Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy
Abstract
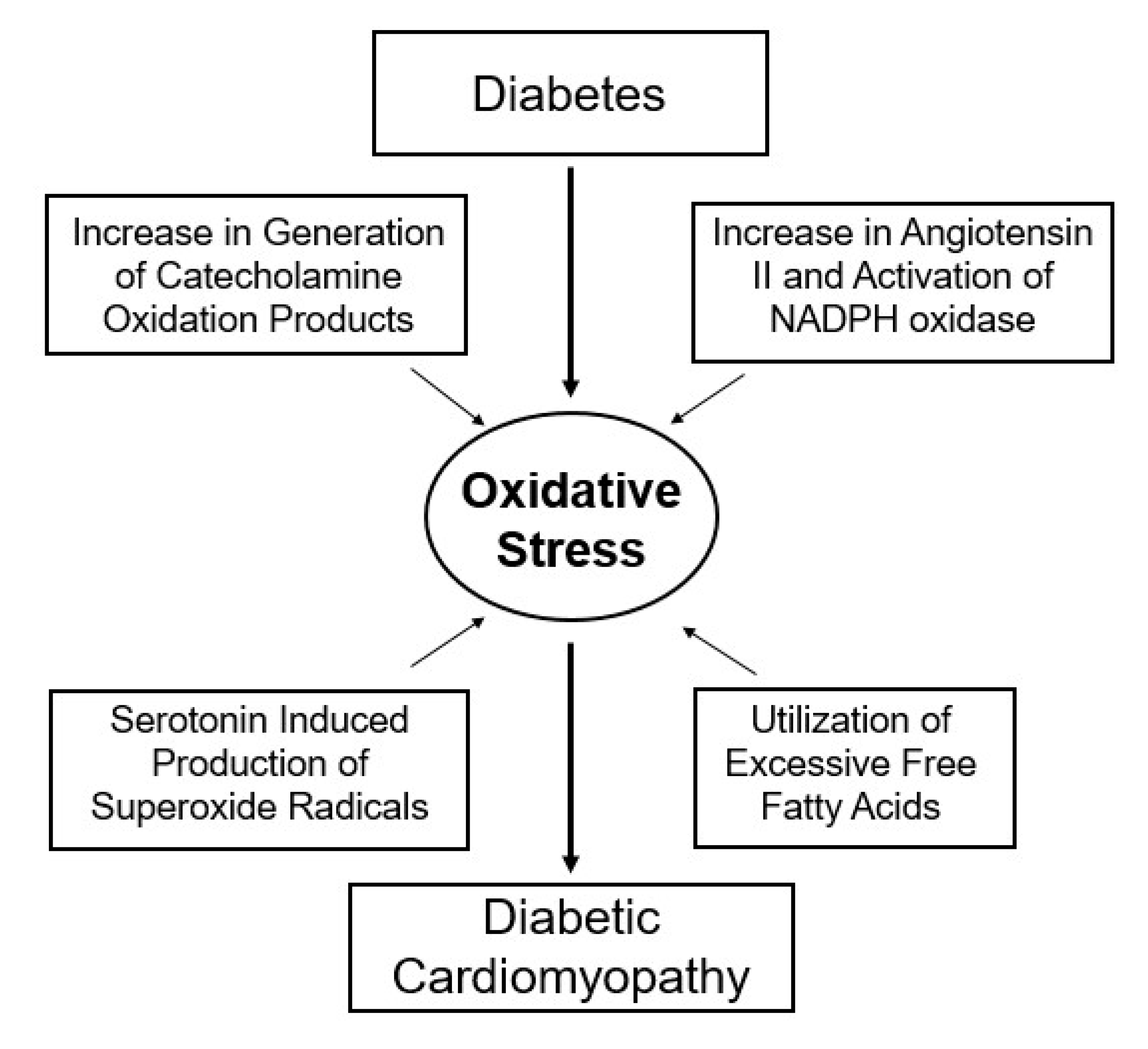
1. Introduction
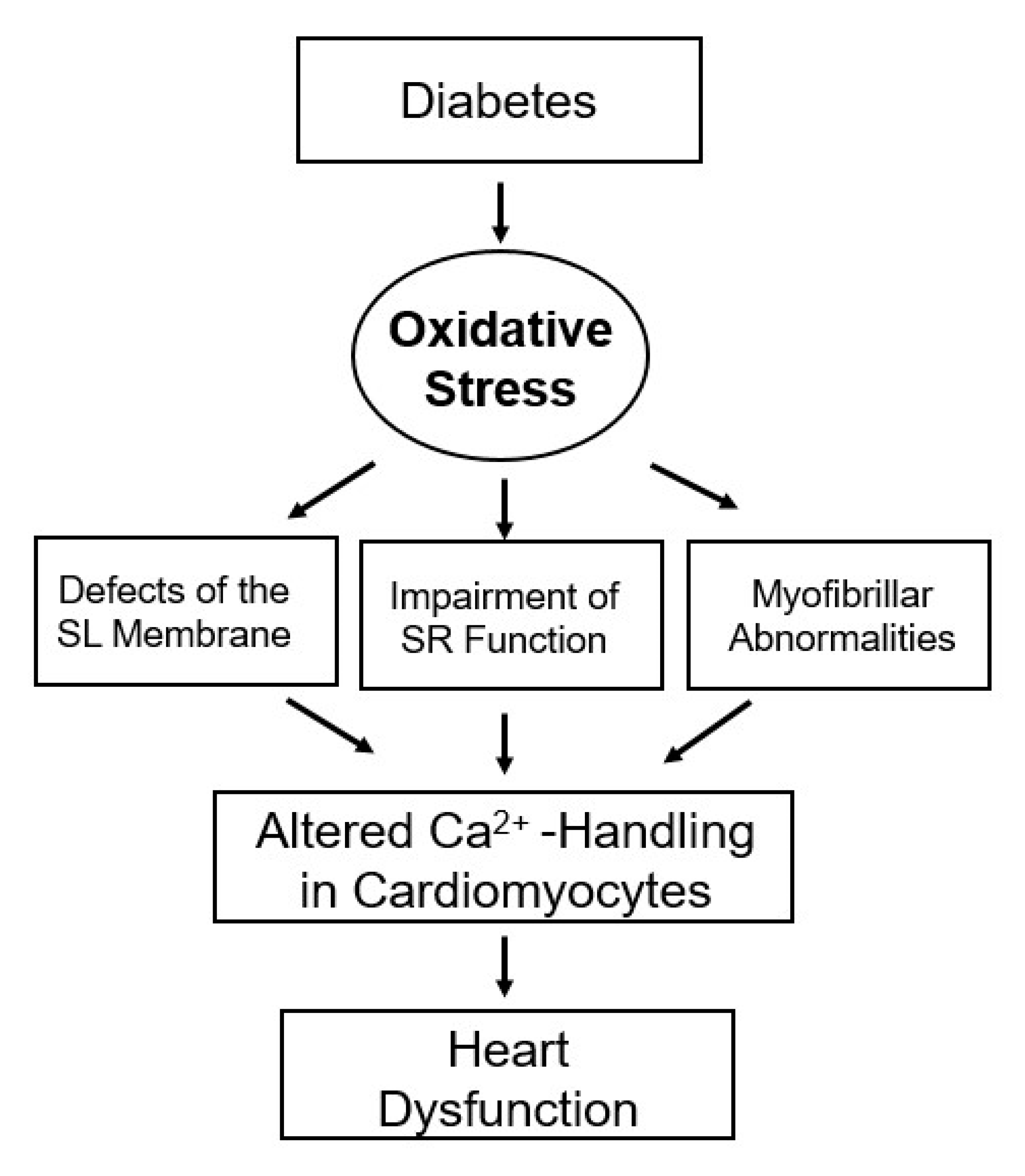
2. Alterations in Cardiac Function, Metabolism and Ultrastructure
3. Subcellular Defects in Ca2+-handling
4. Hormonal Imbalance and Modification of Subcellular Remodeling and Cardiac Dysfunction
5. Modification of Metabolic Defects and Cardiac Dysfunction
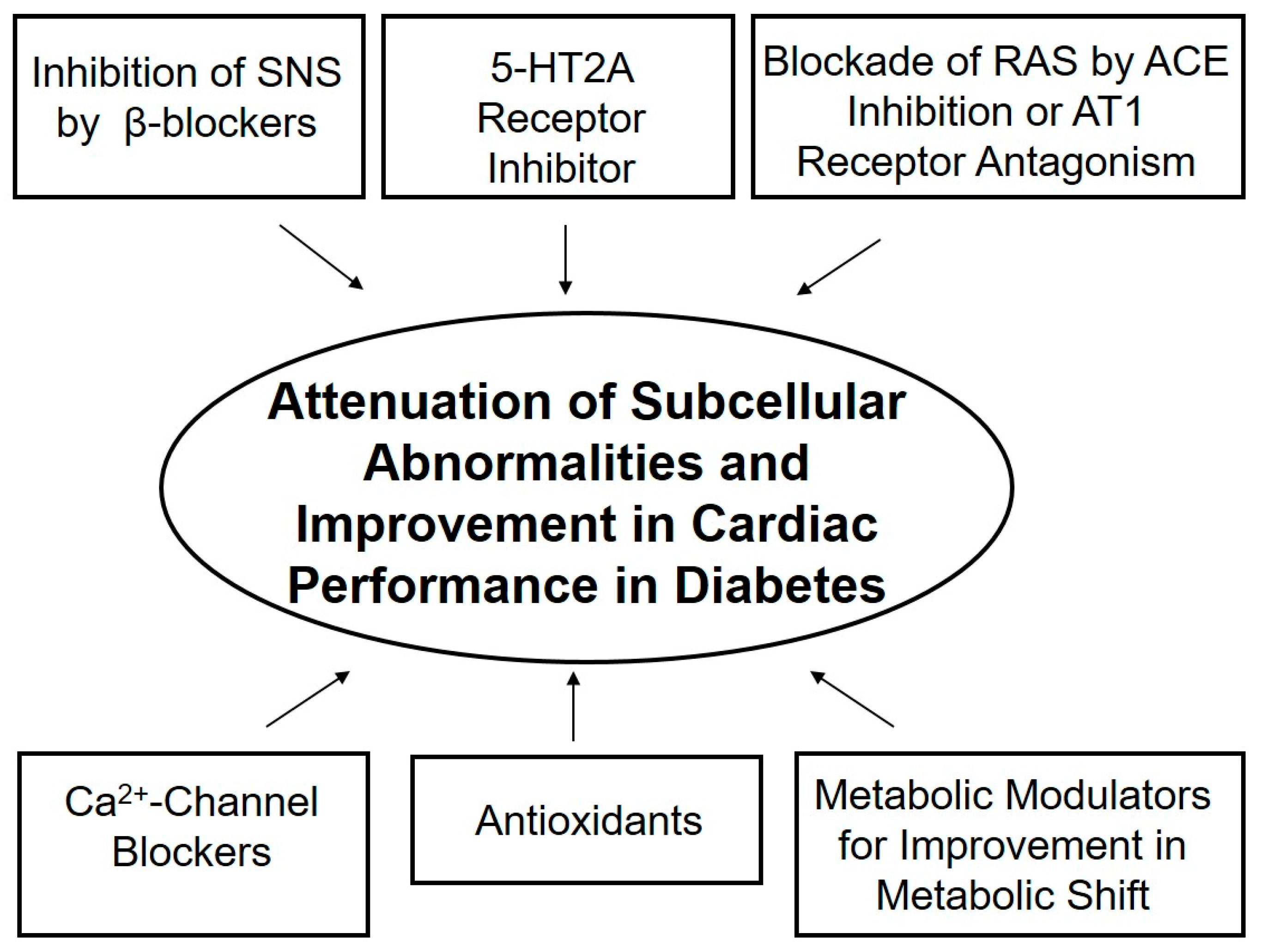
6. Alternative Therapeutic Options
7. Conclusions and Therapeutic Implications
Funding
Acknowledgments
Conflicts of Interest
References
- Regan, T.J.; Ahmed, S.; Haider, B.; Moschos, C.; Weisse, A. Diabetic cardiomyopathy: Experimental and clinical observations. N. Engl. J. Med. 1994, 91, 776–778. [Google Scholar]
- Regan, T.J. Congestive heart failure in the diabetic. Ann. Rev. Med. 1983, 34, 161–168. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Pierce, G.N.; Innes, I.R.; Beamish, R.E. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can. J. Cardiol. 1985, 1, 263–281. [Google Scholar] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies, beyond glycemic control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.C.; Abel, E.D. Heart failure in type 2 diabetes mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Takeda, N.; Rodriguez-Leyva, D.; Elimban, V. Mechanisms of subcellular remodeling in heart failure due to diabetes. Heart Fail. Rev. 2014, 19, 87–99. [Google Scholar] [CrossRef]
- Fein, F.S.; Sonnenblick, E.H. Diabetic cardiomyopathy. Prog. Cardiovasc. Dis. 1985, 25, 255–270. [Google Scholar] [CrossRef]
- Stanley, W.C.; Lopaschuk, G.D.; McCormack, J.G. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc. Res. 1997, 34, 25–33. [Google Scholar] [CrossRef]
- Pierce, G.N.; Russell, J.C. Regulation of intracellular Ca2+ in the heart during diabetes. Cardiovasc. Res. 1997, 34, 41–47. [Google Scholar] [CrossRef]
- Feuvray, D. The regulation of intracellular pH in the diabetic myocardium. Cardiovasc. Res. 1997, 34, 48–54. [Google Scholar] [CrossRef]
- Yu, J.Z.; Rodrigues, B.; McNeill, J.H. Intracellular calcium levels are unchanged in the diabetic heart. Cardiovasc. Res. 1997, 34, 91–98. [Google Scholar] [CrossRef]
- Hayashi, H.; Noda, N. Cystolic Ca2+ concentration in diabetic rat myocytes. Cardiovasc. Res. 1997, 34, 99–103. [Google Scholar] [CrossRef]
- Koltai, M.Z.; Hadhazy, P.; Posa, I.; Kocsis, E.; Winkler, G.; Rosen, P.; Pogátsa, G. Characteristics of coronary endothelial dysfunction in experimental diabetes. Cardiovasc. Res. 1997, 34, 157–167. [Google Scholar] [CrossRef]
- Tappia, P.S.; Dent, M.R.; Dhalla, N.S. Oxidative stress and redox regulation of phospholipase D in myocardial disease. Free Radic. Biol. Med. 2006, 41, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Dhalla, N.S. Role of microangiopathy in diabetic cardiomyopathy. Heart Fail. Rev. 2014, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Adameova, A.; Dhalla, N.S. Attenuation of diabetes-induced cardiac and subcellular defects by sulphur-containing amino acids. Curr. Med. Chem. 2018, 25, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signaling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef]
