Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols
Abstract
1. Introduction
2. Results and Discussion
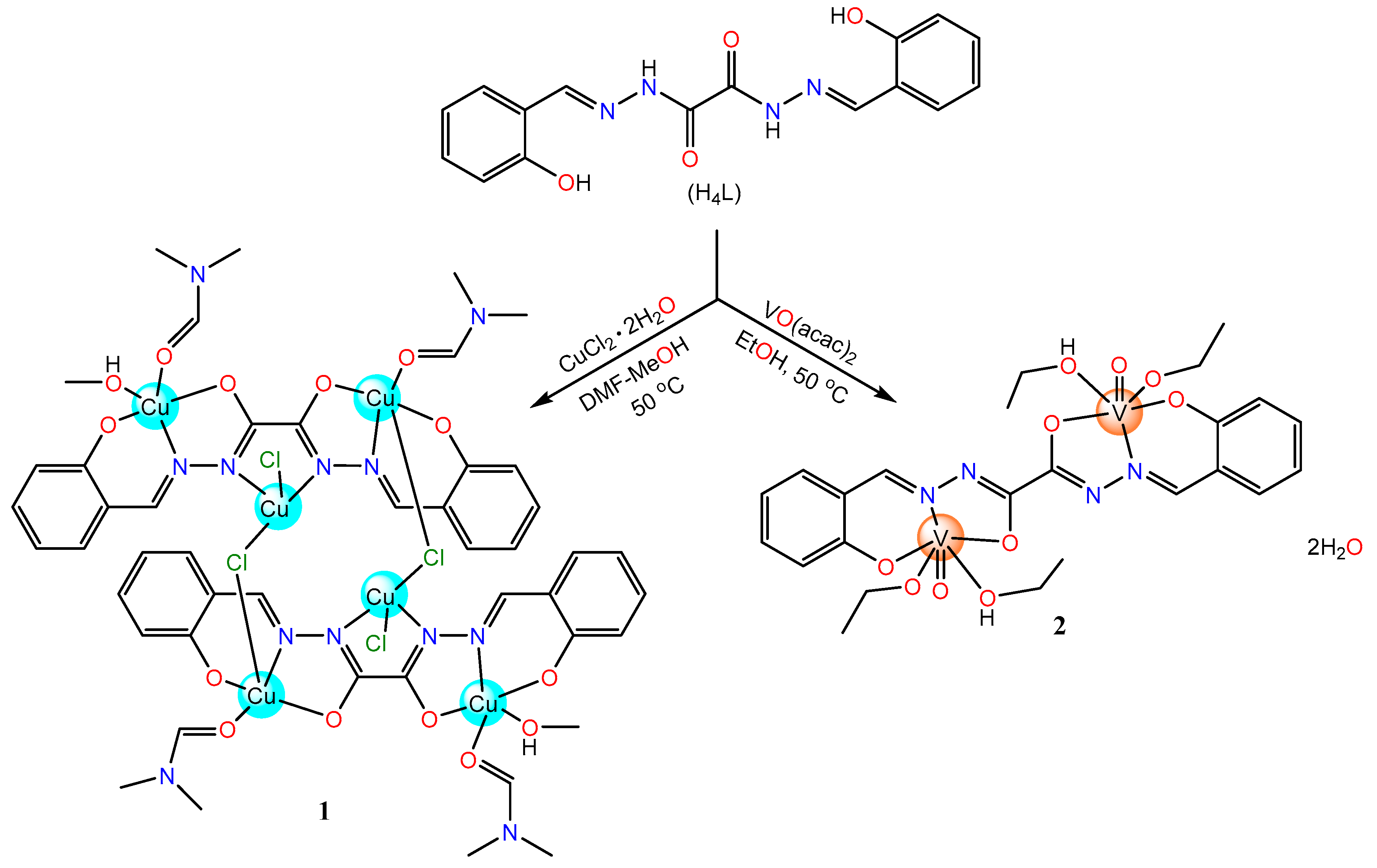
2.1. Synthesis and Structural Characterization
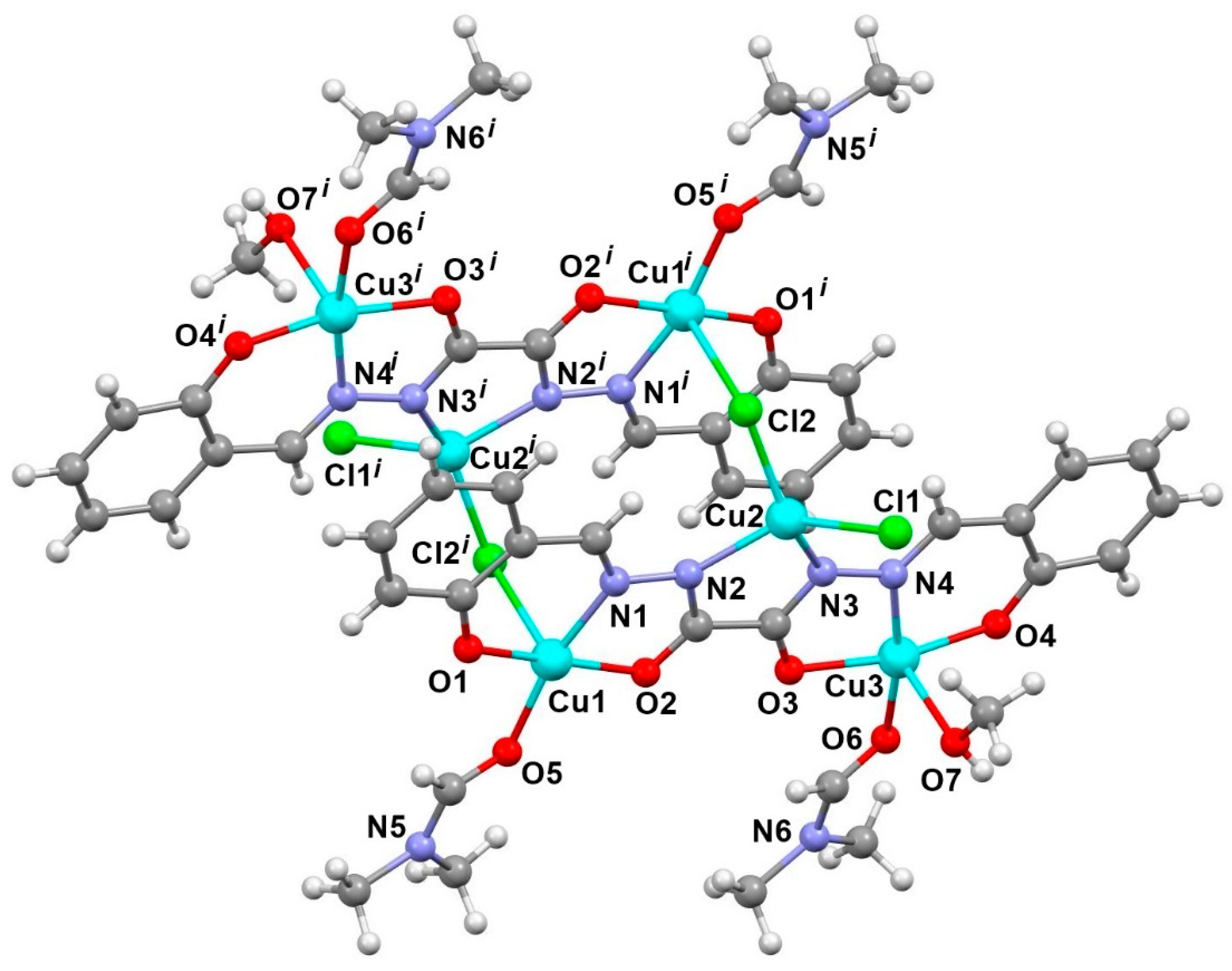
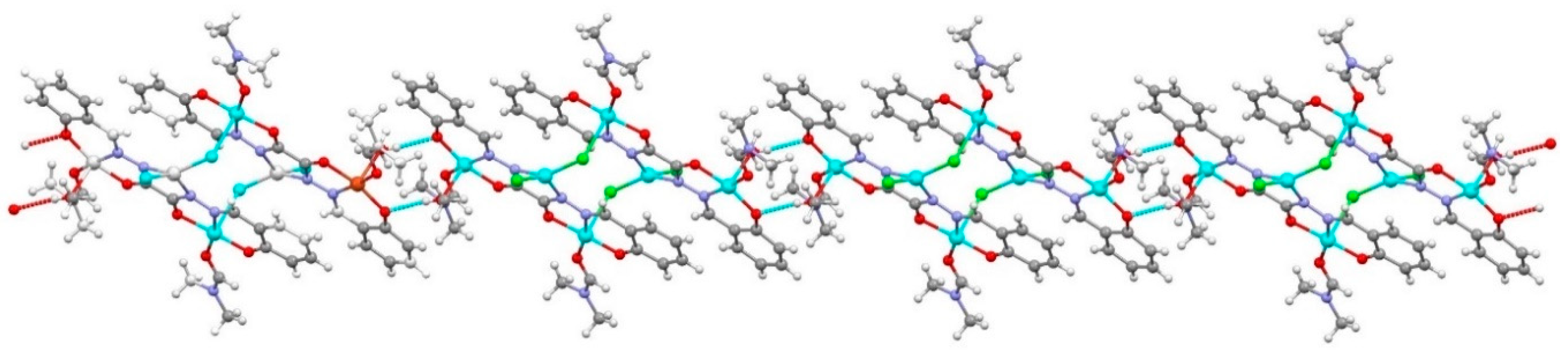
2.2. General Description of the Crystal Structure
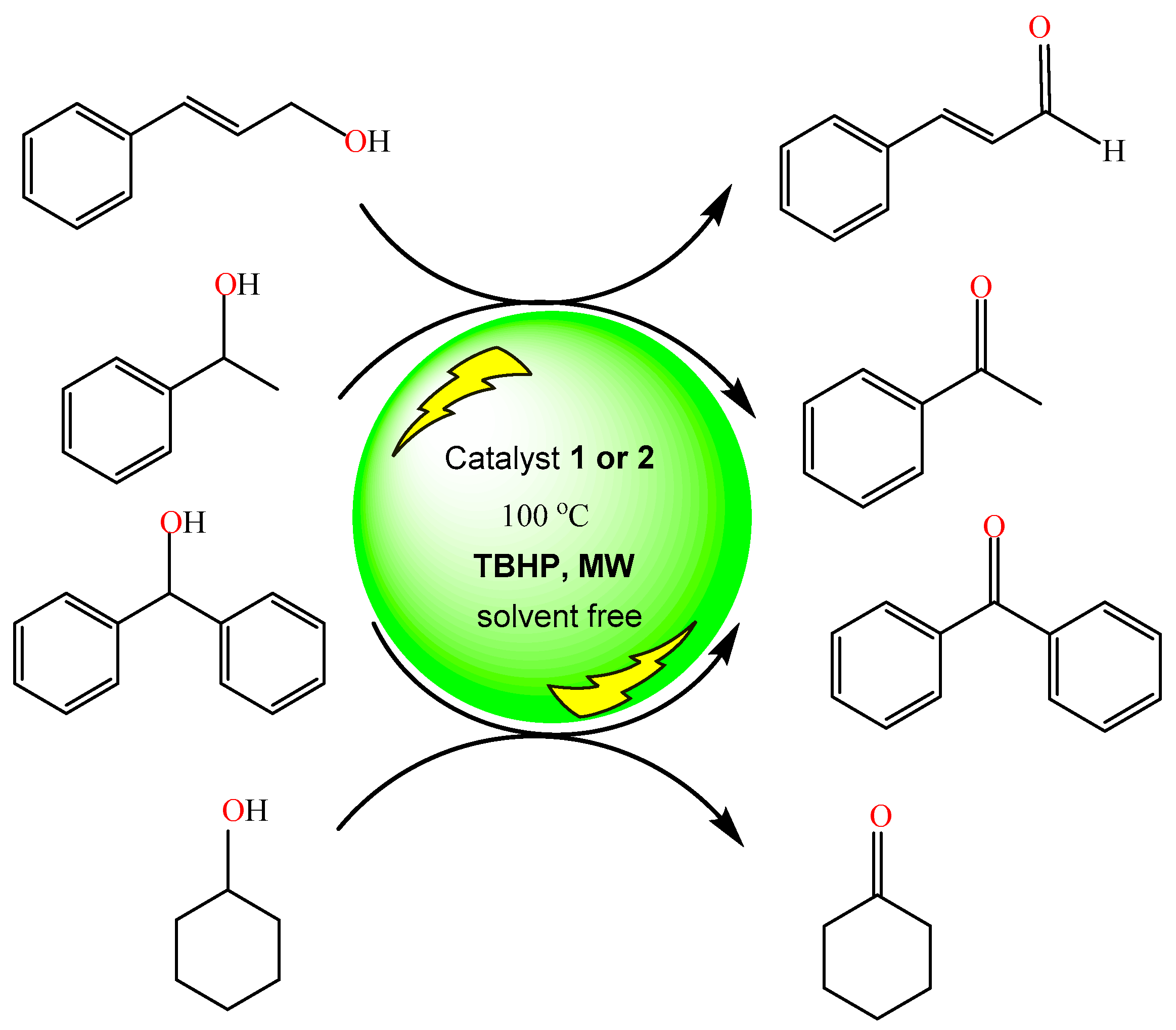
2.3. Catalytic Studies
3. Materials and Methods
3.1. Syntheses of the Pro-Ligand H4L
3.2. Synthesis of [Cu3(μ2-1κNO2,2κNO2-L)(μ-Cl)2(Cl)(MeOH)(DMF)2]2 (1)
3.3. Synthesis of [{VO(OEt)(EtOH)}2(1κNO2,2κNO2-L)]·2H2O (2)
3.4. X-ray Measurements
3.5. Catalytic Studies
Typical Procedures for the Catalytic Oxidation of Alcohols and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301–302, 200–239. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L. Coordination chemistry of non-oxido, oxido and dioxidovanadium(IV/V) complexes with azine fragment ligands. Coord. Chem. Rev. 2014, 265, 89–124. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A hexanuclear mixed-valence oxovanadium (IV,V) complex as a highly efficient alkane oxidation catalyst. Inorg. Chem. 2012, 51, 11229–11231. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) complexes in keto form: Structural characterization and catalytic activity towards cyclohexane oxidation. Molecules 2016, 21, 425. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 2015, 3959–3969. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Tautomeric effect of hydrazone Schiff bases in tetranuclear Cu(II) complexes: Magnetism and catalytic activity towards mild hydrocarboxylation of alkanes. Dalton Trans. 