De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function
Abstract
:1. Introduction
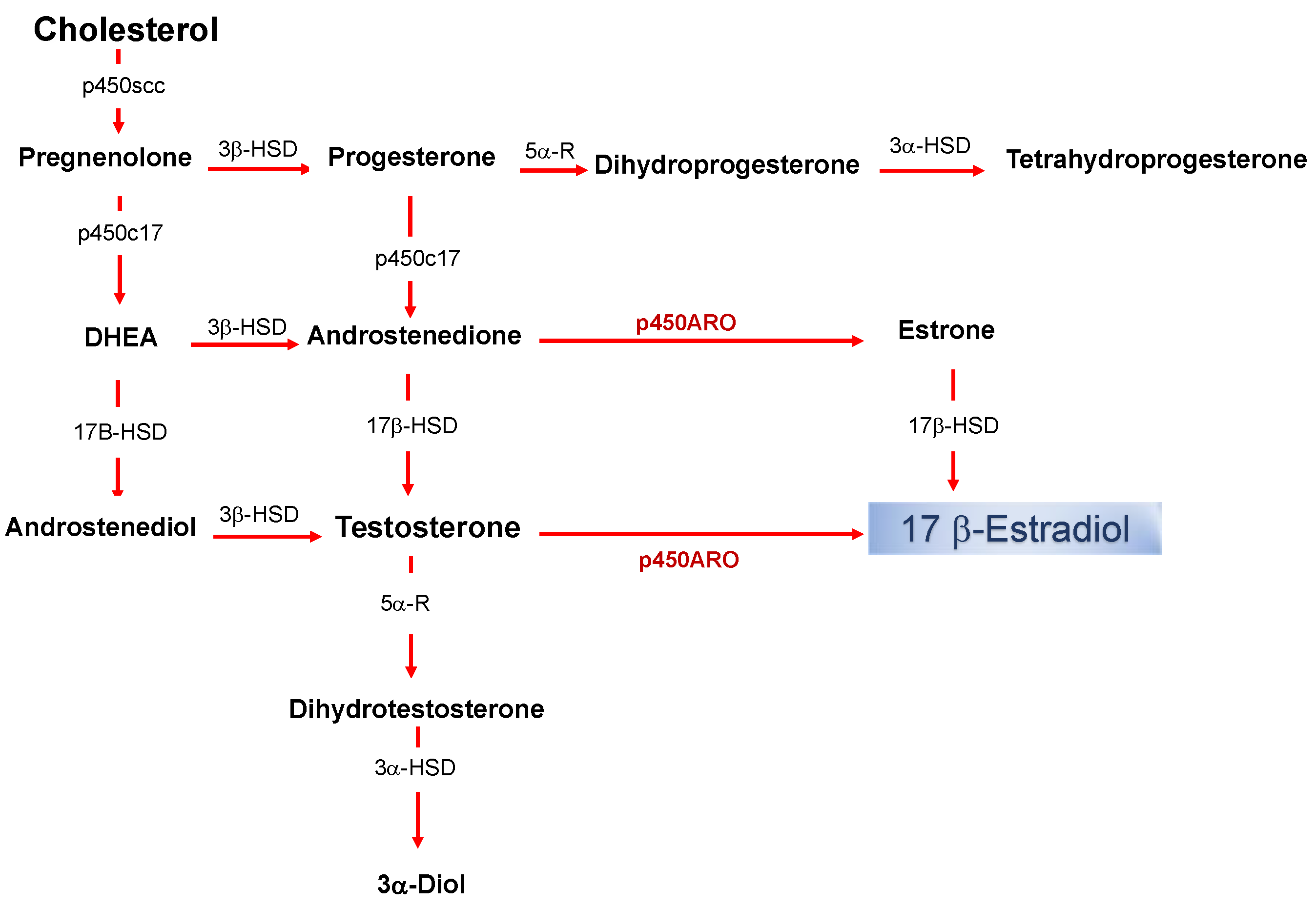
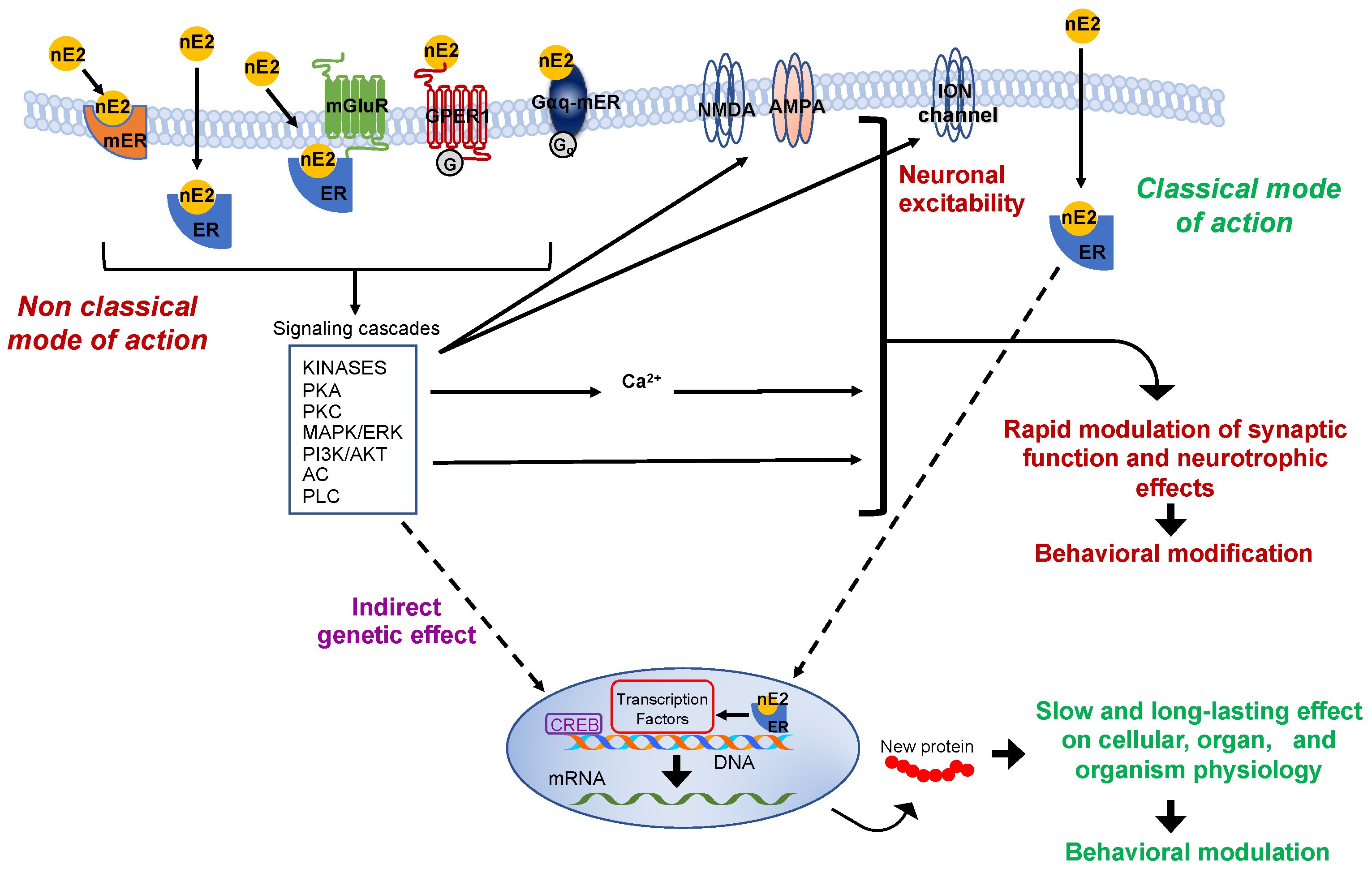
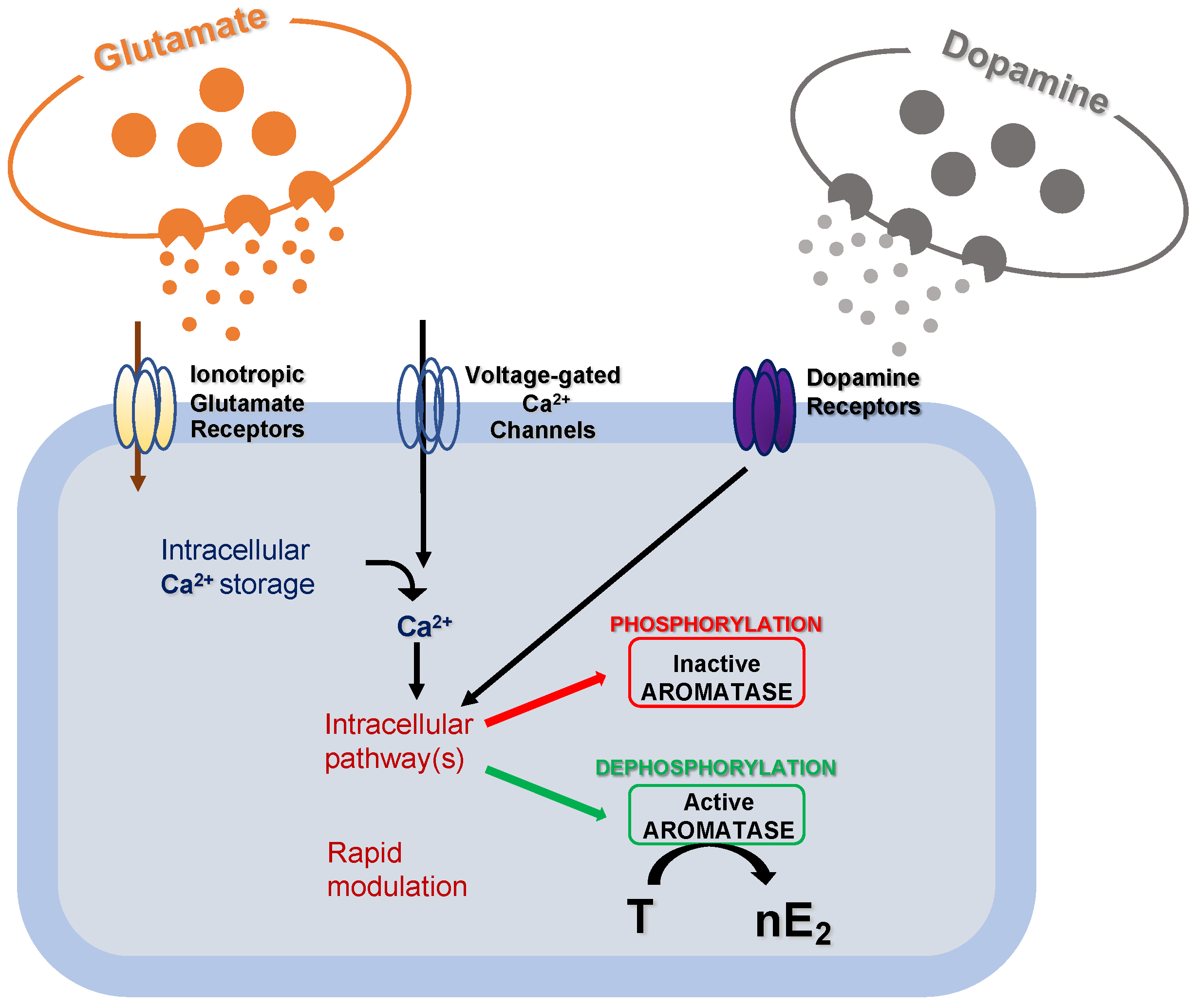
2. The Effectiveness of the De Novo Synthesized Estradiol in Rapidly Influencing the Neuronal Functioning
3. Significance of the nE2 Influence upon Cerebellar Functioning
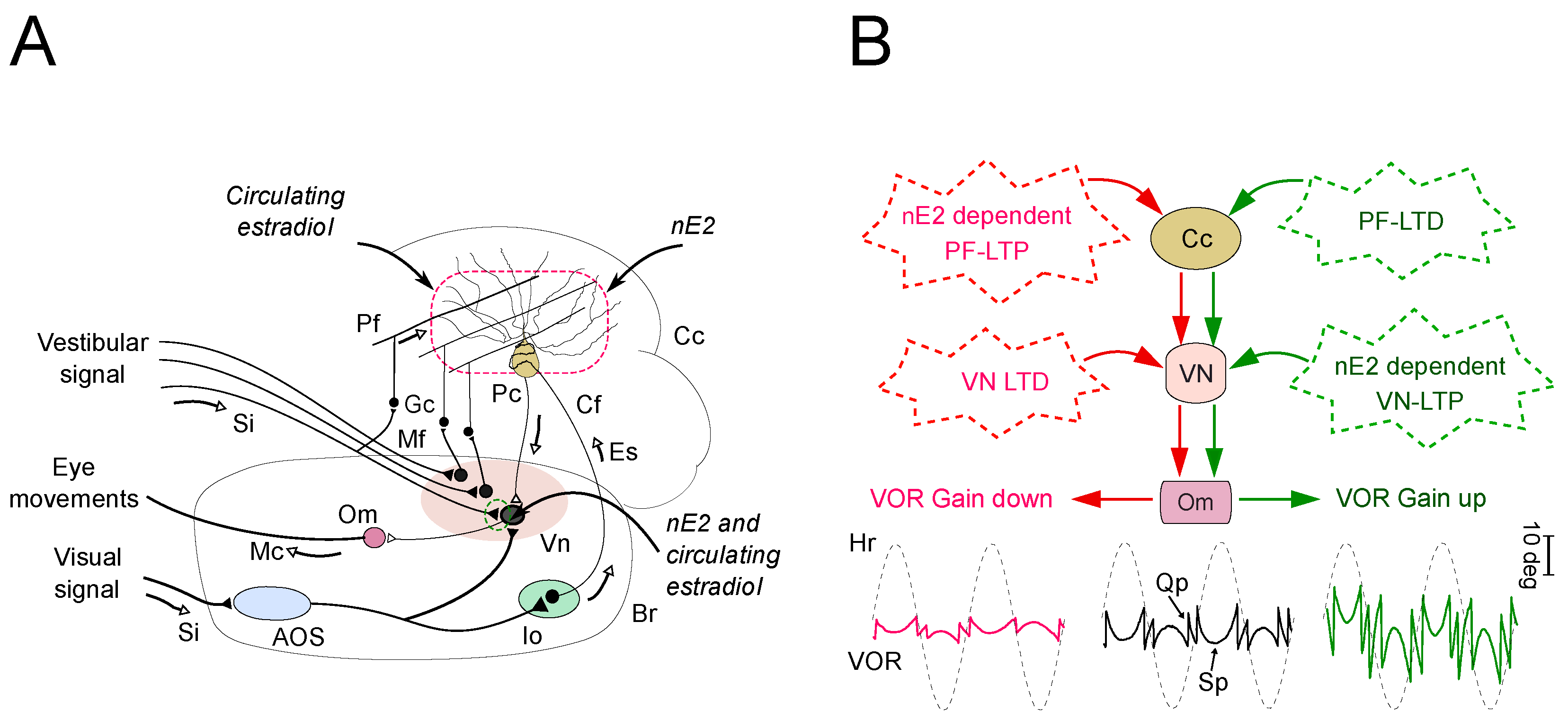
4. The Impact of nE2 upon Cerebellar Functioning in Adult Rodents: Multiple Lines of Evidence
4.1. Implications for the Impact of nE2 on the Cerebellar Function
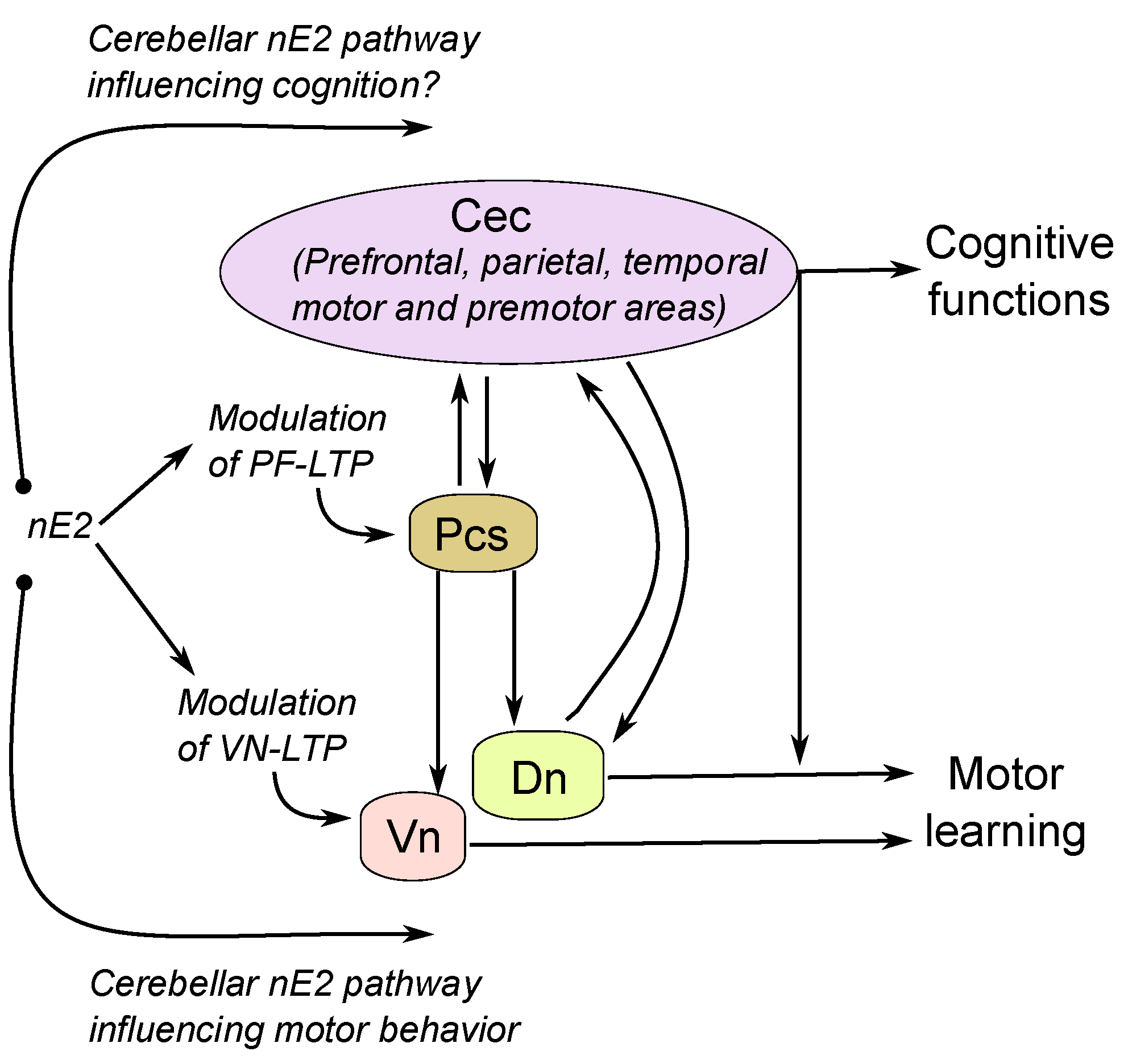
4.2. The Possible Relationship between Cerebellar nE2 Pathway and Other Brain Structures: Implication for Health
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| nE2 | estradiol synthesized in the nervous system |
| ERs | estrogen receptors |
| ERα | estrogen receptors isoform α |
| ERβ | estrogen receptors isoform β |
| GPCRs | G-protein-coupled receptors |
| GPER-1 | estrogen-G-protein-coupled receptors-1 |
| Gαq-mER | Gαq-membrane estrogen receptor |
| ER-X | estrogen receptor-X |
| GABA | Gamma aminobutyric acid |
| VOR | vestibulo-ocular reflex |
| PCs | Purkinje cells |
| BDNF | brain-derived neurotrophic factor; mGluR1a: metabotropic glutamate receptors type 1a |
| PF-LTP | long term potentiation at the parallel fiber-Purkinje cell synapse |
| PF-LTD | long term depression at the parallel fiber-Purkinje cell synapse |
| LTZ | letrozole |
| ICI 182,780 | estrogen receptor antagonist |
| NMDAR | N-methyl-D-aspartate receptor |
| LTP | long term potentiation |
| LTD | long term depression |
References
- Cornil, C.A.; Ball, G.F.; Balthazart, J. Rapid control of male typical behaviors by brain-derived estrogens. Front. Neuroendocr. 2012, 33, 425–446. [Google Scholar] [CrossRef] [Green Version]
- Balthazart, J.; Baillien, M.; Cornil, C.A.; Ball, G.F. Preoptic aromatase modulates male sexual behavior: Slow and fast mechanisms of action. Physiol. Behav. 2004, 83, 247–270. [Google Scholar] [CrossRef]
- Hojo, Y.; Murakami, G.; Mukai, H.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M.; Ishii, H.; Kimoto, T.; Kawato, S. Estrogen synthesis in the brain—Role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 2008, 290, 31–43. [Google Scholar] [CrossRef]
- Woolley, C.S. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 657–680. [Google Scholar] [CrossRef]
- Ervin, K.S.; Phan, A.; Gabor, C.S.; Choleris, E. Rapid oestrogenic regulation of social and nonsocial learning. J. Neuroendocr. 2013, 25, 1116–1132. [Google Scholar] [CrossRef] [Green Version]
- Cooke, B.M.; Woolley, C.S. Gonadal hormone modulation of dendrites in the mammalian CNS. J. Neurobiol. 2005, 64, 34–46. [Google Scholar] [CrossRef]
- McEwen, B.S.; Alves, S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999, 20, 279–307. [Google Scholar] [CrossRef]
- Konkle, A.T.M.; McCarthy, M.M. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 2011, 152, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Hojo, Y.; Hattori, T.A.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.T.; Mukai, H.; Morrison, J.H.; Janssen, W.G.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Baulieu, E.E. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997, 52, 1–32. [Google Scholar]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front. Neuroendocr. 2000, 21, 1–56. [Google Scholar] [CrossRef]
- Kimoto, T.; Tsurugizawa, T.; Ohta, Y.; Makino, J.; Tamura, H.; Hojo, Y.; Takata, N.; Kawato, S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology 2001, 142, 3578–3589. [Google Scholar] [CrossRef]
- Cornil, C.A.; Ball, G.F.; Balthazart, J. The dual action of estrogen hypothesis. Trends Neurosci. 2015, 38, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S.; Shay, D.A.; Vieira-Potter, V.J. Cognitive effects of aromatase and possible role in memory disorders. Front. Endocrinol. (Lausanne) 2018, 9, 610. [Google Scholar] [CrossRef]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Yagi, S.; Chow, C.; Galea, L.A. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm. Behav. 2015, 74, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Clayton, D.F. The genomic action potential. Neurobiol. Learn. Mem. 2000, 74, 185–216. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Sebastian, K.S.; Devassy, S.; Priyadarsini, L.; Farook, M.F.; Shameem, A.; Mathew, D.; Sreeja, S.; Thampan, R.V. Membrane estrogen receptors: Genomic actions and post transcriptional regulation. Mol. Cell. Endocrinol. 2006, 246, 34–41. [Google Scholar] [CrossRef]
- Vasudevan, N.; Pfaff, D.W. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocr. 2008, 29, 238–257. [Google Scholar] [CrossRef]
- Balthazart, J.; Choleris, E.; Remage-Healey, L. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav. 2018, 99, 1–8. [Google Scholar] [CrossRef]
- Rudolph, L.M.; Cornil, C.A.; Mittelman-Smith, M.A.; Rainville, J.R.; Remage-Healey, L.; Sinchak, K.; Micevych, P.E. Actions of steroids: New neurotransmitters. J. Neurosci. 2016, 36, 11449–11458. [Google Scholar] [CrossRef] [Green Version]
- Ronnekleiv, O.K.; Kelly, M.J. Membrane-initiated effects of estradiol in the central nervous system. In Hormones, Brain and Behavior; Pfaff, D.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar]