- Sharma, A.; Tate, M.; Mathew, G.; Vince, J.E.; Ritchie, R.H.; de Haan, J.B. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: Therapeutic implications. Front. Physiol. 2018, 9, 114. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Tappia, P.S.; Neki, N.S.; Dhalla, N.S. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail. Rev. 2014, 19, 113–121. [Google Scholar] [CrossRef]
- Gillery, P.; Monboisse, J.C.; Maquart, F.X.; Borel, J.P. Glycation of proteins as a source of superoxide. Diabetes Metab. 1988, 14, 25–30. [Google Scholar]
- Wolff, S.P.; Dean, R.T. Glucose auto-oxidationand protein modification. The potential role of ‘autooxidative glycosylation’ in diabtes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.V.; Smith, C.C.T.; Wolff, S.P. Autooxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes 1990, 39, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Dillman, W.H. Diabetes and thyroid-hormone-induced changes in cardiac function and their molecular basis. Annu. Rev. Med. 1989, 40, 373–394. [Google Scholar] [CrossRef]
- Schaffer, S.W. Cardiomyopathy associated with non-insulin-dependent diabetes. Mol. Cell. Biochem. 1991, 107, 1–20. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Liu, X.; Panagia, V.; Takeda, N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc. Res. 1998, 40, 239–247. [Google Scholar] [CrossRef]
- Machackova, J.; Barta, J.; Dhalla, N.S. Molecular defects in cardiac myofibrillar proteins due to thyroid hormone imbalance and diabetes. Can. J. Physiol. Pharmacol. 2005, 83, 1071–1091. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Rangi, S.; Zieroth, S.; Xu, Y.-J. Alterations in sarcoplasmic reticulum and mitochondrial functions in diabetic cardiomyopathy. Exptl. Clin. Cardiol. 2012, 17, 115–120. [Google Scholar]
- Golfman, L.S.; Takeda, N.; Dhalla, N.S. Cardiac membrane Ca2+-transport in alloxan-induced diabetes in rats. Diabetes Res. Clin. Prac. 1996, 31 (Suppl. 1), S73–S77. [Google Scholar] [CrossRef]
- Golfman, L.; Dixon, I.M.C.; Takeda, N.; Chapman, D.; Dhalla, N.S. Differential changes in cardiac myofibrillar and sarcoplasmic reticular gene expression in alloxan-induced diabetes. Mol. Cell. Biochem. 1999, 200, 15–25. [Google Scholar] [CrossRef]
- Takeda, N.; Dixon, I.M.C.; Hata, T.; Elimban, V.; Shah, K.R.; Dhalla, N.S. Sequence of alterations in subcellular organelles during the development of heart dysfunction in diabetes. Diabetes Res. Clin. Prac. 1996, 30 (Suppl. 1), S113–S122. [Google Scholar] [CrossRef]
- Tappia, P.S.; Thliveris, J.; Xu, Y.J.; Aroutiounova, N.; Dhalla, N.S. Effects of amino acid supplementation on myocardial cell damage and cardiac function in diabetes. Exp. Clin. Cardiol. 2011, 16, e17–e22. [Google Scholar] [PubMed]
