Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms
Abstract
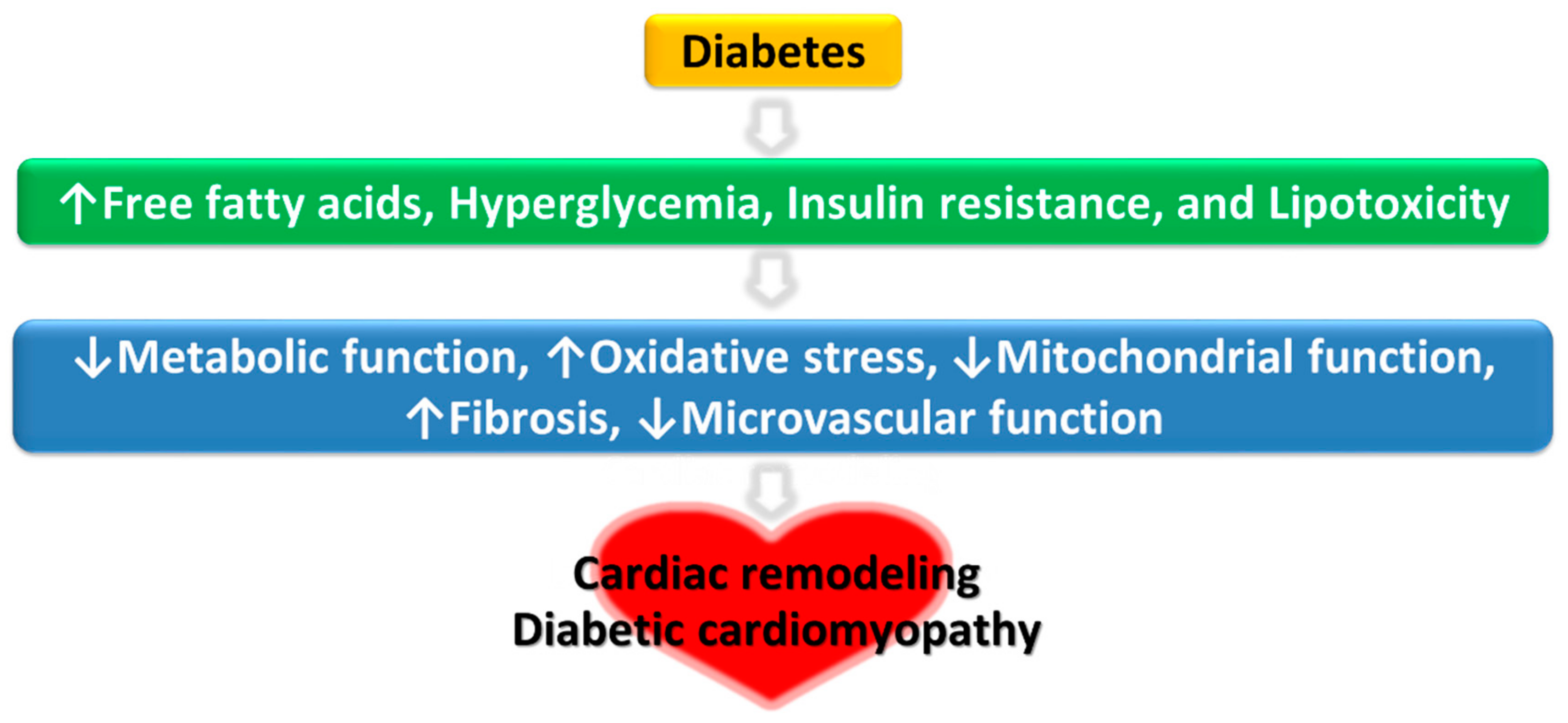
1. Introduction
2. Clinical Aspects of DCM
2.1. Definition and Characteristics of DCM
2.2. Treatment Strategies for DCM
2.3. Clinical Significance of Exercise for the Treatment of DCM
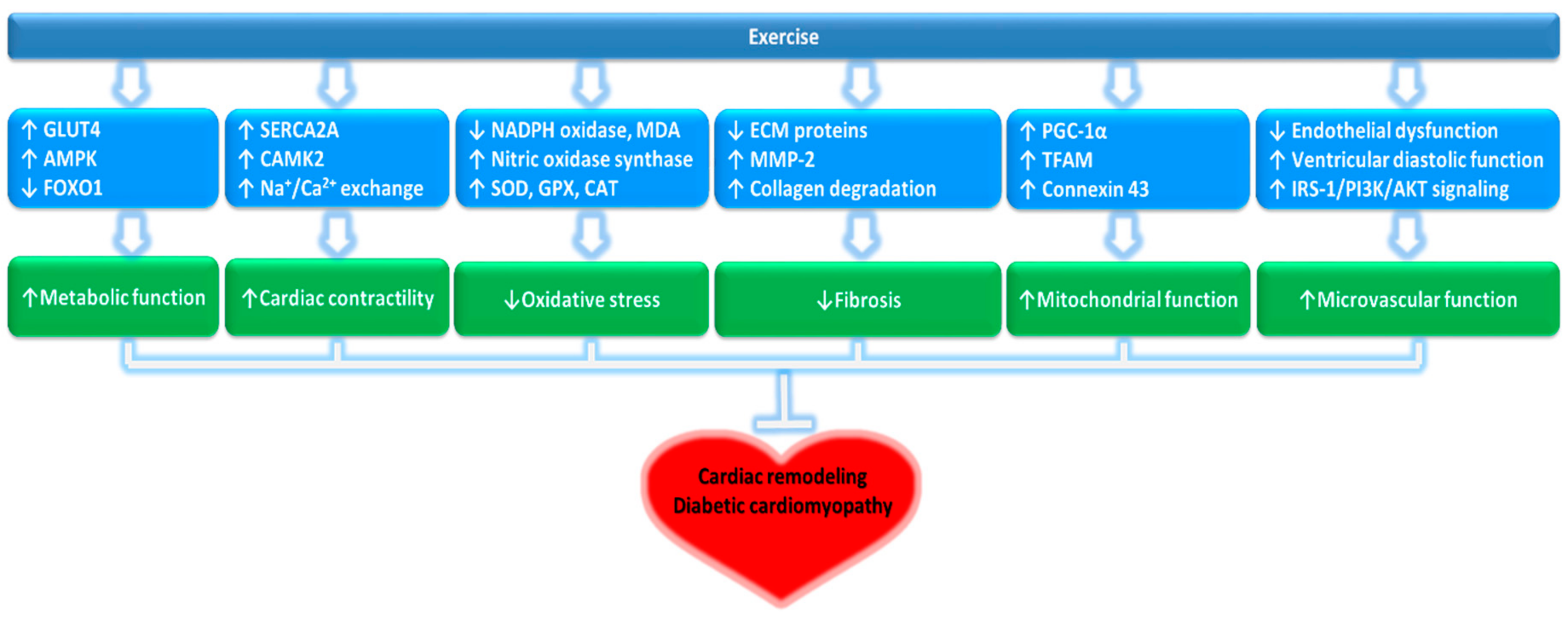
3. Potential Mechanisms of Protective Effects of Exercise Against DCM
3.1. Cardiac Cell Metabolism
3.2. Calcium Regulation in Cardiac Cells
3.3. Mitochondrial Function of Cardiac Cells
3.4. Oxidative Stress in Cardiomyocytes
3.5. Myocardial Fibrosis
3.6. Apoptosis of Cardiomyocytes
3.7. Microvascular Function of Cardiomyocytes
4. Prospective New Biomarkers in DCM
5. Clinical Perspective on the Future Use of Exercise in DCM Prevention
Exercise Is an Early Diagnostic Tool for Prevention and Better Treatment of DCM
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmidt, A.M. Diabetes mellitus and cardiovascular disease. Arter. Thromb Vasc. Biol. 2019, 39, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzen, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Charnogursky, G.A.; Emanuele, N.V.; Emanuele, M.A. Neurologic complications of diabetes. Curr. Neurol. Neurosci. Rep. 2014, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Badhwar, S.; Chandran, D.S.; Jaryal, A.K.; Jyotsna, V.P.; Deepak, K.K. Imbalance between Angiotensin II-Angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab. Syndr. 2019, 13, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.L.; Kapral, M.K.; Fung, K.; Tu, J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet 2006, 368, 29–36. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Bai, S.Z.; Sun, J.; Wu, H.; Zhang, N.; Li, H.X.; Li, G.W.; Li, H.Z.; He, W.; Zhang, W.H.; Zhao, Y.J.; et al. Decrease in calcium-sensing receptor in the progress of diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2012, 95, 378–385. [Google Scholar] [CrossRef]
- Malek, V.; Gaikwad, A.B. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc. Res. 2019, 115, 373–384. [Google Scholar] [CrossRef]
- Chengji, W.; Xianjin, F. Exercise protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway in rats. J. Cell Physiol. 2019, 234, 1682–1688. [Google Scholar] [CrossRef]
- Wang, H.; Bei, Y.; Lu, Y.; Sun, W.; Liu, Q.; Wang, Y.; Cao, Y.; Chen, P.; Xiao, J.; Kong, X. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem. 2015, 35, 2159–2168. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, J.; Zheng, S.; Zhang, L.; Guo, X.; Zhang, J.; Xiao, X. Physical exercise and its protective effects on diabetic cardiomyopathy: What is the evidence? Front. Endocrinol. 2018, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Moe, B.; Eilertsen, E.; Nilsen, T.I. The combined effect of leisure-time physical activity and diabetes on cardiovascular mortality: The Nord-Trondelag Health (HUNT) cohort study, Norway. Diabetes Care 2013, 36, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Adeghate, E.; Singh, J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014, 19, 15–23. [Google Scholar] [CrossRef]
- Sakakibara, M.; Hirashiki, A.; Cheng, X.W.; Bando, Y.; Ohshima, K.; Okumura, T.; Funahashi, H.; Ohshima, S.; Murohara, T. Association of diabetes mellitus with myocardial collagen accumulation and relaxation impairment in patients with dilated cardiomyopathy. Diabetes Res. Clin. Pract. 2011, 92, 348–355. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic cardiomyopathy: Definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular dysfunction and phenotypic derangement in diabetic cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef]
- Lee, M.M.Y.; McMurray, J.J.V.; Lorenzo-Almorós, A.; Kristensen, S.L.; Sattar, N.; Jhund, P.S.; Petrie, M.C. Diabetic cardiomyopathy. Heart 2019, 105, 337–345. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Zhao, Y.; Peng, K.; Li, W.; Wang, Y.; Zhang, J.; Zhou, S.; Liu, Q.; Li, X.; et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes 2014, 63, 3497–3511. [Google Scholar] [CrossRef]
- Mathis, D.R.; Liu, S.S.; Rodrigues, B.B.; McNeill, J.H. Effect of hypertension on the development of diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 2000, 78, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. Diabetic cardiomyopathy. A unique entity or a complication of coronary artery disease? Diabetes Care 1995, 18, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.; Reicher-Reiss, H.; Boyko, V.; Shotan, A.; Mandelzweig, L.; Goldbourt, U.; Behar, S. Usefulness of beta-blocker therapy in patients with non-insulin-dependent diabetes mellitus and coronary artery disease. Bezafibrate Infarction Prevention (BIP) Study Group. Am. J. Cardiol. 1996, 77, 1273–1277. [Google Scholar] [CrossRef]
