A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing
Abstract
1. Introduction
2. Results
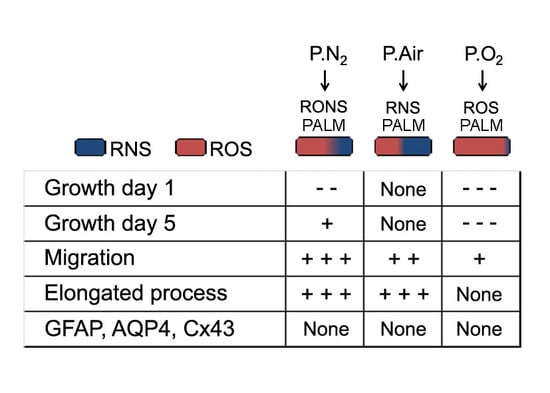
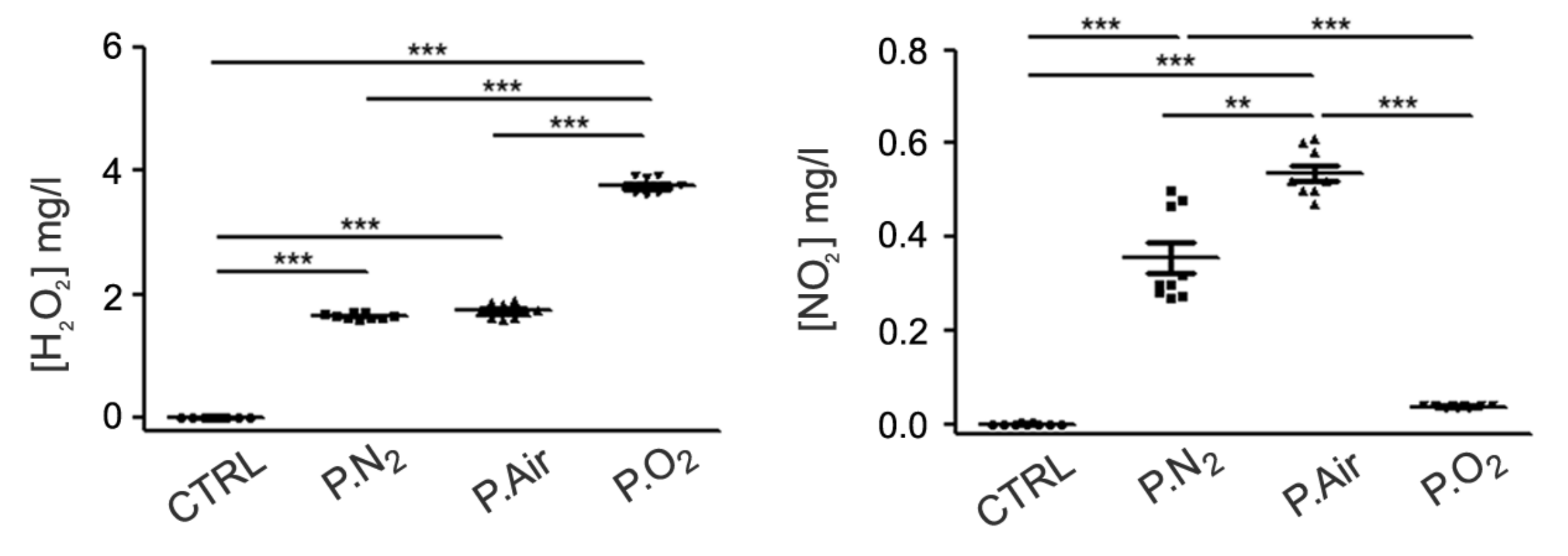
2.1. Different PALM Compositions as a Function of the Gas Feed
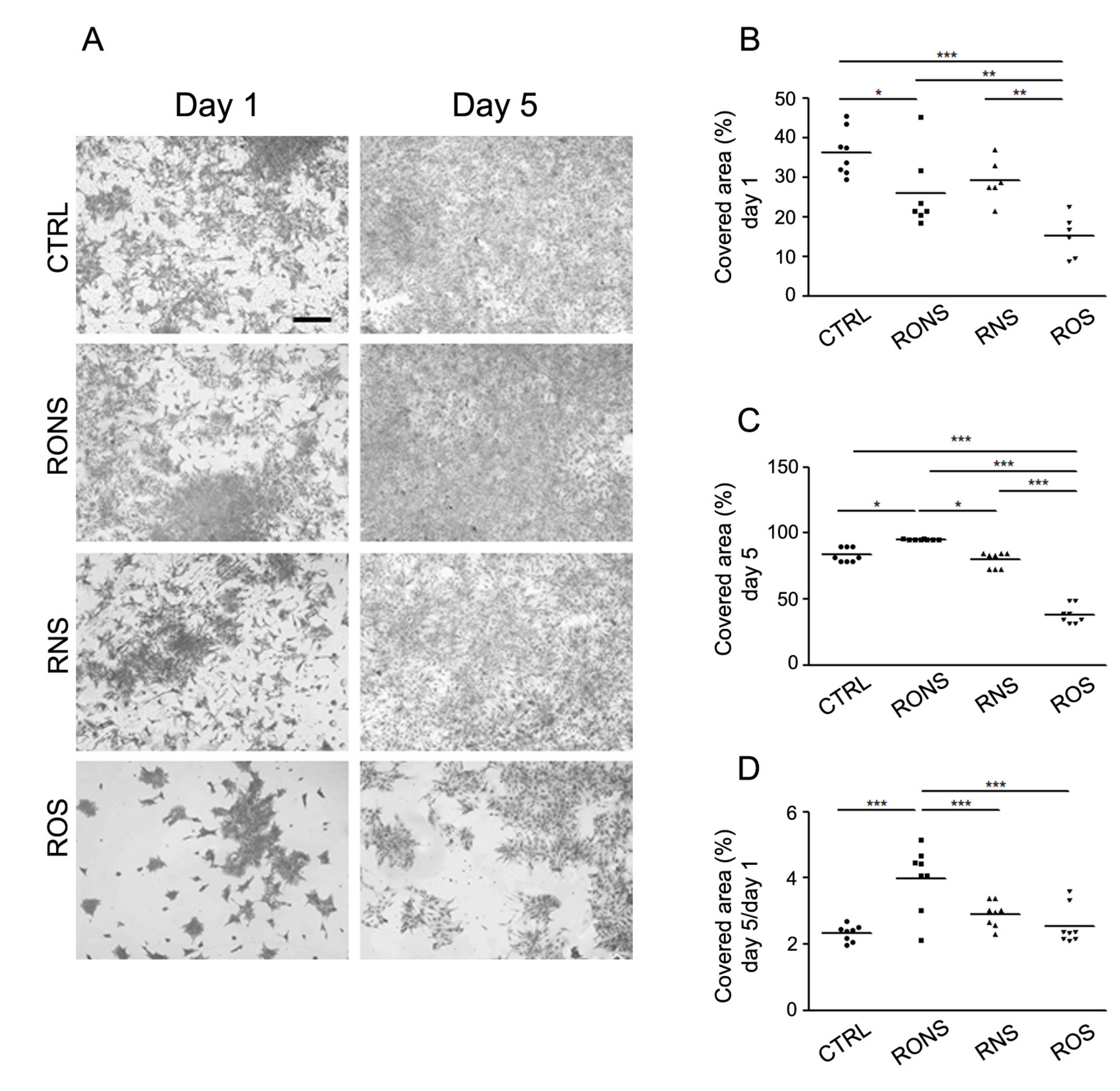
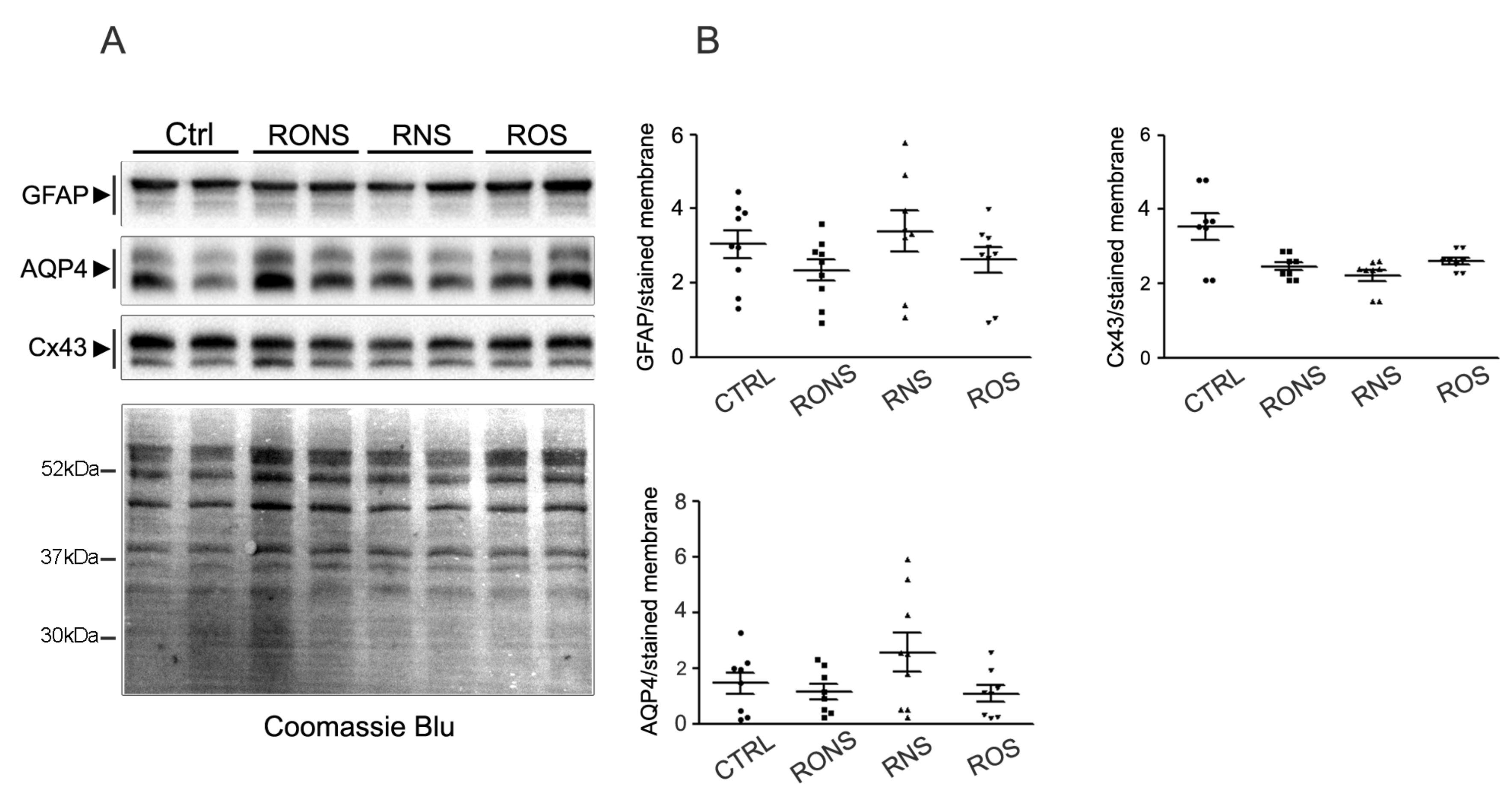
2.2. Effect of PALM Containing Different Ratios of ROS and RNS on Primary Astrocyte Cultures
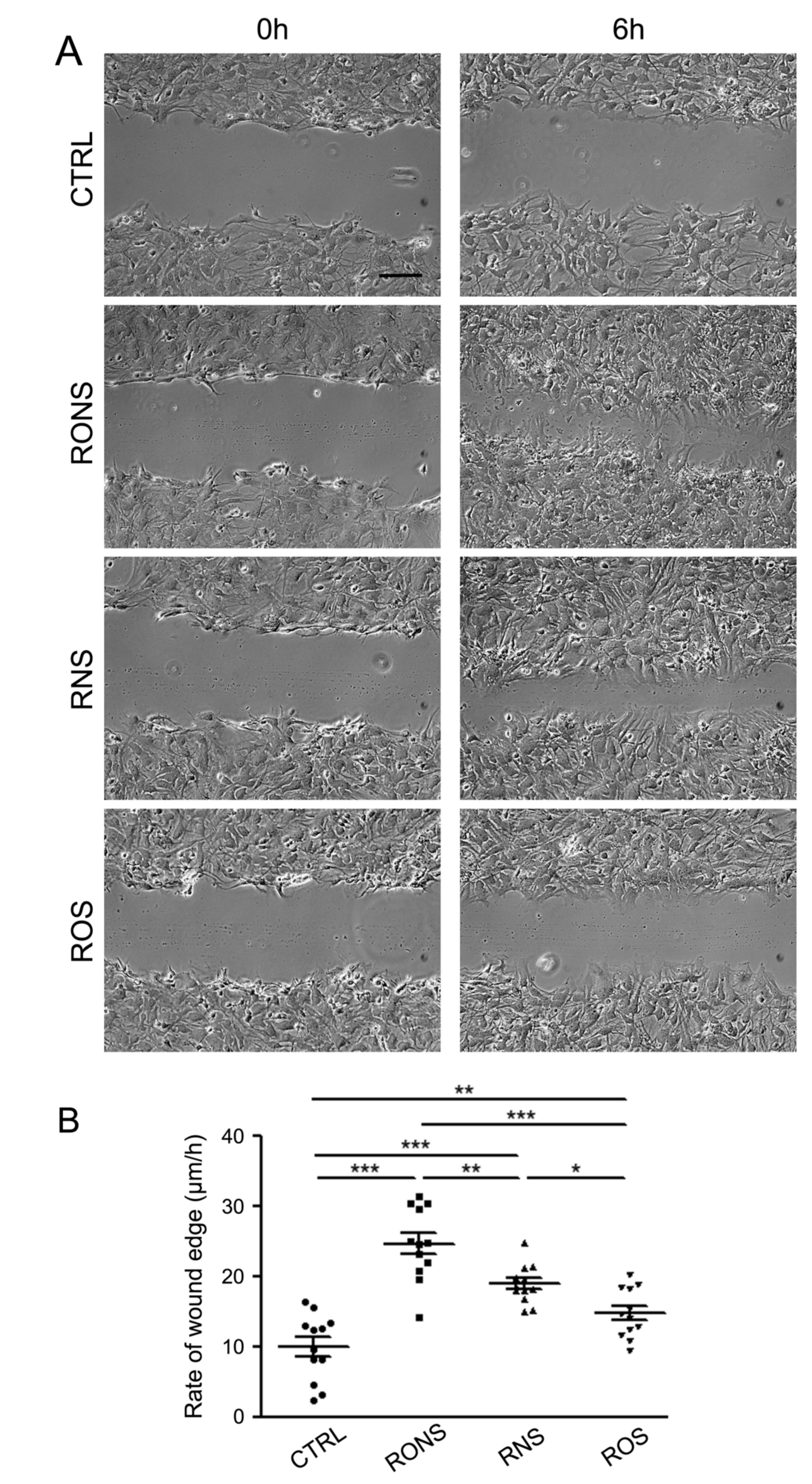
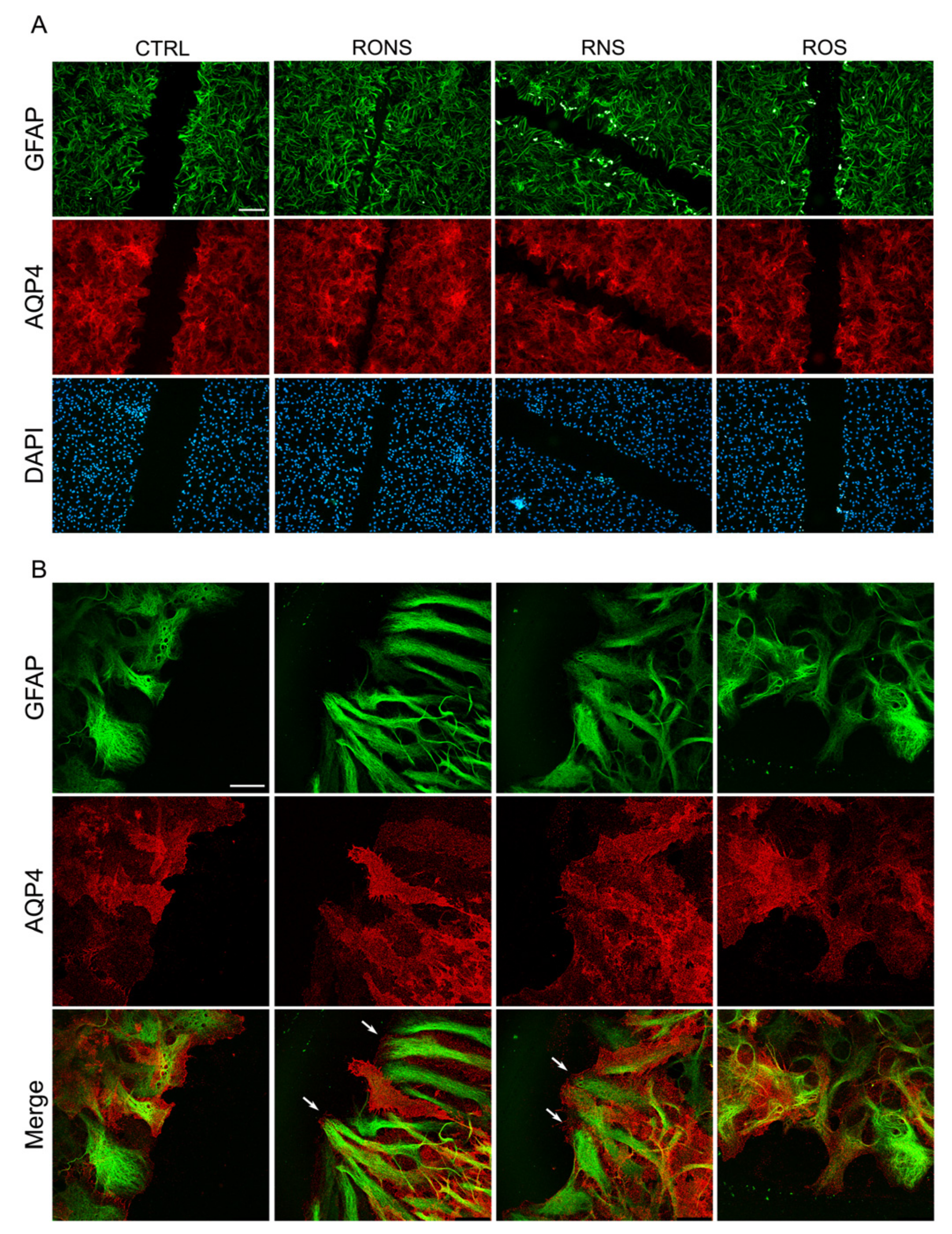
2.3. ROS and RNS Are Able to Improve Astrocyte Migration without Inducing a Gliotic Reaction
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Primary Astrocyte Cultures
4.3. Plasma Source and Plasma Parameters Utilised for the Synthesis of PALM
4.4. H2O2 and NO2− Detection
4.5. PALM Treatment of Primary Astrocyte Cultures
4.6. Coomassie Blue Cell Staining and Surface Area Covered by Cell Calculation
4.7. Scratch-Induced Migration Assay
4.8. SDS-PAGE and Western Blot Analysis
4.9. Antibodies
4.10. Immunofluorescence and Confocal Microscopy Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—more than just brain glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Pisani, F.; Simone, L.; Cibelli, A.; Mola, M.G.; Dal Monte, M.; Frigeri, A.; Bagnoli, P.; Svelto, M. Glio-vascular modifications caused by aquaporin-4 deletion in the mouse retina. Exp. Eye Res. 2016, 146, 259–268. