Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan
Abstract
1. Introduction
2. Results and Discussion
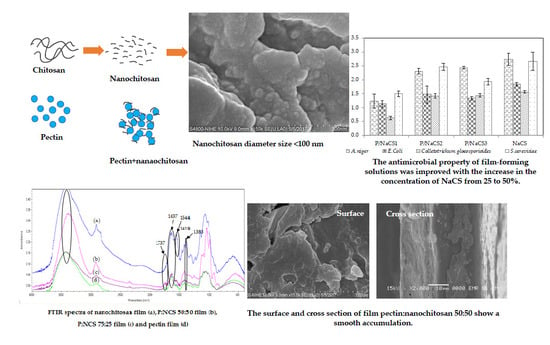
2.1. Preparation and Characterization of Nanochitosan
2.2. Thickness and Mechanical Properties of Films
2.3. Contact Angle (CA) of the Films
2.4. Hydration Properties of the Films
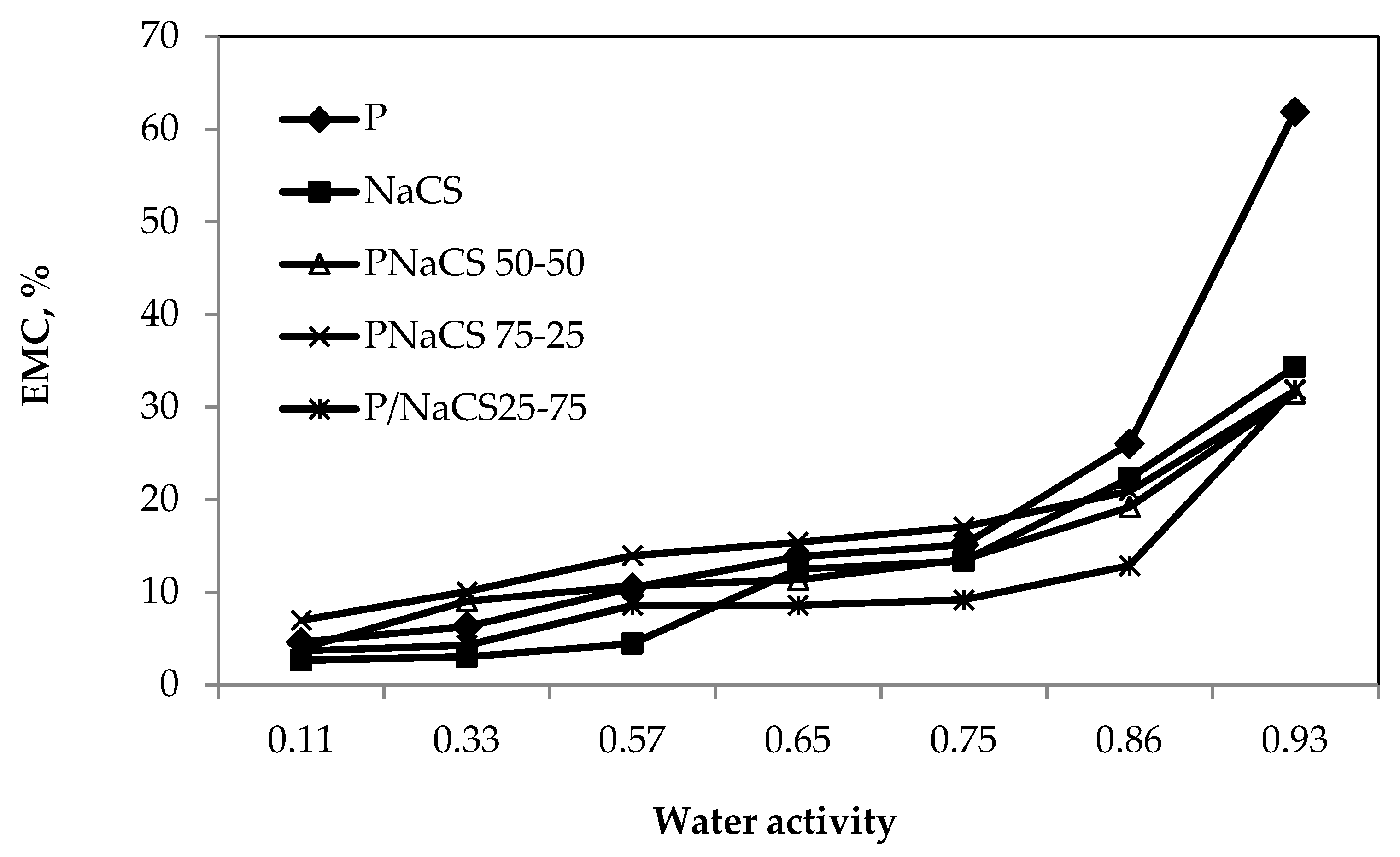
2.5. Moisture Sorption Isotherm of the Films
2.6. Color and Transparency of the Films
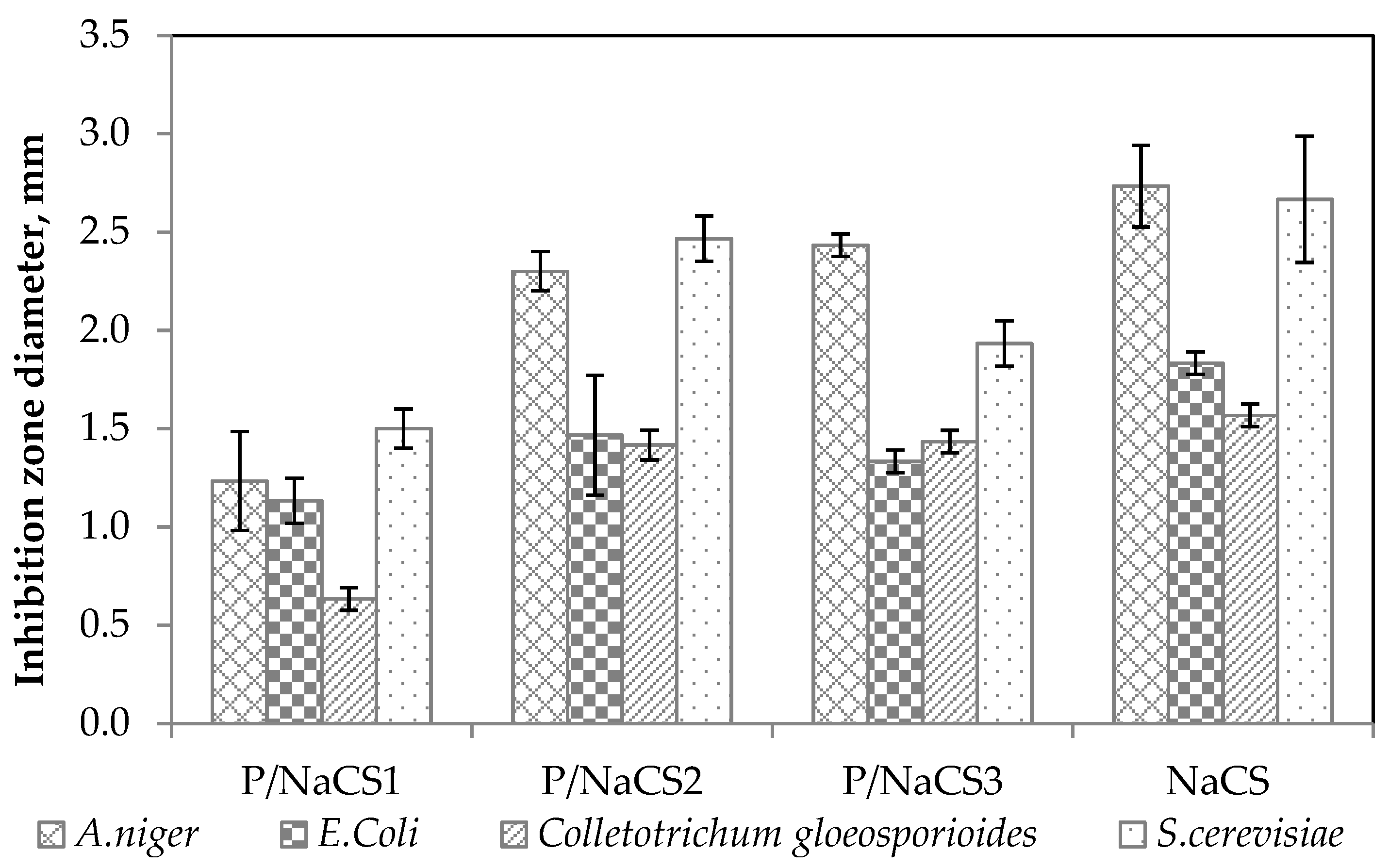
2.7. Antimicrobial Action of Film-Forming Solutions
2.8. Oxygen Barrier Property of the Films
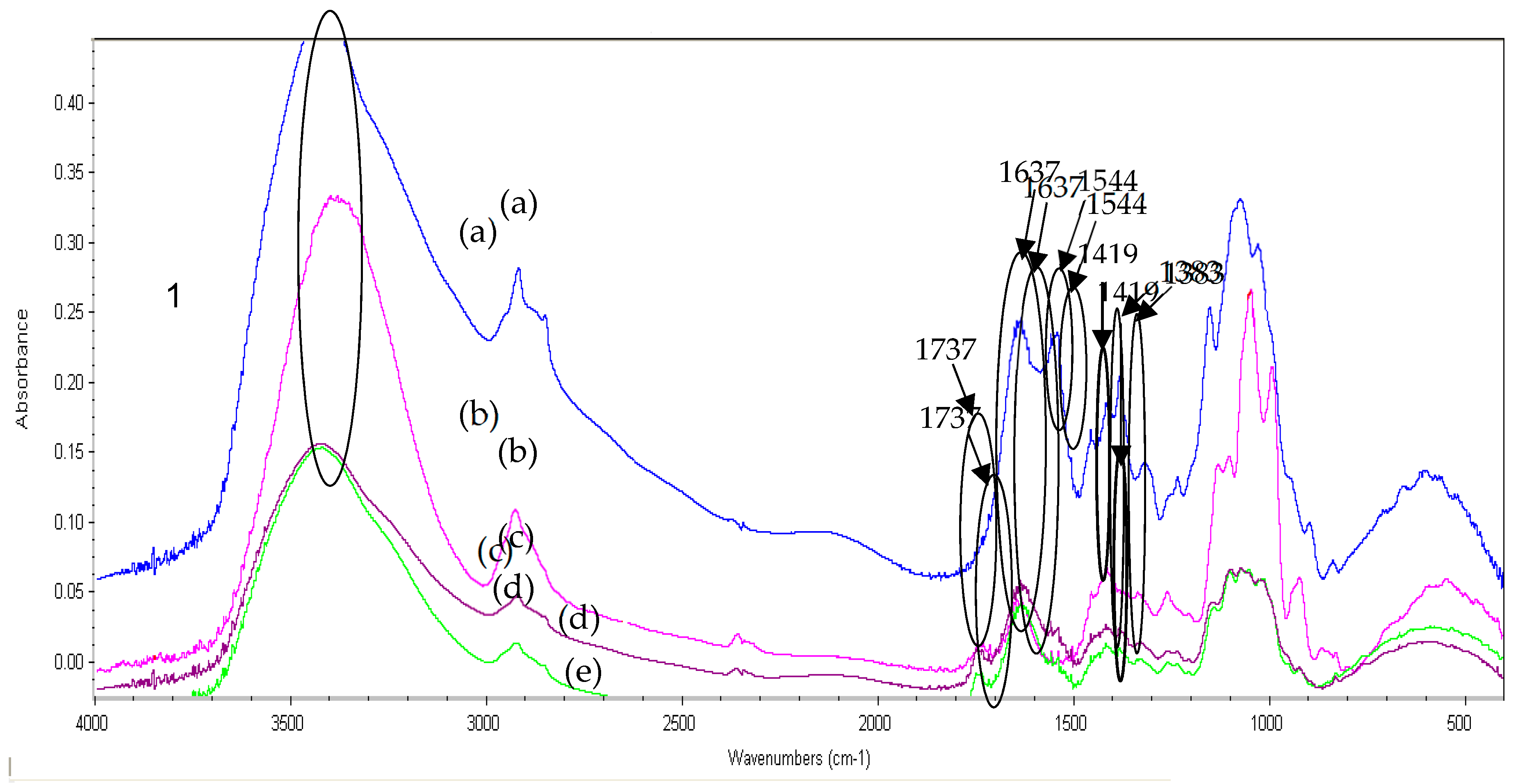
2.9. Fourier Transform Infrared (FTIR) Analysis of Pectin, Pectin:Nanochitosan and Nanochitosan Films
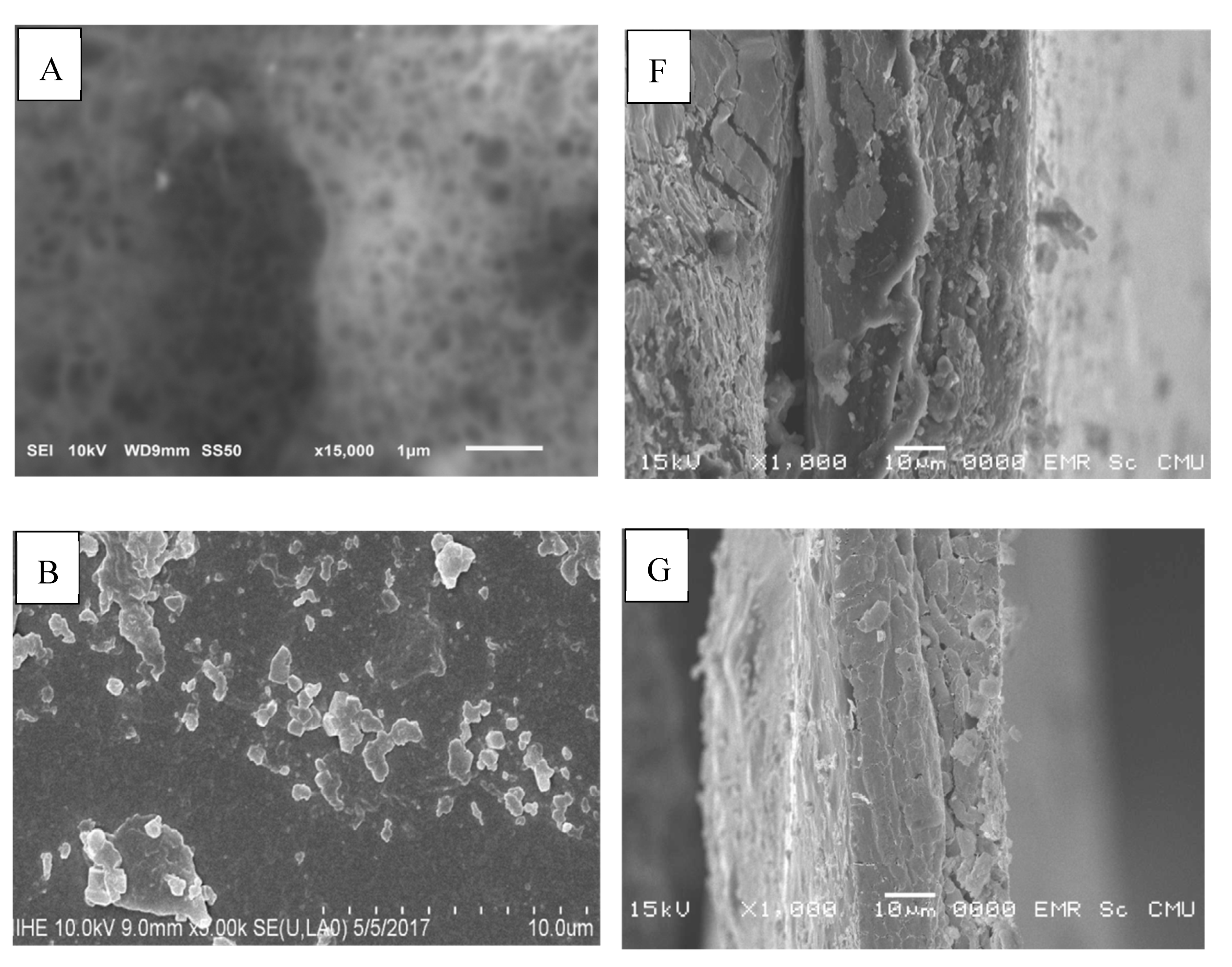
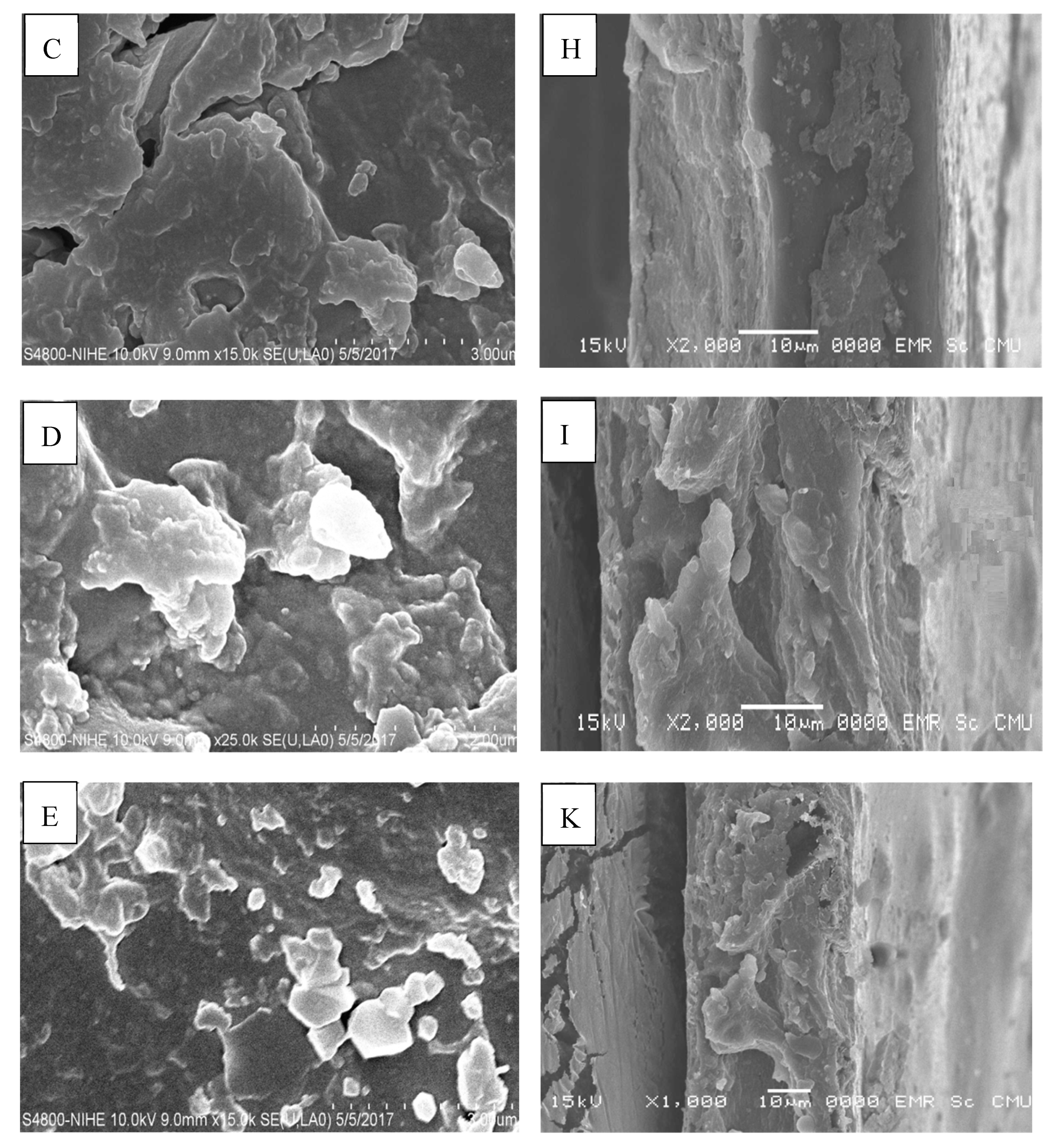
2.10. Scanning Electron Microscopy (SEM)
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Film Thickness
3.4. Film Solubility
3.5. Swelling Degree (SD)
3.6. Water Vapor Permeability (WVP) and Water Vapor Transmission rate (WVTR)
3.7. Moisture Sorption Isotherm
3.8. Mechanical Properties
