrose and Starch Metabolism. Plant Prod. Sci. 2000, 3, 9–16. [Google Scholar] [CrossRef]
- Dantas, B.F.; de Ribeiro, L.S.; da Silva, A.P.; de Luz, S.R.S. Foliar Carbohydrates Content and Invertase Activity in Vines at São Francisco River Valley-Brazil. Rev. Bras. Frutic. 2005, 27, 198–202. [Google Scholar] [CrossRef]
- Blagoveschenski, A.V.; Sossiedov, N.I. The Specific Action of Plant Ferments: The Specific Conditions of Action of Leaf Invertases. Biochem. J. 1925, 19, 350–354. [Google Scholar] [CrossRef]
- Giaquinta, R. Source and Sink Leaf Metabolism in Relation to Phloem Translocation: Carbon Partitioning and Enzymology. Plant Physiol. 1978, 61, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C. Biochemical Mechanism for Regulation of Sucrose Accumulation in Leaves during Photosynthesis. Plant Physiol. 1989, 91, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Von Schaewen, A.; Stitt, M.; Schmidt, R.; Sonnewald, U.; Willmitzer, L. Expression of a Yeast-Derived Invertase in the Cell Wall of Tobacco and Arabidopsis Plants Leads to Accumulation of Carbohydrate and Inhibition of Photosynthesis and Strongly Influences Growth and Phenotype of Transgenic Tobacco Plants. EMBO J. 1990, 9, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U.; Brauer, M.; von Schaewen, A.; Stitt, M.; Willmitzer, L. Transgenic Tobacco Plants Expressing Yeast-Derived Invertase in Either the Cytosol, Vacuole or Apoplast: A Powerful Tool for Studying Sucrose Metabolism and Sink/Source Interactions. Plant J. 1991, 1, 95–106. [Google Scholar] [CrossRef]
- Ni, D.A. Role of Vacuolar Invertase in Regulating Arabidopsis Stomatal Opening. Acta Physiol. Plant. 2012, 34, 2449–2452. [Google Scholar] [CrossRef]
- Chen, S.-F.; Liang, K.; Yin, D.-M.; Ni, D.-A.; Zhang, Z.-G.; Ruan, Y.-L. Ectopic Expression of a Tobacco Vacuolar Invertase Inhibitor in Guard Cells Confers Drought Tolerance in Arabidopsis. J. Enzym. Inhib. Med. Chem. 2016, 31, 1381–1385. [Google Scholar] [CrossRef]
- Christensen, N.M.; Faulkner, C.; Oparka, K. Evidence for Unidirectional Flow through Plasmodesmata. Plant Physiol. 2009, 150, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Leong, B.J.; Lybrand, D.B.; Lou, Y.-R.; Fan, P.; Schilmiller, A.L.; Last, R.L. Evolution of Metabolic Novelty: A Trichome-Expressed Invertase Creates Specialized Metabolic Diversity in Wild Tomato. Sci. Adv. 2019, 5, eaaw3754. [Google Scholar] [CrossRef]
- Pagliarani, C.; Casolo, V.; Ashofteh Beiragi, M.; Cavalletto, S.; Siciliano, I.; Schubert, A.; Gullino, M.L.; Zwieniecki, M.A.; Secchi, F. Priming Xylem for Stress Recovery Depends on Coordinated Activity of Sugar Metabolic Pathways and Changes in Xylem Sap pH. Plant Cell Environ. 2019, 42, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The Possible Role of Non-Structural Carbohydrates in the Regulation of Tree Hydraulics. Int. J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Windt, C.; Vergeldt, F.J.; De Jager, P.A.; Van As, H. MRI of Long-Distance Water Transport: A Comparison of the Phloem and Xylem Flow Characteristics and Dynamics in Poplar, Castor Bean, Tomato and Tobacco. Plant Cell Environ. 2006, 29, 1715–1729. [Google Scholar] [CrossRef]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell Wall-Bound Invertase Limits Sucrose Export and Is Involved in Symptom Development and Inhibition of Photosynthesis during Compatible Interaction between Tomato and Xanthomonas campestris Pv vesicatoria. Plant Physiol. 2008, 148, 1523–1536. [Google Scholar] [CrossRef]