- Ganguly, P.K.; Dhalla, K.S.; Innes, I.R.; Beamish, R.E.; Dhalla, N.S. Altered norepinephrine turnover and metabolism in diabetic cardiomyopathy. Circ. Res. 1986, 59, 684–693. [Google Scholar] [CrossRef]
- Ganguly, P.K.; Beamish, R.E.; Dhalla, K.S.; Innes, I.R.; Dhalla, N.S. Norepinephrine storage, distribution and release in diabetic cardiomyopathy. Am. J. Physiol. 1987, 252, E734–E739. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Umrani, D.N.; Bodiwala, D.N.; Dhalla, N.S. Usefulness of 5-HT2A receptor antagonists in diabetes. In Atherosclerosis, Hypertension and Diabetes; Pierce, G.N., Nagano, M., Zahradka, P., Dhalla, N.S., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 317–326. [Google Scholar]
- Hileeto, D.; Cukiernik, M.; Mukherjee, S.; Evans, T.; Barbin, Y.; Downey, D.; Karmazyn, M.; Chakrabarti, S. Contributions of endothelin-1 and sodium hydrogen exchanger-1 in the diabetic myocardium. Diabetes Metab. Res. Rev. 2002, 18, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Ganguly, P.K.; Bhullar, S.K.; Tappia, P.S. Role of catecholamines in the pathogenesis of diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 2019, 97, 815–819. [Google Scholar] [CrossRef]
- Cook, S.A.; Varela-Carver, A.; Mongillo, M.; Kleinert, C.; Khan, M.T.; Leccisotti, L.; Strickland, N.; Matsui, T.; Das, S.; Rosenzweig, A.; et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur. Heart J. 2010, 31, 100–111. [Google Scholar] [CrossRef]
- Banerjee, S.K.; McGaffin, K.R.; Pastor-Soler, N.M.; Ahmad, F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [Google Scholar] [CrossRef]
- Coort, S.L.; Bonen, A.; van der Vusse, G.J.; Glatz, J.F.; Luiken, J.J. Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: Role of sarcolemmal substrate transporters. Mol. Cell. Biochem. 2007, 299, 5–18. [Google Scholar] [CrossRef]
- Luiken, J.J. Sarcolemmal fatty acid uptake vs. mitochondrial β-oxidation as target to regress cardiac insulin resistance. Appl. Physiol. Nutr. Metab. 2009, 34, 473–480. [Google Scholar] [CrossRef]
- van den Brom, C.E.; Huisman, M.C.; Vlasblom, R.; Boontje, N.M.; Duijst, S.; Lubberink, M.; Molthoff, C.F.; Lammertsma, A.A.; van der Velden, J.; Boer, C.; et al. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: Studies using positron emission tomography. Cardiovasc. Diabetol. 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Afzal, N.; Ganguly, P.K.; Dhalla, K.S.; Pierce, G.N.; Singal, P.K.; Dhalla, N.S. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes 1988, 37, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Louch, W.E.; Stokke, M.K.; Sjaastad, I.; Christensen, G.; Sejersted, O.M. No rest for the weary: Diastolic calcium homeostasis in the normal and failing myocardium. Physiology 2012, 27, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Mozaffari, M.S.; Artman, M.; Wilson, G.L. Basis for myocardial mechanical defects associated with non-insulin-dependent diabetes. Am. J. Physiol. 1989, 256, E25–E30. [Google Scholar] [CrossRef] [PubMed]