2013, 42, 16578–16587. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxido vanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohol. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Alegria, E.C.B.A.; Liu, C.-M.; Pombeiro, A.J.L. Dinuclear Mn(II,II) complexes: Magnetic properties and microwave assisted oxidation of alcohols. Dalton Trans. 2014, 43, 3966–3977. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Iron(III) and cobalt(III) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016, 6, 8079–8088. [Google Scholar] [CrossRef]
- Sutradhar, M.; Carrella, L.M.; Rentschler, E. A discrete μ4-oxido tetranuclear iron(III) cluster. Eur. J. Inorg. Chem. 2012, 2012, 4273–4278. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Roy Barman, T.; Scorcelletti, F.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enol aroylhydrazone Cu(II) complexes. Mol. Catal. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Catalytic activity of polynuclear vs. dinuclear Aroylhydrazone Cu(II) complexes in microwave-assisted oxidation of neat aliphatic and aromatic hydrocarbons. Molecules 2019, 24, 47. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Ni(II)-aroylhydrazone complexes as catalyst Precursors towards efficient solvent-free Nitroaldol condensation Reaction. Catalysts 2019, 9, 554. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Roy Barman, T.; Kuznetsov, M.L.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes of different nuclearities in the catalytic oxidation of cyclohexane and cyclohexanol—An experimental and theoretical investigation. New J. Chem. 2019, 43, 17557–17570. