Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels
Abstract
1. Introduction
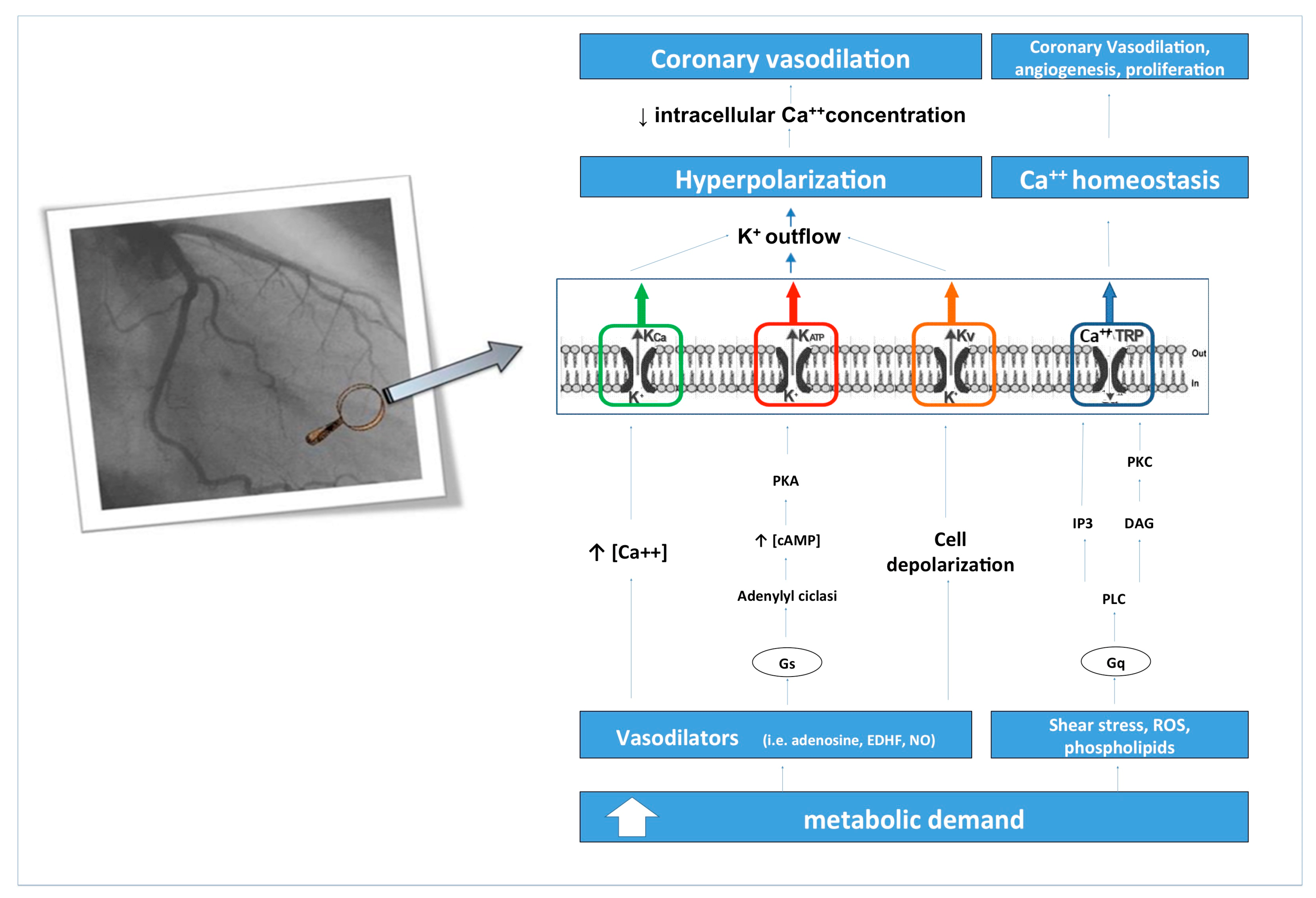
2. Coronary Blood Flow and its Regulation Mechanisms: Role of Ion Channels
3. Coronary Microcirculation in the Pathophysiology of Ischemic Heart Disease and Heart Failure
4. Ion Channels in Ischemic Heart Disease and Heart Failure
4.1. ATP-Sensitive Potassium (KATP) Channel
4.2. Potassium Calcium-Activated Channel (KCa) Channels
4.3. Voltage-Gated Potassium (Kv) Channels
4.4. Transient Receptor Potential (TRP) Channels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaudhry, M.A. Heart Failure. Curr. Hypertens Rev. 2019, 15, 7. [Google Scholar] [CrossRef]
- Gould, K.L.; Lipscomb, K. Effects of coronary stenoses on coronary flow reserve and resistance. Am. J. Cardiol. 1974, 34, 48–55. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; De Marchis, M.; Palmirotta, R.; Volterrani, M.; Mancone, M.; Fedele, F. Diabetes Mellitus and Ischemic Heart Disease: The Role of Ion Channels. Int. J. Mol. Sci. 2018, 19, 802. [Google Scholar] [CrossRef] [PubMed]
- Fedele, F.; Severino, P.; Bruno, N.; Stio, R.; Caira, C.; D’Ambrosi, A.; Brasolin, B.; Ohanyan, V.; Mancone, M. Role of ion channels in coronary microcirculation: A review of the literature. Future Cardiol. 2013, 9, 897–905. [Google Scholar] [CrossRef]
- Ferrari, R.; Fox, K. Heart rate reduction in coronary artery disease and heart failure. Nat. Rev. Cardiol. 2016, 13, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Marzilli, M.; Merz, C.N.; Boden, W.E.; Bonow, R.O.; Capozza, P.G.; Chilian, W.M.; DeMaria, A.N.; Guarini, G.; Huqi, A.; Morrone, D.; et al. Obstructive Coronary Atherosclerosis and Ischemic Heart Disease: An Elusive Link! J. Am. Coll. Cardiol. 2012, 60, 951–956. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J. Diabetes Res. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Chilian, W.M.; Severino, P.; Canali, E.; Logan, S.; De Marchis, M.L.; Volterrani, M.; Palmirotta, R.; Guadagni, F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res. Cardiol. 2013, 108, 387. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gutterman, D.D. Vascular control in humans: Focus on the coronary microcirculation. Basic Res. Cardiol. 2009, 104, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H. Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, Explains the “L-Arginine Paradox” and Acts as a Novel Cardiovascular Risk Factor. J. Nutr. 2004, 134, 2842S–2847S. [Google Scholar] [CrossRef] [PubMed]
- Chilian, W.M.; Harrison, D.G.; Haws, C.W.; Snyder, W.D.; Marcus, M.L. Adrenergic coronary tone during submaximal exercise in the dog is produced by circulating catecholamines. Evidence for adrenergic denervation supersensitivity in the myocardium but not in coronary vessels. Circ. Res. 1986, 58, 68–82. [Google Scholar] [CrossRef]
- Chilian, W.M.; Ackell, P.H. Transmural differences in sympathetic coronary constriction during exercise in the presence of coronary stenosis. Circ. Res. 1988, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Kaski, J.C.; Lerman, A. Coronary microvascular dysfunction in the clinical setting: From mystery to reality. Eur. Heart J. 2012, 33, 2771–2783. [Google Scholar] [CrossRef]
- Tiefenbacher, C.P.; Chilian, W.P. Heterogeneity of coronary vasomotion. Basic Res. Cardiol. 1998, 93, 446–454. [Google Scholar] [CrossRef]
