Short-Term Western Diet Aggravates Non-Alcoholic Fatty Liver Disease (NAFLD) With Portal Hypertension in TGR(mREN2)27 Rats
Abstract
1. Introduction
2. Results
2.1. Western Diet Induced Obesity in TGR(mREN2)27 Rats
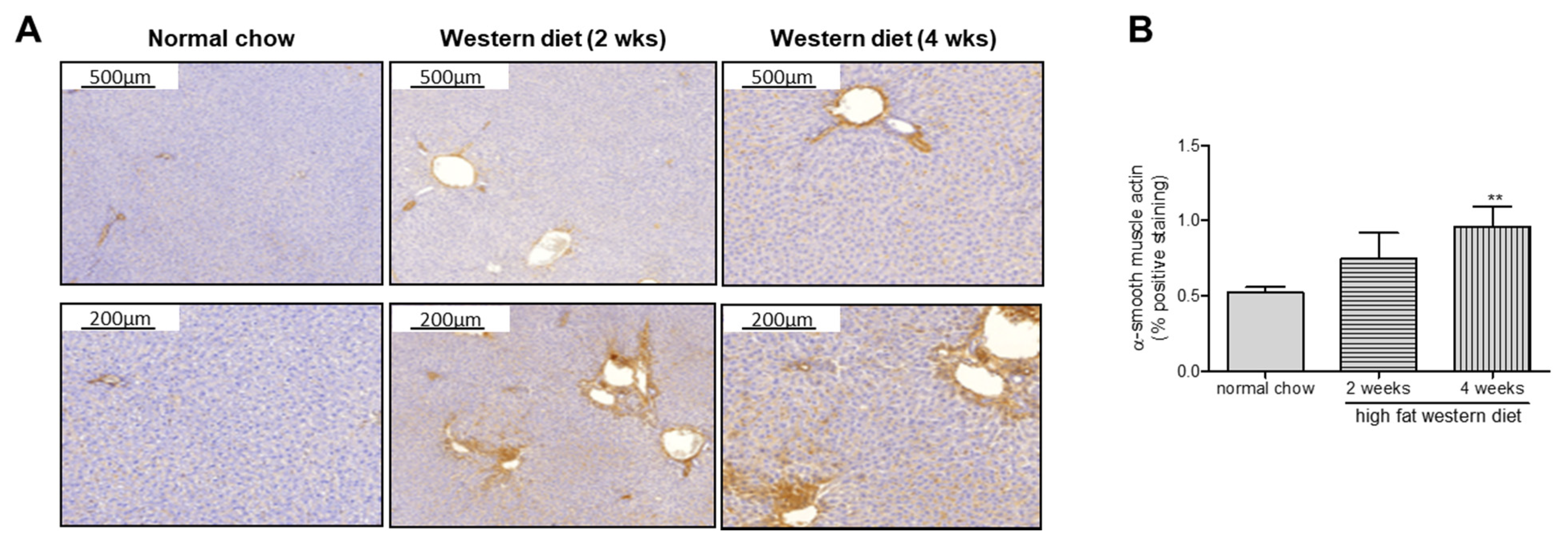
2.2. Western Diet Aggravated Liver Fibrosis in TGR(mREN2)27 Rats
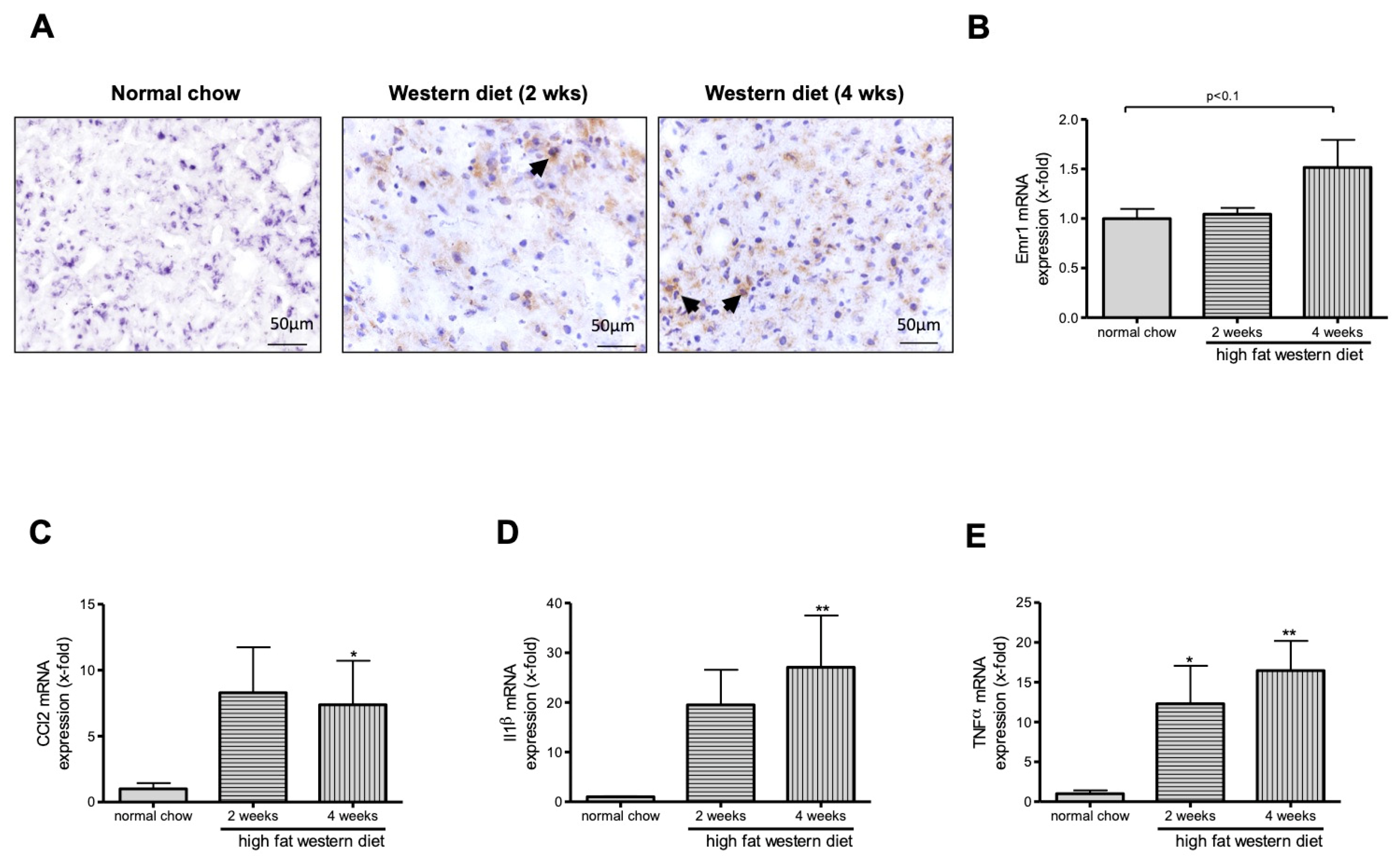
2.3. Western Diet Induced Activation of Macrophages in TGR(mREN2)27 Rats
2.4. Western Diet Induced NAFLD in TGR(mREN2)27 Rats
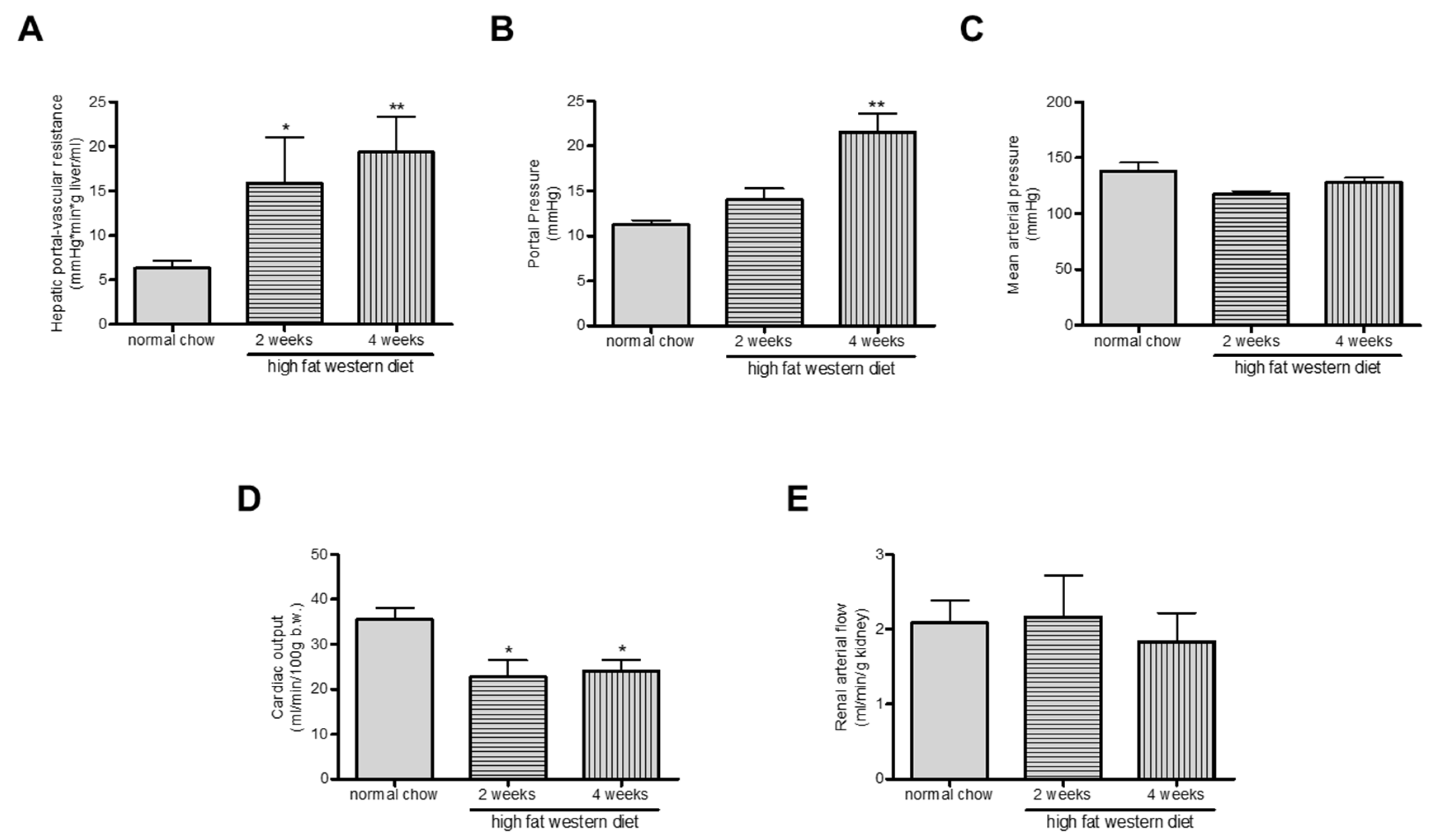
2.5. Western Diet Aggravated Portal Hypertension in TGR(mREN2)27 Rats
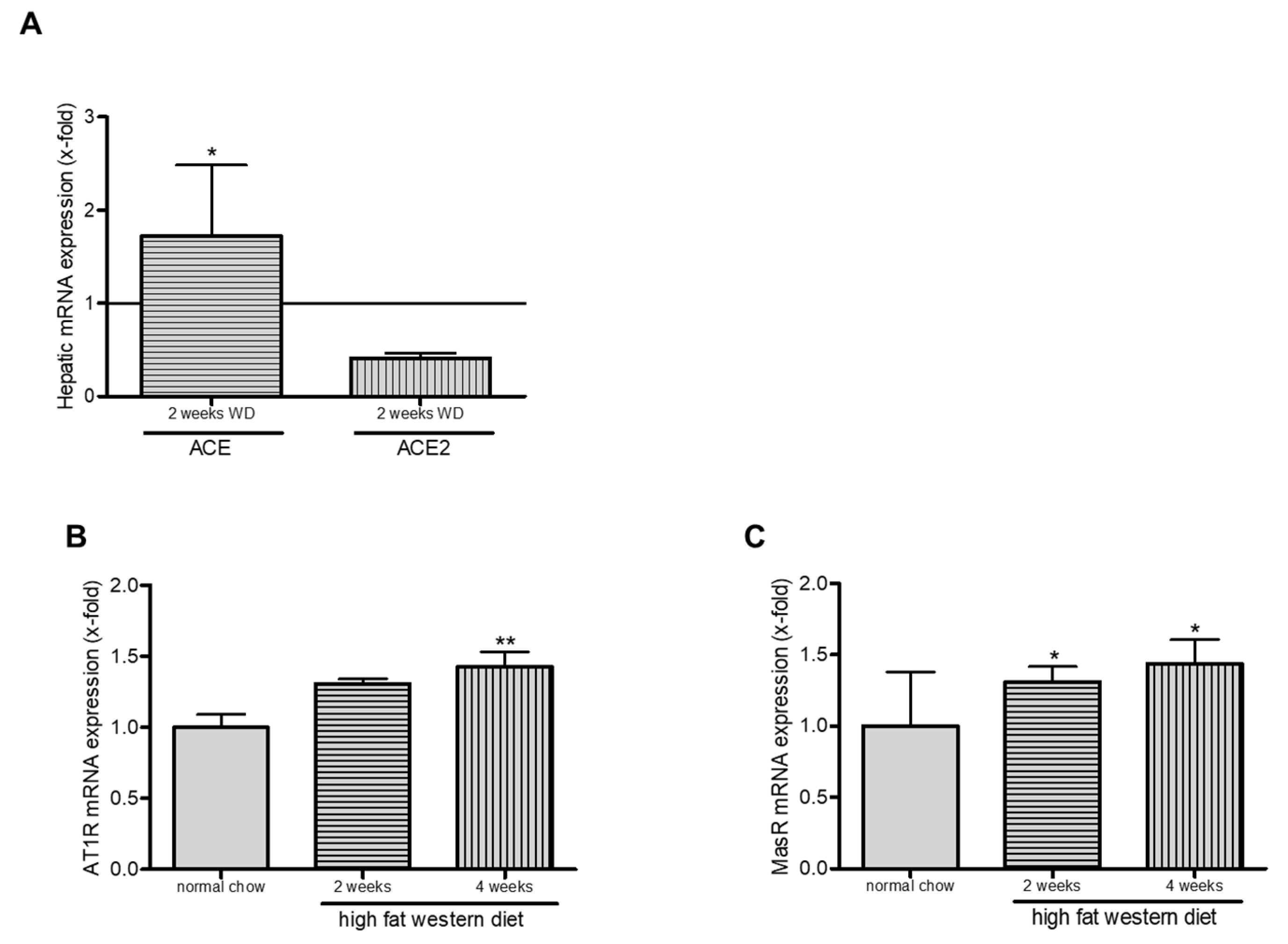
2.6. Western Diet and Role of RAS in TGR(mREN2)27 Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Models of Liver Disease
4.2. Hemodynamic Studies
4.3. Tissue Collection
4.4. Sirius Red Staining and α-Smooth Muscle Actin Immunohistochemistry
4.5. Oil Red O Staining
4.6. F4/80 Staining
4.7. Hepatic Western Blots
4.8. Hepatic Triglyceride Content
4.9. Quantitative Real-Time PCR
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EMR1 | EGF module-containing mucin-like receptor |
| FAS | Fatty acid synthase |
| IL1β | Interleukin 1-β |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NAFLD | Non-alcoholic fatty liver disease |
| RAS | Renin-angiotensin system |
| α-SMA | α-smooth muscle actin |
| SREBP-1c | Sterol regulatory element binding protein-1c |
| TGF-β | Transforming growth factor-β |
| TNFα | Tumor necrosis factor α |
| WD | Western diet |
References
- Kadayifci, A.; Tan, V.; Ursell, P.C.; Merriman, R.; Bass, N.M. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: A comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J. Hepatol. 2008, 49, 595–599. [Google Scholar] [CrossRef]
