Novel Insights into the Pathogenesis of Spinal Sarcopenia and Related Therapeutic Approaches: A Narrative Review
Abstract
1. Introduction
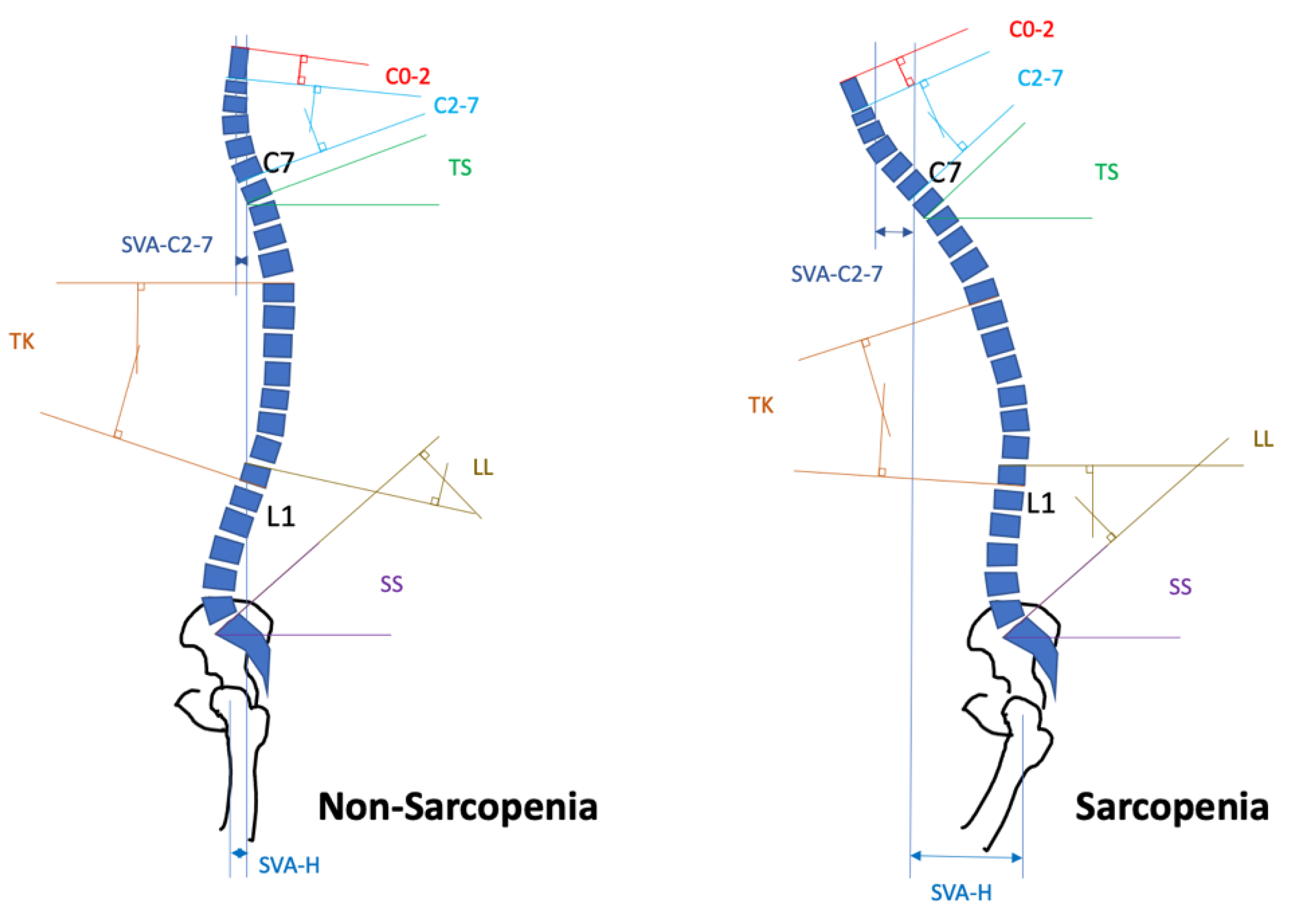
2. Progression and Characteristics of Spinal Sarcopenia
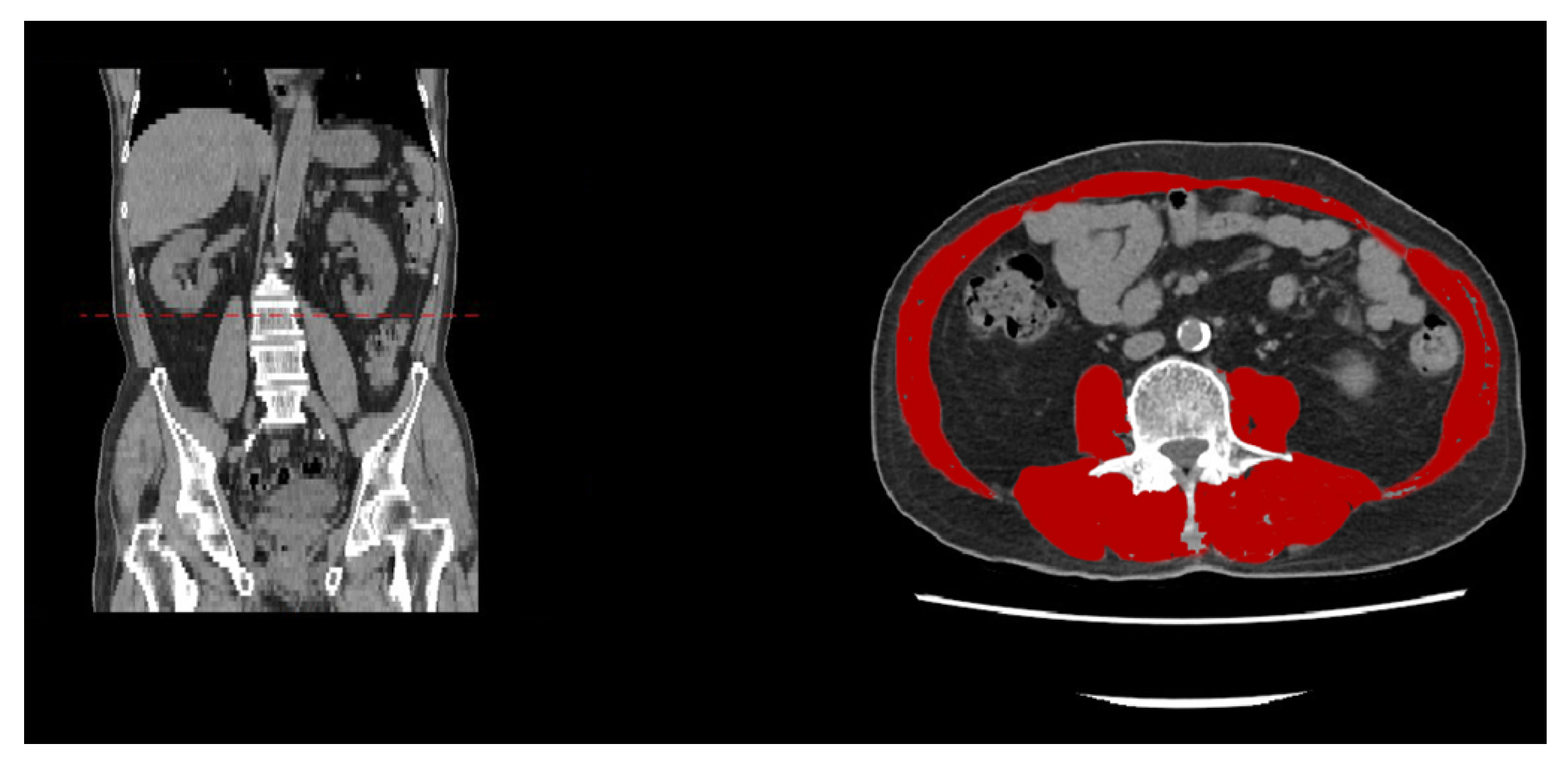
3. Image Detection Methodologies for Spinal Sarcopenia
4. Impact of Spinal Sarcopenia on Surgical Interventions for Spinal Disorders
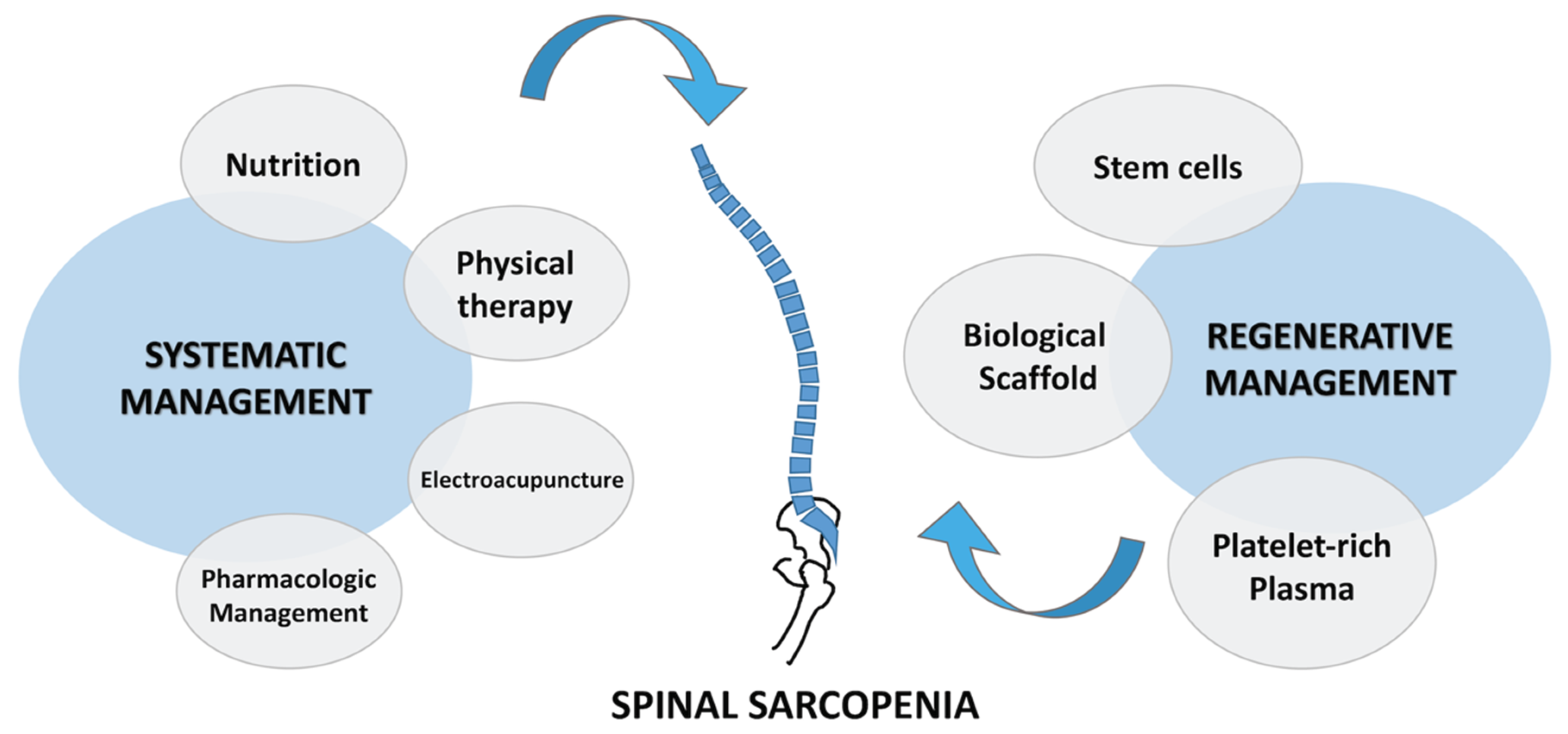
5. Conventional Treatment of Spinal Sarcopenia
5.1. Nutrition Strategy and Antiinflammatory Medication
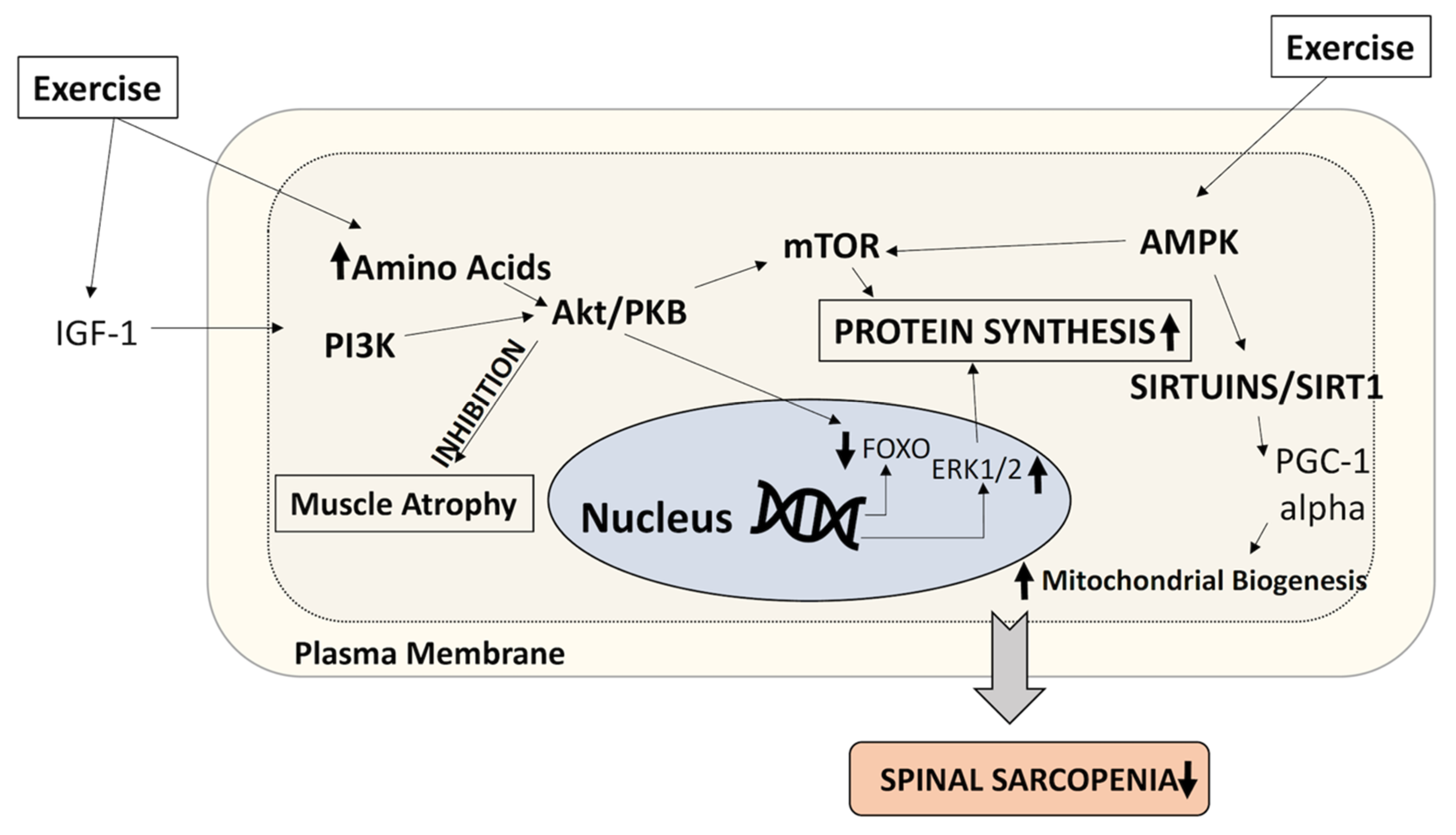
5.2. Physical Therapy and Resistance Excercise
5.3. Aerobic Training
5.4. Electroacupuncture
5.5. Pharmacologic Management
6. Regenerative Therapeutic Approaches for Spinal Sarcopenia
6.1. Stem Cells
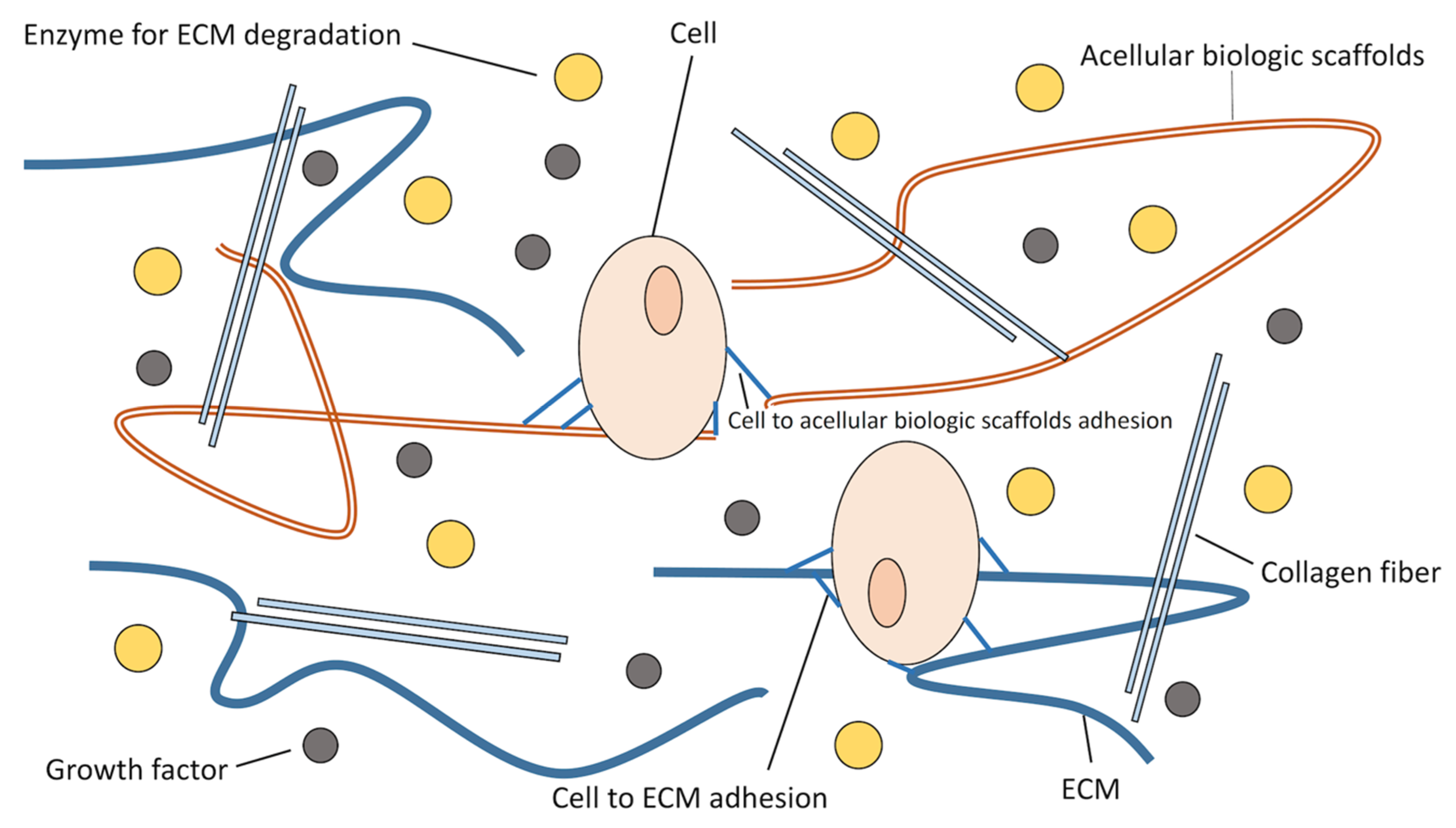
6.2. Biologic Scaffolds
6.3. Platelet-Rich Plasma
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, T.W.; Lee, S.W.; Leung, J.; Kwok, T.; Woo, J. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yuki, A.; Ando, F.; Otsuka, R.; Shimokata, H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all-cause mortality risk in older Japanese adults. Geriatr. Gerontol. Int. 2017, 17, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-J.; Kim, Y.-H. Factors Affecting Sarcopenia in Korean Adults by Age Groups. Osong Public Health Res. Perspect. 2017, 8, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Brzeszczynska, J.; Meyer, A.; McGregor, R.; Schilb, A.; Degen, S.; Tadini, V.; Johns, N.; Langen, R.; Schols, A.; Glass, D.J.; et al. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J. Cachexia Sarcopenia Muscle 2018, 9, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Htun, N.C.; Ishikawa-Takata, K.; Kuroda, A.; Tanaka, T.; Kikutani, T.; Obuchi, S.P.; Hirano, H.; Iijima, K. Screening for Malnutrition in Community Dwelling Older Japanese: Preliminary Development and Evaluation of the Japanese Nutritional Risk Screening Tool (NRST). J. Nutr. Health Aging 2016, 20, 114–120. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Chang, C.K.; Li, T.C.; Lin, C.C. Comparison of height- and weight-adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr. Gerontol. Int. 2015, 15, 45–53. [Google Scholar] [CrossRef]
- Yamada, M.; Nishiguchi, S.; Fukutani, N.; Tanigawa, T.; Yukutake, T.; Kayama, H.; Aoyama, T.; Arai, H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J. Am. Med. Dir. Assoc. 2013, 14, 911–915. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y.; Han, F. Prevalence of sarcopenia in the community-dwelling, elderly Chinese population: A systematic review and meta-analysis. Lancet 2017, 390, S35. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Giles, J.T.; Ling, S.M.; Ferrucci, L.; Bartlett, S.J.; Andersen, R.E.; Towns, M.; Muller, D.; Fontaine, K.R.; Bathon, J.M. Abnormal body composition phenotypes in older rheumatoid arthritis patients: Association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008, 59, 807–815. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol. Biol. Sci. Med. Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef]