- Hayes, M.A.; Feechan, A.; Dry, I.B. Involvement of Abscisic Acid in the Coordinated Regulation of a Stress-Inducible Hexose Transporter (VvHT5) and a Cell Wall Invertase in Grapevine in Response to Biotrophic Fungal Infection. Plant Physiol. 2010, 153, 211–221. [Google Scholar] [CrossRef]
- Murcia, G.; Pontin, M.; Piccoli, P. Role of ABA and Gibberellin A3 on Gene Expression Pattern of Sugar Transporters and Invertases in Vitis vinifera Cv. Malbec during Berry Ripening. Plant Growth Regul. 2018, 84, 275–283. [Google Scholar] [CrossRef]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal Closure, Basal Leaf Embolism, and Shedding Protect the Hydraulic Integrity of Grape Stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Marusig, D.; Tombesi, S. Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef] [PubMed]
- Scholes, J.; Bundock, N.; Wilde, R.; Rolfe, S. The Impact of Reduced Vacuolar Invertase Activity on the Photosynthetic and Carbohydrate Metabolism of Tomato. Planta 1996, 200, 265–272. [Google Scholar] [CrossRef]
- Koroleva, O.A.; Tomos, D.A.; Farrar, J.; Pollock, C.J. Changes in Osmotic and Turgor Pressure in Response to Sugar Accumulation in Barley Source Leaves. Planta 2002, 215, 210–219. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem Loading and Unloading of Sugars and Amino Acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef]
- Haupt, S.; Duncan, G.H.; Holzberg, S.; Oparka, K.J. Evidence for Symplastic Phloem Unloading in Sink Leaves of Barley. Plant Physiol. 2001, 125, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W. Biophysical Limitation of Cell Elongation in Cereal Leaves. Ann. Bot. 2002, 90, 157–167. [Google Scholar] [CrossRef][Green Version]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of Aquaporins in Leaf Physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Buckley, T. The Contributions of Apoplastic, Symplastic and Gas Phase Pathways for Water Transport Outside the Bundle Sheath in Leaves. Plant Cell Environ. 2015, 38, 7–22. [Google Scholar] [CrossRef]
- Ma, S.; Sun, L.; Sui, X.; Li, Y.; Chang, Y.; Fan, J.; Zhang, Z. Phloem Loading in Cucumber: Combined Symplastic and Apoplastic Strategies. Plant J. 2019, 98, 391–404. [Google Scholar] [CrossRef]
- Kingston-Smith, A.H.; Galtier, N.; Pollock, C.J.; Foyer, C.H. Soluble Acid Invertase Activity in Leaves Is Independent of Species Differences in Leaf Carbohydrates, Diurnal Sugar Profiles and Paths of Phloem Loading. New Phytol. 1998, 139, 283–292. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C. Molecular Biology of Sugar and Anthocyanin Accumulation in Grape Berries. In Molecular Biology & Biotechnology of the Grapevine; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 1–33. ISBN 978-94-017-2308-4. [Google Scholar]