- Mendes, F.D.; Suzuki, A.; Sanderson, S.O.; Lindor, K.D.; Angulo, P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 1028.e2–1033.e2. [Google Scholar] [CrossRef]
- Nagpal, S.J.S.; Kabbany, M.N.; Mohamad, B.; Lopez, R.; Zein, N.N.; Alkhouri, N. Portal Hypertension Complications Are Frequently the First Presentation of NAFLD in Patients Undergoing Liver Transplantation Evaluation. Dig. Dis. Sci. 2016, 61, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Benvegnù, L.; Gios, M.; Boccato, S.; Alberti, A. Natural history of compensated viral cirrhosis: A prospective study on the incidence and hierarchy of major complications. Gut 2004, 53, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Sauerbruch, T.; Trebicka, J. Future therapy of portal hypertension in liver cirrhosis–a guess. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ratziu, V.; Oren, R. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World J. Gastroenterol. 2011, 17, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2016, 66, 180–190. [Google Scholar] [CrossRef]
- Mullins, J.J.; Peters, J.; Ganten, D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 1990, 344, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Yamaguchi, T.; Franco-Saenz, R.; Mulrow, P.J.; Ganten, D. Adrenal renin is released into the circulation of the hypertensive transgenic rat TGR (mRen-2)27. Trans. Assoc. Am. Physicians 1992, 105, 123–132. [Google Scholar]
- Wei, Y.; Clark, S.E.; Morris, E.M.M.; Thyfault, J.P.; Uptergrove, G.M.; Whaley-Connell, A.; Ferrario, C.M.; Sowers, J.R.; Ibdah, J.A. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J. Hepatol. 2008, 49, 417–428. [Google Scholar] [CrossRef]