- Oberlander, J.G.; Woolley, C.S. 17beta-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 2016, 36, 2677–2690. [Google Scholar] [CrossRef]
- Kelly, M.J.; Moss, R.L.; Dudley, C.A. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976, 114, 152–157. [Google Scholar] [CrossRef]
- Mittelman-Smith, M.A.; Rudolph, L.M.; Mohr, M.A.; Micevych, P.E. Rodent models of non-classical progesterone action regulating ovulation. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.J.; Su, L.D.; Wang, Y.N.; Yang, D.; Sun, C.L.; Zhou, L.; Wang, X.X.; Shen, Y. Long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses requires presynaptic and postsynaptic signaling cascades. J. Neurosci. 2014, 34, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Morissette, M.; Le Saux, M.; D’Astous, M.; Jourdain, S.; Al Sweidi, S.; Morin, N.; Estrada-Camarena, E.; Mendez, P.; Garcia-Segura, L.M.; Di Paolo, T.; et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J. Steroid Biochem. Mol. Biol. 2008, 108, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Kuo, J.; Hamid, N.; Bondar, G.; Prossnitz, E.R.; Micevych, P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J. Neurosci. 2010, 30, 12950–12957. [Google Scholar] [CrossRef]
- Hazell, G.G.; Yao, S.T.; Roper, J.A.; Prossnitz, E.R.; O’Carroll, A.M.; Lolait, S.J. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009, 202, 223–236. [Google Scholar] [CrossRef]
- Levin, E.R. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2009, 20, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Toran-Allerand, C.D.; Guan, X.; MacLusky, N.J.; Horvath, T.L.; Diano, S.; Singh, M.; Connolly, E.S., Jr.; Nethrapalli, I.S.; Tinnikov, A.A. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002, 22, 8391–8401. [Google Scholar] [CrossRef]
- Toran-Allerand, C.D. Estrogen and the brain—Beyond ER-alpha, ER-beta, and 17 beta-estradiol. Ann. N. Y. Acad. Sci. 2005, 1052, 136–144. [Google Scholar] [CrossRef]
- Qiu, J.; Ronnekleiv, O.K.; Kelly, M.J. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids 2008, 73, 985–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razandi, M.; Pedram, A.; Greene, G.L.; Levin, E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ER alpha and ER beta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999, 13, 307–319. [Google Scholar] [CrossRef]
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142. [Google Scholar]
- Levin, E.R.; Hammes, S.R. Nuclear receptors outside the nucleus: Extranuclear signalling by steroid receptors. Nat. Rev. Mol. Cell Biol. 2016, 17, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Melcangi, R.C.; Panzica, G.; Garcia-Segura, L.M. Neuroactive steroids: Focus on human brain. Neuroscience 2011, 191, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Charlier, T.D.; Cornil, C.A.; Patte-Mensah, C.; Meyer, L.; Mensah-Nyagan, A.G.; Balthazart, J. Local modulation of steroid action: Rapid control of enzymatic activity. Front. Neurosci. 2015, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Hedges, V.L.; Chen, G.; Yu, L.; Krentzel, A.A.; Starrett, J.R.; Zhu, J.N.; Suntharalingam, P.; Remage-Healey, L.; Wang, J.J.; Ebner, T.J.; et al. Local estrogen synthesis regulates parallel fiber-purkinje cell neurotransmission within the cerebellar cortex. Endocrinology 2018, 159, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Dieni, C.V.; Ferraresi, A.; Sullivan, J.A.; Grassi, S.; Pettorossi, V.E.; Panichi, R. Acute inhibition of estradiol synthesis impacts vestibulo-ocular reflex adaptation and cerebellar long-term potentiation in male rats. Brain Struct. Funct. 2018, 223, 837–850. [Google Scholar] [CrossRef] [Green Version]
- Grassi, S.; Frondaroli, A.; Dieni, C.; Scarduzio, M.; Pettorossi, V.E. Long-term potentiation in the rat medial vestibular nuclei depends on locally synthesized 17beta-estradiol. J. Neurosci. 2009, 29, 10779–10783. [Google Scholar] [CrossRef] [Green Version]
- Scarduzio, M.; Panichi, R.; Pettorossi, V.E.; Grassi, S. Synaptic long-term potentiation and depression in the rat medial vestibular nuclei depend on neural activation of estrogenic and androgenic signals. PLoS ONE 2013, 8, e80792. [Google Scholar] [CrossRef]
- Chen, B.S.; Roche, K.W. Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007, 53, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Hojo, Y.; Higo, S.; Ishii, H.; Ooishi, Y.; Mukai, H.; Murakami, G.; Kominami, T.; Kimoto, T.; Honma, S.; Poirier, D.; et al. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 2009, 150, 5106–5112. [Google Scholar] [CrossRef]
- Mukai, H.; Kimoto, T.; Hojo, Y.; Kawato, S.; Murakami, G.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim. Biophys. Acta 2010, 1800, 1030–1044. [Google Scholar] [CrossRef]
- Cornil, C.A.; Ball, G.F.; Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006, 1126, 2–26. [Google Scholar] [CrossRef] [Green Version]
- Storman, E.M.; Liu, N.J.; Wessendorf, M.W.; Gintzler, A.R. Physical linkage of estrogen receptor alpha and aromatase in rat: Oligocrine and endocrine actions of CNS-produced estrogens. Endocrinology 2018, 159, 2683–2697. [Google Scholar] [CrossRef]
