Influence of Diet and Nutrition on Prostate Cancer
Abstract
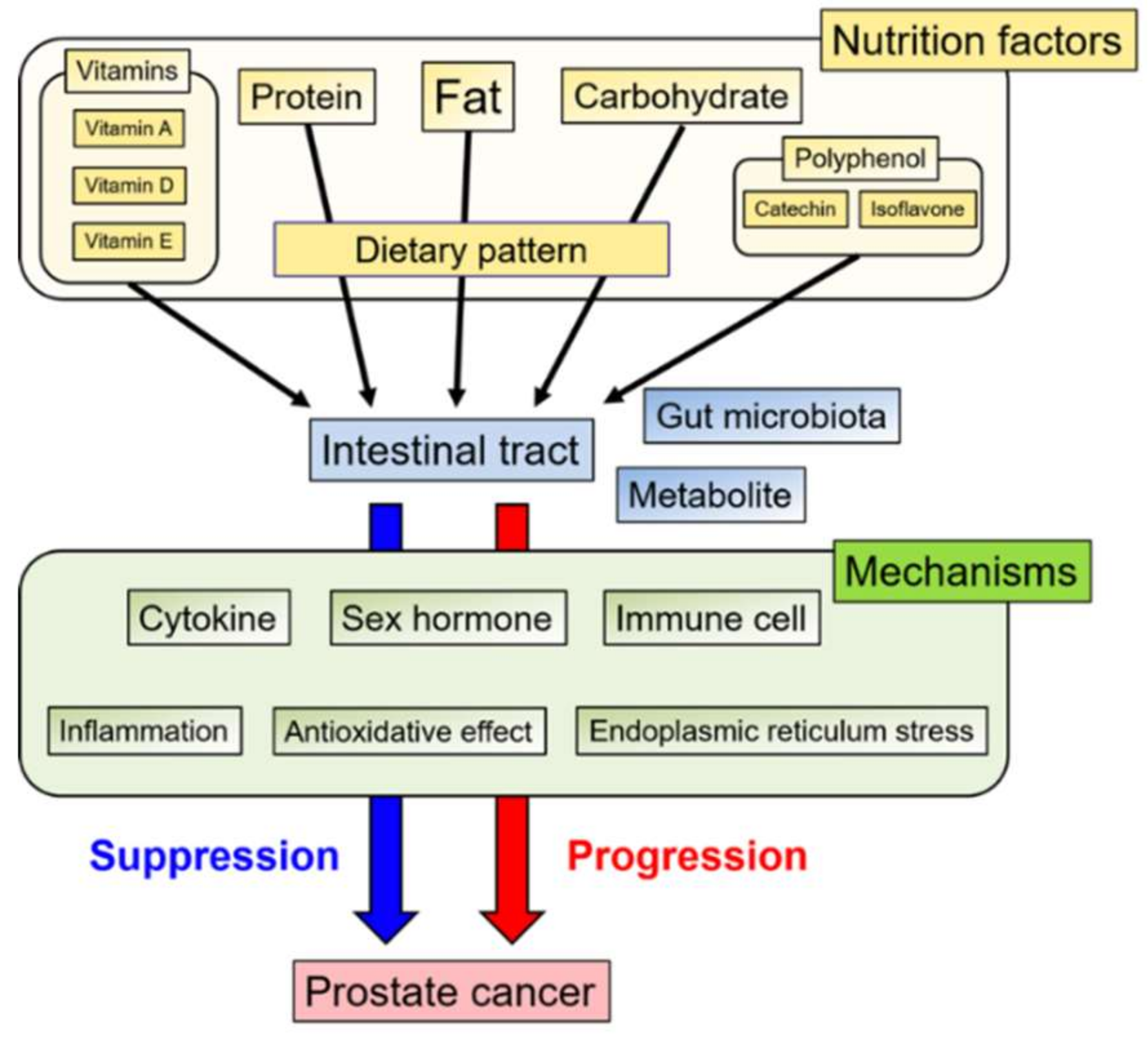
1. Introduction
2. Nutrition factors
2.1. Fat
2.2. Protein
2.3. Carbohydrates
2.4. Vitamins
2.4.1. Vitamin A
2.4.2. Vitamin D
2.4.3. Vitamin E + Selenium
2.5. Polyphenols
2.5.1. Catechin
2.5.2. Isoflavones
3. Dietary Pattern
4. Gut Microbiota
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Lloyd, T.; Hounsome, L.; Mehay, A.; Mee, S.; Verne, J.; Cooper, A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008-2010. BMC Med. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Tsugane, S.; de Souza, J.M.P.; Costa, M.L.; Mirra, A.P.; Gotlieb, S.L.D.; Laurenti, R.; Watanabe, S. Cancer incidence rates among Japanese immigrants in the city of São Paulo, Brazil, 1969–78. Cancer Causes Control 1990, 1, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Henderson, B.E.; Mack, T.M.; Yatani, R. Cancers of the prostate and breast among japanese and white immigrants in los angeles county. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef]
- Darcey, E.; Boyle, T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allen, N.; Bueno-De-Mesquita, H.B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Kaaks, R.; Teucher, B.; Boeing, H.; et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2013, 108, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.A.; Kreiger, N.; Lightfoot, N.; Purdham, J.; Sass-Kortsak, A. Prostate cancer risk and diet, recreational physical activity and cigarette smoking. Chronic Dis. Can. 2007, 27, 145–153. [Google Scholar] [PubMed]
- Liu, F.; Wang, J.; Wu, H.L.; Wang, H.; Wang, J.X.; Zhou, R.; Zhu, Z. Leisure time physical activity and risk of prostate cancer: A dose-response meta-analysis. Minerva Urol. Nefrol. 2018, 70, 152–161. [Google Scholar]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Torner, J.C.; Lynch, C.F.; Rubenstein, L.M.; Lemke, J.H.; Cohen, M.B.; Lubaroff, D.M.; Wallace, R.B. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 1997, 8, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. North Am. 2016, 45, 571–579. [Google Scholar]
- Hayashi, T.; Fujita, K.; Matsushita, M.; Nonomura, N. Main Inflammatory Cells and Potentials of Anti-Inflammatory Agents in Prostate Cancer. Cancers Basel 2019, 11, 1153. [Google Scholar] [CrossRef]
- Albert, B.; Johnson, A.; Lewis, J. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Ohwaki, K.; Endo, F.; Kachi, Y.; Hattori, K.; Muraishi, O.; Nishikitani, M.; Yano, E. Relationship between dietary factors and prostate-specific antigen in healthy men. Urol. Int. 2012, 89, 270–274. [Google Scholar] [CrossRef]
- Aronson, W.J.; Barnard, R.J.; Freedland, S.J.; Henning, S.; Elashoff, D.; Jardack, P.M.; Cohen, P.; Heber, D.; Kobayashi, N. Growth Inhibitory Effect of Low Fat Diet on Prostate Cancer Cells: Results of a Prospective, Randomized Dietary Intervention Trial in Men With Prostate Cancer. J. Urol. 2010, 183, 345–350. [Google Scholar] [CrossRef]
- Fleshner, N.; Bagnell, P.S.; Klotz, L.; Venkateswaran, V.; Kristal, A.R.; Thompson, I.M.; Klein, E.; Denis, L.J.; Ford, L.G. Dietary fat and prostate cancer. J. Urol. 2004, 171, 19–24. [Google Scholar] [CrossRef]
- Liss, M.A.; Al-Bayati, O.; Gelfond, J.; Goros, M.; Ullevig, S.; DiGiovanni, J.; Hamilton-Reeves, J.; O’Keefe, D.; Bacich, D.; Weaver, B.; et al. Higher baseline dietary fat and fatty acid intake is associated with increased risk of incident prostate cancer in the SABOR study. Prostate Cancer Prostatic Dis. 2019, 22, 244–251. [Google Scholar] [CrossRef]