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S. GFAP and astrogliosis. Brain Pathol. 1994, 4, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yamashita, T.; Kumura, E.; Tamatani, M.; Kobayashi, A.; Yokawa, T.; Maruno, M.; Kato, A.; Ohnishi, T.; Kohmura, E.; et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Mol. Brain Res. 2000, 78, 131–137. [Google Scholar] [CrossRef]
- Collignon, F.; Wetjen, N.M.; Cohen-Gadol, A.A.; Cascino, G.D.; Parisi, J.; Meyer, F.B.; Marsh, W.R.; Roche, P.; Weigand, S.D. Altered expression of connexin subtypes in mesial temporal lobe epilepsy in humans. J. Neurosurg. 2006, 105, 77–87. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Feng, A.; Rock, R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013, 38, 2148–2159. [Google Scholar] [CrossRef]
- Schreiner, B.; Romanelli, E.; Liberski, P.; Ingold-Heppner, B.; Sobottka-Brillout, B.; Hartwig, T.; Chandrasekar, V.; Johannssen, H.; Zeilhofer, H.U.; Aguzzi, A.; et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell. Rep. 2015, 12, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Carrillo-de Sauvage, M.-A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Finsterwald, C.; Magistretti, P.J.; Lengacher, S. Astrocytes: New targets for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2015, 21, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An inflammation-centric view of neurological disease: Beyond the neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Sosunov, A.; Olabarria, M.; Goldman, J.E. Alexander disease: An astrocytopathy that produces a leukodystrophy. Brain Pathol. 2018, 28, 388–398. [Google Scholar] [CrossRef]