- Kretz, O.; Fester, L.; Wehrenberg, U.; Zhou, L.; Brauckmann, S.; Zhao, S.; Prange-Kiel, J.; Naumann, T.; Jarry, H.; Frotscher, M.; et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 2004, 24, 5913–5921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.J.; Makeyeva, Y.V.; Paitel, E.R.; Pedersen, A.L.; Hon, A.T.; Gunderson, J.A.; Saldanha, C.J. Hippocampal aromatization modulates spatial memory and characteristics of the synaptic membrane in the male zebra finch. Endocrinology 2017, 158, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Azcoitia, I.; Arevalo, M.A.; Garcia-Segura, L.M. Neural-derived estradiol regulates brain plasticity. J. Chem. Neuroanat. 2018, 89, 53–59. [Google Scholar] [CrossRef]
- Sellers, K.J.; Erli, F.; Raval, P.; Watson, I.A.; Chen, D.; Srivastava, D.P. Rapid modulation of synaptogenesis and spinogenesis by 17beta-estradiol in primary cortical neurons. Front. Cell. Neurosci. 2015, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Brocca, M.E.; Garcia-Segura, L.M. Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell. Mol. Neurobiol. 2019, 39, 473–481. [Google Scholar] [CrossRef]
- Tremere, L.A.; Jeong, J.K.; Pinaud, R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J. Neurosci. 2009, 29, 5949–5963. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Di Mauro, M.; Scarduzio, M.; Panichi, R.; Tozzi, A.; Calabresi, P.; Grassi, S. Modulatory role of androgenic and estrogenic neurosteroids in determining the direction of synaptic plasticity in the CA1 hippocampal region of male rats. Physiol. Rep. 2013, 1, e00185. [Google Scholar] [CrossRef]
- Tozzi, A.; de Iure, A.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Giampa, C.; Di Mauro, M.; Mazzocchetti, P.; Costa, C.; Di Filippo, M.; et al. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: Interaction with the dopaminergic system. Front. Cell. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, M.; Tozzi, A.; Calabresi, P.; Pettorossi, V.E.; Grassi, S. Neo-synthesis of estrogenic or androgenic neurosteroids determine whether long-term potentiation or depression is induced in hippocampus of male rat. Front. Cell. Neurosci. 2015, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Mermelstein, P.G.; Becker, J.B.; Surmeier, D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996, 16, 595–604. [Google Scholar] [CrossRef]
- Li, J.; Siegel, M.; Yuan, M.K.; Zeng, Z.Y.; Finnucan, L.; Persky, R.; Hurn, P.D.; McCullough, L.D. Estrogen enhances neurogenesis and behavioral recovery after stroke. J. Cereb. Blood Flow Metab. 2011, 31, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Fester, L.; von Blittersdorff, B.; Hassu, B.; Nogens, H.; Prange-Kiel, J.; Jarry, H.; Wegscheider, K.; Rune, G.M. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 2010, 151, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Roselli, C.E.; Horton, L.E.; Resko, J.A. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology 1985, 117, 2471–2477. [Google Scholar] [CrossRef]
- Lymer, J.M.; Sheppard, P.A.S.; Kuun, T.; Blackman, A.; Jani, N.; Mahbub, S.; Choleris, E. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 2018, 89, 30–38. [Google Scholar] [CrossRef]
- Li, J.; Gibbs, R.B. Detection of estradiol in rat brain tissues: Contribution of local versus systemic production. Psychoneuroendocrinology 2019, 102, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Yanike, M.; Frank, L.M.; Smith, A.C.; Brown, E.N.; Suzuki, W.A. Single neurons in the monkey hippocampus and learning of new associations. Science 2003, 300, 1578–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Xu, D.; Ashe, J.; Bushara, K. Specificity of inferior olive response to stimulus timing. J. Neurophysiol. 2008, 100, 1557–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeber, S.L.; White, J.J.; George-Jones, N.A.; Sillitoe, R.V. Architecture and development of olivocerebellar circuit topography. Front. Neural Circuits 2012, 6, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatadze, N.; Sato, S.M.; Woolley, C.S. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS ONE 2014, 9, e100628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seredynski, A.L.; Balthazart, J.; Christophe, V.J.; Ball, G.F.; Cornil, C.A. Neuroestrogens rapidly regulate sexual motivation but not performance. J. Neurosci. 2013, 33, 164–174. [Google Scholar] [CrossRef] [Green Version]
- De Groof, G.; Balthazart, J.; Cornil, C.A.; Van der Linden, A. Topography and lateralized effect of acute aromatase inhibition on auditory processing in a seasonal songbird. J. Neurosci. 2017, 37, 4243–4254. [Google Scholar] [CrossRef]