- Nusier, M.; Ozcelikay, A.T.; Shah, A.K.; Dhalla, N.S. Role of intracellular Ca2+-overload in cardiac dysfunction in heart disease. J. Clin. Cardiol. Cardiovasc. Interven. 2020, 3. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Ballard-Croft, C.; Boerth, S.; Allo, S.N. Mechanisms underlying depressed Na+/Ca2+ exchanger activity in diabetic heart. Cardiovasc. Res. 1997, 34, 129–136. [Google Scholar] [CrossRef]
- Makino, N.; Dhalla, K.S.; Elimban, V.; Dhalla, N.S. Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am. J. Physiol. 1987, 253, E202–E207. [Google Scholar] [CrossRef]
- Liu, X.; Suzuki, H.; Sethi, R.; Tappia, P.S.; Takeda, N.; Dhalla, N.S. Blockade of renin angiotensin system attenuates sarcolemma and sarcoplasmic reticulum remodeling in chronic diabetes. Ann. N.Y. Acad. Sci. 2006, 1084, 141–154. [Google Scholar] [CrossRef]
- Borda, E.; Pascual, J.; Wald, M.; Sterin-Borda, L. Hypersensitivity to calcium associated with an increased sarcolemmal Ca2+-ATPase activity in diabetic heart. Can. J. Cardiol. 1988, 4, 97–101. [Google Scholar]
- Pierce, G.N.; Ramjiawan, B.; Dhalla, N.S.; Ferrari, R. Na+-H+ exchange in cardiac sarcolemmal vesicles isolated from diabetic rats. Am. J. Physiol. 1990, 258, H255–H261. [Google Scholar] [CrossRef]
- Dyck, J.R.; Lopaschuk, G.D. Glucose metabolism, H+ production and Na+/H+-exchanger mRNA levels in ischemic hearts from diabetic animals. Mol. Cell. Biochem. 1998, 180, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Ostadalova, I.; Kolar, F.; Dhalla, N.S. Alterations in Ca2+-channels during the development of diabetic cardiomyopathy. Mol. Cell. Biochem. 1992, 109, 173–179. [Google Scholar] [PubMed]
- Clark, T.A.; Maddaford, T.G.; Tappia, P.S.; Heyliger, C.E.; Ganguly, P.K.; Pierce, G.N. Restoration of cardiomyocyte function in streptozotocin-induced diabetic rats after treatment with vanadate in a tea decoction. Curr. Pharm. Biotechnol. 2010, 11, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tibbits, G.F.; McNeill, J.H. Cellular functions of diabetic cardiomyocytes: Contractility, rapid-cooling contracture, and ryanodine binding. Am. J. Physiol. 1994, 266, H2082–H2089. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Zhong, Y.; Hoit, B.D.; Grupp, I.L.; Hahn, H.; Dilly, K.W.; Guatimosim, S.; Lederer, W.J.; Matlib, M.A. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1398–H1408. [Google Scholar] [CrossRef] [PubMed]
- Yaras, N.; Bilginoglu, A.; Vassort, G.; Turan, B. Restoration of diabetes-induced abnormal local Ca2+ release in cardiomyocytes by angiotensin II receptor blockade. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H912–H920. [Google Scholar] [CrossRef][Green Version]
- Netticadan, T.; Temsah, R.M.; Kent, A.; Elimban, V.; Dhalla, N.S. Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 2001, 50, 2133–2138. [Google Scholar] [CrossRef]