- Giacchetti, G.; Sechi, L.A.; Rilli, S.; Carey, R.M. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol. Metab. 2005, 16, 120–126. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef]
- Takasu, T.; Takakura, S. Effect of ipragliflozin, an SGLT2 inhibitor, on cardiac histopathological changes in a non-diabetic rat model of cardiomyopathy. Life Sci. 2019, 230, 19–27. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Neal, B.; Perkovic, V.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Fabbrini, E.; Sun, T.; Li, Q.; et al. Canagliflozin for primary and secondary prevention of cardiovascular events: Results drom the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018, 137, 323–334. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Cavaiola, T.S.; Pettus, J. Cardiovascular effects of sodium glucose cotransporter 2 inhibitors. Diabetes Metab. Syndr. Obes. 2018, 11, 133–148. [Google Scholar] [CrossRef]
- Verma, S. Potential mechanisms of sodium-glucose co-transporter 2 inhibitor-related cardiovascular benefits. Am. J. Cardiol. 2019, 124, S36–S44. [Google Scholar] [CrossRef]
- Le Douairon Lahaye, S.; Bekono, F.R.; Broderick, T. Physical activity and diabetic cardiomyopathy: Myocardial adaptation depending on exercise load. Curr. Diabetes Rev. 2014, 10, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Vered, A.; Battler, A.; Segal, P.; Liberman, D.; Yerushalmi, Y.; Berezin, M.; Neufeld, H.N. Exercise-induced left ventricular dysfunction in young men with asymptomatic diabetes mellitus (diabetic cardiomyopathy). Am. J. Cardiol. 1984, 54, 633–637. [Google Scholar] [CrossRef]
- Broderick, T.L.; Poirier, P.; Gillis, M. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes Metab. Res. Rev. 2005, 21, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mitka, M. Study: Exercise may match medication in reducing mortality associated with cardiovascular disease, diabetes. JAMA 2013, 310, 2026–2027. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.M.; Eves, N.D.; Mandic, S.; Glenn, N.M.; Quinney, H.A.; Haykowsky, M.J. The role of exercise in the treatment of cardiovascular disease associated with type 2 diabetes mellitus. Sports Med. 2004, 34, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Dendale, P.; van Loon, L.J.; Meeusen, R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010, 40, 921–940. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fletcher, J.P.; Mathis, R.A.; Cade, W.T. Effects of moderate- versus high-intensity exercise training on physical fitness and physical function in people with type 2 diabetes: A randomized clinical trial. Phys. Ther. 2014, 94, 1720–1730. [Google Scholar] [CrossRef]
- Hansen, D.; Dendale, P.; Jonkers, R.A.; Beelen, M.; Manders, R.J.; Corluy, L.; Mullens, A.; Berger, J.; Meeusen, R.; van Loon, L.J. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia 2009, 52, 1789–1797. [Google Scholar] [CrossRef]
- da Silva, D.E.; Grande, A.J.; Roever, L.; Tse, G.; Liu, T.; Biondi-Zoccai, G.; de Farias, J.M. High-intensity interval training in patients with type 2 diabetes mellitus: A systematic review. Curr. Atheroscler Rep. 2019, 21, 8. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Barengo, N.C.; Qiao, Q.; Lakka, T.A.; Tuomilehto, J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care 2005, 28, 799–805. [Google Scholar] [CrossRef]
- Karjalainen, J.; Peltonen, M.; Vanhala, M.; Korpi-Hyovalti, E.; Puolijoki, H.; Saltevo, J.; Oksa, H.; Saaristo, T.; Tuomilehto, J.; Kujala, U.M. Leisure time physical activity in individuals with screen-detected type 2 diabetes compared to those with known type 2 diabetes. Diabetes Res. Clin. Pract 2008, 81, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Shigeru, M.; Ryusei, U.; Mitsuru, M.; Shigehiro, K. Adherence to a home-based exercise program and incidence of cardiovascular disease in type 2 diabetes patients. Int. J. Sports Med. 2007, 28, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Scheede-Bergdahl, C.; Benee Olsen, D.; Reving, D.; Boushel, R.; Dela, F. Cardiovascular disease markers in type 2 diabetes: The effects of a moderate home-based exercise training programme. Diab. Vasc. Dis. Res. 2009, 6, 291–296. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Standards of medical care in diabetes. Diabetes Care 2019, 42, S29–S30. [Google Scholar] [CrossRef]