- Cornil, C.A.; Taziaux, M.; Baillien, M.; Ball, G.F.; Balthazart, J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav. 2006, 49, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Sierra, A.; Azcoitia, I.; Garcia-Segura, L. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine 2003, 21, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.G.; Wang, R.M.; Tang, H.; Dong, Y.; Chan, A.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol. 2014, 389, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Luine, V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. J. Steroid Biochem. Mol. Biol. 2016, 160, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid actions in memory and neurologic/neuropsychiatric disorders. Front. Endocrinol. (Lausanne) 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Dieni, C.V.; Sullivan, J.A.; Faralli, M.; Contemori, S.; Biscarini, A.; Pettorossi, V.E.; Panichi, R. 17 beta-estradiol synthesis modulates cerebellar dependent motor memory formation in adult male rats. Neurobiol. Learn. Mem. 2018, 155, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.M.; Milad, M.R. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn. Mem. 2014, 21, 347–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remage-Healey, L.; Dong, S.; Maidment, N.T.; Schlinger, B.A. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci. 2011, 31, 10034–10038. [Google Scholar] [CrossRef] [Green Version]
- Engler-Chiurazzi, E.B.; Brown, C.M.; Povroznik, J.M.; Simpkins, J.W. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog. Neurobiol. 2017, 157, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [Green Version]
- Biegon, A.; Kim, S.W.; Alexoff, D.L.; Jayne, M.; Carter, P.; Hubbard, B.; King, P.; Logan, J.; Muench, L.; Pareto, D.; et al. Unique distribution of aromatase in the human brain: In vivo studies with PET and [N-methyl-11C]vorozole. Synapse 2010, 64, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Azcoitia, I.; Yague, J.G.; Garcia-Segura, L.M. Estradiol synthesis within the human brain. Neuroscience 2011, 191, 139–147. [Google Scholar] [CrossRef]
- Lavaque, E.; Mayen, A.; Azcoitia, I.; Tena-Sempere, M.; Garcia-Segura, L.M. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J. Neurobiol. 2006, 66, 308–318. [Google Scholar] [CrossRef]
- Roselli, C.E.; Ellinwood, W.E.; Resko, J.A. Regulation of brain aromatase activity in rats. Endocrinology 1984, 114, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Munetomo, A.; Hojo, Y.; Higo, S.; Kato, A.; Yoshida, K.; Shirasawa, T.; Shimizu, T.; Barron, A.; Kimoto, T.; Kawato, S.; et al. Aging-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in the cortex, hypothalamus and cerebellum. J. Physiol. Sci. 2015, 65, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Mezaki, Y.; Shikimi, H.; Ukena, K.; Tsutsui, K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology 2003, 144, 4466–4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, K. Neurosteroids in the Purkinje cell: Biosynthesis, mode of action and functional significance. Mol. Neurobiol. 2008, 37, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K. Neurosteroid biosynthesis and action during cerebellar development. Cerebellum 2012, 11, 414–415. [Google Scholar] [CrossRef]
- Hedges, V.L.; Ebner, T.J.; Meisel, R.L.; Mermelstein, P.G. The cerebellum as a target for estrogen action. Front. Neuroendocr. 2012, 33, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Nagai, A. Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Res. 2006, 1083, 39–49. [Google Scholar] [CrossRef]
- Mitra, S.W. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2844. [Google Scholar] [CrossRef]
- Price, R.H.; Handa, R.J. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci. Lett. 2000, 288, 115–118. [Google Scholar] [CrossRef]
- Grassi, S.; Scarduzio, M.; Panichi, R.; Dall’Aglio, C.; Boiti, C.; Pettorossi, V.E. Opposite long-term synaptic effects of 17beta-estradiol and 5alpha-dihydrotestosterone and localization of their receptors in the medial vestibular nucleus of rats. Brain Res. Bull. 2013, 97, 1–7. [Google Scholar] [CrossRef]
- Grassi, S.; Frondaroli, A.; Scarduzio, M.; Dutia, M.B.; Dieni, C.; Pettorossi, V.E. Effects of 17beta-estradiol on glutamate synaptic transmission and neuronal excitability in the rat medial vestibular nuclei. Neuroscience 2010, 165, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.L.; Wikler, K.C. Aromatase in developing sensory systems of the rat brain. J. Neuroendocr. 1999, 11, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; McCarthy, M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, J.M.; Witt, D.M.; Rissman, E.F. Sex differences in the cerebellum and frontal cortex: Roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology 2011, 93, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Biamonte, F.; Assenza, G.; Marino, R.; D’Amelio, M.; Panteri, R.; Caruso, D.; Scurati, S.; Yague, J.G.; Garcia-Segura, L.M.; Cesa, R.; et al. Interactions between neuroactive steroids and reelin haploinsufficiency in Purkinje cell survival. Neurobiol. Dis. 2009, 36, 103–115. [Google Scholar] [CrossRef]
- Andreescu, C.E.; Milojkovic, B.A.; Haasdijk, E.D.; Kramer, P.; De Jong, F.H.; Krust, A.; De Zeeuw, C.I.; De Jeu, M.T. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J. Neurosci. 2007, 27, 10832–10839. [Google Scholar] [CrossRef]
- Yamada, N.M.; Hirata, S.; Kato, J. Distribution and postnatal changes of aromatase mRNA in the female rat brain. J. Steroid Biochem. Mol. Biol. 1994, 48, 529–533. [Google Scholar] [CrossRef]
- Jakab, R.L.; Wong, J.K.; Belcher, S.M. Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J. Comp. Neurol. 2001, 430, 396–409. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Abdel-Rahman, A.A. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur. J. Endocrinol. 2000, 142, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Cai, W.Q.; Zhou, D.S.; Su, B.Y. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002, 935, 73–80. [Google Scholar] [CrossRef]
