Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies
Abstract
1. Introduction
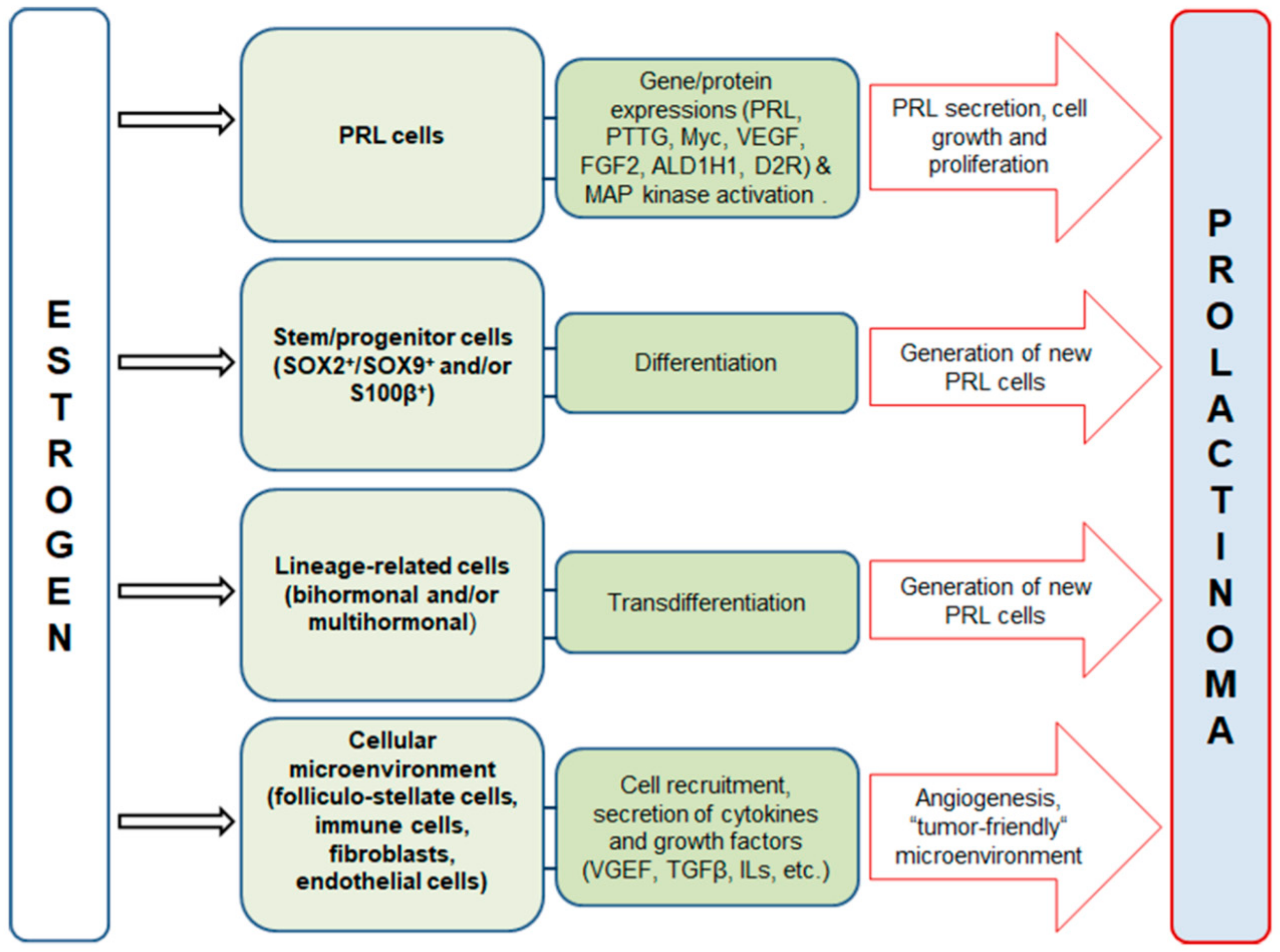
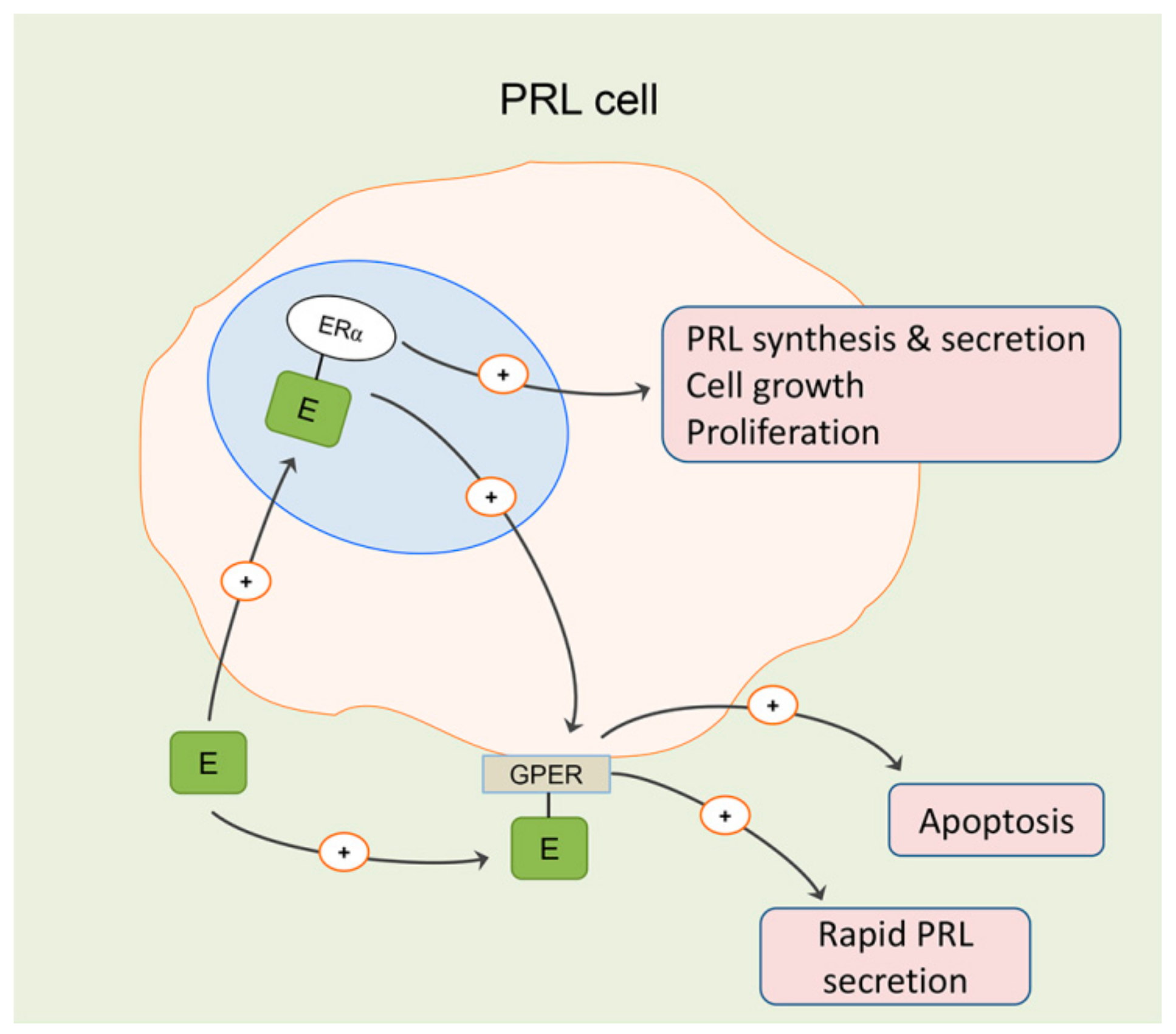
2. Estrogen Signaling in the Anterior Pituitary and Prolactinoma Development in Males
3. Effects of Estradiol on Differentiated Lactotropic Cells in the Anterior Pituitary
4. Effects of Estradiol on the Cellular Microenvironment in the Anterior Pituitary
5. Effects of Estradiol on Stem/Progenitor Cells and Transdifferentiation of Lineage-Related Hormone-Producing Cells in the Anterior Pituitary
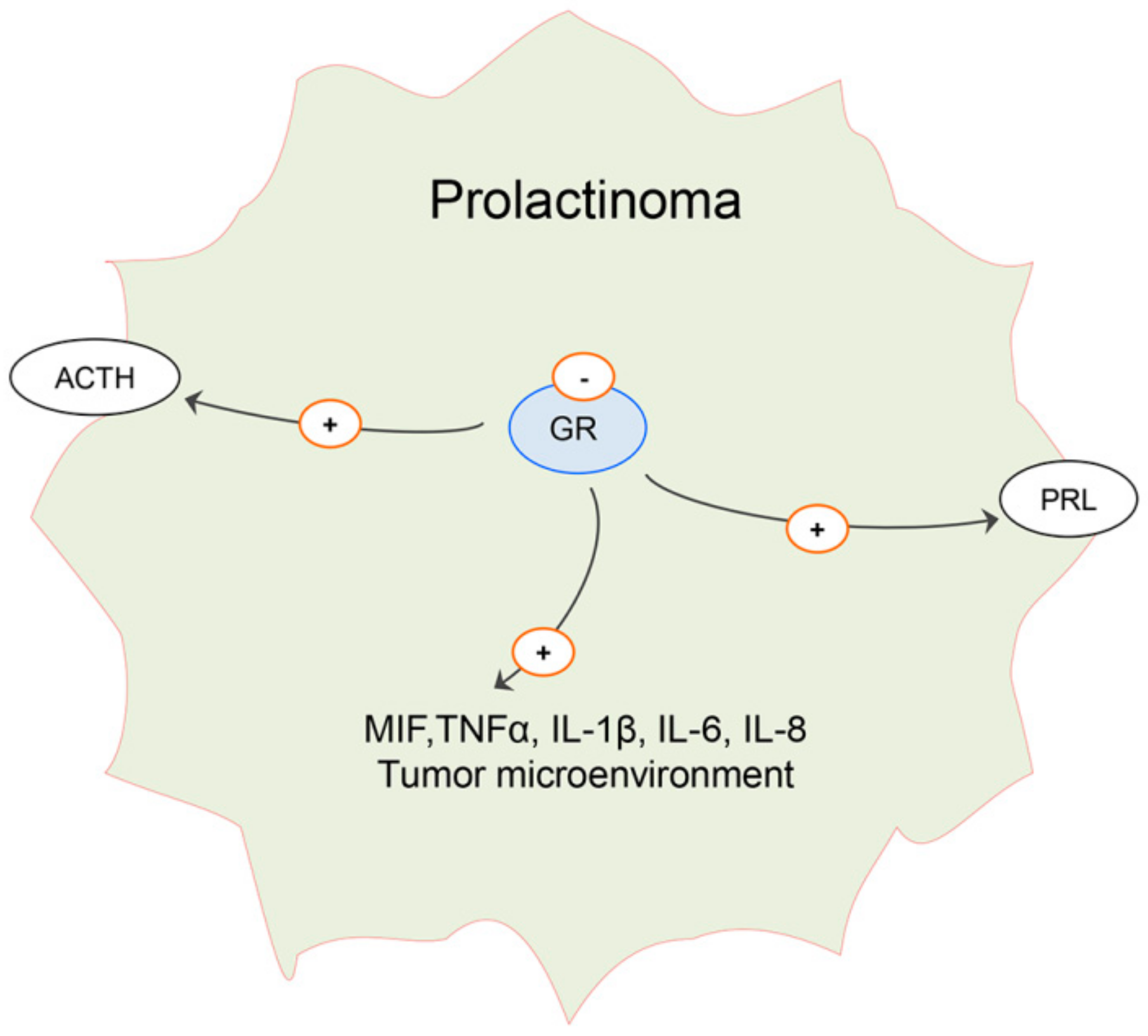
6. Effects of Estradiol on Pituitary ACTH Cells and Glucocorticoid Homeostasis in Males: Possible Implication on Prolactinoma Development
7. Effects of Estradiol on TSH Cells in Males and Putative Changes in Local Thyroid Hormone Metabolism and Action in Prolactinomas
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogasawara, K.; Nogami, H.; Tsuda, M.C.; Gustafsson, J.A.; Korach, K.S.; Ogawa, S.; Harigaya, T.; Hisano, S. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology 2009, 150, 1061–1068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckstrum, K.S.; Weis, K.E.; Baur, N.G.; Yoshihara, Y.; Raetzman, L.T. Icam5 Expression Exhibits Sex Differences in the Neonatal Pituitary and Is Regulated by Estradiol and Bisphenol, A. Endocrinology 2016, 157, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Ishida, M.; Izawa, M.; Arita, J. Differences between rat strains in the development of PRL-secreting pituitary tumors with long-term estrogen treatment: In vitro insulin-like growth factor-1-induced lactotroph proliferation and gene expression are affected in Wistar-Kyoto rats with low estrogen-susceptibility. Endocr. J. 2013, 60, 1251–1259. [Google Scholar] [PubMed]
- Bisson, J.R.; Chan, K.J.; Safer, J.D. Prolactin levels do not rise among transgender women treated with estradiol and spironolactone. Endocr. Pract. 2018, 24, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Vroonen, L.; Daly, A.F.; Beckers, A. Epidemiology and Management Challenges in Prolactinomas. Neuroendocrinology 2019, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Nash, R.; Tangpricha, V.; Brockman, J.; Ward, K.; Goodman, M. Cancer in Transgender People: Evidence and Methodological Considerations. Epidemiol. Rev. 2017, 39, 93–107. [Google Scholar] [CrossRef]
- Agustsson, T.T.; Baldvinsdottir, T.; Jonasson, J.G.; Olafsdottir, E.; Steinthorsdottir, V.; Sigurdsson, G.; Thorsson, A.; Carroll, P.; Korbonits, M.; Benediktsson, R. The epidemiology of pituitary adenomas in Iceland, 1955-2012: A nationwide population-based study. Eur. J. Endocrinol. 2015, 173, 655–664. [Google Scholar] [CrossRef]
- Pekic, S.; Soldatovic, I.; Miljic, D.; Stojanovic, M.; Doknic, M.; Petakov, M.; Popovic, V. Familial Cancer Clustering in Patients with Prolactinoma. Horm. Cancer. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Pekić, S.; Medic Stojanoska, M.; Popovic, V. Hyperprolactinemia/Prolactinomas in the Postmenopausal Period: Challenges in Diagnosis and Management. Neuroendocrinology 2019, 109, 28–33. [Google Scholar] [CrossRef]
- Song, Y.J.; Chen, M.T.; Lian, W.; Xing, B.; Yao, Y.; Feng, M.; Wang, R.Z. Surgical treatment for male prolactinoma: A retrospective study of 184 cases. Medicine (Baltimore) 2017, 96, e5833. [Google Scholar] [CrossRef]
- Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; Francois, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur. J. Endocrinol. 2015, 172, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumours. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef]
- Lim, C.T.; Korbonits, M. Update on the clinicopathology of pituitary adenomas. Endocr. Pract. 2018, 24, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.T.; Ho, Y.; Liebhaber, S.A. Transcriptome Analyses of Female Somatotropes and Lactotropes Reveal Novel Regulators of Cell Identity in the Pituitary. Endocrinology 2018, 159, 3965–3980. [Google Scholar] [CrossRef] [PubMed]
- Wierinckx, A.; Delgrange, E.; Bertolino, P.; François, P.; Chanson, P.; Jouanneau, E.; Lachuer, J.; Trouillas, J.; Raverot, G. Sex-Related Differences in Lactotroph Tumor Aggressiveness Are Associated With a Specific Gene-Expression Signature and Genome Instability. Front. Endocrinol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.A.; Smiljanic, K.; Maso Prévide, R.; Iben, J.R.; Li, T.; Rokic, M.B.; Sherman, A.; Coon, S.L.; Stojilkovic, S. Cell Type- and Sex-Dependent Transcriptome Profiles of Rat Anterior Pituitary Cells. Front. Endocrinol. (Lausanne) 2019, 10, 623. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Reis, L.O.; Zani, E.L.; García-Perdomo, H.A. Estrogen therapy in patients with prostate cancer: A contemporary systematic review. Int. Urol. Nephrol. 2018, 50, 993–1003. [Google Scholar] [CrossRef]