- Park, K.; Knoblauch, J.; Oparka, K.; Jensen, K.H. Controlling Intercellular Flow through Mechanosensitive Plasmodesmata Nanopores. Nat. Commun. 2019, 10, 3564. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.E.H.; Thorpe, M.R.; Farrar, J.F.; Koroleva, O.A. Source–Sink Coupling in Young Barley Plants and Control of Phloem Loading. J. Exp. Bot. 2002, 53, 1671–1676. [Google Scholar] [CrossRef]
- Andersen, P.C.; Brodbeck, B.V. Chemical Composition of Xylem Exudate from Bleeding Spurs of Vitis rotundifolia Noble and Vitis Hybrid Suwannee in Relation to Pruning Date. Am. J. Enol. Vitic. 1989, 40, 155. [Google Scholar]
- Glad, C.; Regnard, J.L.; Querou, Y.; Brun, O.; Morot-Gaudry, J.F. Flux and Chemical Composition of Xylem Exudates from Chardonnay Grapevines: Temporal Evolution and Effect of Recut. Am. J. Enol. Vitic. 1992, 43, 275. [Google Scholar]
- Zhang, Y.; Keller, M. Discharge of Surplus Phloem Water May Be Required for Normal Grape Ripening. J. Exp. Bot. 2017, 68, 585–595. [Google Scholar] [CrossRef][Green Version]
- Pate, J.S. Nutrients and Metabolites of Fluids Recovered from Xylem and Phloem: Significance in Relation to Long-distance Transport in Plants. In Transport and Transfer Process in Plants; Wardlaw, I.F., Passioura, J.B., Eds.; Academic Press: Cambridge, MA, USA, 1976; pp. 253–281. ISBN 978-0-12-734850-6. [Google Scholar]
- Lohaus, G.; Burba, M.; Heldt, H.W. Comparison of the Contents of Sucrose and Amino Acids in the Leaves, Phloem Sap and Taproots of High and Low Sugar-Producing Hybrids of Sugar Beet (Beta vulgaris L.). J. Exp. Bot. 1994, 45, 1097–1101. [Google Scholar] [CrossRef]
- Morandi, B.; Rieger, M.; Grappadelli, L.C. Vascular Flows and Transpiration Affect Peach (Prunus persica Batsch.) Fruit Daily Growth. J. Exp. Bot. 2007, 58, 3941–3947. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Winkler, A.; Knoche, M. Xylem, Phloem, and Transpiration Flows in Developing Sweet Cherry Fruit. Trees 2016, 30, 1821–1830. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Shackel, K.A.; Matthews, M.A. Developmental Changes in the Diurnal Water Budget of the Grape Berry Exposed to Water Deficits. Plant Cell Environ. 1994, 17, 811–820. [Google Scholar] [CrossRef]
- Keller, M.; Smith, J.P.; Bondada, B.R. Ripening Grape Berries Remain Hydraulically Connected to the Shoot. J. Exp. Bot. 2006, 57, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Desnoues, E.; Génard, M.; Quilot-Turion, B.; Baldazzi, V. A Kinetic Model of Sugar Metabolism in Peach Fruit Reveals a Functional Hypothesis of a Markedly Low Fructose-to-Glucose Ratio Phenotype. Plant J. 2018, 94, 685–698. [Google Scholar] [CrossRef]
- Moing, A.; Carbonne, F.; Zipperlin, B.; Svanella, L.; Gaudillère, J.-P. Phloem Loading in Peach: Symplastic or Apoplastic? Physiolol. Plant. 1997, 101, 489–496. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Pharr, D.M.; Madore, M.A. Cucurbits. In Photoassimilates Distributions in Plants and Crops. Source-Sink Interactions; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 729–757. [Google Scholar]