- Bidoli, E.; Talamini, R.; Bosetti, C.; Negri, E.; Maruzzi, D.; Montella, M.; Franceschi, S.; La Vecchia, C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann. Oncol. 2005, 16, 152–157. [Google Scholar] [CrossRef]
- Park, S.-Y.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Fat and meat intake and prostate cancer risk: The multiethnic cohort study. Int. J. Cancer 2007, 121, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Wallström, P.; Bjartell, A.; Gullberg, B.; Olsson, H.; Wirfält, E. A prospective study on dietary fat and incidence of prostate cancer (Malmö, Sweden). Cancer Causes Control 2007, 18, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Key, T.J.; Appleby, P.N.; Travis, R.C.; Overvad, K.; Jakobsen, M.U.; Johnsen, N.F.; Tjønneland, A.; Linseisen, J.; Rohrmann, S.; et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 87, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L.; Dive, C.; Renehan, A.G. Biological Mechanisms Linking Obesity and Cancer Risk: New Perspectives. Annu. Rev. Med. 2010, 61, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Ngo, T.H.; Leung, P.S.; Aronson, W.J.; Golding, L.A. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate 2003, 56, 201–206. [Google Scholar] [CrossRef]
- Ngo, T.H.; Barnard, R.J.; Cohen, P.; Freedland, S.; Tran, C.; DeGregorio, F.; Elshimali, Y.I.; Heber, D.; Aronson, W.J. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin. Cancer Res. 2003, 9, 2734–2743. [Google Scholar]
- Mavropoulos, J.C.; Buschemeyer, W.C.; Tewari, A.K.; Rokhfeld, D.; Pollak, M.; Zhao, Y.; Febbo, P.G.; Cohen, P.; Hwang, D.; Devi, G.; et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev. Res. Phila. 2009, 2, 557–565. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Liu, B.; Huang, C.; Fatima, S.; Su, T.; Zhao, X.; Ho, A.H.M.; Han, Q.; Hu, X.; Gong, R.-H.; et al. Signal transducer and activator of transcription-3 drives the high-fat diet-associated prostate cancer growth. Cell Death Dis. 2019, 10, 637. [Google Scholar] [CrossRef]
- Saha, A.; Ahn, S.; Blando, J.; Su, F.; Kolonin, M.G.; DiGiovanni, J. Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice. Cancer Res. 2017, 77, 5158–5168. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Numakura, K.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; Tsuchiya, N.; Habuchi, T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate 2012, 72, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ishiguro, H.; Kobayashi, N.; Hasumi, H.; Watanabe, M.; Yao, M.; Uemura, H. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. Prostate 2015, 75, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.M.; Kasperzyk, J.L.; Mucci, L.A.; Giovannucci, E.; Price, A.; Wolk, A.; Håkansson, N.; Fall, K.; Andersson, S.O.; Andrén, O. Dietary fatty acid intake and prostate cancer survival in Örebro county, Sweden. Am. J. Epidemiol. 2012, 176, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Lichtenstein, P.; Feychting, M.; Ahlbom, A.; Wolk, A. Fatty fish consumption and risk of prostate cancer. Lancet 2001, 357, 1764–1766. [Google Scholar] [CrossRef]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef]