- Cunha, F.S.; Domenice, S.; Câmara, V.L.; Sircili, M.H.; Gooren, L.J.; Mendonça, B.B.; Costa, E.M. Diagnosis of prolactinoma in two male-to-female transsexual subjects following high-dose cross-sex hormone therapy. Andrologia 2015, 47, 680–684. [Google Scholar] [CrossRef]
- Russell, N.; Grossmann, M. Mechanisms in endocrinology. Estradiol as a male hormone. Eur. J. Endocrinol. 2019. EJE-18-1000.R2. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Shah, S.I.; Duong, T.; Abel, P.; Langley, R.E. Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer. Oncol. Hematol. Rev. 2014, 10, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Hoermann, R.; Cheung, A.S.; Ching, M.; Zajac, J.D.; Handelsman, D.J.; Grossmann, M. Short-term effects of transdermal estradiol in men undergoing androgen deprivation therapy for prostate cancer: A randomized placebo-controlled trial. Eur. J. Endocrinol. 2018, 178, 565–576. [Google Scholar] [CrossRef] [PubMed]
- García-Malpartida, K.; Martín-Gorgojo, A.; Rocha, M.; Gómez-Balaguer, M.; Hernández-Mijares, A. Prolactinoma induced by estrogen and cyproterone acetate in a male-to-female transsexual. Fertil. Steril. 2010, 94, 1097.e13-5. [Google Scholar] [CrossRef] [PubMed]
- Gooren, L.J.; T’Sjoen, G. Endocrine treatment of aging transgender people. Rev. Endocr. Metab. Disord. 2018, 19, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Caimari, F.; Korbonits, M. Novel Genetic Causes of Pituitary Adenomas. Clin. Cancer. Res. 2016, 22, 5030–5042. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M.; European Society of Endocrinology. Clinical Practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Hannen, R.; Steffani, M.; Voellger, B.; Carl, B.; Wang, J.; Bartsch, J.W.; Nimsky, C. Effects of anti-estrogens on cell invasion and survival in pituitary adenoma cells: A systematic study. J. Steroid. Biochem. Mol. Biol. 2019, 187, 88–96. [Google Scholar] [CrossRef]
- Kadioglu, P.; Oral, G.; Sayitoglu, M.; Erensoy, N.; Senel, B.; Gazioglu, N.; Sav, A.; Cetin, G.; Ozbek, U. Aromatase cytochrome P450 enzyme expression in human pituitary. Pituitary 2008, 11, 29–35. [Google Scholar] [CrossRef]
- García Barrado, M.J.; Blanco, E.J.; Carretero Hernández, M.; Iglesias Osma, M.C.; Carretero, M.; Herrero, J.J.; Burks, D.J.; Carretero, J. Local transformations of androgens into estradiol by aromatase P450 is involved in the regulation of prolactin and the proliferation of pituitary prolactin-positive cells. PLoS ONE 2014, 9, e101403. [Google Scholar] [CrossRef]
- Caglar, A.S.; Kapucu, A.; Dar, K.A.; Ozkaya, H.M.; Caglar, E.; Ince, H.; Kadioglu, P. Localization of the aromatase enzyme expression in the human pituitary gland and its effect on growth hormone, prolactin, and thyroid stimulating hormone axis. Endocrine 2015, 49, 761–768. [Google Scholar] [CrossRef]
- Li, X. Aromatase over expression transgenic murine models for aromatase inhibitor studies. Mol. Hum. Reprod. 2010, 16, 80–86. [Google Scholar] [CrossRef] [PubMed]
- García-Barrado, M.J.; Blanco, E.J.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Iglesias-Osma, M.C.; Carretero-Hernández, M.; Rodríguez-Cobos, J.; Burks, D.J.; Carretero, J. Relevance of pituitary aromatase and estradiol on the maintenance of the population of prolactin-positive cells in male mice. Steroids 2016, 111, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lindzey, J.; Wetsel, W.C.; Couse, J.F.; Stoker, T.; Cooper, R.; Korach, K.S. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology 1998, 139, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.H.; Mezzomo, L.C.; Ferreira, N.P.; Roehe, A.V.; Kohek, M.B.; Oliveira, M.C. Aromatase P450 expression in human pituitary adenomas. Neuropathology 2015, 35, 16–23. [Google Scholar] [CrossRef] [PubMed]
- García-Barrado, M.J.; Blanco, E.J.; Iglesias-Osma, M.C.; Carretero-Hernández, M.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Carretero, M.; Herrero, J.J.; Carrero, S.; Carretero, J. Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas. Int. J. Mol. Sci. 2017, 18, E2299. [Google Scholar] [CrossRef]
- Su, Y.X.; Du, G.L.; Shen, H.L.; Wang, W.; Bao, J.L.; Aierken, A.; Wang, B.W.; Jiang, S.; Zhu, J.; Gao, X.M. Increased expression of aromatase cytochrome P450 enzyme is associated with prolactinoma invasiveness in post-menopausal women. J. Int. Med. Res. 2019, 47, 3115–3126. [Google Scholar] [CrossRef]