3.9. Color Measurement
3.10. Water Contact Angle
3.11. Oxygen Permeability
3.12. Fourier transform infrared (FTIR) spectroscopy
3.13. Scanning Electron Microscopy (SEM)
3.14. Antimicrobial Action of Film-Forming Solutions
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NCS | Nanochitosan |
| P | Pectin |
| SEM | Scanning electron microscope |
| FTIR | Fourier Transform infrared |
| MAA | Methacrylic acid |
| CS | Chitosan |
References
- Hosseini, S.F.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties–A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Badii, F.; Shahamirian, M. Recent innovations in the area of edible films and coatings. Recent Pat. FoodNutr. Agric. 2013, 5, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Marcia, P.M.C.; Lourençode, M.F.; Mauricio, T.M.C. New polyelectrolyte complex from pectin/chitosan and montmorillonite clay. Carbohydr. Polym. 2016, 146, 123–130. [Google Scholar]
- de Moura, M.R.; Luiz, F.A.A.; Mattoso, H.C. Preparation of chitosan nanoparticles using methacrylic acid. J. Colloid Interface Sci. 2008, 321, 477–483. [Google Scholar] [CrossRef]
- Odegard, G.M.; Clancy, T.C.; Gates, T.S. Modeling of the mechanical properties of nanoparticle/polymer composites. Polymer 2005, 46, 553–562. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohydr. Polym. 2010, 79, 301–305. [Google Scholar] [CrossRef]
- Parris, N.C.D.R.; Joubran, R.F.; Pessen, H. Composition factors affecting the water vapor permeability and tensile properties of hydrophilic films. J. Agric. Food Chem. 1995, 43, 1432–1435. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; Tijith, K.G.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite film-based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef]
- Verma, A.K. Anti-oxidant activities of biopolymeric nanoparticles: Boon or bane. J. Pharm. Res. 2014, 8, 871–876. [Google Scholar]
- Rungsinee, S.; Pitak, N. Oxygen permeability and mechanical properties of banana films. Food Res. Int. 2007, 40, 365–370. [Google Scholar]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr Polym 2013, 96, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Čopíková, J.; Marounek, M.; Mlčochová, P.; Blafková, P.; Tkadlecová, M.; Havlíček, J. Preparation of N-alkylamides of highly methylated (HM) citrus pectin. Czech. J. Food Sci. 2003, 21, 162–166. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Tran, T.X. Optimization of pectin extraction from yanang leaves and making pectin–alginate films. J. Sci. Technol. Univ. DanangVietnam. 2016, 11, 170–174. [Google Scholar]
- Nguyen, T.M.N.; Nguyen, D.L.; Tran, T.M. Effect of nano chitosan on property of HPMC-Carnauba composite films and quality of the coated bananas. Vietnam J. Sci. Technol. 2016, 54, 105–114. [Google Scholar]
- Nguyen, T.M.N.; Nguyen, T.T.Q.; Tran, T.M.; Nguyen, D.L. Synthesis of nano chitosan by using methacrylic acid and evaluation of antibacterial activity against Colletotrichum Musae associated with banana anthracnose. J. Sci. Technol. Vietnam Acad. Sci. Technol. 2015, 53, 8–14. [Google Scholar]
- Gontard, N.; Guilbert, S.; Cuq, J. Edible wheat gluten films: Influence of the main process variables on film properties using response surface methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Kieckbusch, T.G.; da Silva, M.A. Natamycin release from alginate/pectin films for food packaging applications. J. Food Eng. 2012, 110, 18–25. [Google Scholar]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Effects of zinc oxide nanoparticles on properties of pectin/alginate edible films. Int. J. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Quyen, D.T.M.; Adisak, J.; Rachtanapun, P. Relationship between Solubility, Moisture Sorption Isotherms and Morphology of Chitosan/methylcellulose Films with Different Carbendazim Content. J. Agric. Sci. 2012, 4, 188–196. [Google Scholar] [CrossRef]
- ASTM, Standard test methods for tensile properties of thin plastic sheeting D882-10. In Annual Book of ASTM; American Society for Testing and Materials: Philadelphia, PA, USA, 2010.
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Jantanasakulwong, N.L.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch and polyethylene-graft-maleic anhydride with chitosan as compatibilizer. Carbohydr. Polym. 2016, 153, 89–95. [Google Scholar] [CrossRef]
- ASTM D3985-05, Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor Book of Standards. Astm Int. 2010. Available online: https://standards.globalspec.com/std/10198811/ASTM%20D3985 (accessed on 19 March 2020).