- Westerhof, N.; Boer, C.; Lamberts, R.R.; Sipkema, P. Cross- Talk Between Cardiac Muscle and Coronary Vasculature. Physiol. Rev. 2006, 86, 1263–1308. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.M.; Tune, J.D. Role of potassium channels in coronary vasodilation. Exp. Biol. Med. 2010, 235, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Bao, L.; Kefaloyianni, E.; Taskin, E.; Okorie, U.; Hong, M.; Dhar-Chowdhury, P.; Kaneko, M.; Coetzee, W.A. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J. Mol. Cell. Cardiol. 2012, 52, 410–418. [Google Scholar] [CrossRef]
- Camici, P.G.; D’Amati, G.; Rimoldi, O. Coronary microvascular dysfunction: Mechanisms and functional assessment. Nat. Rev. Cardiol. 2015, 12, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; Berry, C. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020, cvaa006. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Tschöpe, C.; Carli, M.F.D.; Rimoldi, O.; Van Linthout, S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc. Res. 2020, cvaa023. [Google Scholar] [CrossRef] [PubMed]
- Berwick, Z.C.; Moberly, S.P.; Kohr, M.C.; Morrical, E.B.; Kurian, M.M.; Dick, G.M.; Tune, J.D. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic Res. Cardiol. 2012, 107, 264. [Google Scholar] [CrossRef]
- Standen, N.B.; Quayle, J.M. K+ channel modulation in arterial smooth muscle. Acta Physiol. Scand. 1998, 164, 549–557. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium Channels in the Peripheral Microcirculation. Microcirculation 2005, 12, 113–127. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on coronary microvascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2020, cvaa003. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Miličić, D.; Jakuš, N.; Fabijanović, D. Microcirculation and Heart Failure. Curr. Pharm. Des. 2018, 24, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Olson, T.P.; Lam, C.S.; Flood, K.S.; Lerman, A.; Johnson, B.D.; Redfield, M.M. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010, 56, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Shah, K.B.; Kop, W.J.; Christenson, R.H.; Diercks, D.B.; Henderson, S.; Hanson, K.; Li, S.Y.; de Filippi, C.R. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin. Chem. 2011, 57, 874–882. [Google Scholar] [CrossRef]
- Matsubara, J.; Sugiyama, S.; Nozaki, T.; Sugamura, K.; Konishi, M.; Ohba, K.; Matsuzawa, Y.; Akiyama, E.; Yamamoto, E.; Sakamoto, K.; et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 861–869. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef]
- Edelmann, F.; Gelbrich, G.; Düngen, H.D.; Fröhling, S.; Wachter, R.; Stahrenberg, R.; Binder, L.; Töpper, A.; Lashki, D.J.; Schwarz, S.; et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 2011, 58, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Kohr, M.J.; Davis, J.P.; Ziolo, M.T. Peroxynitrite increases protein phosphatase activity and promotes the interaction of phospholamban with protein phosphatase 2a in the myocardium. Nitric Oxide 2009, 20, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Calderone, A.; Thaik, C.M.; Takahashi, N.; Chang, D.L.; Colucci, W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Investig. 1998, 101, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Perino, A.; Ghigo, A.; Hirsch, E.; Shah, A.M. NADPH oxidases in heart failure: Poachers or gamekeepers? Antioxid. Redox Signal. 2013, 18, 1024–1041. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Adamo, F.; Severino, P. Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction: The Misleading Definition of the New Guidelines. Cardiol. Rev. 2017, 25, 4–5. [Google Scholar] [CrossRef]