- Witham, M.D.; Aihie Sayer, A. Introduction to the Age and Ageing sarcopenia collection. Age Ageing 2016, 45, 752–753. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Mayr, R.; Gierth, M.; Zeman, F.; Reiffen, M.; Seeger, P.; Wezel, F.; Pycha, A.; Comploj, E.; Bonatti, M.; Ritter, M.; et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 505–513. [Google Scholar] [CrossRef]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- White, T.; White, T.A.; LeBrasseur, N.K. Myostatin and Sarcopenia: Opportunities and Challenges—A Mini-Review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. The effect of glucocorticoids on bone and muscle. Osteoporosis Sarcopenia 2015, 1, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Sarcopenic Obesity: The Confluence of Two Epidemics. Obes. Res. 2004, 12, 887–888. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Sharma, M.; Kambadur, R.; Matthews, K.G.; Somers, W.G.; Devlin, G.P.; Conaglen, J.V.; Fowke, P.J.; Bass, J.J. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell Physiol. 1999, 180, 1–9. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Bhasin, S.; Sinha-Hikim, I.; Pak-Loduca, J.; Gonzalez-Cadavid, N.F. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J. Nutr. Health Aging 2002, 6, 343–348. [Google Scholar]
- Gonzalez-Freire, M.; Scalzo, P.; D’Agostino, J.; Moore, Z.A.; Diaz-Ruiz, A.; Fabbri, E.; Zane, A.; Chen, B.; Becker, K.G.; Lehrmann, E.; et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 2018, 17. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Sgarioto, N.; Kapchinsky, S.; Purves-Smith, F.; Norris, B.; Pion, C.H.; Barbat-Artigas, S.; Lemieux, F.; Taivassalo, T.; Morais, J.A.; et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014, 28, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Trounce, I.; Byrne, E.; Marzuki, S. Decline in skeletal muscle mitochondrial respiratory chain function: Possible factor in ageing. Lancet 1989, 1, 637–639. [Google Scholar] [CrossRef]
- Tonkonogi, M.; Fernstrom, M.; Walsh, B.; Ji, L.L.; Rooyackers, O.; Hammarqvist, F.; Wernerman, J.; Sahlin, K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003, 446, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.-P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr) 2015, 37, 121. [Google Scholar] [CrossRef] [PubMed]
- Kirchengast, S.; Johannes, H. Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol. Anz. Bericht über die Biol. Anthropol. Lit. 2009, 67, 139–151. [Google Scholar] [CrossRef]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Lenrow, D.A.; Holmes, J.H.; Dlewati, A.; Santanna, J.; Rosen, C.J.; et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999, 84, 2647–2653. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef]
- Bakhshi, V.; Elliott, M.; Gentili, A.; Godschalk, M.; Mulligan, T. Testosterone improves rehabilitation outcomes in ill older men. J. Ame. Geriatr. Soc. 2000, 48, 550–553. [Google Scholar] [CrossRef]
- Mudali, S.; Dobs, A.S. Effects of testosterone on body composition of the aging male. Mech. Ageing Dev. 2004, 125, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Messier, V.; Rabasa-Lhoret, R.; Barbat-Artigas, S.; Elisha, B.; Karelis, A.D.; Aubertin-Leheudre, M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011, 68, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Hoshino, M.; Ohyama, S.; Terai, H.; Suzuki, A.; Yamada, K.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; et al. The association of back muscle strength and sarcopenia-related parameters in the patients with spinal disorders. Eur. Spine J. 2019, 28, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Suzuki, M.; Yamanaka, H.; Tamai, H.; Kobayashi, T.; Orita, S.; Yamauchi, K.; Suzuki, M.; Inage, K.; Fujimoto, K.; et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord. 2017, 12, 9. [Google Scholar] [CrossRef]
- Hiyama, A.; Katoh, H.; Sakai, D.; Sato, M.; Tanaka, M.; Nukaga, T.; Watanabe, M. Correlation analysis of sagittal alignment and skeletal muscle mass in patients with spinal degenerative disease. Sci. Rep. 2018, 8, 15492. [Google Scholar] [CrossRef]