- Maezawa, I.; Swanberg, S.; Harvey, D.; LaSalle, J.M.; Jin, L.-W. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci. 2009, 29, 5051–5061. [Google Scholar] [CrossRef]
- Meunier, C.; Merienne, N.; Jollé, C.; Déglon, N.; Pellerin, L. Astrocytes are key but indirect contributors to the development of the symptomatology and pathophysiology of Huntington’s disease. Glia 2016, 64, 1841–1856. [Google Scholar] [CrossRef]
- Barbeito, L. Astrocyte-based cell therapy: New hope for amyotrophic lateral sclerosis patients? Stem Cell Res. Ther. 2018, 9, 241. [Google Scholar] [CrossRef]
- Graves, D.B. Low temperature plasma biomedicine : A tutorial review. Phys. Plasm. 2017, 21, 080901. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Wende, K.; Vandana, K.W. A comparison of floating-electrode DBD and kINPen jet : Plasma parameters to achieve similar growth reduction in colon cancer cells under standardized conditions. Plasma Chem. Plasma Process. 2018, 38, 1–12. [Google Scholar]
- Von Woedtke, T.; Metelmann, H.R.; Weltmann, K.D. Clinical plasma medicine: State and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Reuter, S.; Von Woedtke, T.; Weltmann, K.D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- 