- Severino, P.; Mather, P.J.; Pucci, M.; D’Amato, A.; Mariani, M.V.; Infusino, F.; Birtolo, L.I.; Maestrini, V.; Mancone, M.; Fedele, F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics 2019, 9, 170. [Google Scholar] [CrossRef]
- Fedele, F.; Severino, P.; Calcagno, S.; Mancone, M. Heart Failure: TNM-like classification. J. Am. Coll. Cardiol. 2014, 63, 1959–1960. [Google Scholar] [CrossRef]
- Severino, P.; Maestrini, V.; Mariani, M.V.; Birtolo, L.I.; Scarpati, R.; Mancone, M.; Fedele, F. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail. Rev. 2020, 25, 9–17. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Priest, B.T.; McDermott, J.S. Cardiac ion channels. Channels 2015, 9, 352–359. [Google Scholar] [CrossRef]
- Zhang, X.; Yoon, J.Y.; Morley, M.; McLendon, J.M.; Mapuskar, K.A.; Gutmann, R.; Mehdi, H.; Bloom, H.L.; Dudley, S.C.; Ellinor, P.T.; et al. A common variant alters SCN5A-miR-24 interaction and associates with heart failure mortality. J. Clin. Investig. 2018, 128, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Doshi, D.; Marx, S.O. Ion channels, transporters, and pumps as targets for heart failure therapy. J. Cardiovasc. Pharmacol. 2009, 54, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Baartscheer, A.; Schumacher, C.A.; van Borren, M.M.; Belterman, C.N.; Coronel, R.; Fiolet, J.W. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc. Res. 2003, 57, 1015–1024. [Google Scholar] [CrossRef]
- Baartscheer, A.; Hardziyenka, M.; Schumacher, C.A.; Belterman, C.N.; van Borren, M.M.; Verkerk, A.O.; Coronel, R.; Fiolet, J.W. Chronic inhibition of the Na+/H+—Exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br. J. Pharmacol. 2008, 154, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Janse, M.J. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004, 61, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Nabauer, M.; Kaab, S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998, 37, 324–334. [Google Scholar] [CrossRef]
- Kraljevic, J.; Høydal, M.A.; Ljubkovic, M.; Moreira, J.B.; Jørgensen, K.; Ness, H.O.; Bækkerud, F.H.; Dujic, Z.; Wisløff, U.; Marinovic, J. Role of KATP Channels in Beneficial Effects of Exercise in Ischemic Heart Failure. Med. Sci. Sports Exerc. 2015, 47, 2504–2512. [Google Scholar] [CrossRef]
- Kane, G.C.; Behfar, A.; Dyer, R.B.; O’Cochlain, D.F.; Liu, X.K.; Hodgson, D.M.; Reyes, S.; Miki, T.; Seino, S.; Terzic, A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum. Mol. Genet. 2006, 15, 2285–2297. [Google Scholar] [CrossRef]
- Olson, T.M.; Terzic, A. Human KATP channelopathies: Diseases of metabolic homeostasis. Pflugers Arch. Eur. J. Physiol. 2010, 460, 295–306. [Google Scholar] [CrossRef]
- Zlatkovic, J.; Arrell, D.K.; Kane, G.C.; Miki, T.; Seino, S.; Terzic, A. Proteomic profiling of KATP channel-deficient hypertensive heart maps risk for maladaptive cardiomyopathic outcome. Proteomics 2009, 9, 1314–1325. [Google Scholar] [CrossRef]