- Klein, S.; Kleine, C.-E.; Pieper, A.; Granzow, M.; Gautsch, S.; Himmit, M.; Kahrmann, K.; Schierwagen, R.; Uschner, F.E.; Magdaleno, F.; et al. TGR(mREN2)27 rats develop non-alcoholic fatty liver disease-associated portal hypertension responsive to modulations of Janus-kinase 2 and Mas receptor. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; García-Pagán, J.C. Complications of cirrhosis. I. Portal hypertension. J. Hepatol. 2000, 32, 141–156. [Google Scholar] [CrossRef]
- Granzow, M.; Schierwagen, R.; Klein, S.; Kowallick, B.; Huss, S.; Linhart, M.; Mazar, I.G.R.; Görtzen, J.; Vogt, A.; Schildberg, F.A.; et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology 2014, 60, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Rick, J.; Lehmann, J.; Schierwagen, R.; Schierwagen, I.G.; Verbeke, L.; Hittatiya, K.; Uschner, F.E.; Manekeller, S.; Strassburg, C.P.; et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut 2015, 66, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Paik, Y.; Lindquist, J.N.; Lemasters, J.J.; Brenner, D.A. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology 2004, 126, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Ramalho, L.N.; Sancho-Bru, P.; Ruiz-Ortega, M.; Ramalho, L.N.; Abraldes, J.G.; Colmenero, J.; Dominguez, M.; Egido, J.; Arroyo, V.; et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am. J. Physiol. Liver Physiol. 2009, 296, G147–G156. [Google Scholar] [CrossRef]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2011, 61, 416–426. [Google Scholar] [CrossRef]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef]