- Okada, S.; Hiuge, A.; Makino, H.; Nagumo, A.; Takaki, H.; Konishi, H.; Goto, Y.; Yoshimasa, Y.; Miyamoto, Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J. Atheroscler. Thromb 2010, 17, 828–833. [Google Scholar] [CrossRef]
- Jonker, J.T.; de Mol, P.; de Vries, S.T.; Widya, R.L.; Hammer, S.; van Schinkel, L.D.; van der Meer, R.W.; Gans, R.O.; Webb, A.G.; Kan, H.E.; et al. Exercise and type 2 diabetes mellitus: Changes in tissue-specific fat distribution and cardiac function. Radiology 2013, 269, 434–442. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- Garvey, W.T.; Hardin, D.; Juhaszova, M.; Dominguez, J.H. Effects of diabetes on myocardial glucose transport system in rats: Implications for diabetic cardiomyopathy. Am. J. Physiol. 1993, 264, H837–H844. [Google Scholar] [CrossRef]
- Zamora, M.; Villena, J.A. Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Huang, X.; Wu, D.; Cheng, Y.; Zhang, X.; Liu, T.; Liu, Q.; Xia, P.; Zhang, G.; Hu, S.; Liu, S. Restoration of myocardial glucose uptake with facilitated myocardial glucose transporter 4 translocation contributes to alleviation of diabetic cardiomyopathy in rats after duodenal-jejunal bypass. J. Diabetes Investig. 2019, 10, 626–638. [Google Scholar] [CrossRef]
- Rohling, M.; Herder, C.; Stemper, T.; Mussig, K. Influence of acute and chronic exercise on gucose uptake. J. Diabetes Res. 2016, 2016, 2868652. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur J. Clin. Invest. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.; Novikova, L.; Boulatnikov, I.G.; Smirnova, I.V. Exercise-induced cardiac performance in autoimmune (type 1) diabetes is associated with a decrease in myocardial diacylglycerol. J. Appl. Physiol. (1985) 2012, 113, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R. Evolving concepts of myocardial energy metabolism: More than just fats and carbohydrates. Circ. Res. 2016, 119, 1173–1176. [Google Scholar] [CrossRef]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014, 171, 2080–2090. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Mokuda, O.; Sakamoto, Y.; Ikeda, T.; Mashiba, H. Effects of anoxia and low free fatty acid on myocardial energy metabolism in streptozotocin-diabetic rats. Ann. Nutr. Metab. 1990, 34, 259–265. [Google Scholar] [CrossRef]
- Wall, S.R.; Lopaschuk, G.D. Glucose oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats. Biochim. Biophys. Acta 1989, 1006, 97–103. [Google Scholar] [CrossRef]
- Suarez, J.; Scott, B.; Dillmann, W.H. Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1439–R1445. [Google Scholar] [CrossRef]
- Ziegelhoffer, A.; Ravingerova, T.; Styk, J.; Tribulova, N.; Volkovova, K.; Sebokova, J.; Breier, A. Diabetic cardiomyopathy in rats: Biochemical mechanisms of increased tolerance to calcium overload. Diabetes Res. Clin. Pract. 1996, 31, S93–S103. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Allo, S.; Schaffer, S.W. The effect of sulfonylurea therapy on defective calcium movement associated with diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 1989, 67, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Wehrens, X.H.; Wyatt, T.A.; Parbhu, S.; Rozanski, G.J.; Patel, K.P.; Bidasee, K.R. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J. Appl. Physiol. (1985) 2009, 106, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Novoa, U.; Arauna, D.; Moran, M.; Nunez, M.; Zagmutt, S.; Saldivia, S.; Valdes, C.; Villasenor, J.; Zambrano, C.G.; Gonzalez, D.R. High-Intensity Exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid. Med. Cell Longev. 2017, 2017, 7921363. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Givvimani, S.; Kundu, S.; Metreveli, N.; Pushpakumar, S.; Tyagi, S.C. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell Cardiol. 2016, 92, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Levine, T.B. Peroxisome proliferator activator receptors (PPAR), insulin resistance, and cardiomyopathy: Friends or foes for the diabetic patient with heart failure? Cardiol. Rev. 2004, 12, 158–170. [Google Scholar] [CrossRef]
- Hafstad, A.D.; Boardman, N.; Aasum, E. How exercise may amend metabolic disturbances in diabetic cardiomyopathy. Antioxid. Redox. Signal. 2015, 22, 1587–1605. [Google Scholar] [CrossRef]