24 Trizio, I.; Trulli, M.G.; Lo Porto, C.; Pignatelli, D.; Camporeale, G.; Palumbo, F.; Sardella, E.; Gristina, R.; Favia, P. Plasma processes for life sciences. Chem. Mol. Sci. Chem. Eng. 2018. [Google Scholar]
- Fiebrandt, M.; Lackmann, J.W.; Stapelmann, K. From patent to product: 50 years of low-pressure plasma sterilization. Plasma Processes Polym. 2018, 15, e1800139. [Google Scholar] [CrossRef]
- Anderson, C.E.; Cha, N.R.; Lindsay, A.D.; Clark, D.S.; Graves, D.B. The role of interfacial reactions in determining plasma-liquid chemistry. Plasma Chem. Plasma Process. 2016, 36, 1393–1415. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Haertel, B.; Weltmann, K.D.; Lindequist, U. Plasma pharmacy—Physical plasma in pharmaceutical applications. Pharmazie 2013, 68, 492–498. [Google Scholar]
- Katiyar, K.S.; Lin, A.; Fridman, A.; Keating, C.E.; Cullen, D.K.; Miller, V. Non-thermal plasma accelerates astrocyte regrowth and neurite regeneration following physical trauma in vitro. Appl. Sci. 2019, 9, 3747. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold atmospheric plasma and plasma-activated medium trigger RONS-based tumor cell apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef]