- Reyes, S.; Park, S.; Johnson, B.D.; Terzic, A.; Olson, T.M. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum. Genet. 2009, 126, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kane, G.C.; Liu, X.K.; Yamada, S.; Olson, T.M.; Terzic, A. Cardiac KATP channels in health and disease. J. Mol. Cell. Cardiol. 2005, 38, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.J.; Steckley, D.C.; Light, P.E. Current status of the E23K Kir6.2 polymorphism: Implications for type-2 diabetes. Hum. Genet. 2005, 116, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Bienengraeber, M.; Olson, T.M.; Selivanov, V.A.; Kathmann, E.C.; O’Cochlain, F.; Gao, F.; Karger, A.B.; Ballew, J.D.; Hodgson, D.M.; Zingman, L.V.; et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat. Genet. 2004, 36, 382–387. [Google Scholar] [CrossRef]
- Wang, X.; Fitts, R.H. Effects of regular exercise on ventricular myocyte biomechanics and KATP channel function. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H885–H896. [Google Scholar] [CrossRef]
- Foster, M.N.; Coetzee, W.A. KATP channels in the cardiovascular system. Physiol. Rev. 2016, 96, 177–252. [Google Scholar] [CrossRef]
- Nichols, C.G. KATP channels as molecular sensors of cellular metabolism. Nature 2006, 440, 470–476. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive Oxygen Species (ROS)-induced ROS Release: A New Phenomenon Accompanying Induction of the Mitochondrial Permeability Transition in Cardiac Myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Kudo, M.; Xu, M.; Ayub, A.; Ashraf, M. Mitochondrial K(ATP) channel as an end effector of cardioprotection during late preconditioning: Triggering role of nitric oxide. J. Mol. Cell. Cardiol. 2001, 33, 2037–2046. [Google Scholar] [CrossRef]
- Guo, Y.; Jones, W.K.; Xuan, Y.T.; Tang, X.L.; Bao, W.; Wu, W.J.; Han, H.; Laubach, V.E.; Ping, P.; Yang, Z.; et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 11507–11512. [Google Scholar] [CrossRef] [PubMed]
- Rakhit, R.D.; Edwards, R.J.; Marber, M.S. Nitric oxide, nitrates and ischaemic preconditioning. Cardiovasc. Res. 1999, 43, 621–627. [Google Scholar] [CrossRef][Green Version]
- Maack, C.; Dabew, E.R.; Hohl, M.; Schäfers, H.J.; Böhm, M. Endogenous activation of mitochondrial KATP channels protects human failing myocardium from hydroxyl radical-induced stunning. Circ. Res. 2009, 105, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Talukder, M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef]
- Bolli, R.; Marban, E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999, 79, 609–634. [Google Scholar] [CrossRef]
- Valgimigli, M.; Merli, E.; Malagutti, P.; Soukhomovskaia, O.; Cicchitelli, G.; Antelli, A.; Canistro, D.; Francolini, G.; Macri, G.; Mastrorilli, F.; et al. Hydroxyl radical generation, levels of tumor necrosis factor alpha, and progression to heart failure after acute myocardial infarction. J. Am. Coll. Cardiol. 2004, 43, 2000–2008. [Google Scholar] [CrossRef]
- Costa, A.D.; Pierre, S.V.; Cohen, M.V.; Downey, J.M.; Garlid, K.D. cGMP signalling in pre- and post-conditioning: The role of mitochondria. Cardiovasc. Res. 2008, 77, 344–352. [Google Scholar] [CrossRef]
- Ardehali, H.; O’Rourke, B. Mitochondrial K (ATP) channels in cell survival and death. J. Mol. Cell. Cardiol. 2005, 39, 7–16. [Google Scholar] [CrossRef]