- Gillam, M.P.; Middler, S.; Freed, D.J.; Molitch, M.E. The novel use of very high doses of cabergoline and a combination of testosterone and an aromatase inhibitor in the treatment of a giant prolactinoma. J. Clin. Endocrinol. Metab. 2002, 87, 4447–4451. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. The forgotten effects of thyrotropin-releasing hormone: Metabolic functions and medical applications. Front Neuroendocrinol. 2019, 52, 29–43. [Google Scholar] [CrossRef]
- Sarkar, D.K. Genesis of prolactinomas: Studies using estrogen-treated animals. Front. Horm. Res. 2006, 35, 32–49. [Google Scholar]
- Ho, Y.; Hu, P.; Peel, M.T.; Chen, S.; Camara, P.G.; Epstein, D.J.; Stephen, H.W. Stephen Single cell transcriptomic analysis of the adult mouse pituitary reveals a novel multi-hormone cell cluster and physiologic demand-induced lineage plasticity. bioRxiv 2018. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kikuchi, M.; Horiguchi, K.; Kusumoto, K.; Kouki, T.; Kawanishi, K.; Takashi, Y. Estrogen receptor alpha regulates retinaldehyde dehydrogenase 1 expression in rat anterior pituitary cells. Endocr. J. 2009, 56, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.P.; Fernando, M.; Melmed, S. Functional role of estrogen in pituitary tumor pathogenesis. J. Clin. Investig. 2002, 109, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, H.M.; Comunoglu, N.; Keskin, F.E.; Oz, B.; Haliloglu, O.A.; Tanriover, N.; Gazioglu, N.; Kadioglu, P. Locally produced estrogen through aromatization might enhance tissue expression of pituitary tumor transforming gene and fibroblast growth factor 2 in growth hormone-secreting adenomas. Endocrine 2016, 52, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xue, Y.; Cao, L.; Liu, Q.; Liu, C.; Shan, X.; Wang, H.; Gu, Y.; Zhang, Y. ESR1 and its antagonist fulvestrant in pituitary adenomas. Mol. Cell. Endocrinol. 2017, 443, 32–41. [Google Scholar] [CrossRef]
- Tong, Y.; Zheng, Y.; Zhou, J.; Oyesiku, N.M.; Koeffler, H.P.; Melmed, S. Genomic characterization of human and rat prolactinomas. Endocrinology 2012, 153, 3679–3691. [Google Scholar] [CrossRef]
- Denef, C. Paracrinicity: The Story of 30 Years of Cellular Pituitary Crosstalk. J. Neuroendocrinol. 2008, 20, 1–70. [Google Scholar]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta. Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef]
- Rizzoti, K.; Akiyama, H.; Lovell-Badge, R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell. Stem. Cell. 2013, 13, 419–432. [Google Scholar] [CrossRef]
- Andoniadou, C.L.; Matsushima, D.; Gharavy, S.N.M.; Signore, M.; Mackintosh, A.I.; Schaeffer, M.; Gaston-Massuet, C.; Mollard, P.; Jacques, T.S.; Le Tissier, P.; et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell. Stem. Cell. 2013, 13, 433–445. [Google Scholar]
- Higuchi, M.; Kanno, N.; Yoshida, S.; Ueharu, H.; Chen, M.; Yako, H.; Shibuya, S.; Sekita, M.; Tsuda, M.; Mitsuishi, H.; et al. GFP-expressing S100beta-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell. Tissue. Res. 2014, 357, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Frawley, L.S.; Boockfor, F.R. Mammosomatotropes: Presence and functions in normal and neoplastic pituitary tissue. Endocr. Rev. 1991, 12, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Núñez, L.; Villalobos, C.; Senovilla, L.; García-Sancho, J. Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J. Physiol. 2003, 549, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V. Effects of estradiol on prolactin and growth hormone messenger RNAs in cultured normal and neoplastic (MtT/W15 and GH3) rat pituitary cells. Cancer. Res. 1989, 49, 1247–1253. [Google Scholar]
- Šošić-Jurjević, B.; Filipović, B.; Renko, K.; Miler, M.; Trifunović, S.; Ajdžanovič, V.; Kӧhrle, J.; Milošević, V. Testosterone and estradiol treatments differently affect pituitary-thyroid axis and liver deiodinase 1 activity in orchidectomized middle-aged rats. Exp. Gerontol. 2015, 72, 85–98. [Google Scholar] [CrossRef]
- Nestorović, N.; Trifunović, S.; Manojlović-Stojanoski, M.; Jarić, I.; Ristić, N.; Filipović, B.; Šošić-Jurjević, B.; Milošević, V. Soy Phytoestrogens Do Not Fully Reverse Changes in Rat Pituitary Castration Cells: Unbiased Stereological Study. Anat. Rec. 2018, 301, 1416–1425. [Google Scholar] [CrossRef]
- Filipović, B.; Sošić-Jurjević, B.; Ajdžanović, V.; Pantelić, J.; Nestorović, N.; Milošević, V.; Sekulić, M. The effects of sex steroids on thyroid C cells and trabecular bone structure in the rat model of male osteoporosis. J. Anat. 2013, 222, 313–320. [Google Scholar] [CrossRef]