- Haskew-Layton, R.E.; Payappilly, J.B.; Smirnova, N.A.; Ma, T.C.; Chan, K.K.; Murphy, T.H.; Guo, H.; Langley, B.; Sultana, R.; Butterfield, D.A.; et al. Controlled Enzymatic Production of Astrocytic Hydrogen Peroxide Protects Neurons From Oxidative Stress via an Nrf2-independent Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 17385–17390. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Graves, D.B.; Chang, H.; Shimizu, T.; Morfill, G. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Hammer, M.U.; Jablonowski, H.; von Woedtke, T.; Reuter, S.; Machala, Z. Fluorescence measurements of peroxynitrite/peroxynitrous acid in cold air plasma treated aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 8883–8896. [Google Scholar] [CrossRef] [PubMed]
- Te Boekhorst, V.; Preziosi, L.; Friedl, P. Plasticity of cell migration in vivo and in silico. Annu. Rev. Cell. Dev. Biol. 2016, 32, 491–526. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Wang, H.H.; Wu, W.B.; Chu, P.J.; Yang, C.M. Transforming growth factor-β1 induces matrix metalloproteinase-9 and cell migration in astrocytes: Roles of ROS-dependent ERK- and JNK-NF-κB pathways. J. Neuroinflamm. 2010, 7, 88. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma activated medium as anticancer tool and immunogenic cell death inducer in metastatic melanoma and pancreatic cancer models. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, S.; Mao, X.; Lu, X.; He, G.; Yang, G.; Chen, M.; Ishaq, M.; Ostrikov, K. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Res. 2014, 12, 387–399. [Google Scholar] [CrossRef]