- Costa, A.D.T.; Garlid, K.D. Intramitochondrial Signaling: Interactions Among mitoKATP, PKCepsilon, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H874–H882. [Google Scholar] [CrossRef]
- Strutynskyi, R.B.; Voronkov, L.G.; Nagibin, V.S.; Mazur, I.D.; Story, D.; Dosenko, V.E. Changes of the Echocardiographic Parameters in Chronic Heart Failure Patients with Ile337val, Glu23lys, and Ser1369ala Polymorphisms of Genes Encoding the ATP-sensitive Potassium Channels Subunits in the Ukrainian Population. Ann. Hum. Gene 2018, 82, 272–279. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Mariani, M.V.; Cimino, S.; Birtolo, L.I.; Infusino, F.; De Orchi, P.; Palmirotta, R.; et al. Susceptibility to ischemic heart disease: Focusing on genetic variants for ATP-sensitive potassium channel beyond traditional risk factors. Eur. J. Prev. Cardiol. (accepted).
- Ahmed, L.A. Nicorandil: A drug with ongoing benefits and different mechanisms in various diseased conditions. Indian J. Pharmacol. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Herpain, A.; Bouchez, S.; Girardis, M.; Guarracino, F.; Knotzer, J.; Levy, L.; Liebregts, T.; Pollesello, P.; Ricksten, S.E.; Riha, H.; et al. Use of Levosimendan in Intensive Care Unit Settings: An Opinion Paper. J. Cardiovasc. Pharmacol. 2019, 73, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Buerke, M.; Parissis, J.; Ben-Gal, T.; Pollesello, P.; Kivikko, M.; Karavidas, A.; Severino, P.; Comín-Colet, J.; Wikström, G.; et al. Pharmaco-economics of levosimendan in cardiology: A European perspective. Int. J. Cardiol. 2015, 199, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Oliván-Viguera, A.; Sofía Valero, M.; Pinilla, E.; Amor, S.; García-Villalón, Á.L.; Coleman, N.; Laría, C.; Calvín-Tienza, V.; García-Otín, Á.L.; Fernández-Fernández, J.M.; et al. Vascular Reactivity Profile of Novel KCa 3.1-Selective Positive-Gating Modulators in the Coronary Vascular Bed. Basic Clin. Pharmacol. Toxicol. 2016, 119, 184–192. [Google Scholar] [CrossRef]
- Mishra, R.C.; Wulff, H.; Cole, W.C.; Braun, A.P. A Pharmacologic Activator of Endothelial KCa Channels Enhances Coronary Flow in the Hearts of Type 2 Diabetic Rats. J. Mol. Cell. Cardiol. 2014, 72, 364–373. [Google Scholar] [CrossRef]
- Barlow, R.S.; White, R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am. J. Physiol. 1998, 275 (Pt 2), H1283–H1289. [Google Scholar]
- Thengchaisri, N.; Kuo, L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: Role of cyclooxygenase and potassium channels. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2255–H2263. [Google Scholar] [CrossRef]
- Borbouse, L.; Dick, G.M.; Asano, S.; Bender, S.B.; Dincer, U.D.; Payne, G.A.; Neeb, Z.P.; Bratz, I.N.; Sturek, M.; Tune, J.D. Impaired function of coronary BKCa channels in metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1629–H1637. [Google Scholar] [CrossRef]
- Cao, C.M.; Xia, Q.; Gao, Q.; Chen, M.; Wong, T.M. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J. Pharmacol. Exp. Ther. 2005, 312, 644–650. [Google Scholar] [CrossRef]