- Vasanji, Z.; Dhalla, N.S.; Netticadan, T. Increased inhibition of SERCA2 by phospholamban in the type 1 diabetic heart. Mol. Cell. Biochem. 2004, 261, 245–249. [Google Scholar] [CrossRef]
- Rastogi, S.; Sentex, E.; Elimban, V.; Dhalla, N.S.; Netticadan, T. Elevated levels of protein phosphatase 1 and phosphatase 2A may contribute to cardiac dysfunction in diabetes. Biochim Biophys Acta 2003, 1638, 273–277. [Google Scholar] [CrossRef][Green Version]
- Lagadic-Gossmann, D.; Buckler, K.J.; Le Prigent, K.; Feuvray, D. Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am. J. Physiol. 1996, 270, H1529–H1537. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kajiwara, H.; Kurihara, S. Alterations in contractile properties and Ca2+ handling in streptozotocin-induced diabetic rat myocardium. Am. J. Physiol. 1999, 277, H2185–H2194. [Google Scholar] [CrossRef] [PubMed]
- Allo, S.N.; Lincoln, T.M.; Wilson, G.L.; Green, F.J.; Watanabe, A.M.; Schaffer, S.W. Non-insulin-dependent diabetes-induced defects in cardiac cellular calcium regulation. Am. J. Physiol. 1991, 260, C1165–C1171. [Google Scholar] [CrossRef] [PubMed]
- Ligeti, L.; Szenczi, O.; Prestia, C.M.; Szabo, C.; Horvath, K.; Marcsek, Z.L.; Marcsek, Z.L.; van Stiphout, R.G.P.M.; van Riel, N.A.W.; den Buijs, J.O.; et al. Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int. J. Mol. Med. 2006, 17, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Afzal, N.; Pierce, G.N.; Elimban, V.; Beamish, R.E.; Dhalla, N.S. Influence of verapamil on some subcellular defects in diabetic cardiomyopathy. Am. J. Physiol. 1989, 256, E453–E458. [Google Scholar] [CrossRef]
- Ganguly, P.K.; Pierce, G.N.; Dhalla, N.S. Diabetic cardiomyopathy: Membrane dysfunction and therapeutic strategies. J. Appl. Cardiol. 1987, 2, 323–338. [Google Scholar]
- Machackova, J.; Liu, X.; Lukas, A.; Dhalla, N.S. Renin-angiotensin blockade attenuates cardiac myofibrillar remodeling in chronic diabetes. Mol. Cell. Biochem. 2004, 261, 271–278. [Google Scholar] [CrossRef]
- Goyal, R.K.; Elimban, V.; Xu, Y.-J.; Kumamoto, H.; Takeda, N.; Dhalla, N.S. Mechanism of sarpogrelate action in improving cardiac function in diabetes. J. Cardiovasc. Pharmacol. Therap. 2011, 16, 380–387. [Google Scholar] [CrossRef]
- Kato, K.; Lukas, A.; Chapman, D.C.; Rupp, H.; Dhalla, N.S. Differential effects of etomoxir treatment on cardiac Na+-K+-ATPase subunits in diabetic heart. Mol. Cell. Biochem. 2002, 232, 57–62. [Google Scholar] [CrossRef]
- Rupp, H.; Elimban, V.; Dhalla, N.S. Modification of myosin isozymes and SR Ca2+-pump ATPase of the diabetic rat heart by lipid lowering interventions. Mol. Cell. Biochem. 1994, 132, 69–80. [Google Scholar] [CrossRef]
- Kato, K.; Chapman, D.C.; Rupp, H.; Lukas, A.; Dhalla, N.S. Alterations of heart function and Na+-K+ ATPase activity by etomoxir in diabetic rats. J. Appl. Physiol. 1999, 86, 812–818. [Google Scholar] [CrossRef]