- Oono, K.; Takahashi, K.; Sukehara, S.; Kurosawa, H.; Matsumura, T.; Taniguchi, S.; Ohta, S. Inhibition of PC3 human prostate cancer cell proliferation, invasion and migration by eicosapentaenoic acid and docosahexaenoic acid. Mol. Clin. Oncol. 2017, 7, 217–220. [Google Scholar]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.S.; Heo, J.Y.; Park, J.H.; Seo, K.S.; Han, J.; Park, J.I.; et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Wang, J.; Hong, Y.; Shao, S.; Zhang, K.; Hong, W. FFAR1-and FFAR4-dependent activation of Hippo pathway mediates DHA-induced apoptosis of androgen-independent prostate cancer cells. Biochem. Biophys. Res. Commun. 2018, 506, 590–596. [Google Scholar]
- Li, J.; Gu, Z.; Pan, Y.; Wang, S.; Chen, H.; Zhang, H.; Chen, W.; Chen, Y.Q. Dietary supplementation of α-linolenic acid induced conversion of n-3 LCPUFAs and reduced prostate cancer growth in a mouse model. Lipids Health Dis. 2017, 16, 136. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J. The effects of diet on circulating sex hormone levels in men. Nutr. Res. Rev. 2000, 13, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Masko, E.M.; Freedland, A.R.; MacIas, E.; Pelton, K.; Solomon, K.R.; Mostaghel, E.A.; Thomas, G.V.; Pizzo, S.V.; Freeman, M.R.; et al. Serum cholesterol levels and tumor growth in a PTEN-null transgenic mouse model of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Akinsete, J.A.; Ion, G.; Witte, T.R.; Hardman, W.E. Consumption of high ω-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 2012, 33, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tie, Y.; Du, J. Association between dietary protein intake and prostate cancer risk: evidence from a meta-analysis. World J. Surg. Oncol. 2018, 16, 152. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, S.A. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr. Cancer 2009, 61, 437–446. [Google Scholar] [CrossRef]
- Koutros, S.; Cross, A.J.; Sandler, D.P.; Hoppin, J.A.; Ma, X.; Zheng, T.; Alavanja, M.C.R.; Sinha, R. Meat and meat mutagens and risk of prostate cancer in the Agricultural Health Study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 80–87. [Google Scholar] [CrossRef]
- Cross, A.J.; Peters, U.; Kirsh, V.A.; Andriole, G.L.; Reding, D.; Hayes, R.B.; Sinha, R. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005, 65, 11779–11784. [Google Scholar] [CrossRef]
- Major, J.M.; Cross, A.J.; Watters, J.L.; Hollenbeck, A.R.; Graubard, B.I.; Sinha, R. Patterns of meat intake and risk of prostate cancer among African-Americans in a large prospective study. Cancer Causes Control 2011, 22, 1691–1698. [Google Scholar] [CrossRef]
- Nakai, Y.; Nelson, W.G.; De Marzo, A.M. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007, 67, 1378–1384. [Google Scholar] [CrossRef]
- Shirai, T.; Sano, M.; Tamano, S.; Takahashi, S.; Hirose, M.; Futakuchi, M.; Hasegawa, R.; Imaida, K.; Matsumoto, K.; Wakabayashi, K.; et al. The prostate: A target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997, 57, 195–198. [Google Scholar]
- Gao, X.; LaValley, M.P.; Tucker, K.L. Prospective studies of dairy product and calcium intakes and prostate cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: A meta-analysis of 26,769 cases from 45 observational studies. Nutr. Cancer 2008, 60, 421–441. [Google Scholar] [CrossRef]
- Tat, D.; Kenfield, S.A.; Cowan, J.E.; Broering, J.M.; Carroll, P.R.; Van Blarigan, E.L.; Chan, J.M. Milk and other dairy foods in relation to prostate cancer recurrence: Data from the cancer of the prostate strategic urologic research endeavor (CaPSURETM). Prostate 2018, 78, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.K.; Batista, J.L.; Mucci, L.A.; Stampfer, M.J.; Epstein, M.M.; Håkansson, N.; Wolk, A.; Johansson, J.E.; Andrén, O.; Fall, K.; et al. Dairy intake in relation to prostate cancer survival. Int. J. Cancer 2017, 140, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Wolk, A.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998, 58, 442–447. [Google Scholar] [PubMed]