- Zhang, B.; Tolstikov, V.; Turnbull, C.; Hicks, L.M.; Fiehn, O. Divergent Metabolome and Proteome Suggest Functional Independence of Dual Phloem Transport Systems in Cucurbits. Proc. Natl. Acad. Sci. USA 2010, 107, 13532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X.; Ayre, B.G.; Turgeon, R. The Origin and Composition of Cucurbit “Phloem” Exudate. Plant Physiol. 2012, 158, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Bondada, B.R.; Matthews, M.A.; Shackel, K.A. Functional Xylem in the Post-Veraison Grape Berry. J. Exp. Bot. 2005, 56, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.A.; Shackel, K.A. Growth and Water Transport in Fleshy Fruit. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Academic Press: Burlington, VT, USA, 2005; pp. 181–197. ISBN 978-0-12-088457-5. [Google Scholar]
- Zhang, Y.; Keller, M. Grape Berry Transpiration Is Determined by Vapor Pressure Deficit, Cuticular Conductance, and Berry Size. Am. J. Enol. Vitic. 2015, 66, 454. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Schultz, H.R.; Matthews, M.A. Field Evaluation of Water Transport in Grape Berries during Water Deficits. Physiol. Plant. 1996, 97, 55–62. [Google Scholar] [CrossRef]
- Carlomagno, A.; Novello, V.; Ferrandino, A.; Genre, A.; Lovisolo, C.; Hunter, J.J. Pre-Harvest Berry Shrinkage in Cv ‘Shiraz’ (Vitis vinifera L.): Understanding Sap Flow by Means of Tracing. Sci. Hortic. 2018, 233, 394–406. [Google Scholar] [CrossRef]
- Knipfer, T.; Eustis, A.; Brodersen, C.; Walker, A.M.; Mcelrone, A.J. Grapevine Species from Varied Native Habitats Exhibit Differences in Embolism Formation/Repair Associated with Leaf Gas Exchange and Root Pressure. Plant Cell Environ. 2015, 38, 1503–1513. [Google Scholar] [CrossRef]
- Ji, X.M.; Raveendran, M.; Oane, R.; Ismail, A.; Lafitte, R.; Bruskiewich, R.; Cheng, S.H.; Bennett, J. Tissue-Specific Expression and Drought Responsiveness of Cell-Wall Invertase Genes of Rice at Flowering. Plant Mol. Biol. 2005, 59, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Geng, M.-T.; Wu, X.-H.; Liu, J.; Li, R.-M.; Hu, X.-W.; Guo, J.-C. Genome-Wide Identification, 3D Modeling, Expression and Enzymatic Activity Analysis of Cell Wall Invertase Gene Family from Cassava (Manihot esculenta Crantz). Int. J. Mol. Sci. 2014, 15, 7313. [Google Scholar] [CrossRef] [PubMed]
- Tanner, W.; Beevers, H. Transpiration, a Prerequisite for Long-Distance Transport of Minerals in Plants? Proc. Natl. Acad. Sci. USA 2001, 98, 9443. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H. Root Pressure and beyond: Energetically Uphill Water Transport into Xylem Vessels? J. Exp. Bot. 2014, 65, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Pfautsch, S.; Renard, J.; Tjoelker, M.G.; Salih, A. Phloem as Capacitor: Radial Transfer of Water into Xylem of Tree Stems Occurs via Symplastic Transport in Ray Parenchyma. Plant Physiol. 2015, 167, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Aubry, E.; Dinant, S.; Vilaine, F.; Bellini, C.; Le Hir, R. Lateral Transport of Organic and Inorganic Solutes. Plants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.-A.; Ruan, Y.