- Tacke, F.; Weiskirchen, R. An update on the recent advances in antifibrotic therapy. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Recent advances in understanding liver fibrosis: Bridging basic science and individualized treatment concepts. F1000Research 2018, 7, 921. [Google Scholar] [CrossRef]
- Schulz, C.; Gomez-Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.A.; Lefevre, L.; Bush, S.J.; Raper, A.; Young, R.; Lisowski, Z.; McCulloch, M.E.B.; Muriuki, C.; Sauter, K.A.; Clark, E.L.; et al. ADGRE1 (EMR1, F4/80) Is a Rapidly-Evolving Gene Expressed in Mammalian Monocyte-Macrophages. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Morillas, R.; Escorsell, A.; García-Pagán, J.C.; Patch, D.; Matloff, D.S.; et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011, 54, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescà, J.; Ardevol, A.; Augustin, S.; Calleja-Panero, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017, 65, 1293–1305. [Google Scholar] [CrossRef]
- Baffy, G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 563–576. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2014, 13, 643.e1–654.e1. [Google Scholar] [CrossRef]
- Negro, F. Natural history of NASH and HCC. Liver Int. 2020, 40, 72–76. [Google Scholar] [CrossRef]
- Lefere, S.; Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019, 1, 30–43. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef]
- Lattin, J.; Zidar, D.A.; Schroder, K.; Kellie, S.; Hume, D.A.; Sweet, M.J. G-protein-coupled receptor expression, function, and signaling in macrophages. J. Leukoc. Boil. 2007, 82, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Cassado, A.D.A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. In Plant Promoters and Transcription Factors; Springer Science and Business Media