- Avtanski, D.; Novaira, H.J.; Wu, S.; Romero, C.J.; Kineman, R.; Luque, R.M.; Wondisford, F.; Radovick, S. Both estrogen receptor α and β stimulate pituitary GH gene expression. Mol. Endocrinol. 2014, 28, 40–52. [Google Scholar] [CrossRef]
- Kansra, S.; Chen, S.; Bangaru, M.L.; Sneade, L.; Dunckley, J.A.; Ben-Jonathan, N. Selective estrogen receptor down-regulator and selective estrogen receptor modulators differentially regulate lactotroph proliferation. PLoS ONE 2010, 5, e10060. [Google Scholar] [CrossRef]
- Baker, B.L.; Yu, Y.Y. An immunocytochemical study of human pituitary mammotropes from fetal life to old age. Am. J. Anat. 1977, 148, 217–239. [Google Scholar] [CrossRef]
- Roelfsema, F.; Pijl, H.; Keenan, D.M.; Veldhuis, J.D. Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS ONE 2012, 7, e31305. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.; Curley, M.; Tedim Ferreira, M.; Cruickshanks, L.; Milne, L.; Smith, L.B. Pituitary Androgen Receptor Signalling Regulates Prolactin but Not Gonadotrophins in the Male Mouse. PLoS ONE 2015, 10, e0121657. [Google Scholar] [CrossRef] [PubMed]
- Bondioni, S.; Angioni, A.R.; Corbetta, S.; Locatelli, M.; Ferrero, S.; Ferrante, E.; Mantovani, G.; Olgiati, L.; Beck-Peccoz, P.; Spada, A.; et al. Effect of 9-cis retinoic acid on dopamine D2 receptor expression in pituitary adenoma cells. Exp. Biol. Med. (Maywood) 2008, 233, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Occhi, G.; Regazzo, D.; Albiger, N.M.; Ceccato, F.; Ferasin, S.; Scanarini, M.; Denaro, L.; Cosma, C.; Plebani, M.; Cassarino, M.F.; et al. Activation of the Dopamine Receptor Type-2 (DRD2) Promoter by 9-Cis Retinoic Acid in a Cellular Model of Cushing’s Disease Mediates the Inhibition of Cell Proliferation and ACTH Secretion Without a Complete Corticotroph-to-Melanotroph Transdifferentiation. Endocrinology 2014, 155, 3538–3549. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Rosenmann, E.; Nylen, E.; Kaufman, M.; Pinsky, L.; Wrogemann, K. The 56 kDa androgen binding protein is an aldehyde dehydrogenase. Biochem. Biophys. Res. Commun. 1991, 175, 831–838. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X. Molecular Network Basis of Invasive Pituitary Adenoma: A Review. Front. Endocrinol. (Lausanne) 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Filippella, M.; Galland, F.; Kujas, M.; Young, J.; Faggiano, A.; Lombardi, G.; Colao, A.; Meduri, G.; Chanson, P. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: A clinical and immunohistochemical study. Clin. Endocrinol. (Oxf.) 2006, 65, 536–543. [Google Scholar] [CrossRef]
- Zárate, S.; Jaita, G.; Ferraris, J.; Eijo, G.; Magri, M.L.; Pisera, D.; Seilicovich, A. Estrogens induce expression of membrane-associated estrogen receptor α isoforms in lactotropes. PLoS. ONE 2012, 7, e41299. [Google Scholar] [CrossRef]
- Saraf, M.K.; Jeng, Y.J.; Watson, C.S. Nongenomic effects of estradiol vs. the birth control estrogen ethinyl estradiol on signaling and cell proliferation in pituitary tumor cells, and differences in the ability of R-equol to neutralize or enhance these effects. Steroids 2019, 108411. [Google Scholar] [CrossRef]
- Camilletti, M.A.; Abeledo-Machado, A.; Ferraris, J.; Pérez, P.A.; Faraoni, E.Y.; Pisera, D.; Gutierrez, S.; Díaz-Torga, G. Role of GPER in the anterior pituitary gland focusing on lactotroph function. J. Endocrinol. 2019, 240, 99–110. [Google Scholar] [CrossRef]
- Cristina, C.; Luque, G.M.; Demarchi, G.; Lopez Vicchi, F.; Zubeldia-Brenner, L.; Perez Millan, M.I.; Perrone, S.; Ornstein, A.M.; Lacau-Mengido, I.M.; Berner, S.I.; et al. Angiogenesis in pituitary adenomas: Human studies and new mutant mouse models. Int. J. Endocrinol. 2014, 2014, 608497. [Google Scholar] [CrossRef]
- Sarwar, K.; Huda, M.; Van de Velde, V.; Hopkins, L.; Luck, S.; Preston, R.; McGowan, B.; Carroll, P.; Powrie, J. The prevalence and natural history of pituitary haemorrhage in prolactinoma. J. Clin. Endocrinol. Metabol. 2013, 98, 2362–2367. [Google Scholar] [CrossRef]
- Yin, C.; Kang, L.; Lai, C.; Zhou, J.; Shi, B.; Zhang, L.; Chen, H. Effects of 17β-estradiol on leptin signaling in anterior pituitary of ovariectomized rats. Exp. Anim. 2017, 66, 159–166. [Google Scholar] [CrossRef]
- Lv, H.; Li, C.; Gui, S.; Zhang, Y. Expression of estrogen receptor alpha and growth factors in human prolactinoma and its correlation with clinical features and gender. J. Endocrinol. Investig. 2012, 35, 174–180. [Google Scholar]