- Eguchi, Y.; Toyoguchi, T.; Inage, K.; Fujimoto, K.; Orita, S.; Suzuki, M.; Kanamoto, H.; Abe, K.; Norimoto, M.; Umimura, T.; et al. Analysis of skeletal muscle mass in women over 40 with degenerative lumbar scoliosis. Eur. Spine J. 2018. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, S.-U.; Jung, S.H.; Lim, J.-Y.; Kim, D.H.; Lee, S.Y. Natural aging course of paraspinal muscle and back extensor strength in community-dwelling older adults (sarcopenia of spine, SarcoSpine): A prospective cohort study protocol. BMJ Open 2019, 9, e032443. [Google Scholar] [CrossRef]
- Sakai, Y.; Matsui, H.; Ito, S.; Hida, T.; Ito, K.; Koshimizu, H.; Harada, A. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia 2017, 3, 195–200. [Google Scholar] [CrossRef]
- Sasaki, E.; Sasaki, S.; Chiba, D.; Yamamoto, Y.; Nawata, A.; Tsuda, E.; Nakaji, S.; Ishibashi, Y. Age-related reduction of trunk muscle torque and prevalence of trunk sarcopenia in community-dwelling elderly: Validity of a portable trunk muscle torque measurement instrument and its application to a large sample cohort study. PLoS ONE 2018, 13, e0192687. [Google Scholar] [CrossRef]
- Takayama, K.; Kita, T.; Nakamura, H.; Kanematsu, F.; Yasunami, T.; Sakanaka, H.; Yamano, Y. New Predictive Index for Lumbar Paraspinal Muscle Degeneration Associated With Aging. Spine 2016, 41, E84–E90. [Google Scholar] [CrossRef]
- Aftzoglou, P. Sarcopenia and Falls in Patients with Adult Scoliosis. J. Frailty Sarcopenia Falls 2017, 2, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Storheim, K.; Holm, I.; Gunderson, R.; Brox, J.I.; Bo, K. The effect of comprehensive group training on cross-sectional area, density, and strength of paraspinal muscles in patients sick-listed for subacute low back pain. J. Spinal Disord. Tech. 2003, 16, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Danneels, L.A.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; De Cuyper, H.J. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J. 2000, 9, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lim, W.H.; Park, J.-W.; Kwon, B.S.; Ryu, K.H.; Lee, J.H.; Park, Y.G. The Relationship between Cross Sectional Area and Strength of Back Muscles in Patients with Chronic Low Back Pain. Ann. Rehabil. Med. 2012, 36, 173–181. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Ko, B.G.; Chung, J.W.; Kim, S.H.; Park, S.H.; Lee, M.H.; Yeom, J.S. The prevalence and impact of sarcopenia on degenerative lumbar spinal stenosis. Bone Jt. J. 2016, 98-b, 1093–1098. [Google Scholar] [CrossRef]
- Kumagai, G.; Wada, K.; Kudo, H.; Asari, T.; Chiba, D.; Ota, S.; Takeda, O.; Koyama, K.; Nakaji, S.; Ishibashi, Y. Associations between cervical disc degeneration and muscle strength in a cross-sectional population-based study. PLoS ONE 2019, 14, e0210802. [Google Scholar] [CrossRef]
- Ignasiak, D.; Valenzuela, W.; Reyes, M.; Ferguson, S.J. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur. Spine J. 2018, 27, 2650–2659. [Google Scholar] [CrossRef]
- Ohyama, S.; Hoshino, M.; Terai, H.; Toyoda, H.; Suzuki, A.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; Nakamura, H. Sarcopenia is related to spinal sagittal imbalance in patients with spinopelvic mismatch. Eur. Spine J. 2019, 28, 1929–1936. [Google Scholar] [CrossRef]