- Searls, Y.M.; Smirnova, I.V.; Fegley, B.R.; Stehno-Bittel, L. Exercise attenuates diabetes-induced ultrastructural changes in rat cardiac tissue. Med. Sci. Sports Exerc. 2004, 36, 1863–1870. [Google Scholar] [CrossRef]
- Mahmoud, A.M. Exercise amaliorates metabolic disturbances and oxidative stress in diabetic cardiomyopathy: Possible underlying mechanisms. Adv. Exp. Med. Biol. 2017, 999, 207–230. [Google Scholar]
- Joubert, M.; Manrique, A.; Cariou, B.; Prieur, X. Diabetes-related cardiomyopathy: The sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. 2019, 45, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ascensao, A.; Magalhaes, J.; Soares, J.M.; Ferreira, R.; Neuparth, M.J.; Marques, F.; Oliveira, P.J.; Duarte, J.A. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H722–H731. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, J.M.; Kurdys, J.G.; Muntean, D.M.; Rosca, M.G. Mitochondrial NAD(+)/NADH redox state and diabetic cardiomyopathy. Antioxid. Redox. Signal. 2019, 30, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, G.; He, Y.; Da, J.; Xie, B. Obeticholic acid protects against diabetic cardiomyopathy by activation of FXR/Nrf2 signaling in db/db mice. Eur. J. Pharm. 2019, 858, 172393. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- He, X.; Ma, Q. Disruption of Nrf2 synergizes with high glucose to cause heightened myocardial oxidative stress and severe cardiomyopathy in diabetic mice. J. Diabetes Metab. 2012, 7. [Google Scholar]
- Guan, Y.; Zhou, L.; Zhang, Y.; Tian, H.; Li, A.; Han, X. Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway. Cell Signal. 2019, 62, 109339. [Google Scholar] [CrossRef]
- Ge, Z.D.; Lian, Q.; Mao, X.; Xia, Z. Current status and challenges of NRF2 as a potential therapeutic target for dabetic cardiomyopathy. Int. Heart J. 2019, 60, 512–520. [Google Scholar] [CrossRef]
- Quinteiro, H.; Buzin, M.; Conti, F.F.; Dias Dda, S.; Figueroa, D.; Llesuy, S.; Irigoyen, M.C.; Sanches, I.C.; De Angelis, K. Aerobic exercise training promotes additional cardiac benefits better than resistance exercise training in postmenopausal rats with diabetes. Menopause 2015, 22, 534–541. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Ye, J.M.; Fu, X.M.; Wang, X.C.; Ye, J.Y.; Wu, X.R.; Hua, P.; Liao, Y.Q.; Xuan, W.; Duan, J.L.; et al. DDAH2 alleviates myocardial fibrosis in diabetic cardiomyopathy through activation of the DDAH/ADMA/NOS/NO pathway in rats. Int. J. Mol. Med. 2019, 43, 749–760. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, H.; Xu, F.; Wang, J.; Liu, Y.; Xing, X.; Guo, L.; Zhang, M.; Lu, Q. Liraglutide alleviates cardiac fibrosis through inhibiting P4halpha-1 expression in STZ-induced diabetic cardiomyopathy. Acta Biochim. Biophys. Sin. (Shanghai) 2019, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin ameliorates diabetic cardiomyopathy via inhibiting TGF-beta1/Smad signaling. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, X.; Zhao, Z.; Zhang, L.; Zhong, L.; Li, L.; Kang, W.; Zhang, Y.; Ge, Z. Irbesartan ameliorates diabetic cardiomyopathy by regulating protein kinase D and ER stress activation in a type 2 diabetes rat model. Pharm. Res. 2015, 93, 43–51. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid. Med. Cell Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Esposito, K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: Systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J. Am. Heart Assoc. 2019, 8, e01235. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, S.; Huang, J.; Cai, L.; Keller, B.B. Right ventricular dysfunction and remodeling in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H113–H122. [Google Scholar] [CrossRef]
- Zuo, G.; Ren, X.; Qian, X.; Ye, P.; Luo, J.; Gao, X.; Zhang, J.; Chen, S. Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy. J. Cell Physiol. 2019, 234, 1925–1936. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, G. Troxerutin protects against diabetic cardiomyopathy through NFkappaB/AKT/IRS1 in a rat model of type 2 diabetes. Mol. Med. Rep. 2017, 15, 3473–3478. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chavez, A.O.; Gastaldelli, A.; Perego, L.; Tripathy, D.; Saad, M.J.; Velloso, L.; Folli, F. The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr. Vasc. Pharm. 2008, 6, 301–312. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Xue, M.; Qiao, Y.; Chen, Y.; Long, G.; Tian, X.; Hu, Y.; Zhou, P.; Dong, X.; et al. Spleen tyrosine kinaseinduced JNKdependent NLRP3 activation is involved in diabetic cardiomyopathy. Int. J. Mol. Med. 2019, 43, 2481–2490. [Google Scholar]