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Ten Brink, G.J.; Dijksman, A.G. Catalytic Oxidations of Alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition MetalCatalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-scorpionate complexes: Ever young catalytic tools. Coord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic Oxidation of Cyclohexane with Hydrogen Peroxide and a Tetracopper(II) Complex in an Ionic Liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Gupta, S.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Highly efficient divanadium(V) pre-catalyst for mild oxidation of liquid and gaseous alkanes. Appl. Catal. A 2013, 460–461, 82–89. [Google Scholar] [CrossRef]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel Coordination Polymers with (Pyrazolato)-based Tectons: Catalytic Activity in the Peroxidative Oxidation of Alcohols and Cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M.; Omran, A.A. Copper(II)-oxaloyldihydrazone complexes: Physico-chemical studies: Energy band gap and inhibition evaluation of free oxaloyldihydrazones toward the corrosion of copper metal in acidic medium. Arabian J. Chem. 2019, 12, 4287–4302. [Google Scholar] [CrossRef]
- Marko, I.E.; Giles, P.R.; Tsukazaki, M.; Brown, S.M. Copper-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones: An E_cient, Aerobic Alternative. Science 1996, 274, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C. Comprehensive Organic Transformations; Wiley-VCH: New York, NY, USA, 1999. [Google Scholar]