- Shimizu, T.; Lehman, R.A.; Sielatycki, J.A.; Pongmanee, S.; Cerpa, M.; Takemoto, M.; Lenke, L.G. Reciprocal change of sagittal profile in unfused spinal segments and lower extremities after complex adult spinal deformity surgery including spinopelvic fixation: A full-body X-ray analysis. Spine J. 2020, 20, 380–390. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Visser, M.; Ma, R.; Baumgartner, R.N.; Kotler, D.; Gallagher, D.; Heymsfield, S.B. Skeletal muscle mass: Evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J. Appl. Physiol. 1996, 80, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Lee, E.J.; Chung, H.S.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, K.M. Comparisons of three different methods for defining sarcopenia: An aspect of cardiometabolic risk. Sci. Rep. 2017, 7, 6491. [Google Scholar] [CrossRef] [PubMed]
- Albanese, C.V.; Diessel, E.; Genant, H.K. Clinical applications of body composition measurements using DXA. J. Clin. Densitom. 2003, 6, 75–85. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Dumler, F. Use of bioelectric impedance analysis and dual-energy X-ray absorptiometry for monitoring the nutritional status of dialysis patients. ASAIO J. 1997, 43, 256–260. [Google Scholar]
- Kang, S.H.; Cho, K.H.; Park, J.W.; Yoon, K.W.; Do, J.Y. Comparison of bioimpedance analysis and dual-energy X-ray absorptiometry body composition measurements in peritoneal dialysis patients according to edema. Clin. Nephrol. 2013, 79, 261–268. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Morrell, G.R.; Ikizler, T.A.; Chen, X.; Heilbrun, M.E.; Wei, G.; Boucher, R.; Beddhu, S. Psoas Muscle Cross-sectional Area as a Measure of Whole-body Lean Muscle Mass in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2016, 26, 258–264. [Google Scholar] [CrossRef]
- Rutten, I.J.G.; Ubachs, J.; Kruitwagen, R.; Beets-Tan, R.G.H.; Olde Damink, S.W.M.; Van Gorp, T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Psoas as a sentinel muscle for sarcopenia: A flawed premise. J. Cachexia Sarcopenia Muscle 2017, 8, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Baracos, V.; Kazemi-Bajestani, S.M. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int. J. Biochem. Cell Biol. 2013, 45, 2302–2308. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef]
- Lee, K.; Shin, Y.; Huh, J.; Sung, Y.S.; Lee, I.S.; Yoon, K.H.; Kim, K.W. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J. Radiol. 2019, 20, 205–217. [Google Scholar] [CrossRef]
- Lee, S.J.; Janssen, I.; Heymsfield, S.B.; Ross, R. Relation between whole-body and regional measures of human skeletal muscle. Am. J. Clin. Nutr. 2004, 80, 1215–1221. [Google Scholar] [CrossRef]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Loftin, M.; Fukunaga, T. Prevalence of site-specific thigh sarcopenia in Japanese men and women. Age (Dordr) 2014, 36, 417–426. [Google Scholar] [CrossRef]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Gluer, C.C.; Bosy-Westphal, A.; Muller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.; Debigare, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Cauley, J.A.; Tylavsky, F.; Bauer, D.; Cummings, S.; Harris, T.B.; Health, A.B.C.S. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The health, aging, and body composition study. J. Bone Miner. Res. 2010, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Kohara, K.; Tabara, Y.; Kido, T.; Uetani, E.; Ochi, N.; Igase, M.; Miki, T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 2010, 212, 327–332. [Google Scholar] [CrossRef]
- Gu, D.H.; Kim, M.Y.; Seo, Y.S.; Kim, S.G.; Lee, H.A.; Kim, T.H.; Jung, Y.K.; Kandemir, A.; Kim, J.H.; An, H.; et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin. Mol. Hepatol. 2018, 24, 319–330. [Google Scholar] [CrossRef]
- Hsu, J.; Krishnan, A.; Lin, C.T.; Shah, P.D.; Broderick, S.R.; Higgins, R.S.D.; Merlo, C.A.; Bush, E.L. Sarcopenia of the Psoas Muscles Is Associated With Poor Outcomes Following Lung Transplantation. Ann. Thorac. Surg. 2019, 107, 1082–1088. [Google Scholar] [CrossRef]