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 930–937. [Google Scholar] [CrossRef]
- Bernichtein, S.; Pigat, N.; Capiod, T.; Boutillon, F.; Verkarre, V.; Camparo, P.; Viltard, M.; Méjean, A.; Oudard, S.; Souberbielle, J.C.; et al. High milk consumption does not affect prostate tumor progression in two mouse models of benign and neoplastic lesions. PLoS ONE 2015, 10, e0125423. [Google Scholar] [CrossRef]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef]
- Freedland, S.J.; Mavropoulos, J.; Wang, A.; Darshan, M.; Demark-Wahnefried, W.; Aronson, W.J.; Cohen, P.; Hwang, D.; Peterson, B.; Fields, T.; et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008, 68, 11–19. [Google Scholar] [CrossRef]
- Masko, E.M.; Thomas, J.A.; Antonelli, J.A.; Lloyd, J.C.; Phillips, T.E.; Poulton, S.H.; Dewhirst, M.W.; Pizzo, S.V.; Freedland, S.J. Low-carbohydrate diets and prostate cancer: how low is “low enough”? Cancer Prev. Res. Phila. 2010, 3, 1124–1131. [Google Scholar] [CrossRef]
- Makarem, N.; Bandera, E.V.; Lin, Y.; Jacques, P.F.; Hayes, R.B.; Parekh, N. Carbohydrate nutrition and risk of adiposity-related cancers: Results from the Framingham Offspring cohort (1991-2013). Br. J. Nutr. 2017, 117, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Bandera, E.V.; Lin, Y.; Jacques, P.F.; Hayes, R.B.; Parekh, N. Consumption of Sugars, Sugary Foods, and Sugary Beverages in Relation to Adiposity-Related Cancer Risk in the Framingham Offspring Cohort (1991–2013). Cancer Prev. Res. Phila. 2018, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Neuhouser, M.L.; Zhang, Z.-F. Concentrated sugars and incidence of prostate cancer in a prospective cohort. Br. J. Nutr. 2018, 120, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Powell, L.M. Consumption Patterns of Sugar-Sweetened Beverages in the United States. J. Acad. Nutr. Diet. 2013, 113, 43–53. [Google Scholar] [CrossRef]
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-sweetened Beverage Consumption Among U.S. Youth, 2011–2014. NCHS Data Brief. 2017, 1, 1–8. [Google Scholar]
- Mondul, A.M.; Weinstein, S.J.; Albanes, D. Vitamins, metabolomics, and prostate cancer. World J. Urol. 2017, 35, 883–893. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Styer, L. Biochemistry, 5th ed.; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef]
- Etminan, M.; Takkouche, B.; Caamaño-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 340–345. [Google Scholar]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Med. Baltim. 2015, 94, e1260. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Thomas-Ahner, J.M.; Moran, N.E.; Cooperstone, J.L.; Erdman, J.W.; Young, G.S.; Clinton, S.K. B-Carotene 90,100 oxygenase modulates the anticancer activity of dietary tomato or lycopene on prostate carcinogenesis in the TRAMP model. Cancer Prev. Res. 2017, 10, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, M.; Pedersen, S.; Bastani, N.E.; Carlsen, H.; Blomhoff, R.; Paur, I. Tomato paste alters NF-κB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment. Nutr. Cancer 2015, 67, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tan, H.-L.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W.; Moran, N.E.; Clinton, S.K. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev. Res. Phila. 2014, 7, 1228–1239. [Google Scholar] [CrossRef]