-L. Posttranslational Elevation of Cell Wall Invertase Activity by Silencing Its Inhibitor in Tomato Delays Leaf Senescence and Increases Seed Weight and Fruit Hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Falchi, R.; Petrussa, E.; Braidot, E.; Sivilotti, P.; Boscutti, F.; Vuerich, M.; Calligaro, C.; Filippi, A.; Herrera, J.C.; Sabbatini, P.; et al. Analysis of Non-Structural Carbohydrates and Xylem Anatomy of Leaf Petioles Offers New Insights in the Drought Response of Two Grapevine Cultivars. Int. J. Mol. Sci. 2020, 21, 1457. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.-H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone Fruits: Growth and Nitrogen and Organic Acid Metabolism in the Fruits and Seeds-A Review. Front. Plant Sci. 2020, 11, 572601. [Google Scholar] [CrossRef] [PubMed]
- Wächter, R.; Langhans, M.; Aloni, R.; Götz, S.; Weilmünster, A.; Koops, A.; Temguia, L.; Mistrik, I.; Pavlovkin, J.; Rascher, U.; et al. Vascularization, High-Volume Solution Flow, and Localized Roles for Enzymes of Sucrose Metabolism during Tumorigenesis by Agrobacterium Tumefaciens. Plant Physiol. 2003, 133, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Lin, I.W.; Qu, X.-Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A Cascade of Sequentially Expressed Sucrose Transporters in the Seed Coat and Endosperm Provides Nutrition for the Arabidopsis Embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Palmer, W.M.; Ru, L.; Jin, Y.; Patrick, J.W.; Ruan, Y.-L. Tomato Ovary-to-Fruit Transition Is Characterized by a Spatial Shift of mRNAs for Cell Wall Invertase and Its Inhibitor with the Encoded Proteins Localized to Sieve Elements. Mol. Plant 2015, 8, 315–328. [Google Scholar] [CrossRef]
- Corelli Grappadelli, L.; Morandi, B.; Manfrini, L.; O’Connell, M. Apoplasmic and Simplasmic Phloem Unloading Mechanisms: Do They Co-Exist in Angeleno Plums under Demanding Environmental Conditions? J. Plant Physiol. 2019, 237, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of Major Ripening Events during Softening in Grape: Turgor, Sugar Accumulation, Abscisic Acid Metabolism, Colour Development, and Their Relationship with Growth. J. Exp. Bot. 2016, 67, 709–722. [Google Scholar] [CrossRef]
- Kühn, C.; Hajirezaei, M.-R.; Fernie, A.R.; Roessner-Tunali, U.; Czechowski, T.; Hirner, B.; Frommer, W.B. The Sucrose Transporter StSUT1 Localizes to Sieve Elements in Potato Tuber Phloem and Influences Tuber Physiology and Development. Plant Physiol. 2003, 131, 102–113. [Google Scholar] [CrossRef]
- Zanon, L.; Falchi, R.; Santi, S.; Vizzotto, G. Sucrose Transport and Phloem Unloading in Peach Fruit: Potential Role of Two Transporters Localized in Different Cell Types. Physiol. Plant. 2015, 154, 179–193. [Google Scholar] [CrossRef]
- Patrick, J.W. Does Don Fisher’s High-Pressure Manifold Model Account for Phloem Transport and Resource Partitioning? Front. Plant Sci. 2013, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.; Thorpe, M.R.; Minchin, P.E.H.; Pritchard, J.; White, P.J. Solute Is Imported to Elongating Root Cells of Barley as a Pressure Driven-Flow of Solution. Funct. Plant Biol. 2004, 31, 391–397. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli-Grappadelli, L. The Positive Effect of Skin Transpiration in Peach Fruit Growth. J. Plant Physiol. 2010, 167, 1033–1037. [Google Scholar] [CrossRef]