- Hanaoka, M.; Yasuno, M.; Ishiguro, M.; Yamauchi, S.; Kikuchi, A.; Tokura, M.; Ishikawa, T.; Nakatani, E.; Uetake, H. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int. J. Colorectal Dis. 2017, 32, 847–856. [Google Scholar] [CrossRef]
- Peng, P.D.; van Vledder, M.G.; Tsai, S.; de Jong, M.C.; Makary, M.; Ng, J.; Edil, B.H.; Wolfgang, C.L.; Schulick, R.D.; Choti, M.A.; et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2011, 13, 439–446. [Google Scholar] [CrossRef]
- Valero, V., 3rd; Amini, N.; Spolverato, G.; Weiss, M.J.; Hirose, K.; Dagher, N.N.; Wolfgang, C.L.; Cameron, A.A.; Philosophe, B.; Kamel, I.R.; et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J. Gastrointest. Surg. 2015, 19, 272–281. [Google Scholar] [CrossRef]
- McCusker, A.; O’Keeffe, T.; Khan, M.; Ahmed, F.S.; Kulvatunyou, N.; Tang, A.L.; Gries, L.M.; Joseph, B. Sarcopenia Defined by Computed Tomography (CT) Psoas Muscle Area Does Not Predict Frailty in Trauma Patients. J. Am. Coll. Surg. 2017, 225, S61. [Google Scholar] [CrossRef][Green Version]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Suzuki, M.; Yamanaka, H.; Tamai, H.; Kobayashi, T.; Orita, S.; Yamauchi, K.; Suzuki, M.; Inage, K.; Fujimoto, K.; et al. Influence of Skeletal Muscle Mass and Spinal Alignment on Surgical Outcomes for Lumbar Spinal Stenosis. Asian Spine J. 2018, 12, 556–562. [Google Scholar] [CrossRef]
- Inose, H.; Yamada, T.; Hirai, T.; Yoshii, T.; Abe, Y.; Okawa, A. The impact of sarcopenia on the results of lumbar spinal surgery. Osteoporos. Sarcopenia 2018, 4, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Charest-Morin, R.; Street, J.; Zhang, H.; Roughead, T.; Ailon, T.; Boyd, M.; Dvorak, M.; Kwon, B.; Paquette, S.; Dea, N.; et al. Frailty and sarcopenia do not predict adverse events in an elderly population undergoing non-complex primary elective surgery for degenerative conditions of the lumbar spine. Spine J. 2018, 18, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, H.; Anderson, D.E. The Role of Trunk Musculature in Osteoporotic Vertebral Fractures: Implications for Prediction, Prevention, and Management. Curr. Osteoporos. Rep. 2016, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Park, Y.; Ha, J.W.; Zhang, H.Y.; Lee, S.H.; Hong, T.H.; Lee, S.H. Paraspinal Lean Muscle Mass Measurement Using Spine MRI as a Predictor of Adjacent Segment Disease After Lumbar Fusion: A Propensity Score-Matched Case-Control Analysis. AJR. Am. J. Roentgenol. 2019, 1–8. [Google Scholar] [CrossRef]
- Koshimizu, H.; Sakai, Y.; Harada, A.; Ito, S.; Ito, K.; Hida, T. The Impact of Sarcopenia on Cervical Spine Sagittal Alignment After Cervical Laminoplasty. Clin. Spine Surg. 2018, 31, E342–E346. [Google Scholar] [CrossRef]
- Moskven, E.; Bourassa-Moreau, E.; Charest-Morin, R.; Flexman, A.; Street, J. The impact of frailty and sarcopenia on postoperative outcomes in adult spine surgery. A systematic review of the literature. Spine J. 2018, 18, 2354–2369. [Google Scholar] [CrossRef]
- Gakhar, H.; Dhillon, A.; Blackwell, J.; Hussain, K.; Bommireddy, R.; Klezl, Z.; Williams, J. Study investigating the role of skeletal muscle mass estimation in metastatic spinal cord compression. Spine J. 2015, 24, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Lattanzio, F.; Abbatecola, A.M.; Carpia, D.L.; Tosato, M.; Marzetti, E.; Calvani, R.; Onder, G.; Landi, F. Treating sarcopenia in older and oldest old. Curr. Pharm. Des. 2015, 21, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.Y.; Khow, K.S.F.; Jadczak, A.D.; Visvanathan, R. Clinical Screening Tools for Sarcopenia and Its Management. Curr. Gerontol. Geriatr. Res. 2016, 2016, 5978523. [Google Scholar] [CrossRef]