- Maine, E.A.; Westcott, J.M.; Prechtl, A.M.; Dang, T.T.; Whitehurst, A.W.; Pearson, G.W. The cancer-testis antigens SPANX-A/C/D and CTAG2 promote breast cancer invasion. Oncotarget 2016, 7, 14708–14726. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.B.; Zhao, Y.; Liu, X.A.; Ding, Q.; Zha, X.M.; Wang, S. Cancer/testis antigen SSX2 enhances invasiveness in MCF-7 cells by repressing ERalpha signaling. Int. J. Oncol. 2012, 40, 1986–1994. [Google Scholar] [PubMed]
- Bublik, D.R.; Bursać, S.; Sheffer, M.; Oršolić, I.; Shalit, T.; Tarcic, O.; Kotler, E.; Mouhadeb, O.; Hoffman, Y.; Fuchs, G.; et al. Regulatory module involving FGF13, miR-504, and p53 regulates ribosomal biogenesis and supports cancer cell survival. Proc. Natl. Acad. Sci. USA 2017, 114, E496–E505. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, Y.; Zhan, X. The MAPK Pathway-Based Drug Therapeutic Targets in Pituitary Adenomas. Front. Endocrinol. (Lausanne) 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Rusz, O.; Kószó, R.; Dobi, Á.; Csenki, M.; Valicsek, E.; Nikolényi, A.; Uhercsák, G.; Cserháti, A.; Kahán, Z. Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: A case series. Onco. Targets Ther. 2018, 11, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; De Marco, P.; De Francesco, E.M.; Chimento, A.; Pezzi, V.; Maggiolini, M. Cross-talk between GPER and growth factor signaling. J. Steroid. Biochem. Mol. Biol. 2013, 137, 50–56. [Google Scholar] [CrossRef]
- Gaudet, H.M.; Cheng, S.B.; Christensen, E.M.; Filardo, E.J. The G-protein coupled estrogen receptor, GPER: The inside and inside-out story. Mol. Cell. Endocrinol. 2015, 418, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.D.; Limbird, L.E. GPER (GPR30): A Nongenomic Receptor (GPCR) for Steroid Hormones with Implications for Cardiovascular Disease and Cancer. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Ishida, M.; Izawa, M.; Arita, J. Activation of G protein-coupled estrogen receptor 1 mimics, but does not mediate, the anti-proliferative action of estradiol on pituitary lactotrophs in primary culture. Endocr. J. 2017, 64, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Hazell, G.G.; Yao, S.T.; Roper, J.A.; Prossnitz, E.R.; O’Carroll, A.M.; Lolait, S.J. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009, 202, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Mitchner, N.A.; Garlick, C.; Ben-Jonathan, N. Cellular distribution and gene regulation of estrogen receptors alpha and beta in the rat pituitary gland. Endocrinology 1998, 139, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Le Tissier, P.R.; Hodsoncd, D.J.; Lafontcd, C.; Fontanaud, P.; Schaeffer, M.; Mollard, P. Anterior pituitary cell networks. Front. Neuroendocrinol. 2012, 33, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Raverot, G.; Bertolino, P. The Microenvironment of Pituitary Tumors-Biological and Therapeutic Implications. Cancers (Basel) 2019, 11, 1605. [Google Scholar] [CrossRef]
- Devnath, S.; Inoue, K. An insight to pituitary folliculo-stellate cells. J. Neuroendocrinol. 2008, 20, 687–691. [Google Scholar] [CrossRef]
- Oomizu, S.; Chaturvedi, K.; Sarkar, D.K. Folliculostellate cells determine the susceptibility of lactotropes to estradiol’s mitogenic action. Endocrinology 2004, 145, 1473–1480. [Google Scholar] [CrossRef]
- Dennison, K.L.; Chack, A.C.; Hickman, M.P.; Harenda, Q.E.; Shull, J.D. Ept7, a quantitative trait locus that controls estrogen-induced pituitary lactotroph hyperplasia in rat, is orthologous to a locus in humans that has been associated with numerous cancer types and common diseases. PLoS ONE 2018, 13, e0204727. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Camilletti, M.A.; Rifkin, D.B.; Díaz-Torga, G. The pituitary TGFβ1 system as a novel target for the treatment of resistant prolactinomas. J. Endocrinol. 2016, 228, R73–R83. [Google Scholar] [CrossRef]
- Faraoni, E.Y.; Camilletti, M.A.; Abeledo-Machado, A.; Ratner, L.D.; De Fino, F.; Huhtaniemi, I.; Rulli, S.B.; Díaz-Torga, G. Sex differences in the development of prolactinoma in mice overexpressing hCGβ: Role of TGFβ1. J. Endocrinol. 2017, 232, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Recouvreux, M.V.; Lapyckyj, L.; Camilletti, M.A.; Guida, M.C.; Ornstein, A.; Rifkin, D.B.; Becu-Villalobos, D.; Díaz-Torga, G. Sex differences in the pituitary transforming growth factor-β1 system: Studies in a model of resistant prolactinomas. Endocrinology 2013, 154, 4192–4205. [Google Scholar] [CrossRef] [PubMed]