- Marques-Aleixo, I.; Santos-Alves, E.; Oliveira, P.J.; Moreira, P.I.; Magalhaes, J.; Ascensao, A. The beneficial role of exercise in mitigating doxorubicin-induced Mitochondrionopathy. Biochim. Biophys. Acta. Rev. Cancer 2018, 1869, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.M.; Hamlin, R.L.; Unverferth, D.V.; Davis, H.W.; Merola, A.J. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. (1985) 1985, 59, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Khakdan, S.; Delfan, M.; Heydarpour Meymeh, M.; Kazerouni, F.; Ghaedi, H.; Shanaki, M.; Kalaki-Jouybari, F.; Gorgani-Firuzjaee, S.; Rahimipour, A. High-intensity interval training (HIIT) effectively enhances heart function via miR-195 dependent cardiomyopathy reduction in high-fat high-fructose diet-induced diabetic rats. Arch. Physiol. Biochem. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Tu, X.; Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta. Pharmacol. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Woods, R.L. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: A brief review. Clin. Exp. Pharmacol. Physiol. 2004, 31, 791–794. [Google Scholar] [CrossRef]
- Gutkowska, J.; Broderick, T.L.; Bogdan, D.; Wang, D.; Lavoie, J.M.; Jankowski, M. Downregulation of oxytocin and natriuretic peptides in diabetes: Possible implications in cardiomyopathy. J. Physiol. 2009, 587, 4725–4736. [Google Scholar] [CrossRef]
- Broderick, T.L.; Wang, D.; Jankowski, M.; Gutkowska, J. Unexpected effects of voluntary exercise training on natriuretic peptide and receptor mRNA expression in the ob/ob mouse heart. Regul. Pept. 2014, 188, 52–59. [Google Scholar] [CrossRef]
- Stewart, K.J. Role of exercise training on cardiovascular disease in persons who have type 2 diabetes and hypertension. Cardiol. Clin. 2004, 22, 569–586. [Google Scholar] [CrossRef]
- Paulson, D.J.; Mathews, R.; Bowman, J.; Zhao, J. Metabolic effects of treadmill exercise training on the diabetic heart. J. Appl. Physiol. (1985) 1992, 73, 265–271. [Google Scholar] [CrossRef]
- Shearer, J.; Ross, K.D.; Hughey, C.C.; Johnsen, V.L.; Hittel, D.S.; Severson, D.L. Exercise training does not correct abnormal cardiac glycogen accumulation in the db/db mouse model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E31–E39. [Google Scholar] [CrossRef]
- Hafstad, A.D.; Lund, J.; Hadler-Olsen, E.; Hoper, A.C.; Larsen, T.S.; Aasum, E. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes 2013, 62, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Issad, T.; Masson, E.; Pagesy, P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab. 2010, 36, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Belke, D.; Suarez, J.; Swanson, E.; Clark, R.; Hoshijima, M.; Dillmann, W.H. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 2005, 96, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Moien-Afshari, F.; Ghosh, S.; Elmi, S.; Rahman, M.M.; Sallam, N.; Khazaei, M.; Kieffer, T.J.; Brownsey, R.W.; Laher, I. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia 2008, 51, 1327–1337. [Google Scholar] [CrossRef]
- Sallam, N.; Khazaei, M.; Laher, I. Effect of moderate-intensity exercise on plasma C-reactive protein and aortic endothelial function in type 2 diabetic mice. Mediat. Inflamm. 2010, 2010, 149678. [Google Scholar] [CrossRef][Green Version]
- Khazaei, M.; Moien-Afshari, F.; Kieffer, T.J.; Laher, I. Effect of exercise on augmented aortic vasoconstriction in the db/db mouse model of type-II diabetes. Physiol. Res. 2008, 57, 847–856. [Google Scholar]
- Li, S.; Liang, M.; Gao, D.; Su, Q.; Laher, I. Changes in titin and collagen modulate effects of aerobic and resistance exercise on diabetic cardiac function. J. Cardiovasc. Transl. Res. 2019, 12, 404–414. [Google Scholar] [CrossRef]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Yip, D.; Broderick, T.L.; Chiasson, J.L.; Jankowski, M.; Gutkowska, J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 2015, 156, 1416–1428. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Smuder, A.J.; Min, K.; Tumer, N.; Powers, S.K. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1515–H1524. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Rogan, K.J.; Sung, M.M.; Zordoky, B.N.; Haykowsky, M.J.; Young, M.E.; Jones, L.W.; Dyck, J.R. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E243–E253. [Google Scholar] [CrossRef]