LLC: Luxemburg, 2017; Volume 62, pp. 161–179. [Google Scholar]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Inokuchi, S.; Taura, K.; Miyai, K.; Van Rooijen, N.; Schwabe, R.F.; Brenner, D.A. CCR2 promotes hepatic fibrosis in mice. Hepatology 2009, 50, 185–197. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R. Expanding consensus in portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Van Der Graaff, D.; Kwanten, W.J.; Couturier, F.J.; Govaerts, J.S.; Verlinden, W.; Brosius, I.; D’Hondt, M.; Driessen, A.; De Winter, B.Y.; De Man, J.G.; et al. Severe steatosis induces portal hypertension by systemic arterial hyporeactivity and hepatic vasoconstrictor hyperreactivity in rats. Lab. Investig. 2018, 98, 1263–1275. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.-J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162.e14–175.e14. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.-C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.; Eisenbarth, S.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Sigmund, C.D.; Gross, K.W. Structure, expression, and regulation of the murine renin genes. Hypertension 1991, 18, 446–457. [Google Scholar] [CrossRef]
- Schierwagen, R.; Maybüchen, L.; Zimmer, S.; Hittatiya, K.; Bäck, C.; Klein, S.; Uschner, F.E.; Reul, W.; Boor, P.; Nickenig, G.; et al. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci. Rep. 2015, 5, 12931. [Google Scholar] [CrossRef]
- Ramadori, P.; Weiskirchen, R.; Trebicka, J.; Streetz, K. Mouse models of metabolic liver injury. Lab. Anim. 2015, 49, 47–58. [Google Scholar] [CrossRef]