- Masko, E.M.; Freedland, S.J. Prostate cancer and diet: Food for thought? BJU Int. 2011, 107, 1359–1360. [Google Scholar] [CrossRef] [PubMed]
- Newsom-Davis, T.E.; Kenny, L.M.; Ngan, S.; King, J.; Waxman, J. The promiscuous receptor. BJU Int. 2009, 104, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Goodman, P.J.; Tangen, C.; Torkko, K.C.; Schenk, J.M.; Song, X.; Pollak, M.; Thompson, I.M.; Neuhouser, M.L. Interactions of the Insulin-Like Growth Factor Axis and Vitamin D in Prostate Cancer Risk in the Prostate Cancer Prevention Trial. Nutrients 2017, 9, 378. [Google Scholar] [CrossRef]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; Dupre, N.; Platz, E.A.; et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef]
- Ahn, J.; Peters, U.; Albanes, D.; Purdue, M.P.; Abnet, C.C.; Chatterjee, N.; Horst, R.L.; Hollis, B.W.; Huang, W.Y.; Shikany, J.M.; et al. Serum vitamin D concentration and prostate cancer risk: A nested case-control study. J. Natl. Cancer Inst. 2008, 100, 796–804. [Google Scholar] [CrossRef]
- Deschasaux, M.; Souberbielle, J.C.; Latino-Martel, P.; Sutton, A.; Charnaux, N.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Le Clerc, S.; Kesse-Guyot, E.; et al. A prospective study of plasma 25-hydroxyvitamin D concentration and prostate cancer risk. Br. J. Nutr. 2016, 115, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Moy, K.A.; Mannisto, S.; Albanes, D. Circulating 25-hydroxyvitamin D and prostate cancer survival. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Martin, R.M.; Beynon, R.; Harris, R.; Savovic, J.; Zuccolo, L.; Bekkering, G.E.; Fraser, W.D.; Sterne, J.A.C.; Metcalfe, C. Associations of circulating and dietary vitamin D with prostate cancer risk: A systematic review and dose-response meta-analysis. Cancer Causes Control 2011, 22, 319–340. [Google Scholar] [CrossRef]
- Ajibade, A.A.; Kirk, J.S.; Karasik, E.; Gillard, B.; Moser, M.T.; Johnson, C.S.; Trump, D.L.; Foster, B.A. Early growth inhibition is followed by increased metastatic disease with vitamin D (calcitriol) treatment in the TRAMP model of prostate cancer. PLoS ONE 2014, 9, e89555. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Kaddoumi, A. Vitamin E Transporters in Cancer Therapy. AAPS J. 2015, 17, 313–322. [Google Scholar] [CrossRef] [PubMed]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [CrossRef] [PubMed]
- Lawson, K.A.; Wright, M.E.; Subar, A.; Mouw, T.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and health study. J. Natl. Cancer Inst. 2007, 99, 754–764. [Google Scholar] [CrossRef]
- Chan, J.M.; Stampfer, M.J.; Ma, J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Supplemental Vitamin E Intake and Prostate Cancer Risk in a Large Cohort of Men in the United States. Cancer Epidemiol. Prev. Biomarkers 1999, 8, 893–899. [Google Scholar]
- Rodriguez, C.; Jacobs, E.J.; Mondul, A.M.; Calle, E.E.; McCullough, M.L.; Thun, M.J. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 378–382. [Google Scholar]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). JAMA J. Am. Med. Assoc. 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Liu, A.; Wang, G.; Bosland, M.C.; Yang, C.S. δ-Tocopherol inhibits the development of prostate adenocarcinoma in prostate specific Pten−/− mice. Carcinogenesis 2018, 39, 158–169. [Google Scholar] [CrossRef]