- Cardona-Gomez, G.P.; DonCarlos, L.; Garcia-Segura, L.M. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience 2000, 99, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Perez, S.E.; Chen, E.Y.; Mufson, E.J. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res. Dev. Brain Res. 2003, 145, 117–139. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Merchenthaler, I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001, 436, 64–81. [Google Scholar] [CrossRef]
- Boyden, E.S.; Raymond, J.L. Active reversal of motor memories reveals rules governing memory encoding. Neuron 2003, 39, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Lisberger, S.G.; Miles, F.A. Role of primate medial vestibular nucleus in long-term adaptive plasticity of vestibuloocular reflex. J. Neurophysiol. 1980, 43, 1725–1745. [Google Scholar] [CrossRef]
- Robinson, D.A. Adaptive gain control of vestibuloocular reflex by the cerebellum. J. Neurophysiol. 1976, 39, 954–969. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cogn. Sci. 1998, 2, 313–321. [Google Scholar] [CrossRef]
- Kimpo, R.R.; Boyden, E.S.; Katoh, A.; Ke, M.C.; Raymond, J.L. Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J. Neurophysiol. 2005, 94, 3092–3100. [Google Scholar] [CrossRef] [Green Version]
- Titley, H.K.; Heskin-Sweezie, R.; Chung, J.Y.; Kassardjian, C.D.; Razik, F.; Broussard, D.M. Rapid consolidation of motor memory in the vestibuloocular reflex. J. Neurophysiol. 2007, 98, 3809–3812. [Google Scholar] [CrossRef] [Green Version]
- Titley, H.K.; Heskin-Sweezie, R.; Broussard, D.M. The bidirectionality of motor learning in the vestibulo-ocular reflex is a function of cerebellar mGluR1 receptors. J. Neurophysiol. 2010, 104, 3657–3666. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Katoh, A.; Pyle, J.L.; Chatila, T.A.; Tsien, R.W.; Raymond, J.L. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 2006, 51, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasahara, K.; Shikimi, H.; Haraguchi, S.; Sakamoto, H.; Honda, S.; Harada, N.; Tsutsui, K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J. Neurosci. 2007, 27, 7408–7417. [Google Scholar] [CrossRef] [PubMed]
- Coesmans, M.; Weber, J.T.; De Zeeuw, C.I.; Hansel, C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 2004, 44, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Zeeuw, C.I.; Yeo, C.H. Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 2005, 15, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.C.; Kim, S.J. Plasticity leading to cerebellum-dependent learning: Two different regions, two different types. Pflugers Arch. 2019, 471, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Kassardjian, C.D.; Tan, Y.F.; Chung, J.Y.; Heskin, R.; Peterson, M.J.; Broussard, D.M. The site of a motor memory shifts with consolidation. J. Neurosci. 2005, 25, 7979–7985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisberger, S.G. Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J. Neurophysiol. 1994, 72, 974–998. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006, 78, 272–303. [Google Scholar] [CrossRef]
- Boyden, E.S.; Katoh, A.; Raymond, J.L. Cerebellum-dependent learning: The role of multiple plasticity mechanisms. Annu. Rev. Neurosci. 2004, 27, 581–609. [Google Scholar] [CrossRef] [Green Version]
- Broussard, D.M.; Titley, H.K.; Antflick, J.; Hampson, D.R. Motor learning in the VOR: The cerebellar component. Exp. Brain Res. 2011, 210, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, P.M.; Hirata, Y.; Highstein, S.M. The vestibulo-ocular reflex as a model system for motor learning: What is the role of the cerebellum? Cerebellum 2004, 3, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gittis, A.H.; du Lac, S. Intrinsic and synaptic plasticity in the vestibular system. Curr. Opin. Neurobiol. 2006, 16, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Broussard, D.M.; Kassardjian, C.D. Learning in a simple motor system. Learn. Mem. 2004, 11, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.S. Sensorimotor-correlated discharge recorded from ensembles of cerebellar Purkinje cells varies across the estrous cycle of the rat. J. Neurophysiol. 1995, 74, 1095–1108. [Google Scholar] [CrossRef]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef] [Green Version]
- Manto, M.; Oulad Ben Taib, N. The contributions of the cerebellum in sensorimotor control: What are the prevailing opinions which will guide forthcoming studies? Cerebellum 2013, 12, 313–315. [Google Scholar] [CrossRef]
- Doya, K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr. Opin. Neurobiol. 2000, 10, 732–739. [Google Scholar] [CrossRef]
- Ma, Q.; Zeng, L.L.; Shen, H.; Liu, L.; Hu, D. Altered cerebellar-cerebral resting-state functional connectivity reliably identifies major depressive disorder. Brain Res. 2013, 1495, 86–94. [Google Scholar] [CrossRef]