- Refojo, D.; Liberman, A.C.; Giacomini, D.; Carbia Nagashima, A.; Graciarena, M.; Echenique, C.; Paez Pereda, M.; Stalla, G.; Holsboer, F.; Arzt, E. Integrating systemic information at the molecular level: Cross-talk between steroid receptors and cytokine signaling on different target cells. Ann. NY. Acad. Sci. 2003, 992, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yatabe, M.; Tofrizal, A.; Jindatip, D.; Yashiro, T.; Nagai, R. Identification of M2 macrophages in anterior pituitary glands of normal rats and rats with estrogen-induced prolactinoma. Cell. Tissue. Res. 2017, 368, 371–378. [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Pituitary tumour fibroblast-derived cytokines influence tumour aggressiveness. Endocr. Relat. Cancer. 2019. ERC-19-0327.R2. [Google Scholar] [CrossRef]
- Garcia-Lavandeira, M.; Diaz-Rodriguez, E.; Bahar, D.; Garcia-Rendueles, A.R.; Rodrigues, J.S.; Dieguez, C.; Alvarez, C.V. Pituitary cell turnover: From adult stem cell recruitment through differentiation to death. Neuroendocrinology 2015, 101, 175–192. [Google Scholar] [CrossRef]
- Yoshida, S.; Kato, T.; Yako, H.; Susa, T.; Cai, L.Y.; Osuna, M.; Inoue, K.; Kato, Y. Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J. Neuroendocrinol. 2011, 23, 933–943. [Google Scholar] [CrossRef]
- Trifunović, S.; Manojlović-Stojanoski, M.; Ajdžanović, V.; Nestorović, N.; Ristić, N.; Medigović, I.; Milošević, V. Effects of genistein on stereological and hormonal characteristics of the pituitary somatotrophs in rats. Endocrine 2014, 47, 869–877. [Google Scholar] [CrossRef]
- Abdullah, L.N.; Chow, E.K. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef]
- Haston, S.; Manshaei, S.; Martinez-Barbera, J.P. Stem/progenitor cells in pituitary organ homeostasis and tumourigenesis. J. Endocrinol. 2018, 236, R1–R13. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell. Stem. Cell. 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, L.B.; Konovalov, P.V.; Krylova, J.S.; Polyakova, V.O.; Kvetnoy, I.M. Plurihormonal cells of normal anterior pituitary: Facts and conclusions. Oncotarget 2017, 8, 29282–29299. [Google Scholar] [CrossRef] [PubMed]
- Castrique, E.; Fernandez-Fuente, M.; Le Tissier, P.; Herman, A.; Levy, A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J. Endocrinol. 2010, 205, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Vankelecom, H.; Chen, J. Pituitary stem cells: Where do we stand? Mol. Cell. Endocrinol. 2014, 385, 2–17. [Google Scholar] [CrossRef]
- Rizzoti, K. Adult pituitary progenitors/stem cells: From in vitro characterization to in vivo function. Eur. J. Neurosci. 2010, 32, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.T.; Androulakis, I.P. Modeling the Sex Differences and Interindividual Variability in the Activity of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology 2017, 158, 4017–4037. [Google Scholar] [CrossRef]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Ajdžanović, V.Z.; Šošić-Jurjević, B.T.; Filipović, B.R.; Trifunović, S.L.; Milošević, V.Lj. Daidzein effects on ACTH cells: Immunohistomorphometric and hormonal study in an animal model of the andropause. Histol. Histopathol. 2011, 26, 1257–1264. [Google Scholar]
- Ogura, E.; Kageyama, K.; Hanada, K.; Kasckow, J.; Suda, T. Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type beta in hypothalamic 4B cells. Peptides 2008, 29, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, T.; Afonso-Oramas, D.; Cruz-Muros, I.; Barroso-Chinea, P.; Abreu, P.; del Mar Perez-Delgad, M.; Rancel-Torres, N.; del Carmen González, M. Interleukin-6 and nitric oxide synthase expression in the vasopressin and corticotrophin-releasing factor systems of the rat hypothalamus. J. Histochem. Cytochem. 2006, 54, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Lukkarinen, O.; Hammond, G.L.; Kontturi, M.; Vihko, R. Long-term effects of endocrine treatment on serum pituitary hormones in advanced prostatic carcinoma patients. Scand. J. Urol. Nephrol. 1981, 153, 207–211. [Google Scholar] [CrossRef]
- Tarle, M. Plasma osteocalcin values and related hormonal parameters in patients subjected to a variety of prostate anticancer agents. Urol. Res. 1991, 19, 39–44. [Google Scholar] [CrossRef]
- Komesaroff, P.A.; Fullerton, M.; Esler, M.D.; Jennings, G.; Sudhir, K. Oestrogen supplementation attenuates responses to psychological stress in elderly men rendered hypogonadal after treatment for prostate cancer. Clin. Endocrinol. (Oxf.) 2002, 56, 745–753. [Google Scholar] [CrossRef]
- Fuss, J.; Claro, L.; Ising, M.; Biedermann, S.V.; Wiedemann, K.; Stalla, G.K.; Briken, P.; Auer, M.K. Does sex hormone treatment reverse the sex-dependent stress regulation? A longitudinal study on hypothalamus-pituitary-adrenal (HPA) axis activity in transgender individuals. Psychoneuroendocrinol 2019, 104, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Anno, T.; Kawasaki, F.; Shigemoto, R.; Irie, S.; Mune, T.; Kaku, K.; Kaneto, H. Alteration of ACTH and cortisol levels after estradiol valerate treatment in a male subject with gender dysphoria: A case report. Front. Endocrinol. (Lausanne) 2019, 10, 751. [Google Scholar] [CrossRef]