- Wang, H.; Hong, J.; Yang, C.S. δ-Tocopherol inhibits receptor tyrosine kinase-induced AKT activation in prostate cancer cells. Mol. Carcinog. 2016, 55, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.K.; Mo, H. Synergistic Impact of d-δ-Tocotrienol and Geranylgeraniol on the Growth and HMG CoA Reductase of Human DU145 Prostate Carcinoma Cells. Nutr. Cancer 2017, 69, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, 52, e12576. [Google Scholar] [CrossRef]
- Kaneko, S.; Sato, C.; Shiozawa, N.; Sato, A.; Sato, H.; Virgona, N.; Yano, T. Suppressive Effect of Delta-Tocotrienol on Hypoxia Adaptation of Prostate Cancer Stem-like Cells. Anticancer Res. 2018, 38, 1391–1399. [Google Scholar] [PubMed]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhi, F.; Chen, P.; Zhao, K.; Xiang, H.; Mao, Q.; Wang, X.; Zhang, X. Green tea and the risk of prostate cancer: A systematic review and meta-analysis. Med. Baltim. 2017, 96, e6426. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Adhami, V.M.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int. J. Cancer 2005, 113, 660–669. [Google Scholar] [CrossRef]
- Harper, C.E.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Lamartiniere, C.A. Epigallocatechin-3-gallate suppresses early stage, but not late stage prostate cancer inTRAMP mice: Mechanisms of action. Prostate 2007, 67, 1576–1589. [Google Scholar] [CrossRef]
- Ellison, L.F. Tea and other beverage consumption and prostate cancer risk: A Canadian retrospective cohort study. Eur. J. Cancer Prev. 2000, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; West, D.W. Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States). Cancer Causes Control 1993, 4, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, C.L.; Negri, E.; D’Avanzo, B.; Franceschi, S.; Boyle, P. Tea Consumption and Cancer Risk. Nutr. Cancer 1992, 17, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.G.; Hislop, G.T.; Howe, G.R.; Burch, J.D.; Ghadirian, P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int. J. Cancer 1998, 78, 707–711. [Google Scholar] [CrossRef]
- Heilbrun, L.K.; Nomura, A.; Stemmermann, G.N. Black tea consumption and cancer risk: A prospective study. Br. J. Cancer 1986, 54, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Sasazuki, S.; Iwasaki, M.; Inoue, M.; Tsugane, S.; JPHC Study Group. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am. J. Epidemiol. 2008, 167, 71–77. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar]
- Lakshman, M.; Xu, L.; Ananthanarayanan, V.; Cooper, J.; Takimoto, C.H.; Helenowski, I.; Pelling, J.C.; Bergan, R.C. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008, 68, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.E.; Greenberg, N.M.; Elgavish, A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 2001, 61, 6777–6782. [Google Scholar] [PubMed]
- El Touny, L.H.; Banerjee, P.P. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, N.; Akaza, H.; Hinotsu, S.; Fujioka, T.; Naito, S.; Namiki, M.; Takahashi, S.; Hirao, Y.; Horie, S.; Tsukamoto, T.; et al. Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012, 103, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef]
- Lazarevic, B.; Hammarström, C.; Yang, J.; Ramberg, H.; Diep, L.M.; Karlsen, S.J.; Kucuk, O.; Saatcioglu, F.; Taskèn, K.A.; Svindland, A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br. J. Nutr. 2012, 108, 2138–2147. [Google Scholar] [CrossRef]