- Keller, M.; Zhang, Y.; Shrestha, P.M.; Biondi, M.; Bondada, B.R. Sugar Demand of Ripening Grape Berries Leads to Recycling of Surplus Phloem Water via the Xylem. Plant Cell Environ. 2015, 38, 1048–1059. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Hatfield, J.M.; Jaudzems, V.G.; White, R.G.; Keller, M. Grape Berry Cv. Shiraz Epicuticular Wax and Transpiration during Ripening and Preharvest Weight Loss. Am. J. Enol. Vitic. 2004, 55, 121. [Google Scholar]
- Porter, G.A.; Knievel, D.P.; Shannon, J.C. Assimilate Unloading from Maize (Zea mays L.) Pedicel Tissues: I. Evidence for Regulation of Unloading by Cell Turgor. Plant Physiol. 1987, 83, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, A.H.; Jiang, J. Gene Expression Patterns of Invertase Gene Families and Modulation of the Inhibitor Gene in Tomato Sucrose Metabolism. Genet. Mol. Res. 2013, 12, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Ruan, Y.-L.; Fernie, A.R. An Update on Source-to-Sink Carbon Partitioning in Tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural Genetic Variation for Expression of a SWEET Transporter among Wild Species of Solanum lycopersicum (Tomato) Determines the Hexose Composition of Ripening Tomato Fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. Plant Fructokinases: Evolutionary, Developmental, and Metabolic Aspects in Sink Tissues. Front. Plant Sci. 2018, 9, 339. [Google Scholar] [CrossRef]
- Oparka, K.J.; Prior, D.A.M. Movement of Lucifer Yellow CH in Potato Tuber Storage Tissues: A Comparison of Symplastic and Apoplastic Transport. Planta 1988, 176, 533–540. [Google Scholar] [CrossRef]
- Cairns, A.J.; Turner, L.B.; Gallagher, J.A. Ryegrass Leaf Fructan Synthesis Is Oxygen Dependent and Abolished by Endomembrane Inhibitors. New Phytol. 2008, 180, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Lammens, W.; Claupein, W.; Van den Ende, W. Freezing Tolerance by Vesicle-Mediated Fructan Transport. Trends Plant Sci. 2008, 13, 409–414. [Google Scholar] [CrossRef]
- Kim, S.-J.; Brandizzi, F. The Plant Secretory Pathway: An Essential Factory for Building the Plant Cell Wall. Plant Cell Physiol. 2014, 55, 687–693. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.-Q. The Sucrose-Cleaving Enzymes of Plants Are Crucial for Development, Growth and Carbon Partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Li, J.-F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and Homologous Recombination–Mediated Genome Editing in Arabidopsis and Nicotiana benthamiana Using Guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, H.; Xu, N.; Zhang, B.; Gou, F.; Zhu, J.-K. Application of the CRISPR–Cas System for Efficient Genome Engineering in Plants. Mol. Plant 2013, 6, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Ghogare, R.; Williamson-Benavides, B.; Ramírez-Torres, F.; Dhingra, A. CRISPR-Associated Nucleases: The Dawn of a New Age of Efficient Crop Improvement. Transgenic Res. 2020, 29, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, L.; Piazza, S.; Campa, M.; Flachowsky, H.; Hanke, M.-V.; Malnoy, M. Efficient Heat-Shock Removal of the Selectable Marker Gene in Genetically Modified Grapevine. Plant Cell Tissue Organ Cult. 2016, 124, 471–481. [Google Scholar] [CrossRef]