- Piroli, G.; Grillo, C.; Lux de Lantos, V.; Libertun, C.; De Nicola, A.F. Glucocorticoid receptors and inhibition of serum prolactin by dexamethasone are reduced in rats with estrogen-induced pituitary tumors. Neuroendocrinol. Lett. 1991, 13, 75–81. [Google Scholar]
- Cassarino, M.F.; Sesta, A.; Pagliardini, L.; Losa, M.; Lasio, G.; Cavagnini, F.; Pecori Giraldi, F. Proopiomelanocortin, glucocorticoid, and CRH receptor expression in human ACTH-secreting pituitary adenomas. Endocrine 2017, 55, 853–860. [Google Scholar] [CrossRef][Green Version]
- Calandra, T.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF): A Glucocorticoid Counter-Regulator within the Immune System. Crit. Rev. Immunol. 2017, 37, 359–370. [Google Scholar] [CrossRef]
- Capen, C.C. Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol. Pathol. 1997, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Brändli-Baiocco, A.; Balme, E.; Bruder, M.; Chandra, S.; Hellmann, J.; Hoenerhoff, M.J.; Kambara, T.; Landes, C.; Lenz, B.; Mense, M.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Endocrine System. J. Toxicol. Pathol. 2018, 31, 1S–95S. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Sex Specificity in Age-Related Thyroid Hormone Responsiveness. Rinsho Byori. 2016, 64, 72–77. [Google Scholar] [PubMed]
- Christoffolete, M.A.; Ribeiro, R.; Singru, P.; Fekete, C.; da Silva, W.S.; Gordon, D.F.; Huang, S.A.; Crescenzi, A.; Harney, J.W.; Ridgway, E.C.; et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology 2006, 147, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Fliers, E.; Unmehopa, U.A.; Alkemade, A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol. Cell. Endocrinol. 2006, 251, 1–8. [Google Scholar] [CrossRef]
- Alkemade, A.; Friesema, E.C.; Kuiper, G.G.; Wiersinga, W.M.; Swaab, D.F.; Visser, T.J.; Fliers, E. Novel neuroanatomical pathways for thyroid hormone action in the human anterior pituitary. Eur. J. Endocrinol. 2006, 154, 491–500. [Google Scholar] [CrossRef][Green Version]
- Sosic-Jurjevic, B.; Filipovic, B.; Renko, K.; Ajdzanovic, V.; Manojlovic-Stojanoski, M.; Milosevic, V.; Köhrle, J. Orchidectomy of middle-aged rats decreases liver deiodinase 1 and pituitary deiodinase 2 activity. J. Endocrinol. 2012, 215, 247–256. [Google Scholar] [CrossRef][Green Version]
- Bisschop, P.H.; Toorians, A.W.; Endert, E.; Wiersinga, W.M.; Gooren, L.J.; Fliers, E. The effects of sex-steroid administration on the pituitary-thyroid axis in transsexuals. Eur. J. Endocrinol. 2006, 155, 11–16. [Google Scholar] [CrossRef]
- Shukla, P.; Bulsara, K.R.; Luthra, P. Pituitary Hyperplasia in Severe Primary Hypothyroidism: A Case Report and Review of the Literature. Case. Rep. Endocrinol. 2019, 2019, 2012546. [Google Scholar] [CrossRef]
- Jentoft, M.E.; Osamura, R.Y.; Kovacs, K.; Lloyd, R.V.; Scheithauer, B.W. Transdifferentiation of, pituitary thyrotrophs to lactothyrotrophs in primary hypothyroidism: Case report. Virchows. Arch. 2012, 461, 221–225. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. (Lausanne) 2019, 10, 59. [Google Scholar] [CrossRef]
- Tannahill, L.A.; Visser, T.J.; McCabe, C.J.; Kachilele, S.; Boelaert, K.; Sheppard, M.C.; Franklyn, J.A.; Gittoes, N.J. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours. Clin. Endocrinol. (Oxf.) 2002, 56, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Baur, A.; Buchfelder, M.; Köhrle, J. Expression of 5′-deiodinase enzymes in normal pituitaries and in various human pituitary adenomas. Eur. J. Endocrinol. 2002, 147, 263–268. [Google Scholar]
- Alarid, E.T.; Preisler-Mashek, M.T.; Solodin, N.M. Thyroid hormone is an inhibitor of estrogen-induced degradation of estrogen receptor-alpha protein: Estrogen-dependent proteolysis is not essential for receptor transactivation function in the pituitary. Endocrinology 2003, 144, 3469–3476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ying, H.; Furuya, F.; Zhao, L.; Araki, O.; West, B.L.; Hanover, J.A.; Willingham, M.C.; Cheng, S.Y. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J. Clin. Investig. 2006, 116, 2972–2984. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, M.R.; Veirana, N.; Derome, P.; Peillon, F.; Li, J.Y. Adenomatous transformation of the human anterior pituitary is associated with alterations in integrin expression. Int. J. Cancer. 1996, 67, 45–53. [Google Scholar] [CrossRef]