- Lorenzo-Almoros, A.; Tunon, J.; Orejas, M.; Cortes, M.; Egido, J.; Lorenzo, O. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; King, K.L.; Shaw, K.J.; Luis, E.; Rullamas, J.; Luoh, S.M.; Darbonne, W.C.; Knutzon, D.S.; Yen, R.; Chien, K.R.; et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 1995, 92, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, G.; Gomez-Hurtado, N.; Fernandez-Velasco, M.; Calderon, E.; Smani, T.; Ordonez, A.; Cachofeiro, V.; Bosca, L.; Diez, J.; Gomez, A.M.; et al. Cardiotrophin-1 induces sarcoplasmic reticulum Ca(2+) leak and arrhythmogenesis in adult rat ventricular myocytes. Cardiovasc Res. 2012, 96, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jougasaki, M. Cardiotrophin-1 in cardiovascular regulation. Adv. Clin. Chem. 2010, 52, 41–76. [Google Scholar] [PubMed]
- Limongelli, G.; Calabro, P.; Maddaloni, V.; Russo, A.; Masarone, D.; D’Aponte, A.; Roselli, T.; Bonauro, R.; D’Alessandro, R.; D’Andrea, A.; et al. Cardiotrophin-1 and TNF-alpha circulating levels at rest and during cardiopulmonary exercise test in athletes and healthy individuals. Cytokine 2010, 50, 245–247. [Google Scholar] [CrossRef]
- Nyman, K.; Graner, M.; Pentikainen, M.O.; Lundbom, J.; Hakkarainen, A.; Siren, R.; Nieminen, M.S.; Taskinen, M.R.; Lundbom, N.; Lauerma, K. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J. Cardiovasc. Magn. Reson. 2013, 15, 103. [Google Scholar] [CrossRef]
- Chen, W.J.; Greulich, S.; van der Meer, R.W.; Rijzewijk, L.J.; Lamb, H.J.; de Roos, A.; Smit, J.W.; Romijn, J.A.; Ruige, J.B.; Lammertsma, A.A.; et al. Activin A is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 150. [Google Scholar] [CrossRef]
- Perakakis, N.; Mougios, V.; Fatouros, I.; Siopi, A.; Draganidis, D.; Peradze, N.; Ghaly, W.; Mantzoros, C.S. Physiology of activins/follistatins: Associations with metabolic and anthropometric variables and response to exercise. J. Clin. Endocrinol. Metab. 2018, 103, 3890–3899. [Google Scholar] [CrossRef]
- Carley, A.N.; Severson, D.L. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim. Biophys. Acta 2005, 1734, 112–126. [Google Scholar] [CrossRef]
- Hoffmann, U.; Espeter, F.; Weiss, C.; Ahmad-Nejad, P.; Lang, S.; Brueckmann, M.; Akin, I.; Neumaier, M.; Borggrefe, M.; Behnes, M. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure-diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc. Disord. 2015, 15, 50. [Google Scholar] [CrossRef]
- Akbal, E.; Ozbek, M.; Gunes, F.; Akyurek, O.; Ureten, K.; Delibasi, T. Serum heart type fatty acid binding protein levels in metabolic syndrome. Endocrine 2009, 36, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hsu, H.C.; Lee, B.C.; Lin, H.J.; Chen, Y.H.; Huang, H.C.; Ho, Y.L.; Chen, M.F. Exercise training improves cardiac function in infarcted rabbits: Involvement of autophagic function and fatty acid utilization. Eur. J. Heart Fail. 2010, 12, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Chew, P.G.; Dobson, R.; Wootton, A.; Ashrafi, R.; Khand, A. The prognostic value of heart type fatty acid binding protein in patients with suspected acute coronary syndrome: A systematic review. Curr. Cardiol. Rev. 2017, 13, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Asbun, J.; Villarreal, F.J. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Lai, M. Insulin-like growth factor binding protein: A possible marker for the metabolic syndrome? Acta Diabetol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.U.; Gaggin, H.K.; Sheftel, A.D.; Belcher, A.M.; Weiner, R.B.; Baggish, A.L.; Motiwala, S.R.; Liu, P.P.; Januzzi Jr, J.L. Prognostic usefulness of insulin-like growth factor-binding protein 7 in heart failure with reduced ejection fraction: A novel biomarker of myocardial diastolic function? Am. J. Cardiol. 2014, 114, 1543–1549. [Google Scholar] [CrossRef]
- Guo, X.H.; Liu, L.X.; Zhang, H.Y.; Zhang, Q.Q.; Li, Y.; Tian, X.X.; Qiu, Z.H. Insulin-like growth factor binding protein-related protein 1 contributes to hepatic fibrogenesis. J. Dig. Dis. 2014, 15, 202–210. [Google Scholar] [CrossRef]