- Wang, X.; Wu, G.; Wei, W.; Sun, Y. Solvent-free oxidation of alcohols by hydrogen peroxide over chromium Schiff base complexes immobilized on MCM-41. Trans. Met. Chem. 2010, 35, 213–220. [Google Scholar] [CrossRef]
- Farhadi, S.; Zaidi, M. Polyoxometalate–zirconia (POM/ZrO2) nanocomposite prepared by sol-gel process: A green and recyclable photocatalyst for efficient and selective aerobic oxidation of alcohols into aldehydes and ketones. Appl. Catal. A 2009, 354, 119–126. [Google Scholar] [CrossRef]
- Brühne, F.; Wright, E. Benzaldehyde, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 5, pp. 223–233. [Google Scholar]
- Lee, D.G.; Spitzer, U.A. Aqueous dichromate oxidation of primary alcohols. J. Org. Chem. 1970, 35, 3589–3590. [Google Scholar] [CrossRef]
- Prabhakaran, P.V.; Venkatachalam, S.; Ninan, K.N. Permanganate ion supported over crosslinked polyvinylamine as an oxidising agent for alcohols. Eur. Polym. J. 1999, 35, 1743–1746. [Google Scholar] [CrossRef]
- Abad, A.; Almela, C.; Corma, A.; García, H. E_cient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 2006, 62, 6666–6672. [Google Scholar] [CrossRef]
- Tojo, G.; Fernandez, M. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer: New York, NY, USA, 2006. [Google Scholar]
- Bailey, J.E.; Bohnet, M. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 1999–2013. [Google Scholar]