- Robinson, S.; Cooper, C.; Sayer, A.A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. In Clinical Nutrition and Aging; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 3–15. [Google Scholar]
- Van Pelt, R.E.; Dinneno, F.A.; Seals, D.R.; Jones, P.P. Age-related decline in RMR in physically active men: Relation to exercise volume and energy intake. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E633–E639. [Google Scholar] [CrossRef]
- Raguso, C.A.; Kyle, U.; Kossovsky, M.P.; Roynette, C.; Paoloni-Giacobino, A.; Hans, D.; Genton, L.; Pichard, C. A 3-year longitudinal study on body composition changes in the elderly: Role of physical exercise. Clin. Nutr. 2006, 25, 573–580. [Google Scholar] [CrossRef]
- Mitchell, D.; Haan, M.N.; Steinberg, F.M.; Visser, M. Body composition in the elderly: The influence of nutritional factors and physical activity. J. Nutr. Health Aging 2003, 7, 130–139. [Google Scholar]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef]
- Hughes, V.A.; Roubenoff, R.; Wood, M.; Frontera, W.R.; Evans, W.J.; Fiatarone Singh, M.A. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004, 80, 475–482. [Google Scholar] [CrossRef]
- Johnston, A.P.; De Lisio, M.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 2008, 33, 191–199. [Google Scholar] [CrossRef]
- Kanis, J.A.; Gluer, C.C. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000, 11, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. (Seoul) 2018, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-W.; No, M.-H.; Min, D.-H.; Kang, J.-H.; Kwak, H.-B. Aging-induced Sarcopenia and Exercise. J. Korean Acad. Kinesiol. 2017, 19, 43–59. [Google Scholar] [CrossRef]
- Erlich, A.T.; Tryon, L.D.; Crilly, M.J.; Memme, J.M.; Moosavi, Z.S.M.; Oliveira, A.N.; Beyfuss, K.; Hood, D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr. Med. Res. 2016, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Singh, H.; Carter, S.J.; Bryan, D.R.; Fisher, G. Sarcopenia and Its Implications for Metabolic Health. J. Obes. 2019, 2019, 10. [Google Scholar] [CrossRef]
- Martinez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Lopez Saez de Asteasu, M.; Lucia, A.; Galbete, A.; Garcia-Baztan, A.; Alonso-Renedo, J.; Gonzalez-Glaria, B.; Gonzalo-Lazaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern. Med. 2018. [Google Scholar] [CrossRef]
- De Souza Orlandi, F.; Brochine Lanzotti, R.; Gomes Duarte, J.; Novais Mansur, H.; Zazzetta, M.S.; Iost Pavarini, S.C.; Cominetti, M.R.; Matumoto, S. Translation, Adaptation and Validation of Rapid Geriatric Assessment to the Brazilian context. J. Nutr. Health Aging 2018, 22, 1115–1121. [Google Scholar] [CrossRef]
- Morley, J.E.; Little, M.O.; Berg-Weger, M. Rapid Geriatric Assessment: A Tool for Primary Care Physicians. J. Am. Med. Dir. Assoc. 2017, 18, 195–199. [Google Scholar] [CrossRef]
- Little, M.O. The Rapid Geriatric Assessment: A Quick Screen for Geriatric Syndromes. Mol. Med. 2017, 114, 101–104. [Google Scholar]