| Films | Thickness, µm | Tensile Strength, MPa | Elongation, % |
|---|---|---|---|
| P:NCS 100:0 | 54.0 ± 2.50 b | 7.10 ± 0.22 b | 7.17 ± 0.35 b |
| P:NCS 75:25 | 46.5 ± 2.05 c | 10.84 ± 0.38 a | 17.22 ± 0.9 a |
| P:NCS 50:50 | 47.7 ± 1. 8 c | 8.96 ± 0.19 b | 10.60 ± 0.29 b |
| P:NCS 25:75 | 61.2 ± 2.46 a | 2.86 ± 0.09 c | 8.65 ± 0.42 b |
| P:NCS 0:100 | 43.0 ± 1.46 c | 3.57 ± 0.15 c | 10.52 ± 0.43 b |
| Films | Contact angle, ° | Image | ||
|---|---|---|---|---|
| Initial CA | After 12 s | Initial CA | After 12 s | |
| P:NCS100:0 | 62.1 ± 2.12 c | 45.20 ± 2.96 d | 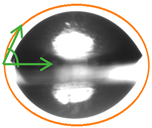 |  |
| P:NCS 75:25 | 89.3 ± 6.86 ab | 82.60 ± 6.85 b |  | 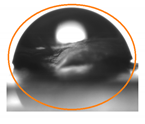 |
| P:NCS 50:50 | 95.4 ± 10.74 ab | 86.23 ± 3.01 ab | 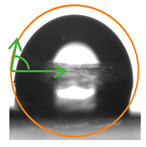 |  |
| P:NCS 25:75 | 97.1 ± 2.9 a | 92.5 ± 3.8 a | 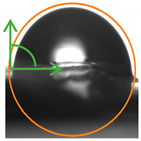 |  |
| P:NCS0:100 | 84.80 ± 1.75 b | 73.37 ± 1.90 c |  | 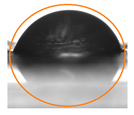 |