- Sutradhar, M.; Roy Barman, T.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Liu, C.-M.; Kou, H.-Z.; Pombeiro, A.J.L. Cu(II) complexes of N-rich aroylhydrazone: Magnetism and catalytic activity towards microwave-assisted oxidation of xylenes. Dalton Trans. 2019, 48, 12839–12849. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Cu(II) and Fe(III) Complexes Derived from N-Acetylpyrazine-2-Carbohydrazide as Efficient Catalysts Towards Neat Microwave Assisted Oxidation of Alcohols. Catalysts 2019, 9, 1053. [Google Scholar] [CrossRef]
- Mardani, H.R.; Golchoubian, H. E_ective oxidation of benzylic and aliphatic alcohols with hydrogen peroxide catalyzed by a manganese(III) Schiff-base complex under solvent-free conditions. Tetrahedron Lett. 2006, 47, 2349–2352. [Google Scholar] [CrossRef]
- Moiseeva, I.N.; Gekham, A.E.; Minin, V.V.; Larin, G.M.; Bashtanov, M.E.; Krasnovskii, A.A.; Moiseev, I.I. Free radical/singlet dioxygen system under the conditions of catalytic hydrogen peroxide decomposition. Kinet. Catal. 2000, 41, 170–177. [Google Scholar] [CrossRef]
- Figiel, P.J.; Leskelä, M.; Repo, T. TEMPO-Copper(II) Diimine-Catalysed Oxidation of Benzylic Alcohols in Aqueous Media. Adv. Synth. Catal. 2007, 349, 1173–1179. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Figiel, P.J.; Räisänen, M.T.; Leskelä, M.; Repo, T. Aerobic oxidation of benzylic alcohols with bis(3,5-di-tert-butylsalicylaldimine)copper(II) complexes. Appl. Catal. A 2009, 371, 17–21. [Google Scholar] [CrossRef]
- Bruker, APEX2 & SAINT; AXS Inc.: Madison, WI, USA, 2004.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A New Tool for Crystal Structure Determination and Refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]

| Parameters | 1 |
|---|---|
| Empirical formula | C23H28Cl2Cu3N6O7 |
| Formula weight | 762.03 |