- Hsieh, M.T.; Wang, L.M.; Changou, C.A.; Chin, Y.T.; Yang, Y.S.H.; Lai, H.Y.; Lee, S.Y.; Yang, Y.N.; Whang-Peng, J.; Liu, L.F.; et al. Crosstalk between integrin αvβ3 and ERα contributes to thyroid hormone-induced proliferation of ovarian cancer cells. Oncotarget 2017, 8, 24237–24249. [Google Scholar] [CrossRef]
- Köhrle, J. The Colorful Diversity of Thyroid Hormone Metabolites. Eur. Thyroid. J. 2019, 8, 115–129. [Google Scholar] [CrossRef]
- Pinna, G.; Meinhold, H.; Hiedra, L.; Thoma, R.; Hoell, T.; Gräf, K.J.; Stoltenburg-Didinger, G.; Eravci, M.; Prengel, H.; Brödel, O.; et al. Elevated 3,5-diiodothyronine concentrations in the sera of patients with nonthyroidal illnesses and brain tumors. J. Clin. Endocrinol. Metab. 1997, 82, 1535–1542. [Google Scholar] [CrossRef]
- Pietzner, M.; Lehmphul, I.; Friedrich, N.; Schurmann, C.; Ittermann, T.; Dörr, M.; Nauck, M.; Laqua, R.; Völker, U.; Brabant, G.; et al. Translating pharmacological findings from hypothyroid rodents to euthyroid humans: Is there a functional role of endogenous 3,5-T2? Thyroid 2015, 25, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; de Lange, P.; Petito, G.; Moreno, M.; Goglia, F.; Lanni, A. 3,5-Diiodothyronine: A Novel Thyroid Hormone Metabolite and Potent Modulator of Energy Metabolism. Front. Endocrinol. (Lausanne) 2018, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological actions of ‘nonclassical’ thyroid hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef]
- Lorenzini, L.; Nguyen, N.M.; Sacripanti, G.; Serni, E.; Borsò, M.; Saponaro, F.; Cecchi, E.; Simoncini, T.; Ghelardoni, S.; Zucchi, R.; et al. Assay of Endogenous 3,5-diiodo-L-thyronine (3,5-T2) and 3,3′-diiodo-L-thyronine (3,3′-T2) in Human Serum: A Feasibility Study. Front. Endocrinol (Lausanne) 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Padron, A.S.; Neto, R.A.; Pantaleão, T.U.; de Souza dos Santos, M.C.; Araujo, R.L.; de Andrade, B.M.; da Silva Leandro, M.; de Castro, J.P.; Ferreira, A.C.; de Carvalho, D.P. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J. Endocrinol. 2014, 221, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.; Lietzow, J.; Wohlgemuth, F.; Hoefig, C.S.; Wiedmer, P.; Schweizer, U.; Köhrle, J.; Schürmann, A. 3,5- Diiodo-L-thyronine (3,5-t2) exerts thyromimetic effects on hypothalamus-pituitarythyroid axis, body composition, and energy metabolism in male diet-induced obese mice. Endocrinology. 2015, 156, 389–399. [Google Scholar] [CrossRef]
- Baur, A.; Bauer, K.; Jarry, H.; Köhrle, J. 3,5-diiodo-L-thyronine stimulates type 1 5’deiodinase activity in rat anterior pituitaries in vivo and in reaggregate cultures and GH3 cells in vitro. Endocrinology 1997, 138, 3242–3248. [Google Scholar] [CrossRef]
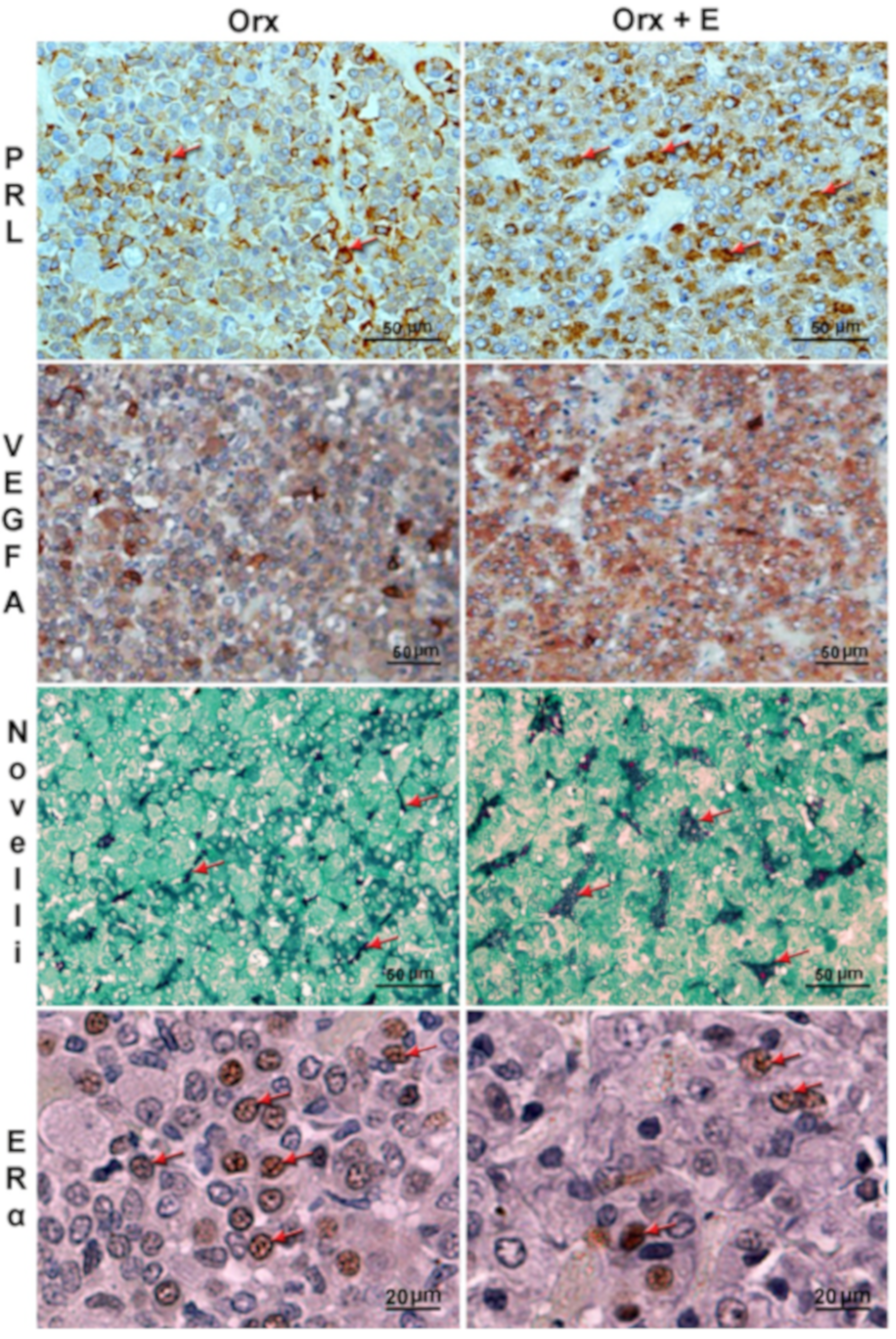
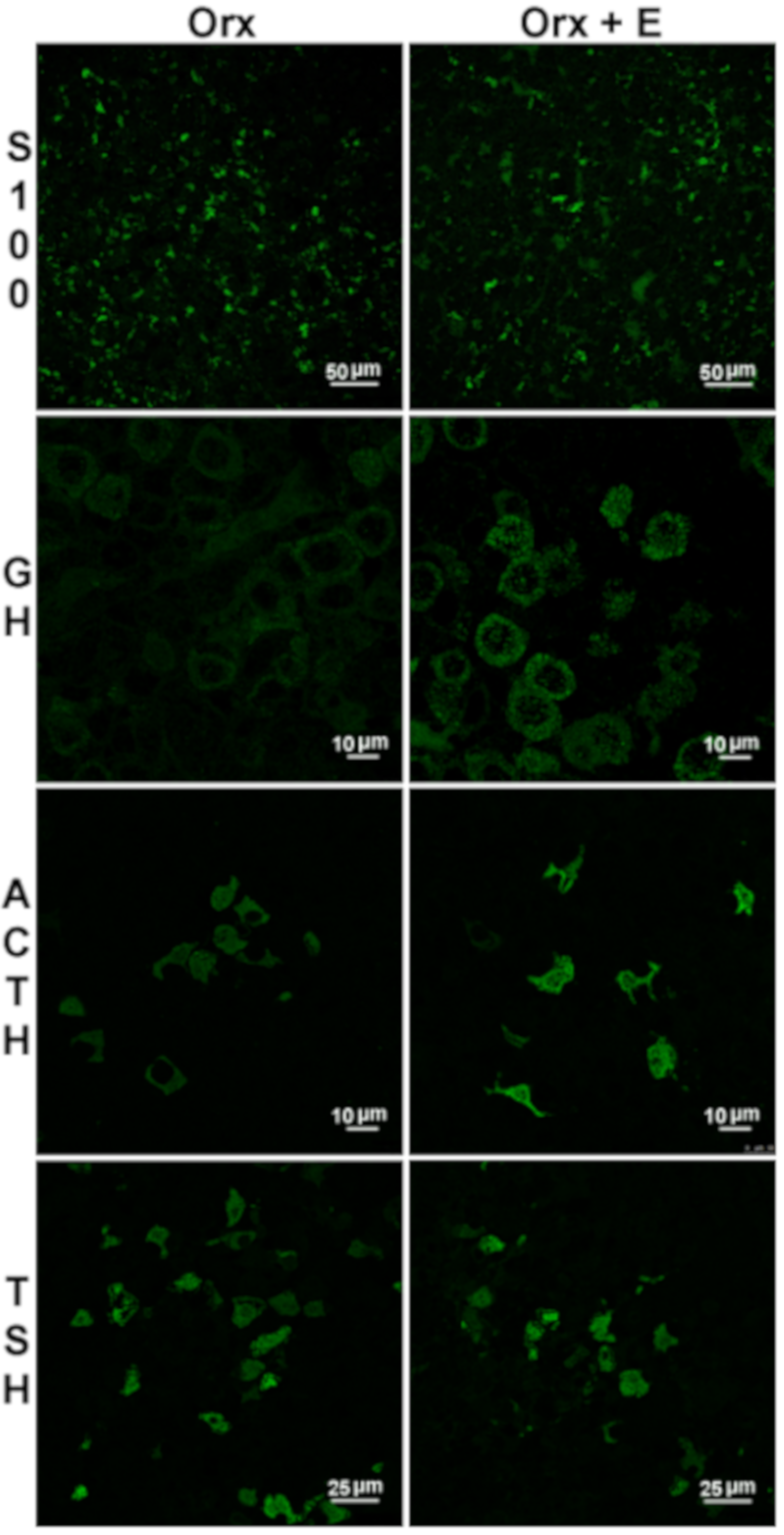

| Measured Parameter | Orx+E vs.Orx |
|---|---|
| Pituitary weight | ↑ |
| VEGFA immunopositivity | ↑ |
| PRL immunopositivity | ↑ |
| ERα immunopositivity | ↑ |
| S100β immunopositivity | ↓ |
| Pituitary GH immunopositivity | ↑ |
| Pituitary ACTH immunopositivity | ↑ |
| Pituitary TSH immunopositivity | ↓ |
| Serum PRL | ↑ |
| Serum GH | ↑ |
| Serum ACTH | ↑ |
| Serum TSH | n.s. |
| Serum corticosterone | n.s. |
| Serum L-thyroxine | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šošić-Jurjević, B.; Ajdžanović, V.; Miljić, D.; Trifunović, S.; Filipović, B.; Stanković, S.; Bolevich, S.; Jakovljević, V.; Milošević, V. Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies. Int. J. Mol. Sci. 2020, 21, 2024. https://doi.org/10.3390/ijms21062024
Šošić-Jurjević B, Ajdžanović V, Miljić D, Trifunović S, Filipović B, Stanković S, Bolevich S, Jakovljević V, Milošević V. Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies. International Journal of Molecular Sciences. 2020; 21(6):2024. https://doi.org/10.3390/ijms21062024
Chicago/Turabian StyleŠošić-Jurjević, Branka, Vladimir Ajdžanović, Dragana Miljić, Svetlana Trifunović, Branko Filipović, Sanja Stanković, Sergey Bolevich, Vladimir Jakovljević, and Verica Milošević. 2020. "Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies" International Journal of Molecular Sciences 21, no. 6: 2024. https://doi.org/10.3390/ijms21062024
APA StyleŠošić-Jurjević, B., Ajdžanović, V., Miljić, D., Trifunović, S., Filipović, B., Stanković, S., Bolevich, S., Jakovljević, V., & Milošević, V. (2020). Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies. International Journal of Molecular Sciences, 21(6), 2024. https://doi.org/10.3390/ijms21062024