- Klein, S.; Schierwagen, R.; Uschner, F.E.; Trebicka, J. Mouse and Rat Models of Induction of Hepatic Fibrosis and Assessment of Portal Hypertension. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Luxemburg, 2017; Volume 1627, pp. 91–116. [Google Scholar]
- Trebicka, J.; Leifeld, L.; Hennenberg, M.; Biecker, E.; Eckhardt, A.; Fischer, N.; Pröbsting, A.S.; Clemens, C.; Lammert, F.; Sauerbruch, T.; et al. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology 2007, 47, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Hennenberg, M.; Odenthal, M.; Shir, K.; Klein, S.; Granzow, M.; Vogt, A.; Dienes, H.-P.; Lammert, F.; Reichen, J.; et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J. Hepatol. 2010, 53, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Rácz, I.; Siegmund, S.V.; Cara, E.; Granzow, M.; Schierwagen, R.; Klein, S.; Wojtalla, A.; Hennenberg, M.; Huss, S.; et al. Role of cannabinoid receptors in alcoholic hepatic injury: Steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011, 31, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Rat treatment | Body Weight (g) | Liver Weight (g) | Spleen Weight (g) |
|---|---|---|---|
| Normal chow | 279.3 ± 13.2 | 11.4 ± 2.3 | 0.6 ± 0.7 |
| High fat Western diet (2 wks) | 315.4 ± 6.7 *** | 12.1 ± 1.4 | 0.6 ± 0.2 |
| High fat Western diet (4 wks) | 341.0 ± 6.4 ***# | 13.7 ± 1.7 * | 0.6 ± 0.1 |
| TGR(mREN2)27 Groups | Portal Pressure (mmHg) | Hepatic-Vascular Resistance (mmHg*min*g Liver/mL) | Splanchnic-Vascular Resistance (mmHg*min*100g/mL) | Mean Arterial Pressure (mmHg) | Systemic-Vascular Resistance (mmHg*min*100g/mL) |
|---|---|---|---|---|---|
| normal chow | 11.24 ± 0.53 | 6.31 ± 0.83 | 49.92 ± 11.12 | 129.73 ± 6.68 | 4.68 ± 0.89 |
| 2 weeks Western diet | 14.00 ± 1.29 * | 15.91 ± 5.11 * | 23.05 ± 4.42 * | 117.40 ± 2.91 | 5.54 ± 0.65 |