- Shaver, A.; Nichols, A.; Thompson, E.; Mallick, A.; Payne, K.; Jones, C.; Manne, N.D.; Sundaram, S.; Shapiro, J.I.; Sodhi, K. Role of serum biomarkers in early dtection of diabetic cardiomyopathy in the west virginian population. Int. J. Med. Sci. 2016, 13, 161–168. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, D.; Chen, P.; Xu, T.; Fu, S.; Li, J.; Bei, Y. MicroRNAs in diabetic cardiomyopathy and clinical perspectives. Front. Genet. 2014, 5, 185. [Google Scholar] [CrossRef]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [CrossRef]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Awe, O.; Metreveli, N.; Qipshidze, N.; Munjal, C.; Tyagi, N.; Tyagi, S.C. Exercise ameliorates diabetic cardiomyopathy by inducing beta2-adrenergic receptors and miR-133a, and attenuating MMP-9. FASEB J. 2011, 25. [Google Scholar]
- Rawal, S.; Manning, P.; Katare, R. Cardiovascular microRNAs: As modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc. Diabetol. 2014, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Regensteiner, J.G.; Bauer, T.A.; Reusch, J.E.; Quaife, R.A.; Chen, M.Y.; Smith, S.C.; Miller, T.M.; Groves, B.M.; Wolfel, E.E. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med. Sci. Sports Exerc. 2009, 41, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.E.; Gusso, S.; Hofman, P.L.; Derraik, J.G.; Hornung, T.S.; Cutfield, W.S.; Baldi, J.C. Systolic and diastolic abnormalities reduce the cardiac response to exercise in adolescents with type 2 diabetes. Diabetes Care 2014, 37, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Teupe, C.; Rosak, C. Diabetic cardiomyopathy and diastolic heart failure difficulties with relaxation. Diabetes Res. Clin. Pract. 2012, 97, 185–194. [Google Scholar] [CrossRef]
- Murtaza, G.; Virk, H.U.H.; Khalid, M.; Lavie, C.J.; Ventura, H.; Mukherjee, D.; Ramu, V.; Bhogal, S.; Kumar, G.; Shanmugasundaram, M.; et al. Diabetic cardiomyopathy-A comprehensive updated review. Prog Cardiovasc. Dis. 2019, 62, 315–326. [Google Scholar] [CrossRef]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef]
- Zhao, M.; Veeranki, S.P.; Li, S.; Steffen, L.M.; Xi, B. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: A national cohort study of 88,140 US adults. Br. J. Sports Med. 2019, 53, 1405–1411. [Google Scholar] [CrossRef]
- Khanna, N.N.; Jamthikar, A.D.; Araki, T.; Gupta, D.; Piga, M.; Saba, L.; Carcassi, C.; Nicolaides, A.; Laird, J.R.; Suri, H.S.; et al. Nonlinear model for the carotid artery disease 10-year risk prediction by fusing conventional cardiovascular factors to carotid ultrasound image phenotypes: A Japanese diabetes cohort study. Echocardiography 2019, 36, 345–361. [Google Scholar] [CrossRef]
- Verboven, M.; Van Ryckeghem, L.; Belkhouribchia, J.; Dendale, P.; Eijnde, B.O.; Hansen, D.; Bito, V. Effect of exercise intervention on cardiac function in type 2 diabetes mellitus: A systematic review. Sports Med. 2019, 49, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.J.; Burns, A.T.; MacIsaac, R.J.; MacIsaac, A.I.; Prior, D.L.; La Gerche, A. Exercise capacity in diabetes mellitus is predicted by activity status and cardiac size rather than cardiac function: A case control study. Cardiovasc. Diabetol. 2018, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Shenoy, S.; Hussain, M.E. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: A systematic review. Diabetes Metab. Syndr. 2018, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
| Study Type (Design) | Subjects | Exercise Intervention | Effects | Ref | |||
|---|---|---|---|---|---|---|---|
| Type | Frequency | Time (min) | Duration | ||||
| Human (non-RCT) | Patients with T2DM | Home-based exercise training (Rowing ergo meter) | 3–4 days/wk | 30 m every other day for a total of 28 sessions | 8 wks | ↑Insulin sensitivity ↑Forearm endothelial function ↓Plasma adhesion molecules (ICAM-1 & VCAM-1) | [43] |
| Human (RCT) | Patients with T2DM | Aerobic & Resistance Exercise | 3–5 days/wk | 75 min/session | 12 wks | ↑Anti-atherosclerotic effects ↓Cardiovascular events ↑Flow-mediated endothelium-dependent vasodilation | [45] |
| Human (non-RCT) | Patients with T2DM | Aerobic Exercise | 4 days/wk | 4–7 h/day | 24 wks | ↓Hepatic triglyceride ↓Paracardial fat volume | [46] |
| Human (RCT) | Patients with T2DM | High intensity interval training | NA | NA | 12 wks | ↑Left ventricular mass (g) ↑Early diastolic filling rate change (ml/s) ↑Peak torsion change ↓Body weight (kg) ↓Liver fat (%) ↓ALP (U/I) ↓HbA1c (%) ↓2–hr glucose (pmol/l) ↓2–hr AUGC (mmol/l) | [47] |
| Subjects | Exercise Intervention | Effects | Ref | |||
|---|---|---|---|---|---|---|
| Type | Frequency | Time (min) | Duration | |||
| STZ-diabetic rats (single injection of STZ: 40mg/kg) | Aerobic exercise | 5 days/wk | 60 min/day | 12 wks | ↑Ejection fractions (%) ↑Left ventricular end-systolic volume ↓Serum cTn-I levels ↓Apoptotic myocardial cells ↓GRP78, CHOP, cleaved caspase-12 protein | [9] |