- Stoodley, C.J.; D’Mello, A.M.; Ellegood, J.; Jakkamsetti, V.; Liu, P.; Nebel, M.B.; Gibson, J.M.; Kelly, E.; Meng, F.; Cano, C.A.; et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci. 2017, 20, 1744–1751. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Hopkins, D.A.; Mayrhofer, H.C.; Bruner, E.; van Leeuwen, F.W.; Raaijmakers, W.; Schmahmann, J.D. The cerebellum in Alzheimer’s disease: Evaluating its role in cognitive decline. Brain 2018, 141, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.T.; Alvina, K.; Womack, M.D.; Chevez, C.; Khodakhah, K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 2006, 9, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Firozan, B.; Goudarzi, I.; Elahdadi Salmani, M.; Lashkarbolouki, T.; Rezaei, A.; Abrari, K. Estradiol increases expression of the brain-derived neurotrophic factor after acute administration of ethanol in the neonatal rat cerebellum. Eur. J. Pharmacol. 2014, 732, 1–11. [Google Scholar] [CrossRef]
- Mirzatoni, A.; Spence, R.D.; Naranjo, K.C.; Saldanha, C.J.; Schlinger, B.A. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J. Neurotrauma 2010, 27, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, K.; Ukena, K.; Sakamoto, H.; Okuyama, S.; Haraguchi, S. Biosynthesis, mode of action, and functional significance of neurosteroids in the purkinje cell. Front. Endocrinol. (Lausanne) 2011, 2, 61. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.S.; Waterhouse, B.D.; Woodward, D.J. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988, 475, 272–282. [Google Scholar] [CrossRef]
- Smith, S.S.; Woodward, D.J.; Chapin, J.K. Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Res. 1989, 476, 307–316. [Google Scholar] [CrossRef]
- Dewing, P.; Boulware, M.I.; Sinchak, K.; Christensen, A.; Mermelstein, P.G.; Micevych, P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci. 2007, 27, 9294–9300. [Google Scholar] [CrossRef] [Green Version]
- Boulware, M.I.; Weick, J.P.; Becklund, B.R.; Kuo, S.P.; Groth, R.D.; Mermelstein, P.G. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005, 25, 5066–5078. [Google Scholar] [CrossRef]
- Luine, V.N.; Beck, K.D.; Bowman, R.E.; Frankfurt, M.; Maclusky, N.J. Chronic stress and neural function: Accounting for sex and age. J. Neuroendocr. 2007, 19, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Remage-Healey, L. Frank beach award winner: Steroids as neuromodulators of brain circuits and behavior. Horm. Behav. 2014, 66, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balthazart, J.; Baillien, M.; Ball, G.F. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology 2006, 147, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocr. 2009, 30, 259–301. [Google Scholar] [CrossRef]
- Charlier, T.D.; Harada, N.; Balthazart, J.; Cornil, C.A. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology 2011, 152, 4199–4210. [Google Scholar] [CrossRef] [Green Version]
- Remage-Healey, L.; Oyama, R.K.; Schlinger, B.A. Elevated aromatase activity in forebrain synaptic terminals during song. J. Neuroendocr. 2009, 21, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, S.; Hara, S.; Ubuka, T.; Mita, M.; Tsutsui, K. Possible role of pineal allopregnanolone in Purkinje cell survival. Proc. Natl. Acad. Sci. USA 2012, 109, 21110–21115. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, K. Kobayashi award: Discovery of cerebellar and pineal neurosteroids and their biological actions on the growth and survival of Purkinje cells during development (review). Gen. Comp. Endocrinol. 2019, 284, 113051. [Google Scholar] [CrossRef]
- Gu, Q.; Moss, R.L. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 1996, 16, 3620–3629. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Mahecha, G.A.; Carnes, K.; Prins, G.S.; Saunders, P.T.; Franca, L.R.; Hess, R.A. Differential hormonal regulation of estrogen receptors ERalpha and ERbeta and androgen receptor expression in rat efferent ductules. Reproduction 2004, 128, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Wempe, M.F.; Buchanan, C.M.; Buchanan, N.L.; Edgar, K.J.; Hanley, G.A.; Ramsey, M.G.; Skotty, J.S.; Rice, P.J. Pharmacokinetics of letrozole in male and female rats: Influence of complexation with hydroxybutenyl-beta cyclodextrin. J. Pharm. Pharmacol. 2007, 59, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.J. Organization of olivocerebellar activity in the absence of excitatory glutamatergic input. J. Neurosci. 2001, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Mathy, A.; Clark, B.A.; Hausser, M. Synaptically induced long-term modulation of electrical coupling in the inferior olive. Neuron 2014, 81, 1290–1296. [Google Scholar] [CrossRef] [Green Version]
- Hoge, G.J.; Davidson, K.G.; Yasumura, T.; Castillo, P.E.; Rash, J.E.; Pereda, A.E. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J. Neurophysiol. 2011, 105, 1089–1101. [Google Scholar] [CrossRef] [Green Version]
- Llinas, R.R. The Noncontinuous nature of movement execution. Mot. Control Concepts Issues 1991, 50, 223–242. [Google Scholar]
- Smith, S.S. Oestrogen effects in olivo-cerebellar and hippocampal circuits. In Novartis Foundation Symposium; Chadwick, D.J., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Cavallotti, C.; Iacopino, L.; Amenta, F. Stimulatory effect of beta-estradiol treatment on GABA-degradative enzymes within rat cerebellar cortex. Neurosci. Lett. 1983, 39, 205–209. [Google Scholar] [CrossRef]