| Crystal system | Triclinic |
| Space group | P¯1 |
| Temperature/K | 296 (2) |
| a/Å | 7.7742 (9) |
| b/Å | 11.9744 (12) |
| c/Å | 17.287 (2) |
| α/° | 104.505 (6) |
| β/° | 98.430 (6) |
| γ/° | 106.176 (4) |
| V (Å3) | 1455.2 (3) |
| Z | 2 |
| Dcalc (g cm−3) | 1.739 |
| μ (Mo Kα) (mm−1) | 2.41 |
| Rfls. collected/unique/observed | 28371/7251/4657 |
| Rint | 0.082 |
| Final R1 a, wR2 b (I ≥ 2σ) | 0.044, 0.087 |
| Goodness-of-fit on F2 | 1.01 |
| Cu2—N3 | 1.978 (3) | Cu3—O6 | 1.983 (2) |
| Cu2—N2 | 2.009 (3) | Cu3—O7 | 2.321 (3) |
| Cu2—Cl1 | 2.2258 (10) | Cu1—O1 | 1.887 (2) |
| Cu2—Cl2 | 2.2307 (9) | Cu1—N1 | 1.937 (3) |
| Cu3—O4 | 1.904 (2) | Cu1—O5 | 1.953 (2) |
| Cu3—N4 | 1.933 (3) | Cu1—O2 | 1.962 (2) |
| Cu3—O3 | 1.977 (2) | ||
| N3—Cu2—N2 | 80.89 (10) | O3—Cu3—O6 | 90.12 (9) |
| N3—Cu2—Cl1 | 97.27 (8) | O4—Cu3—O7 | 92.69 (11) |
| N2—Cu2—Cl1 | 142.23 (9) | N4—Cu3—O7 | 93.64 (11) |
| N3—Cu2—Cl2 | 147.92 (9) | O3—Cu3—O7 | 98.84 (10) |
| N2—Cu2—Cl2 | 103.72 (8) | O6—Cu3—O7 | 92.74 (11) |
| Cl1—Cu2—Cl2 | 97.50 (4) | O1—Cu1—N1 | 94.45 (10) |
| O4—Cu3—N4 | 93.33 (10) | O1—Cu1—O5 | 93.38 (10) |
| O4—Cu3—O3 | 167.56 (11) | N1—Cu1—O5 | 168.00 (11) |
| N4—Cu3—O3 | 81.34 (10) | O1—Cu1—O2 | 173.56 (11) |
| O4—Cu3—O6 | 94.00 (10) | N1—Cu1—O2 | 81.41 (10) |
| N4—Cu3—O6 | 170.04 (11) | O5—Cu1—O2 | 89.97 (10) |
| Entry | Catalyst | Substrate | Temperature (°C) | Reaction Time (h) | Additive | Yield (%) b | TON (TOF (h−1)) c |
|---|---|---|---|---|---|---|---|
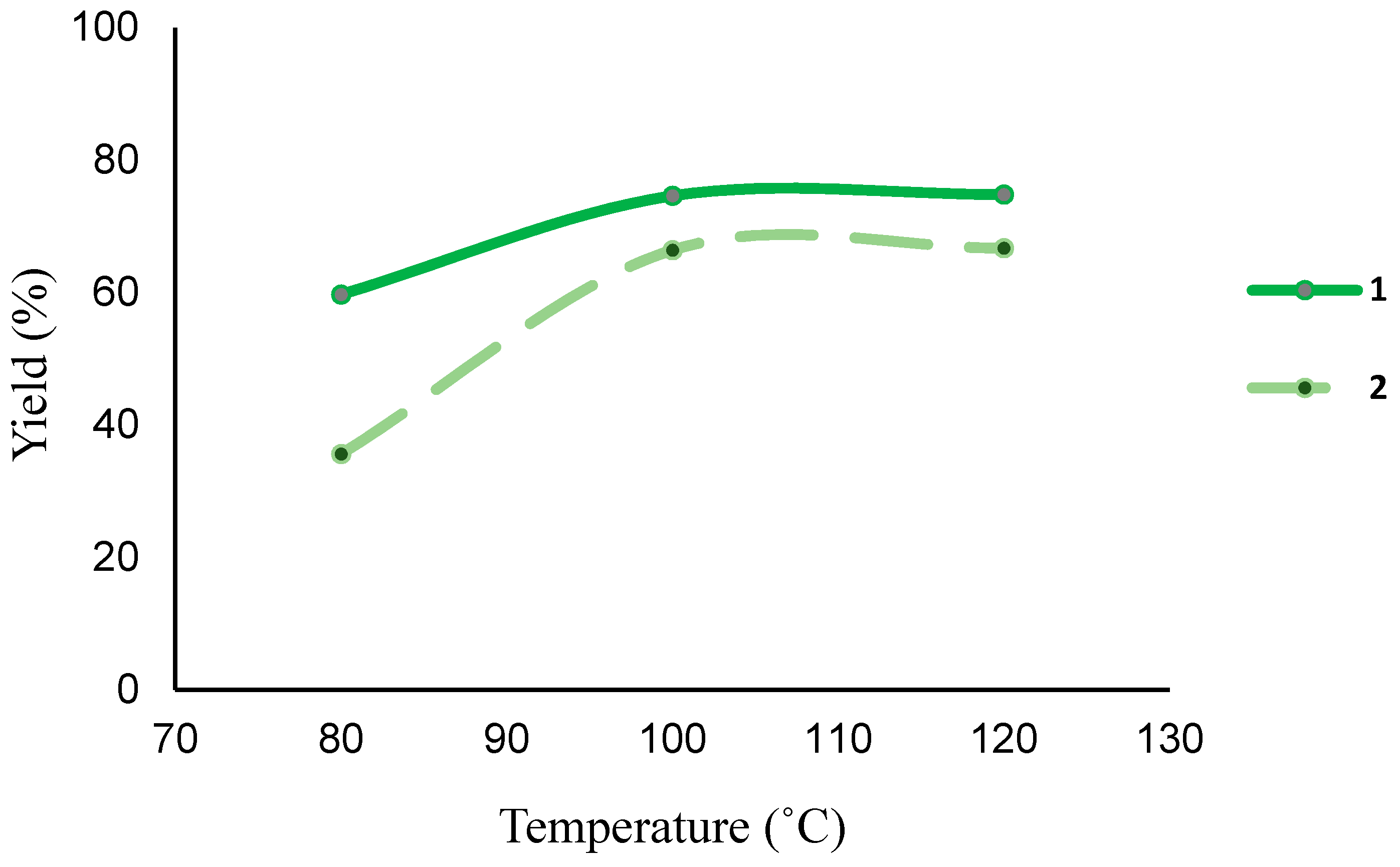
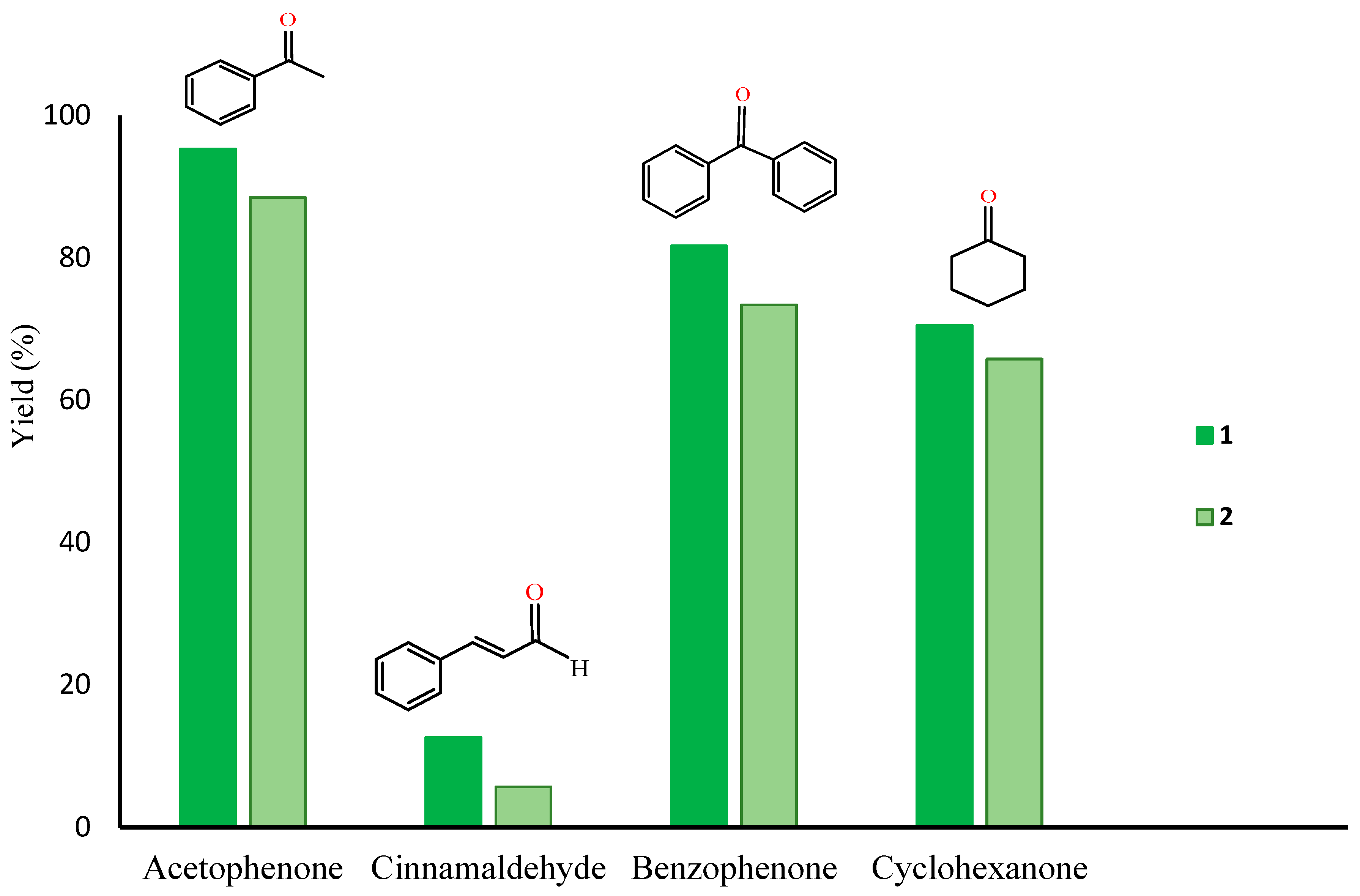
| 1 | 1 | 1-phenyl ethanol | 80 | 0.5 | - | 59.7 | 299 (598) |