| 4 weeks Western diet | 21.50 ± 2.18 **## | 19.36 ± 3.94 ** | 23.48 ± 4.70 * | 127.80 ± 4.49 | 5.57 ± 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremonese, C.; Schierwagen, R.; Uschner, F.E.; Torres, S.; Tyc, O.; Ortiz, C.; Schulz, M.; Queck, A.; Kristiansen, G.; Bader, M.; et al. Short-Term Western Diet Aggravates Non-Alcoholic Fatty Liver Disease (NAFLD) With Portal Hypertension in TGR(mREN2)27 Rats. Int. J. Mol. Sci. 2020, 21, 3308. https://doi.org/10.3390/ijms21093308
Cremonese C, Schierwagen R, Uschner FE, Torres S, Tyc O, Ortiz C, Schulz M, Queck A, Kristiansen G, Bader M, et al. Short-Term Western Diet Aggravates Non-Alcoholic Fatty Liver Disease (NAFLD) With Portal Hypertension in TGR(mREN2)27 Rats. International Journal of Molecular Sciences. 2020; 21(9):3308. https://doi.org/10.3390/ijms21093308
Chicago/Turabian StyleCremonese, Carla, Robert Schierwagen, Frank Erhard Uschner, Sandra Torres, Olaf Tyc, Cristina Ortiz, Martin Schulz, Alexander Queck, Glen Kristiansen, Michael Bader, and et al. 2020. "Short-Term Western Diet Aggravates Non-Alcoholic Fatty Liver Disease (NAFLD) With Portal Hypertension in TGR(mREN2)27 Rats" International Journal of Molecular Sciences 21, no. 9: 3308. https://doi.org/10.3390/ijms21093308
APA StyleCremonese, C., Schierwagen, R., Uschner, F. E., Torres, S., Tyc, O., Ortiz, C., Schulz, M., Queck, A., Kristiansen, G., Bader, M., Sauerbruch, T., Weiskirchen, R., Walther, T., Trebicka, J., & Klein, S. (2020). Short-Term Western Diet Aggravates Non-Alcoholic Fatty Liver Disease (NAFLD) With Portal Hypertension in TGR(mREN2)27 Rats. International Journal of Molecular Sciences, 21(9), 3308. https://doi.org/10.3390/ijms21093308