- Rudick, C.N.; Woolley, C.S. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci. 2001, 21, 6532–6543. [Google Scholar] [CrossRef] [Green Version]
- Jorntell, H.; Hansel, C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 2006, 52, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, J.; Chen, S.; Brinton, R.D. Dual action of estrogen on glutamate-induced calcium signaling: Mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002, 930, 216–234. [Google Scholar] [CrossRef]
- Wang, Y.T.; Linden, D.J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 2000, 25, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Tempia, F.; Alojado, M.E.; Strata, P.; Knopfel, T. Characterization of the mGluR(1)-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J. Neurophysiol. 2001, 86, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.E.; Canepari, M. On the induction of postsynaptic granule cell-Purkinje neuron LTP and LTD. Cerebellum 2010, 9, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, C.; de Jeu, M.; Belmeguenai, A.; Houtman, S.H.; Buitendijk, G.H.; Andreev, D.; De Zeeuw, C.I.; Elgersma, Y. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron 2006, 51, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonewille, M.; Belmeguenai, A.; Koekkoek, S.K.; Houtman, S.H.; Boele, H.J.; van Beugen, B.J.; Gao, Z.; Badura, A.; Ohtsuki, G.; Amerika, W.E.; et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 2010, 67, 618–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiana, H.L. A new approach to understanding adaptive visual-vestibular interactions in the central nervous system. J. Neurophysiol. 1986, 55, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.L.; Lisberger, S.G.; Mauk, M.D. The cerebellum: A neuronal learning machine? Science 1996, 272, 1126–1131. [Google Scholar] [CrossRef]
- Anzai, M.; Kitazawa, H.; Nagao, S. Effects of reversible pharmacological shutdown of cerebellar flocculus on the memory of long-term horizontal vestibulo-ocular reflex adaptation in monkeys. Neurosci. Res. 2010, 68, 191–198. [Google Scholar] [CrossRef]
- Menzies, J.R.; Porrill, J.; Dutia, M.; Dean, P. Synaptic plasticity in medial vestibular nucleus neurons: Comparison with computational requirements of VOR adaptation. PLoS ONE 2010, 5, e13182. [Google Scholar] [CrossRef] [Green Version]
- Evrard, H.C.; Harada, N.; Balthazart, J. Immunocytochemical localization of aromatase in sensory and integrating nuclei of the hindbrain in Japanese quail (Coturnix japonica). J. Comp. Neurol. 2004, 473, 194–212. [Google Scholar] [CrossRef]
- Smith, P.F.; Agrawal, Y.; Darlington, C.L. Sexual dimorphism in vestibular function and dysfunction. J. Neurophysiol. 2019, 121, 2379–2391. [Google Scholar] [CrossRef]
- Panichi, R.; Botti, F.M.; Ferraresi, A.; Faralli, M.; Kyriakareli, A.; Schieppati, M.; Pettorossi, V.E. Self-motion perception and vestibulo-ocular reflex during whole body yaw rotation in standing subjects: The role of head position and neck proprioception. Hum. Mov. Sci. 2011, 30, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Kyriakareli, A.; Ferraresi, A.; Faralli, M.; Schieppati, M.; Bronstein, A.M. Prolonged asymmetric vestibular stimulation induces opposite, long-term effects on self-motion perception and ocular responses. J. Physiol. 2013, 591, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Panichi, R.; Occhigrossi, C.; Ferraresi, A.; Faralli, M.; Lucertini, M.; Pettorossi, V.E. Adaptive changes in the perception of fast and slow movement at different head positions. Aerosp. Med. Hum. Perform. 2017, 88, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Biscarini, A.; Filippi, G.M.; Schieppati, M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin. Neurophysiol. 2015, 126, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Scarduzio, M.; Panichi, R.; Pettorossi, V.E.; Grassi, S. The repetition timing of high frequency afferent stimulation drives the bidirectional plasticity at central synapses in the rat medial vestibular nuclei. Neuroscience 2012, 223, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Panichi, R.; Faralli, M.; Bruni, R.; Kiriakarely, A.; Occhigrossi, C.; Ferraresi, A.; Bronstein, A.M.; Pettorossi, V.E. Asymmetric vestibular stimulation reveals persistent disruption of motion perception in unilateral vestibular lesions. J. Neurophysiol. 2017, 118, 2819–2832. [Google Scholar] [CrossRef]
- Gunther, L.; Beck, R.; Xiong, G.; Potschka, H.; Jahn, K.; Bartenstein, P.; Brandt, T.; Dutia, M.; Dieterich, M.; Strupp, M.; et al. N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS ONE 2015, 10, e0120891. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Miall, R.C.; Ivry, R.B. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn. Sci. 2017, 21, 313–332. [Google Scholar] [CrossRef] [Green Version]
- Morton, S.M.; Bastian, A.J. Cerebellar control of balance and locomotion. Neuroscientist 2004, 10, 247–259. [Google Scholar] [CrossRef]
- Cangussu, L.M.; Nahas-Neto, J.; Petri Nahas, E.A.; Rodrigues Barral, A.B.; Buttros Dde, A.; Uemura, G. Evaluation of postural balance in postmenopausal women and its relationship with bone mineral density—A cross sectional study. BMC Musculoskelet. Disord. 2012, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I.A.; Abramson, V.G.; Isakoff, S.J.; Forero, A.; Balko, J.M.; Kuba, M.G.; Sanders, M.E.; Yap, J.T.; Van den Abbeele, A.D.; Li, Y.; et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2014, 32, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Manni, A.; Harvey, H.; Redmond, C. Endocrine treatment of breast cancer in women. Endocr. Rev. 1990, 11, 221–265. [Google Scholar] [CrossRef]