- Ferrari, R.; Shah, K.R.; Hata, T.; Beamish, R.E.; Dhalla, N.S. Subcellular defects in diabetic myocardium: Influence of propionyl L-carnitine on Ca2+-transport. In The Diabetic Heart; Nagano, M., Dhalla, N.S., Eds.; Raven Pres: New York, NY, USA, 1991; pp. 167–181. [Google Scholar]
- Dhalla, N.S.; Dixon, I.M.C.; Shah, K.R.; Ferrari, R. Beneficial effects of L-carnitine and derivatives on heart membranes in experimental diabetes. In L-Carnitine and its Role in Medicine: From Function to Therapy; Ferrari, R., DiMauro, S., Sherwood, G., Eds.; Academic Press: London, UK, 1992; pp. 411–426. [Google Scholar]
- Zamora, M.; Villena, J.A. Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 2833. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Carbonara, O.; Cozzolino, D.; Rambaldi, P.; Mansi, L.; Torella, D.; Gentile, S.; Turco, S.; Torella, R.; Salvatore, T. Effects of insulin-glucose infusion on left ventricular function at rest and during exercise in healthy subjects and noninsulin dependent diabetic patients: A radionuclide ventriculographic study. J. Am. Coll. Cardiol. 2000, 36, 219–226. [Google Scholar] [CrossRef]
- Cozzolino, D.; Sasso, F.C.; Salvatore, T.; Torella, M.; Gentile, S.; Torella, R.; Giugliano, D. Acute effects of β-endorphin on cardiovascular function in patients with mild to moderate chronic heart failure. Am. Heart J. 2004, 148, E13. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Natali, A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 657–669. [Google Scholar] [CrossRef]
- Molavi, B.; Rassouli, N.; Bagwe, S.; Rasouli, N. A review of thiazolidinediones and metformin in the treatment of type 2 diabetes with focus on cardiovascular complications. Vasc. Health Risk Manag. 2007, 3, 967–973. [Google Scholar]
- Lambadiari, V.; Pavlidis, D.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef]
| Parameter | Control | Time after Alloxan Treatment (Weeks) | ||||
|---|---|---|---|---|---|---|
| 1 Week | 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | ||
| Plasma glucose (mg/dl) | 158 ± 15 | 523 ± 28 * | 525 ± 19 * | 495 ± 26 * | 520 ± 24 * | 512 ± 27 * |
| Plasma insulin (mU/dl) | 2.95 ± 0.20 | 0.84 ± 0.17 * | 0.91 ± 016 * | 0.88 ± 0.14 * | 0.82 ± 0.20 * | 0.93 ± 0.18 * |
| Heart rate (beats/min) | 383 ± 19 | 378 ± 15 | 368 ± 15 | 313 ± 21 * | 280 ± 17 * | 277 ± 14 * |
| MAP (mmHg) | 123 ± 9 | 117 ± 2 | 117 ± 5 | 99 ± 4 * | 93 ± 5 * | 98 ± 7 * |
| LVEDP (mmHg) | 2.4 ± 1.2 | 3.0 ± 1.1 | 3.5 ± 1.2 | 6.2 ± 1.1 * | 6.1 ± 1.0 * | 6.2 ± 1.4 * |
| LVSP (mmHg) | 129 ± 7 | 130 ± 7 | 125 ± 8 | 110 ± 5 * | 104 ± 4 * | 107 ± 7 * |
| SL Na + -K+-ATPase (µmol Pi/mg/h) | 24.6 ± 1.1 | 20.1 ± 0.9 | 16.8 ± 1.3 * | 17.1 ± 0.7 * | 16.5 ± 1.2 * | 17.3 ± 1.1 * |
| SL Na+- Ca2+-exchanger (nmol Ca2+/mg/2 s) | 5.01 ± 0.81 | 4.15 ± 0.81 | 2.80 ± 0.55 * | 2.99 ± 0.67 * | 2.75 ± 0.63 * | 1.90 ± 0.80 * |
| SR Ca2+-stimulated ATPase (µmol Pi/mg/h) | 10.07 ± 0.42 | 9.24 ± 0.30 | 6.95 ± 0.25 * | 6.85 ± 0.33 * | 6.04 ± 0.57 * | 5.95 ± 0.39 * |