- Jin, W.S.; Choi, E.J.; Lee, S.Y.; Bae, E.J.; Lee, T.H.; Park, J. Relationships among Obesity, Sarcopenia, and Osteoarthritis in the Elderly. J. Obes. Metab. Syndr. 2017, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, Y.; Ohta, M.; Ito, A.; Takamatsu, K.; Sugano, A.; Funakoshi, K.; Takaoka, N.; Sato, N.; Yokozaki, H.; Arizono, N.; et al. Electroacupuncture suppresses myostatin gene expression: Cell proliferative reaction in mouse skeletal muscle. Physiol. Genom. 2007, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Robinson, A.; Hu, L.; Klein, J.D.; Hassounah, F.; Li, M.; Wang, H.; Cai, H.; Wang, X.H. Acupuncture plus Low-Frequency Electrical Stimulation (Acu-LFES) Attenuates Diabetic Myopathy by Enhancing Muscle Regeneration. PLoS ONE 2015, 10, e0134511. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Klein, J.D.; Hassounah, F.; Cai, H.; Zhang, C.; Xu, P.; Wang, X.H. Low-frequency electrical stimulation attenuates muscle atrophy in CKD—A potential treatment strategy. J. Am. Soc. Nephrol. 2015, 26, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Soares Mendes Damasceno, G.; Teixeira, T.H.M.M.; de Souza, V.C.; Neiva, T.S.; Prudente Pereira, K.; Teles Landim, M.d.F.; de Melo, G.F.; Romão, J.d.F.F.E.; Tolêdo Nóbrega, O.; de Azevedo Carvalho, G. Acupuncture Treatment in Elderly People with Sarcopenia: Effects on the Strength and Inflammatory Mediators. J. Aging Res. 2019, 2019, 8483576. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and age-related endocrine function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Novel intriguing strategies attenuating to sarcopenia. J. Aging Res. 2012, 2012, 251217. [Google Scholar] [CrossRef]
- Maggio, M.; Lauretani, F.; Ceda, G.P. Sex hormones and sarcopenia in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 3–13. [Google Scholar] [CrossRef]
- Kim, K.; Ahn, N.; Jung, S.; Ju, Y.; Lee, G.; Kim, M.; Jeong, Y. Effects of Resistance Exercise and Fermented Soybean Consumption on Glucose Tolerance and Expressions of Immune Senescence-Related Myokines in Middle-Aged Obese Rats. J. Obes. Metab. Syndr. 2018, 27, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, R.T.; Donnelly, E.; Cody, D.B.; Bauer, M.B.; Isfort, R.J. Urocortin II treatment reduces skeletal muscle mass and function loss during atrophy and increases nonatrophying skeletal muscle mass and function. Endocrinology 2003, 144, 4939–4946. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.D.; Dziki, J.L.; Badylak, S.F. Regenerative Medicine Approaches for Age-Related Muscle Loss and Sarcopenia: A Mini-Review. Gerontology 2017, 63, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef]
- Montarras, D.; Morgan, J.; Collins, C.; Relaix, F.; Zaffran, S.; Cumano, A.; Partridge, T.; Buckingham, M. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005, 309, 2064–2067. [Google Scholar] [CrossRef]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef]
- Mueller, G.M.; O’Day, T.; Watchko, J.F.; Ontell, M. Effect of injecting primary myoblasts versus putative muscle-derived stem cells on mass and force generation in mdx mice. Hum. Gene Ther. 2002, 13, 1081–1090. [Google Scholar] [CrossRef]
- Dellavalle, A.; Maroli, G.; Covarello, D.; Azzoni, E.; Innocenzi, A.; Perani, L.; Antonini, S.; Sambasivan, R.; Brunelli, S.; Tajbakhsh, S.; et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2011, 2, 499. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Shadrach, J.L.; Wagers, A.J. Stem cells for skeletal muscle repair. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kang, J.; Baik, S.H. Treatment of faecal incontinence using allogeneic-adipose-derived mesenchymal stem cells: A study protocol for a pilot randomised controlled trial. BMJ Open 2016, 6, e010450. [Google Scholar] [CrossRef] [PubMed]
- Wilschut, K.J.; Ling, V.B.; Bernstein, H.S. Concise review: Stem cell therapy for muscular dystrophies. Stem Cells Transl. Med. 2012, 1, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Von Roth, P.; Duda, G.N.; Radojewski, P.; Preininger, B.; Strohschein, K.; Rohner, E.; Perka, C.; Winkler, T. Intra-Arterial MSC Transplantation Restores Functional Capacity After Skeletal Muscle Trauma. Open Orthop. J. 2012, 6, 352–356. [Google Scholar] [CrossRef][Green Version]