| Films | Solubility, % | SD, 50 m % | WVP, g·mm/m2·day·kPa | WVTR, g/m2·d |
|---|---|---|---|---|
| P: 100:0 | 100 ± 0 a | −100 ± 0 d | 1.33 ± 0.0285 a | 38.77 ± 0.83 a |
| P:NCS 75:25 | 45.65 ± 1.69 b | 249.45 ± 12.73 b | 0.2699 ± 0.0089 c | 9.91 ± 0.33 c |
| P:NCS 50:50 | 37.5 ± 1.69 c | 117.8 ± 8.77 c | 0.2052 ± 0.0083 d | 8.10 ± 0.33 d |
| P:NCS 25:75 | 11.11 ± 1.13 d | 230.98 ± 11.38 b | 0.5922 ± 0.0204 b | 17.26 ± 0.60 b |
| P:NCS 0:100 | 37.84 ± 1.98 c | 443.15 ± 21.28 a | 0.1755 ± 0.0085 d | 9.24 ± 0.45 cd |
| Films | Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | Chroma | Hue | |
| P:NCS 100:0 | 96.24 ± 0.31 a | 0.14 ± 0.01 b | 2.57 ± 0.12 d | 2.57 ± 0.11 d | 86.81 ± 0.5 b |
| P:NCS 75:25 | 95.76 ± 0.2 a | -0.23 ± 0.01 d | 5.98 ± 0.31 c | 5.99 ± 0.25 c | 87.09 ± 0.73 b |
| P:NCS 50:50 | 95.69 ± 0.31 a | -0.13 ± 0.02 c | 6.59 ± 0.63 c | 6.60 ± 0.63 c | 88.12 ± 0.11 ab |
| P:NCS 25:75 | 95.48 ± 0.25 a | 0.10 ± 0.01 b | 7.49 ± 0.32 b | 7.49 ± 0.32 b | 89.25 ± 0.26 a |
| P:NCS 0:100 | 94.51 ± 0.18 b | 0.22 ± 0.01 a | 9.27 ± 0.43 a | 9.28 ± 0.42 a | 88.62 ± 0.35 a |
| Films | OP, cc·mm/m2·d | OTR, cc/m2·d |