- Kelly, R.M.; Strick, P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003, 23, 8432–8444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmi, J.; Pallesen, K.J.; Neuvonen, T.; Brattico, E.; Korvenoja, A.; Salonen, O.; Carlson, S. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010, 22, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.X.; Beckmann, C.F.; Tomassini, V.; Ramnani, N.; Johansen-Berg, H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex 2010, 20, 953–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dum, R.P.; Strick, P.L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.A.; Erb, M.; Grodd, W.; Pavlova, M.A. Structural loop between the cerebellum and the superior temporal sulcus: Evidence from diffusion tensor imaging. Cereb. Cortex 2014, 24, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Schmahmann, J.D. The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int. Rev. Psychiatr. 2001, 13, 247–260. [Google Scholar] [CrossRef]
- Besnard, S.; Lopez, C.; Brandt, T.; Denise, P.; Smith, P.F. Editorial: The vestibular system in cognitive and memory processes in mammalians. Front. Integr. Neurosci. 2015, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Mast, F.W.; Preuss, N.; Hartmann, M.; Grabherr, L. Spatial cognition, body representation and affective processes: The role of vestibular information beyond ocular reflexes and control of posture. Front. Integr. Neurosci. 2014, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.F.; Darlington, C.L. Personality changes in patients with vestibular dysfunction. Front. Hum. Neurosci. 2013, 7, 678. [Google Scholar] [CrossRef] [Green Version]
- Dean, S.L.; McCarthy, M.M. Steroids, sex and the cerebellar cortex: Implications for human disease. Cerebellum 2008, 7, 38–47. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tomatsu, S.; Izawa, J.; Kakei, S. The cerebro-cerebellum: Could it be loci of forward models? Neurosci. Res. 2016, 104, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Ito, M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hore, J.; Wild, B.; Diener, H.C. Cerebellar dysmetria at the elbow, wrist, and fingers. J. Neurophysiol. 1991, 65, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Blatt, G.J. Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. 2015, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.L.; Narayanan, N.S.; Andreasen, N.C. The therapeutic potential of the cerebellum in schizophrenia. Front. Syst. Neurosci. 2014, 8, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreasen, N.C.; Paradiso, S.; O’Leary, D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998, 24, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Crider, A.; Pillai, A. Estrogen signaling as a therapeutic target in neurodevelopmental disorders. J. Pharmacol. Exp. Ther. 2017, 360, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A role for estrogen in schizophrenia: Clinical and preclinical findings. Int. J. Endocrinol. 2015, 2015, 615356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoxha, E.; Lippiello, P.; Zurlo, F.; Balbo, I.; Santamaria, R.; Tempia, F.; Miniaci, M.C. The emerging role of altered cerebellar synaptic processing in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, M.; Costa, L.; Piacentini, R.; Grassi, C.; Lanzone, A.; Gulino, A. 17beta-estradiol protects cerebellar granule cells against beta-amyloid-induced toxicity via the apoptotic mitochondrial pathway. Neurosci. Lett. 2014, 561, 134–139. [Google Scholar] [CrossRef] [PubMed]
| Cerebellar Cortex Layer | Aromatase | ERα | ERβ | GPER-1 | ||||
|---|---|---|---|---|---|---|---|---|
| Childhood | Adult | Childhood | Adult | Childhood | Adult | Childhood | Adult | |
| Gc | 1, 7 | 1 | 11, 12, 20 | 8, 11, 14, 15, 19, 21 | 18 | |||
| PC | 1, 7 | 1 | 11, 20 | 11 | 7, 11, 12 | 11, 8, 14, 15–17, 19, 21 | 18 | |
| Ml | 11, 12 | 11, 19 | 18 | |||||
| NI | 4, 5, 10 | 2, 3, 4, 9, | 3, 6, 13 | 3, 6 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieni, C.V.; Contemori, S.; Biscarini, A.; Panichi, R. De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function. Int. J. Mol. Sci. 2020, 21, 3316. https://doi.org/10.3390/ijms21093316
Dieni CV, Contemori S, Biscarini A, Panichi R. De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function. International Journal of Molecular Sciences. 2020; 21(9):3316. https://doi.org/10.3390/ijms21093316
Chicago/Turabian StyleDieni, Cristina V., Samuele Contemori, Andrea Biscarini, and Roberto Panichi. 2020. "De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function" International Journal of Molecular Sciences 21, no. 9: 3316. https://doi.org/10.3390/ijms21093316
APA StyleDieni, C. V., Contemori, S., Biscarini, A., & Panichi, R. (2020). De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function. International Journal of Molecular Sciences, 21(9), 3316. https://doi.org/10.3390/ijms21093316