- Liu, Y.; Terata, K.; Chai, Q.; Li, H.; Kleinman, L.H.; Gutterman, D.D. Peroxynitrite Inhibits Ca2-Activated K Channel Activity in Smooth Muscle of Human Coronary Arterioles. Circ. Res. 2002, 91, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Cao, S.; Chabowski, D.S.; Korishettar, A.; Ge, A.; Zheng, X.; Sparapani, R.; Gutterman, D.D.; Zhang, D.X. Contribution of Kv 1.5 Channel to Hydrogen Peroxide-Induced Human Arteriolar Dilation and Its Modulation by Coronary Artery Disease. Circ. Res. 2017, 120, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Alex, I.M.; Scott, M.B.; Bysani, C.; Douglas, K.B.; Shawn, B.B. Aldosterone Impairs Coronary Adenosine-Mediated Vasodilation via Reduced Functional Expression of Ca 2+-activated K + Channels. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H357–H363. [Google Scholar] [CrossRef] [PubMed]
- Idres, S.; Perrin, G.; Domergue, V.; Lefebvre, F.; Gomez, S.; Varin, A.; Fischmeister, R.; Leblais, V.; Manoury, B. Contribution of BKCa channels to vascular tone regulation by PDE3 and PDE4 is lost in heart failure. Cardiovasc. Res. 2019, 115, 130–144. [Google Scholar] [CrossRef]
- Inoue, T.; Sakai, Y.; Morooka, S.; Hayashi, T.; Takayanagi, K.; Yamaguchi, H.; Kakoi, H.; Takabatake, Y. Vasodilatory capacity of coronary resistance vessels in dilated cardiomyopathy. Am. Heart J. 1994, 127, 376–381. [Google Scholar] [CrossRef]
- Kiuchi, K.; Sato, N.; Shannon, R.P.; Vatner, D.E.; Morgan, K.; Vatner, S.F. Depressed betaadrenergic receptor-and endothelium-mediated vasodilation in conscious dogs with heart failure. Circ. Res. 1993, 73, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, L. Function and regulation of large conductance Ca2þ-activated Kþ channel in vascular smooth muscle cells. Drug Discov. Today 2012, 17, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular determinants of BK channel functional diversity and functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef]
- Wan, E.; Kushner, J.S.; Zakharov, S.; Nui, X.W.; Chudasama, N.; Kelly, C.; Waase, M.; Doshi, D.; Liu, G.; Iwata, S.; et al. Reduced vascular smooth muscle BK channel current underlies heart failure-induced vasoconstriction in mice. FASEB J. 2013, 27, 1859–1867. [Google Scholar] [CrossRef]
- Wang, X.; Fisher, P.W.; Xi, L.; Kukreja, R.C. Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 105–113. [Google Scholar] [CrossRef]
- Sasaki, N.; Sato, T.; Ohler, A.; O’Rourke, B.; Marban, E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation 2000, 101, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Dwenger, M.M.; Ohanyan, V.; Navedo, M.F.; Nystoriak, M.A. Coronary microvascular Kv1 channels as regulatory sensors of intracellular pyridine nucleotide redox potential. Microcirculation 2018, 25. [Google Scholar] [CrossRef]
- Rogers, P.A.; Dick, G.M.; Chilian, W.M. Hydrogen Peroxide (H2O2) Activates Voltage-Gated K+ (KV) Channels in Coronary Smooth Muscle Cells. FASEB J. 2006, 20, A1397. [Google Scholar]
- Guarini, G.; Kiyooka, T.; Ohanyan, V.; Pung, Y.F.; Marzilli, M.; Chen, Y.R.; Chen, C.L.; Kang, P.T.; Hardwick, J.P.; Kolz, C.L.; et al. Impaired Coronary Metabolic Dilation Leads to Heart Failure During Pressure Overload. Circulation 2012, 126, A18543. [Google Scholar] [CrossRef]
- Ohanyan, V.A.; Bratz, I.N.; Guarini, G.; Yin, L.; Chilian, W.M. Kv 1.5 Channels Play a Critical Role in Coronary Metabolic Dilation. Circulation 2010, 122, A16997. [Google Scholar]
- Li, H.; Gutterman, D.D.; Rusch, N.J.; Bubolz, A.; Liu, Y. Nitration and Functional Loss of Voltage-Gated K Channels in Rat Coronary Microvessels Exposed to High Glucose. Diabetes 2004, 53, 2436–2442. [Google Scholar] [CrossRef]