| 2 | 100 | 0.5 | - | 74.6 | 373 (746) | ||
| 3 | 120 | 0.5 | - | 74.8 | 374 (748) | ||
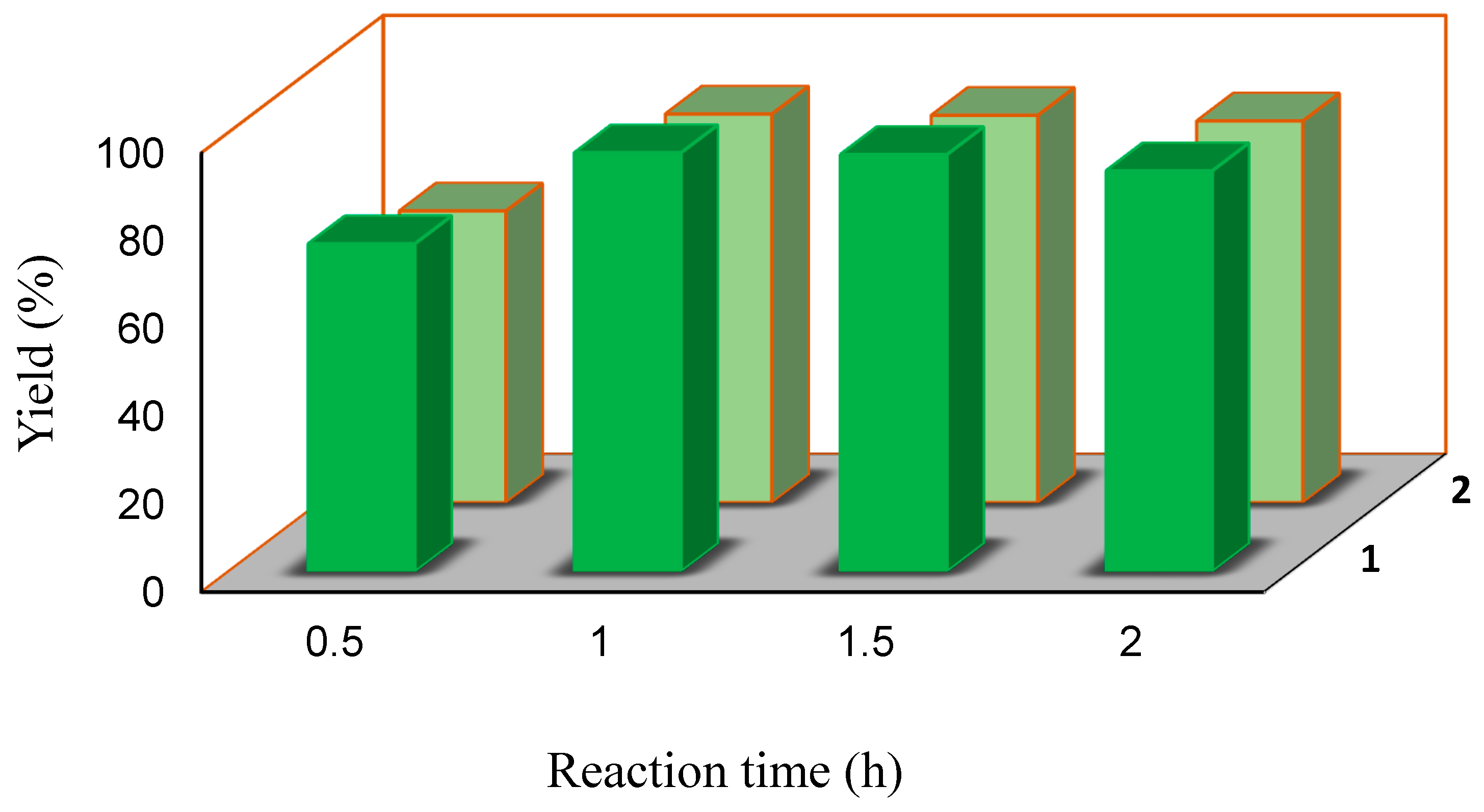
| 4 | 100 | 1.0 | - | 95.3 | 476 (476) | ||
| 5 | 100 | 1.5 | - | 94.9 | 475 (317) | ||
| 6 | 100 | 2.0 | - | 91.2 | 327 (164) | ||
| 7 d | 100 | 1 | - | 76.6 | 383 (383) | ||
| 8 d | 100 | 6 | 96.8 | 484 (81) | |||
| 9 e | 100 | 1 | HNO3 | 27.2 | 136 (136) | ||
| 10 f | 100 | 1 | HPCA | 54.8 | 274 (274) | ||
| 11 g | 100 | 1 | TEMPO | 94.7 | 474 (474) | ||
| 12 h | 100 | 1 | Ph2NH | 7.9 | 40 (40) | ||
| 13 | cinnamyl alcohol | 100 | 1 | - | 12.6 | 63 (63) | |
| 14 g | 100 | 1 | TEMPO | 12.0 | 60 (60) | ||
| 15 h | 100 | 1 | Ph2NH | 1.6 | 8 (8) | ||
| 16 | benzhydrol | 100 | 1 | - | 81.7 | 409 (409) | |
| 17 g | 100 | 1 | TEMPO | 80.9 | 405 (405) | ||
| 18 h | 100 | 1 | Ph2NH | 3.2 | 16 (16) | ||
| 19 | cyclohexanol | 100 | 1 | - | 70.5 | 353 (353) | |
| 20 g | 100 | 1 | TEMPO | 71.0 | 355 (355) | ||
| 21h | 100 | 1 | Ph2NH | 3.4 | 178 (356) | ||
| 22 | 2 | 1-phenyl ethanol | 80 | 0.5 | - | 35.6 | 178 (356) |
| 23 | 100 | 0.5 | - | 66.4 | 332 (664) | ||
| 24 | 120 | 0.5 | - | 66.7 | 326 (652) | ||
| 25 | 100 | 1.0 | - | 88.5 | 443 (443) | ||
| 26 | 100 | 1.5 | - | 88.2 | 441 (294) | ||
| 27 | 100 | 2.0 | - | 86.9 | 435 (218) | ||
| 28 d | 100 | 1.0 | - | 67.8 | 339 (339) | ||
| 29 e | 100 | 1.0 | HNO3 | 16.7 | 84 (84) | ||
| 30 f | 100 | 1.0 | HPCA | 45.6 | 228 (228) | ||
| 31 g | 100 | 1.0 | TEMPO | 92.1 | 461 (461) | ||
| 32 h | 100 | 1.0 | Ph2NH | 6.7 | 34 (34) | ||
| 33 | cinnamyl alcohol | 100 | 1.0 | - | 5.7 | 133 (133) | |