- Omar, S.A.; Artime, E.; Webb, A.J. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide 2012, 26, 229–240. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Processes Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Gupta, S.; Joshi, N.; Sharma, S.; Singh, S. Astrocyte activation and neurotoxicity: A study in different rat brain regions and in rat C6 astroglial cells. Environ. Toxicol. Pharmacol. 2015, 40, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Judée, F.; Fongia, C.; Ducommun, B.; Yousfi, M. Short and long time effects of low temperature plasma activated media on 3D multicellular tumor spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar]
- Mola, M.G.; Sparaneo, A.; Gargano, C.D.; Spray, D.C.; Svelto, M.; Frigeri, A.; Scemes, E.; Nicchia, G.P. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins. Glia 2016, 64, 139–154. [Google Scholar] [CrossRef]
- De Bellis, M.; Pisani, F.; Mola, M.G.; Basco, D.; Catalano, F.; Nicchia, G.P.; Svelto, M.; Frigeri, A. A novel human aquaporin-4 splice variant exhibits a dominant-negative activity: A new mechanism to regulate water permeability. Mol. Biol. Cell 2014, 25, 470–480. [Google Scholar] [CrossRef]
| PALM | Gas | Flow Rate(slm) | V (kV) | Distance (mm) | Period (DC%) | Frequency (kHz) | Treatment Time (s) | Dissipated Energy (J) |
|---|---|---|---|---|---|---|---|---|
| P.O2 | O2 | 0.5 | 13 | 3 | 100 (25) | 6 | 30 | 51 ± 2 |