- Baltes, N.J.; Hummel, A.W.; Konecna, E.; Cegan, R.; Bruns, A.N.; Bisaro, D.M.; Voytas, D.F. Conferring Resistance to Geminiviruses with the CRISPR–Cas Prokaryotic Immune System. Nat. Plants 2015, 1, 15145. [Google Scholar] [CrossRef]
- Čermák, T.; Curtin, S.J.; Gil-Humanes, J.; Čegan, R.; Kono, T.J.Y.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Bruetschy, C. The EU Regulatory Framework on Genetically Modified Organisms (GMOs). Transgenic Res. 2019, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR–Cas9-Mediated Genome Editing in Apple and Grapevine. Nat. Protoc. 2018, 13, 2844–2863. [Google Scholar] [CrossRef] [PubMed]
- Escudier, J.L. Vins de Qualite? À Teneur Reduite En Alcool; Agence Nationale de la Recherche: Bordeaux, France, 2009; pp. 55–59. [Google Scholar]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A New Version of the Grapevine Reference Genome Assembly (12X.v2) and of Its Annotation (VCost.V3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
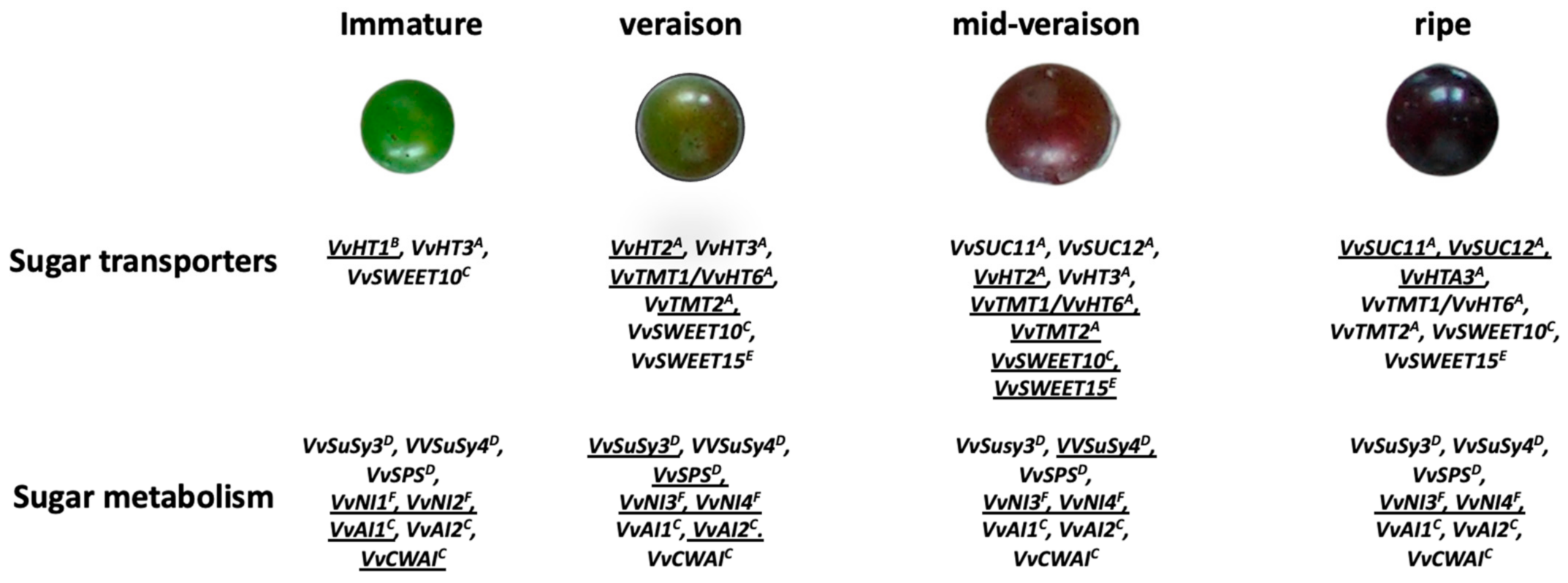
| Stage I–II | ||||
|---|---|---|---|---|
| Sucrose | Fructose | Glucose | References | |
| Grape—Pinot Noir | ||||
| Skin | <1 | 2 | 3 | [21] |
| Flesh | <1 | 1 | 4 | [21] |
| Grape—Muscat Bailey A | ||||
| Skin | <1 | <1 | 3 | [43] |
| Flesh | <1 | <1 | 3 | [43] |
| Grape—Steuben | ||||
| Skin | <1 | <1 | 3 | [43] |
| Flesh | <1 | <1 | 3 | [43] |
| Apricot—common | 2 | 1 | 3 | [44] |
| Apricot—Japanese | 1.3 | 1.1 | 2.0 | [45] |
| Cherry—sweet | ˂1 | 9 | 23 | [46] |
| Peach | 3 | 13 | 14 | [47,48] |
| Plum—Japanese | 7 | 11 | 14 | [49,50] |
| Tomato | ||||
| Hexose accumulator (Solanum lycopersicon) | 0 | 13 | 12 | [51] |
| Sucrose accumulator (Solanum peruvianum) | 2 | 15 | 14 | [51] |
| Stage III-Ripe | ||||
| Grape (Pinot Noir) | ||||
| Skin | 8 | 66 | 72 | [21] |
| Flesh | 9 | 81 | 84 | [21] |
| Grape (Muscat Bailey A) | ||||
| Skin | 17 | 47 | 43 | [43] |
| Flesh | 2 | 50 | 54 | [43] |
| Grape (Steuben) | ||||
| Skin | 41 | 47 | 43 | [43] |
| Flesh | 29 | 63 | 65 | [43] |
| Apricot (common) | 65 | 6 | 18 | [44] |
| Apricot (Japanese) | 9.0 | 0.9 | 0.5 | [45] |
| Cherry (sweet) | ˂1 | 65 | 75 | [46,52] |
| Peach | 48 | 9 | 7 | [47,53] |
| Plum (Japanese) | 92 | 21 | 27 | [49,50] |
| Tomato | ||||
| Hexose accumulator (Solanum lycopersicon) | <2 | 32 | 26 | [51] |
| Sucrose accumulator (Solanum peruvianum) | 73 | 16 | 8 | [51] |
| Grape—mature leaves | ||||
| Riesling × Silvaner | 7 | [54] | ||
| Thompson seedless | 7–10 | 4.5–7 | 5–8 | [55] |
| Stage I–II | ||||
|---|---|---|---|---|
| Sucrose Synthase (Cleavage) | Neutral Invertase | Total Acid Invertase | References | |
| Grape | ||||
| Muscat Bailey A | <0.25 | 180–370 | [43] | |
| Skin | 0.2 | 70–160 | [43] | |
| Flesh | 0.05–2 | 190–230 | [43] | |
| Steuben | <0.2 | <4 | [43] | |
| Skin | 0.2–0.5 | 2.5–5.0 | [43] | |
| Flesh | 0.15–0.2 | 2.3–4.2 | [43] | |
| High sucrose cultivars | 2.5 | 2 | <10 | [94] |