| 34 g | 100 | 1.0 | TEMPO | 7.2 | 36 (36) | ||
| 35 | benzhydrol | 100 | 1.0 | - | 73.4 | 367 (367) | |
| 36 g | 100 | 1.0 | TEMPO | 79.8 | 399 (399) | ||
| 37 | cyclohexanol | 100 | 1.0 | - | 65.8 | 329 (329) | |
| 38 g | 100 | 1.0 | TEMPO | 67.9 | 340 (340) | ||
| 39 | CuCl2·2H2O | 1-phenyl ethanol | 100 | 1.0 | - | 6.2 | 31 (31) |
| 40 | cinnamyl alcohol | 100 | 1.0 | - | 1.7 | 9 (9) | |
| 41 | benzhydrol | 100 | 1.0 | - | 4.4 | 22 (22) | |
| 42 | cyclohexanol | 100 | 1.0 | - | 3.5 | 18 (18) | |
| 43 | VO(acac)2 | 1-phenyl ethanol | 100 | 1.0 | - | 4.9 | 25 (25) |
| 44 | cinnamyl alcohol | 100 | 1.0 | - | 1.1 | 6 (6) | |
| 45 | benzhydrol | 100 | 1.0 | - | 3.6 | 18 (18) | |
| 46 | cyclohexanol | 100 | 1.0 | - | 2.8 | 14 (14) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. Int. J. Mol. Sci. 2020, 21, 2832. https://doi.org/10.3390/ijms21082832
Sutradhar M, Roy Barman T, Pombeiro AJL, Martins LMDRS. Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. International Journal of Molecular Sciences. 2020; 21(8):2832. https://doi.org/10.3390/ijms21082832
Chicago/Turabian StyleSutradhar, Manas, Tannistha Roy Barman, Armando J. L. Pombeiro, and Luísa M. D. R. S. Martins. 2020. "Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols" International Journal of Molecular Sciences 21, no. 8: 2832. https://doi.org/10.3390/ijms21082832
APA StyleSutradhar, M., Roy Barman, T., Pombeiro, A. J. L., & Martins, L. M. D. R. S. (2020). Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. International Journal of Molecular Sciences, 21(8), 2832. https://doi.org/10.3390/ijms21082832