| P.Air | Air | 0.5 | 13 | 3 | 100 (25) | 6 | 30 | 46 ± 5 |
| P.N2 | N2 | 0.5 | 13 | 3 | 100 (25) | 6 | 30 | 43 ± 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardella, E.; Mola, M.G.; Gristina, R.; Piccione, M.; Veronico, V.; De Bellis, M.; Cibelli, A.; Buttiglione, M.; Armenise, V.; Favia, P.; et al. A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing. Int. J. Mol. Sci. 2020, 21, 3343. https://doi.org/10.3390/ijms21093343
Sardella E, Mola MG, Gristina R, Piccione M, Veronico V, De Bellis M, Cibelli A, Buttiglione M, Armenise V, Favia P, et al. A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing. International Journal of Molecular Sciences. 2020; 21(9):3343. https://doi.org/10.3390/ijms21093343
Chicago/Turabian StyleSardella, Eloisa, Maria Grazia Mola, Roberto Gristina, Monica Piccione, Valeria Veronico, Manuela De Bellis, Antonio Cibelli, Maura Buttiglione, Vincenza Armenise, Pietro Favia, and et al. 2020. "A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing" International Journal of Molecular Sciences 21, no. 9: 3343. https://doi.org/10.3390/ijms21093343
APA StyleSardella, E., Mola, M. G., Gristina, R., Piccione, M., Veronico, V., De Bellis, M., Cibelli, A., Buttiglione, M., Armenise, V., Favia, P., & Nicchia, G. P. (2020). A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing. International Journal of Molecular Sciences, 21(9), 3343. https://doi.org/10.3390/ijms21093343