| SR Ca2+ uptake (nmol Ca2+/mg/min) | 128 ± 10 | 119 ± 15 | 65 ± 7 * | 50 ± 12 * | 51 ± 9 * | 64 ± 11 * |
| Myofibrillar Ca2+-stimulated ATPase (µmol Pi/mg/h) | 12.7 ± 1.0 | 12.1 ± 0.9 | 8.0 ± 0.7 * | 6.9 ± 0.7 * | 6.6 ± 0.7 * | 6.4 ± 0.7 * |
| Parameter | Control | Time after STZ Treatment | ||||
|---|---|---|---|---|---|---|
| 15 Days | 18 Days | 21 Days | 24 Days | 27 Days | ||
| Plasma glucose (mg/dl) | 130 ± 8 | 388 ± 21 * | 425 ± 22 * | 412 ± 20 * | 448 ± 24 * | 435 ± 24 * |
| Plasma insulin (µU/mL) | 31.2 ± 1.7 | 12.4 ± 0.6 * | 13.2 ± 0.5 * | 12.7 ± 0.6 * | 12.5 ± 0.7 * | 13.1 ± 0.4 * |
| Heart rate (beats/min) | 412 ± 5 | 424 ± 6 | 409 ± 6 | 401 ± 5 | 376 ± 5 * | 362 ± 5 * |
| LVEDP (mmHg) | 2.3 ± 1.4 | 4.7 ± 0.8 | 5.2 ± 1.6 | 8.6 ± 0.7 * | 9.6 ± 0.9 * | 9.8 ± 0.6 * |
| LVSP (mmHg) | 150 ± 4 | 144 ± 4 | 138 ± 5 | 136 ± 3 * | 131 ± 3 * | 122 ± 3 * |
| SL Na+-K+-ATPase (µmol Pi/mg/h) | 25.2 ± 2.4 | 24.7 ± 3.1 | 21.2 ± 5.2 | 18.3 ± 3.4 * | 17.2 ± 2.1 * | 15.2 ± 1.9 * |
| SL Na+-Ca2+-exchanger (nmol Ca2+/mg/10 s) | 21.4 ± 2.1 | 18.5 ± 2.2 | 17.6 ± 3.1 | 16.5 ± 3.2 * | 16.0 ± 1.2 * | 15.1 ± 1.1 * |
| SR Ca2+-stimulated ATPase (nmol Pi/mg/min) | 158 ± 7 | 160 ± 4 | 149 ± 5 | 132 ± 6 * | 124 ± 3 * | 115 ± 6 * |
| SR Ca2+-uptake (nmol Ca2+/mg/min) | 58 ± 5 | 52 ± 3 | 54 ± 5 | 44 ± 3 * | 42 ± 3 * | 39 ± 4 * |
| Myofibrillar Ca2+-stimulated ATPase (nmol Pi/mg/min) | 192 ± 7 | 190 ± 14 | 182 ± 21 | 162 ± 16 * | 149 ± 13 * | 138 ± 12 * |
| Parameter | Control | Diabetes | Diabetes + Verapamil |
|---|---|---|---|
| Plasma glucose (mg/dl) | 190 ± 5 | 706 ± 56 * | 661 ± 9 * |
| Plasma insulin (mU/dl) | 3.10 ± 0.25 | 0.90 ± 0.10 * | 1.0 ± 0.15 * |
| Heart rate (beats/min) | 357 ± 6 | 283 ± 7 * | 334 ± 9 # |
| LVEDP (mmHg) | 3.0 ± 2.0 | 19.0 ± 1.0 * | 3.0 ± 0.7 # |
| LVSP (mmHg) | 151 ± 2 | 123 ± 3 * | 151 ± 3 # |
| + dP/dt (mmHg/s) | 6137 ± 176 | 4332 ± 226 * | 5415 ± 90 # |
| − dP/dt (mmHg/s) | 5415 ± 158 | 3610 ± 173 * | 4693 ± 8 |
| Myofibrillar Ca2+-stimulated ATPase (nmol Pi/mg/min) | 148 ± 7 | 95 ± 5 * | 134 ± 11 # |
| SR Ca2+-stimulated ATPase† (µmol Pi/mg/5 min) | 0.89 ± 0.08 | 0.52 ± 0.05 * | 0.92 ± 0.06 # |
| SR Ca2+-uptake† (nmol Ca2+/mg/min) | 56 ± 6 | 30 ± 4 * | 49 ± 6 # |
| Control | Diabetes | Diabetes + Enalapril | Diabetes + Losartan | |
|---|---|---|---|---|
| Plasma glucose (U/mL) | 154 ± 9 | 489 ± 17 * | 464 ± 12 * | 471 ± 9 * |
| Plasma insulin (mg/mL) | 29 ± 2 | 12 ± 2 * | 13 ± 1 * | 13 ± 1 # |
| +dP/dt (mmHg/s) | 5840 ± 265 | 3780 ± 218 * | 4764 ± 196 *# | 4780 ± 225 *# |
| − dP/dt (mmHg/s) | 5560 ± 164 | 3376 ± 187 * | 4580 ± 208 *# | 4548 ± 192 *# |
| LVSP (mmHg) | 140 ± 12 | 85 ± 8 * | 119 ± 8 # | 116 ± 7 # |
| LVEDP (mmHg) | 3.4 ± 0.2 | 3.9 ± 0.3 | 3.9 ± 0.2 | 4.1 ± 0.3 |
| Myofibrillar Ca2+ -stimulated ATPase (nmol Pi/mg/5 min) | 870 ± 21 | 524 ± 23 * | 720 ± 26 *# | 708 ± 16 *# |
| SL Na+-K+-ATPase (µmol Pi/mg/h) | 23.2 ± 3.5 | 13.1 ± 1.8 * | 18.2 ± 1.6 # | 18.3 ± 1.5 # |