- Liu, Q.; Hua, B.; Su, W.; Di, B.; Yu, S.; Gao, S.; Liu, H.; Zhao, X.; Li, W.; Li, H. AGEs Impair Kv Channel-Mediated Vasodilation of Coronary Arteries by Activating the NF-κB Signaling Pathway in ZDF Rats. Biomed. Pharmacother. 2019, 120, 109527. [Google Scholar] [CrossRef]
- Ohanyan, V.; Yin, L.; Bardakjian, R.; Kolz, C.; Enrick, M.; Hakobyan, T.; Luli, J.; Graham, K.; Khayata, M.; Logan, S.; et al. Kv1.3 Channels Facilitate the Connection Between Metabolism and Blood Flow in the Heart. Microcirculation 2017, 24. [Google Scholar] [CrossRef]
- Berwick, Z.; Dick, G.M.; O’Leary, H.A.; Bender, S.B.; Goodwill, A.G.; Moberly, S.P.; Kohr Owen, M.; Miller, S.J.; Obukhov, A.G.; Tune, J.D. Contribution of Electromechanical Coupling Between Kv and Ca v1.2 Channels to Coronary Dysfunction in Obesity. Basic Res. Cardiol. 2013, 108, 370. [Google Scholar] [CrossRef]
- Morales-Cano, D.; Moreno, L.; Barreira, B.; Pandolfi, R.; Chamorro, V.; Jimenez, R.; Villamor, E.; Duarte, J.; Perez-Vizcaino, F.; Cogolludo, A. Kv7 Channels Critically Determine Coronary Artery Reactivity: Left-Right Differences and Down-Regulation by Hyperglycaemia. Cardiovasc. Res. 2015, 106, 98–108. [Google Scholar] [CrossRef]
- Lavalle, C.; Mariani, M.V.; Piro, A.; Straito, M.; Severino, P.; Della Rocca, D.G.; Forleo, G.B.; Romero, J.; Di Biase, L.; Fedele, F. Electrocardiographic features, mapping and ablation of idiopathic outflow tract ventricular arrhythmias. J. Interv. Card. Electrophysiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Mariani, M.V.; Maraone, A.; Piro, A.; Ceccacci, A.; Tarsitani, L.; Maestrini, V.; Mancone, M.; Lavalle, C.; Pasquini, M.; et al. Triggers for Atrial Fibrillation: The Role of Anxiety. Cardiol. Res. Pract. 2019, 18, 1208505. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Nishijima, Y.; Terentyev, D.; Khan, M.; Terentyeva, R.; Hamlin, R.L.; Nakayama, T.; Gyorke, S.; Cardounel, A.J.; Carnes, C.A. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc. Res. 2009, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shioya, T.; Murayama, T.; Sugihara, M.; Odagiri, F.; Nakazato, Y.; Nishizawa, H.; Chugun, A.; Sakurai, T.; Daida, H.; et al. Multistep ion channel remodeling and lethal arrhythmia precede heart failure in a mouse model of inherited dilated cardiomyopathy. PLoS ONE 2012, 7, e35353. [Google Scholar] [CrossRef] [PubMed]
- Falcón, D.; Galeano-Otero, I.; Martín-Bórnez, M.; Fernández-Velasco, M.; Gallardo-Castillo, I.; Rosado, J.A.; Ordóñez, A.; Smani, T. TRPC Channels: Dysregulation and Ca 2+ Mishandling in Ischemic Heart Disease. Cells 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. Role of TRP Channels in the Cardiovascular System. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef]
- Kochukov, M.Y.; Balasubramanian, A.; Noel, R.C.; Marrelli, S.P. Role of TRPC1 and TRPC3 Channels in Contraction and Relaxation of Mouse Thoracic Aorta. J. Vasc. Res. 2013, 50, 11–20. [Google Scholar] [CrossRef]
- Willette, R.N.; Weike, B.; Nerurkar, S.; Yue, T.L.; Doe, C.P.; Stankus, G.; Turner, G.H.; Ju, H.; Thomas, H.; Fishman, C.E.; et al. Systemic Activation of the Transient Receptor Potential Vanilloid Subtype 4 Channel Causes Endothelial Failure and Circulatory Collapse: Part 2. J. Pharmacol. Exp. Ther. 2008, 326, 443–452. [Google Scholar] [CrossRef]