| Thompson seedless | 1–3 | 110–350 | [92] | |
| Asian pear | 2–10 | 8–33 | [95] | |
| Cherry (sweet) | 22 | [96] | ||
| Grapefruit | ||||
| Juice sacs | 15 | 3.9 | 25 | [97] |
| Major vascular bundles | 12 | 2.0 | 49 | [97] |
| Albedo of peel | 4 | 1.6 | 91 | [97] |
| Kiwifruit | 40 | 3 | 8 | [98] |
| Peach | 10–20 | 2–12 | 6–30 | [47,99] |
| Strawberry | 6 | 2.5 | 25 | [100] |
| Tomato | ||||
| Hexose accumulator (Solanum lycopersicon) | 30 | 240 | [101] | |
| Sucrose accumulator (Solanum chmielewskii) | 18 | 4 | [101] | |
| Stage III—Ripe | ||||
| Grape | ||||
| Muscat Bailey A | <0.4 | 310–360 | [43] | |
| Skin | [43] | |||
| Flesh | [43] | |||
| Steuben | <0.5 | <2 | [43] | |
| Skin | [43] | |||
| Flesh | [43] | |||
| High sucrose cultivars | 4 | 10 | 15 | [94] |
| Thompson seedless | 1.3 | 110–350 | [92] | |
| Asian pear | 1–6 | 1–7 | [95] | |
| Cherry (sweet) | 180 | [96] | ||
| Grapefruit | ||||
| Juice sacs | 0.1 | 0.6 | 0.4 | [97] |
| Major vascular bundles | 1.0 | 0.3 | 1.3 | [97] |
| Albedo of peel | 0.1 | 0.5 | 1.9 | [97] |
| Kiwifruit | 4 | 1 | 4 | [98] |
| Peach | 1–14 | 0–1.5 | 2–6 | [47,99] |
| Increased from | 5–25 | <3 | 0 | [100] |
| Large number of genotypes | average | |||
| 3.6 | 1.8 | 1.5 | [102] | |
| range | ||||
| 0–13 | 0–11 | 0–6 | [102] | |
| Strawberry | 6 | 10 | 3 | [100] |
| Tomato | ||||
| Hexose accumulator (Solanum lycopersicon) | 1–3 | 1200 | [101] | |
| Sucrose accumulator (Solanum chmielewskii) | 0.06–2.5 | <0.3 | [101] | |
| Grape young leaves | ||||
| Riesling × Silvaner | 90 | [54] | ||
| Shiraz | 322 | [103] | ||
| Grape mature leaves | ||||
| Riesling × Silvaner | 10 | [54] | ||
| Shiraz | 78 | [103] | ||
| Several cultivars | 1–5 | 2–6 | 5–35 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, R.P.; Bonghi, C.; Varotto, S.; Battistelli, A.; Burbidge, C.A.; Castellarin, S.D.; Chen, Z.-H.; Darriet, P.; Moscatello, S.; Rienth, M.; et al. Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison. Int. J. Mol. Sci. 2021, 22, 7794. https://doi.org/10.3390/ijms22157794
Walker RP, Bonghi C, Varotto S, Battistelli A, Burbidge CA, Castellarin SD, Chen Z-H, Darriet P, Moscatello S, Rienth M, et al. Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison. International Journal of Molecular Sciences. 2021; 22(15):7794. https://doi.org/10.3390/ijms22157794
Chicago/Turabian StyleWalker, Robert P., Claudio Bonghi, Serena Varotto, Alberto Battistelli, Crista A. Burbidge, Simone D. Castellarin, Zhi-Hui Chen, Philippe Darriet, Stefano Moscatello, Markus Rienth, and et al. 2021. "Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison" International Journal of Molecular Sciences 22, no. 15: 7794. https://doi.org/10.3390/ijms22157794
APA StyleWalker, R. P., Bonghi, C., Varotto, S., Battistelli, A., Burbidge, C. A., Castellarin, S. D., Chen, Z.-H., Darriet, P., Moscatello, S., Rienth, M., Sweetman, C., & Famiani, F. (2021). Sucrose Metabolism and Transport in Grapevines, with Emphasis on Berries and Leaves, and Insights Gained from a Cross-Species Comparison. International Journal of Molecular Sciences, 22(15), 7794. https://doi.org/10.3390/ijms22157794