|---|---|---|
| P:NCS 100:0 | 1320.89 ± 88.29 a | 671.01 ± 47.11 a |
| P:NCS 75:25 | 836.89 ± 56.12 c | 350.54 ± 18.99 bc |
| P:NCS 50:50 | 47.67 ± 5.11 d | 18.63 ± 2.17 d |
| P:NCS 25:75 | 898.75 ± 61.44 b | 409.03 ± 35.63 b |
| P:NCS 0:100 | 832.23 ± 49.89 c | 320.8 ± 25.88 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. https://doi.org/10.3390/ijms21062224
Ngo TMP, Nguyen TH, Dang TMQ, Tran TX, Rachtanapun P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. International Journal of Molecular Sciences. 2020; 21(6):2224. https://doi.org/10.3390/ijms21062224
Chicago/Turabian StyleNgo, Thi Minh Phuong, Thanh Hoi Nguyen, Thi Mong Quyen Dang, Thi Xo Tran, and Pornchai Rachtanapun. 2020. "Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan" International Journal of Molecular Sciences 21, no. 6: 2224. https://doi.org/10.3390/ijms21062224
APA StyleNgo, T. M. P., Nguyen, T. H., Dang, T. M. Q., Tran, T. X., & Rachtanapun, P. (2020). Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. International Journal of Molecular Sciences, 21(6), 2224. https://doi.org/10.3390/ijms21062224