| SL Na+- Ca2+-exchanger (nmol Ca2+/mg/10 s) | 21.3 ± 1.2 | 12.1 ± 0.9 * | 17.3 ± 1.2 *# | 16.1 ± 1.5 *# |
| SR Ca2+- stimulated ATPase (nmol Pi/mg/5 min) | 165 ± 7 | 115 ± 10 * | 154 ± 5 # | 153 ± 6 # |
| SR Ca2+-uptake (nmol Ca2+/mg/2 min) | 62.7 ± 2.3 | 36.5 ± 3.1 * | 50.3 ± 2.1 *# | 53.4 ± 2.7 *# |
| SR Ca2+-release (nmol Ca2+/mg/15 s) | 9.3 ± 0.4 | 5.8 ± 0.3 * | 8.7 ± 0.5 # | 8.5 ± 0.4 # |
| MDA (nmol/mg tissue lipids) | 3.8 ± 0.13 | 7.1 ± 0.49 * | 4.9 ± 0.5 *# | 5.4 ± 0.6 *# |
| Control | Diabetes | Diabetes + PPLC | |
|---|---|---|---|
| Plasma glucose (mg/dL) | 137 ± 3 | 445 ± 18 * | 215 ± 38 # |
| Plasma insulin (µU/mL) | 55 ± 6 | 20 ± 2 * | 23 ± 2 # |
| +dP/dt (mmHg/s) | 5300 ± 150 | 4150 ± 200 * | 5200 ± 175 # |
| -dP/dt (mmHg/s) | 4600 ± 120 | 3700 ± 150 * | 4800 ± 200 # |
| LVSP (mmHg) | 160 ± 5 | 120 ± 7 * | 165 ± 7 # |
| Myofibrillar Ca2+ -stimulated ATPase (nmol Pi/mg/5 min) | 0.48 ± 0.015 | 0.33 ± 0.025 * | 0.35 ± 0.021 * |
| SL Na+-K+-ATPase (µmol Pi/mg/h) | 19.8 ± 2.1 | 10.7 ± 1.6 * | 15.7 ± 1.6 # |
| SL Na+-Ca2+-exchanger (nmol Ca2+/mg/30 s) | 8.8 ± 1.6 | 3.2 ± 1.4 * | 5.1 ± 1.2 * |
| SR Ca2+-stimulated ATPase (nmol Pi/mg/5 min) | 814 ± 74 | 428 ± 50 * | 784 ± 65 # |
| SR Ca2+-uptake (nmol Ca2+/mg/5 min) | 224 ± 11 | 135 ± 15 * | 205 ± 9 # |
| Control | Diabetes | Diabetes + Vitamin E | |
|---|---|---|---|
| Plasma glucose (mg/dl) | 151 ± 8 | 487 ± 9 * | 478 ± 9 * |
| Plasma insulin (µU/mL) | 28 ± 2 | 11 ± 1 * | 12 ± 1 * |
| +dP/dt (mmHg/s) | 5722 ± 254 | 4210 ± 145 * | 5450 ± 180 # |
| -dP/dt (mmHg/s) | 5525 ± 129 | 4155 ± 135 * | 5341 ± 182 # |
| Myofibrillar Ca2+ -stimulated ATPase (µmol Pi/mg/h) | 11.6 ± 0.9 | 5.8 ± 0.5 * | 8.4 ± 0.4 # |
| SL Na+-K+-ATPase (µmol Pi/mg/h) | 24.7 ± 6 | 16.1 ± 2.7 * | 23.6 ± 2.9 # |
| SL Na+- Ca2+-exchanger (nmol Ca2+/mg/2 s) | 3.9 ± 0.2 | 2.1 ± 0.2 * | 3.7 ± 0.4 # |
| SR Ca2+-release (nmol Ca2+/mg/3 min) | 20.5 ± 2.1 | 10.2 ± 1.3 * | 19.1 ± 1.8 # |
| SR Ca2+-uptake (nmol Ca2+/mg/min) | 79.5 ± 7.1 | 43.7 ± 4.3 * | 62.8 ± 3.2 *# |
| MDA (nmol/mg tissue lipids) | 4.2 ± 0.3 | 6.9 ± 0.4 * | 4.3 ± 0.5 # |
| Conjugated dienes (nmol/mg tissue lipids) | 39.6 ± 3.2 | 68.3 ± 7.1 * | 46.7 ± 5.4 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhalla, N.S.; Shah, A.K.; Tappia, P.S. Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 2413. https://doi.org/10.3390/ijms21072413
Dhalla NS, Shah AK, Tappia PS. Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. International Journal of Molecular Sciences. 2020; 21(7):2413. https://doi.org/10.3390/ijms21072413
Chicago/Turabian StyleDhalla, Naranjan S., Anureet K. Shah, and Paramjit S. Tappia. 2020. "Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy" International Journal of Molecular Sciences 21, no. 7: 2413. https://doi.org/10.3390/ijms21072413
APA StyleDhalla, N. S., Shah, A. K., & Tappia, P. S. (2020). Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. International Journal of Molecular Sciences, 21(7), 2413. https://doi.org/10.3390/ijms21072413