| Obese diabetic mice (db/db) | Aerobic Exercise | 5 days/wk | 330m run at speed of 10 m/min (2 wks) + 330m run at speed of 11 m/min (3 wks) | 5 wks | ↑Body weight (gm) ↑Mean blood pressure (mmHg) ↑Heart weight (gm) ↑Blood glucose (mg/dL) ↑Stroke volume (µL) ↑Ejection fraction (%) ↑Fractional Shortening (%) | [65] |
| STZ-diabetic rats (single injection of STZ: 55 g/kg) | Aerobic Exercise | 5 days/wk | 10–15 min/day, total 60 min (speed 2m/min at grade 5%) | 7 wks | ↑Citrate synthase activity (µmol/tissue(g)/min) ↑Heart Rate (bpm) ↓Left ventricular end diastolic diameter & Left ventricular end systolic diameter (mm) ↑Fractional shortening (%) ↑Ejection fraction (%) ↑Cardiac output (ml/min) ↓QRS interval (m/sec) ↑Myocyte contractile kinetics velocity(µm/sec), extent of cell shortening (µm), and relengthening velocity (µm/sec) ↓Myocyte contractile kinetics time to 50% peak contractile velocity (m/sec) & time to 50% peak relaxation velocity (m/sec) | [63] |
| 7 wk old diabetic rats (Injection STZ 65 mg) | Aerobic Exercise | 5 days/wk | 60 min/day (20 m/min pace) | 9 wks | ↑Cytoplasmic area (% of intracellular area) ↑Lipid droplets(µm2) ↑Mitochondria area, myofibrillar area, mitochondria quality index, cytoplasm area, and collagen fiber circumference | [69] |
| 3 month old male Sprague Dwaley rat (Single dose of alloxan; 200 mg/kg) | High intensity of Aerobic Exercise | 5 days/wk | NA | 4 wks | ↓Body weight (g) ↑Plasma glucose (mg/dL) ↑NOX2 & NOX4, p67phox ↓SERCA2 ↓eNOS & BH4 | [64] |
| BBDR (Biomedical Research Models) male rats | Aerobic Exercise | 5 days/wk | 50 min/day | 8 wks | ↑Plasma glucose (mg/dl) & HbA1c (%) ↑LV end-systolic volume (µl), LV end-diastolic volume (µl), and LV – dp/dt (mmHg/s) ↑Myocardial mitochondria fractional area (%) | [54] |
| Adult Sprague-dawley male rats + doxorubicin (DOX: 20 mg/kg body weight) | Aerobic Exercise | 5 days/wk | 60 min/day (30 m/min) | NA | ↑Protect against Dox-mediated damage in mitochondria ↓Caspase 3 & calpain ↑SOD1, SOD2, Catalase, GPX1, catalase, and HSP72 ↓Hydrogen peroxide (pmol/mg/min) | [109] |
| 8 wks old female C57BL6 mice | Aerobic Exercise + Resveratrol supplementation | 5 days/wk | 30 min/day | 8 wks | ↑Left ventricle posterior wall (mm) ↑Intraventricular septum (mm) ↑Left ventricle internal dimension (mm) ↑Fractional shortening ↓Citrate synthase activity (mmol/mg/min) ↓ANF & UCP3 | [110] |
| Sprague-dawley (SD) diabetic rats | Aerobic Exercise | 5 days/wk | 60 min/day Low intensity: 20m/min High intensity: 34 m/min | 12 wks | ↓Left ventricular & diastolic volume ↓caspase 3, cTn-1 (lg/l), Grp78, CHOP, and Caspase 12 | [9] |
| Sprague-Dawley rats (intravenous injection of streptozotocin: 60mg/kg | Aerobic Exercise | 5 days/wk | 60 min/day (32 m/min) | 10 wks | ↓Body weight (g) ↓Glucose (mmol/L) ↓Triglycerides (mmol/L) ↑Glucose oxidation (nmol/g/min) ↑Glycolysis (nmol/g/min) ↑Aortic flow (ml/min) | [33] |
| 6-8 wk male Wistar rat + Doxorubicin (20 mg/kg) | Aerobic Exercise | 5 days/wk | 60 min/day | 14 wks | ↑Mitochondrial respiration, calcium tolerance, oxidative damage, and heat shock proteins ↓State 3 respiration, respiratory control ratio, uncoupled respiration, aconitase activity, and protein sulfhydryl content | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.Y.; Ko, J.R.; Jang, J.E.; Kim, T.N.; Youm, J.B.; Kwak, H.-B.; Bae, J.H.; Kim, A.H.; Ko, K.S.; Rhee, B.D.; et al. Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 6284. https://doi.org/10.3390/ijms20246284
Seo DY, Ko JR, Jang JE, Kim TN, Youm JB, Kwak H-B, Bae JH, Kim AH, Ko KS, Rhee BD, et al. Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. International Journal of Molecular Sciences. 2019; 20(24):6284. https://doi.org/10.3390/ijms20246284
Chicago/Turabian StyleSeo, Dae Yun, Jeong Rim Ko, Jung Eun Jang, Tae Nyun Kim, Jae Boum Youm, Hyo-Bum Kwak, Jun Hyun Bae, Amy Hyein Kim, Kyung Soo Ko, Byoung Doo Rhee, and et al. 2019. "Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms" International Journal of Molecular Sciences 20, no. 24: 6284. https://doi.org/10.3390/ijms20246284
APA StyleSeo, D. Y., Ko, J. R., Jang, J. E., Kim, T. N., Youm, J. B., Kwak, H.-B., Bae, J. H., Kim, A. H., Ko, K. S., Rhee, B. D., & Han, J. (2019). Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. International Journal of Molecular Sciences, 20(24), 6284. https://doi.org/10.3390/ijms20246284