- Freichel, M.; Suh, S.H.; Pfeifer, A.; Schweig, U.; Trost, C.; Weissgerber, P.; Biel, M.; Philipp, S.; Freise, D.; Droogmans, G.; et al. Lack of an Endothelial Store-Operated Ca2+ Current Impairs Agonist-Dependent Vasorelaxation in TRP4-/- Mice. Nat. Cell Biol. 2001, 3, 121–127. [Google Scholar] [CrossRef]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 Forms a Novel Ca2+ Signaling Complex with Ryanodine Receptors and BKCa Channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef]
- Inoue, R.; Jian, Z.; Kawarabayashi, Y. Mechanosensitive TRP Channels in Cardiovascular Pathophysiology. Pharmacol. Ther. 2009, 123, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Mathar, I.; Vennekens, R.; Meissner, M.; Kees, F.; Van der Mieren, G.; Camacho, L.; Juan, E.; Uhl, S.; Voets, T.; Hummel, B.; et al. Increased Catecholamine Secretion Contributes to Hypertension in TRPM4-deficient Mice. J. Clin. Investig. 2010, 120, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient Receptor Potential Channels Regulate Myogenic Tone of Resistance Arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ching, L.C.; Zhao, J.F.; Shyue, S.K.; Lee, H.F.; Kou, Y.R.; Lee, T.S. Essential Role of Transient Receptor Potential Vanilloid Type 1 in Evodiamine-Mediated Protection Against Atherosclerosis. Acta Physiol. 2013, 207, 299–307. [Google Scholar] [CrossRef]
- Jung, C.; Gené, G.G.; Tomás, M.; Plata, C.; Selent, J.; Pastor, M.; Fandos, C.; Senti, M.; Lucas, G.; Elosua, R.; et al. A Gain-Of-Function SNP in TRPC4 Cation Channel Protects Against Myocardial Infarction. Cardiovasc. Res. 2011, 91, 465–471. [Google Scholar] [CrossRef]
- Severino, P.; Netti, L.; Mariani, M.V.; Maraone, A.; D’Amato, A.; Scarpati, R.; Infusino, F.; Pucci, M.; Lavalle, C.; Maestrini, V.; et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019, 2, 4874921. [Google Scholar] [CrossRef]
- Severino, P.; Mariani, M.V.; Fedele, F. Futility in cardiology: The need for a change in perspectives. Eur. J. Heart Fail. 2019, 21, 1483–1484. [Google Scholar] [CrossRef]
- Basoli, A.; Cametti, C.; Satriani, F.G.; Mariani, P.; Severino, P. Hemocompatibility of stent materials: Alterations in electrical parameters of erythrocyte membranes. Vasc. Health Risk Manag. 2012, 8, 197–204. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Birtolo, L.I.; Mariani, M.V.; Lavalle, C.; Maestrini, V.; Mancone, M.; Fedele, F. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. Int. J. Mol. Sci. 2020, 21, 3167. https://doi.org/10.3390/ijms21093167
Severino P, D’Amato A, Pucci M, Infusino F, Birtolo LI, Mariani MV, Lavalle C, Maestrini V, Mancone M, Fedele F. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. International Journal of Molecular Sciences. 2020; 21(9):3167. https://doi.org/10.3390/ijms21093167
Chicago/Turabian StyleSeverino, Paolo, Andrea D’Amato, Mariateresa Pucci, Fabio Infusino, Lucia Ilaria Birtolo, Marco Valerio Mariani, Carlo Lavalle, Viviana Maestrini, Massimo Mancone, and Francesco Fedele. 2020. "Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels" International Journal of Molecular Sciences 21, no. 9: 3167. https://doi.org/10.3390/ijms21093167
APA StyleSeverino, P., D’Amato, A., Pucci, M., Infusino, F., Birtolo, L. I., Mariani, M. V., Lavalle, C., Maestrini, V., Mancone, M., & Fedele, F. (2020). Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. International Journal of Molecular Sciences, 21(9), 3167. https://doi.org/10.3390/ijms21093167