- Rybalko, V.; Hsieh, P.L.; Ricles, L.M.; Chung, E.; Farrar, R.P.; Suggs, L.J. Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair. Regen Med. 2017, 12, 153–167. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef]
- Barberi, L.; Scicchitano, B.M.; De Rossi, M.; Bigot, A.; Duguez, S.; Wielgosik, A.; Stewart, C.; McPhee, J.; Conte, M.; Narici, M.; et al. Age-dependent alteration in muscle regeneration: The critical role of tissue niche. Biogerontology 2013, 14, 273–292. [Google Scholar] [CrossRef]
- Fuoco, C.; Petrilli, L.L.; Cannata, S.; Gargioli, C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. Res. 2016, 11, 86. [Google Scholar] [CrossRef][Green Version]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra258. [Google Scholar] [CrossRef] [PubMed]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tarnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, K.; Villarreal, A.; Gottschalk, A.; Tokish, J.M.; Choate, W.S. Treatment of Muscle Injuries with Platelet-Rich Plasma: A Review of the Literature. Curr. Rev. Musculoskelet Med. 2018, 11, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.S.A.; Yusof, A.; Mohamed Ali, M.R. Platelet-rich plasma (PRP) for acute muscle injury: A systematic review. PLoS ONE 2014, 9, e90538. [Google Scholar] [CrossRef] [PubMed]
- Sheth, U.; Dwyer, T.; Smith, I.; Wasserstein, D.; Theodoropoulos, J.; Takhar, S.; Chahal, J. Does Platelet-Rich Plasma Lead to Earlier Return to Sport When Compared With Conservative Treatment in Acute Muscle Injuries? A Systematic Review and Meta-analysis. Arthroscopy 2018, 34, 281–288.e281. [Google Scholar] [CrossRef]
- Harmon, K.G.; Rao, A.L. The use of platelet-rich plasma in the nonsurgical management of sports injuries: Hype or hope? Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 620–626. [Google Scholar] [CrossRef]
- Dimauro, I.; Grasso, L.; Fittipaldi, S.; Fantini, C.; Mercatelli, N.; Racca, S.; Geuna, S.; Di Gianfrancesco, A.; Caporossi, D.; Pigozzi, F.; et al. Platelet-rich plasma and skeletal muscle healing: A molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS ONE 2014, 9, e102993. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-K.; Lin, Y.-C.; Lee, C.-Y.; Chen, C.-Y.; Tani, J.; Huang, T.-J.; Chang, H.; Wu, M.-H. Novel Insights into the Pathogenesis of Spinal Sarcopenia and Related Therapeutic Approaches: A Narrative Review. Int. J. Mol. Sci. 2020, 21, 3010. https://doi.org/10.3390/ijms21083010
Kuo Y-K, Lin Y-C, Lee C-Y, Chen C-Y, Tani J, Huang T-J, Chang H, Wu M-H. Novel Insights into the Pathogenesis of Spinal Sarcopenia and Related Therapeutic Approaches: A Narrative Review. International Journal of Molecular Sciences. 2020; 21(8):3010. https://doi.org/10.3390/ijms21083010
Chicago/Turabian StyleKuo, Yu-Kai, Yu-Ching Lin, Ching-Yu Lee, Chih-Yu Chen, Jowy Tani, Tsung-Jen Huang, Hsi Chang, and Meng-Huang Wu. 2020. "Novel Insights into the Pathogenesis of Spinal Sarcopenia and Related Therapeutic Approaches: A Narrative Review" International Journal of Molecular Sciences 21, no. 8: 3010. https://doi.org/10.3390/ijms21083010
APA StyleKuo, Y.-K., Lin, Y.-C., Lee, C.-Y., Chen, C.-Y., Tani, J., Huang, T.-J., Chang, H., & Wu, M.-H. (2020). Novel Insights into the Pathogenesis of Spinal Sarcopenia and Related Therapeutic Approaches: A Narrative Review. International Journal of Molecular Sciences, 21(8), 3010. https://doi.org/10.3390/ijms21083010