- Kumar, N.B.; Cantor, A.; Allen, K.; Riccardi, D.; Besterman-Dahan, K.; Seigne, J.; Helal, M.; Salup, R.; Pow-Sang, J. The specific role of isoflavones in reducing prostate cancer risk. Prostate 2004, 59, 141–147. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-Term Soy Isoflavone Intervention in Patients with Localized Prostate Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Shin, S.; Saito, E.; Sawada, N.; Ishihara, J.; Takachi, R.; Nanri, A.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; et al. Dietary patterns and prostate cancer risk in Japanese: the Japan Public Health Center-based Prospective Study (JPHC Study). Cancer Causes Control 2018, 29, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Niclis, C.; Román, M.D.; Osella, A.R.; Eynard, A.R.; Díaz, M.D.P. Traditional Dietary Pattern Increases Risk of Prostate Cancer in Argentina: Results of a Multilevel Modeling and Bias Analysis from a Case-Control Study. J. Cancer Epidemiol. 2015, 2015, 179562. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.L.; Deneo-Pellegrini, H.; Boffetta, P.; Aune, D.; Acosta, G.; Brennan, P.; Ferro, G.; Mendilaharsu, M. Dietary patterns and risk of advanced prostate cancer: a principal component analysis in Uruguay. Cancer Causes Control 2010, 21, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Fritschi, L.; de Klerk, N.H.; Mackerras, D.; Leavy, J. Dietary patterns identified using factor analysis and prostate cancer risk: a case control study in Western Australia. Ann. Epidemiol. 2008, 18, 364–370. [Google Scholar] [CrossRef]
- Askari, F.; Parizi, M.K.; Jessri, M.; Rashidkhani, B. Dietary patterns in relation to prostate cancer in Iranian men: a case-control study. Asian Pac. J. Cancer Prev. 2014, 15, 2159–2163. [Google Scholar] [CrossRef]
- Muller, D.C.; Severi, G.; Baglietto, L.; Krishnan, K.; English, D.R.; Hopper, J.L.; Giles, G.G. Dietary patterns and prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3126–3129. [Google Scholar] [CrossRef]
- Wu, K.; Hu, F.B.; Willett, W.C.; Giovannucci, E. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 167–171. [Google Scholar] [CrossRef]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef]
- Zhou, H.; Tai, J.; Xu, H.; Lu, X.; Meng, D. Xanthoceraside Could Ameliorate Alzheimer’s Disease Symptoms of Rats by Affecting the Gut Microbiota Composition and Modulating the Endogenous Metabolite Levels. Front. Pharmacol. 2019, 10, 1035. [Google Scholar] [CrossRef]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Picchianti-Diamanti, A.; Panebianco, C.; Salemi, S.; Sorgi, M.L.; Di Rosa, R.; Tropea, A.; Sgrulletti, M.; Salerno, G.; Terracciano, F.; D’Amelio, R.; et al. Analysis of Gut Microbiota in Rheumatoid Arthritis Patients: Disease-Related Dysbiosis and Modifications Induced by Etanercept. Int. J. Mol. Sci. 2018, 19, 2938. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2017, 35, 199–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Liu, F.; Riordan, S.M.; Lee, C.S.; Zhang, L. Global Investigations of Fusobacterium nucleatum in Human Colorectal Cancer. Front. Oncol. 2019, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Hsu, R.Y.C.; Chan, C.H.F.; Spicer, J.D.; Rousseau, M.C.; Giannias, B.; Rousseau, S.; Ferri, L.E. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011, 71, 1989–1998. [Google Scholar] [CrossRef]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
| Subject | Type | Source Examples | Risk | Reported Mechanisms | References |
|---|---|---|---|---|---|
| Fat | Total fat | Animal meat, butter | Increased PCa risk | IL-6/STAT3 pathway MDSC, Macrophage infiltration GF-I signaling pathway Increase in local androgen | [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Unsaturated fatty acid | Fish, vegetable oil | Decreased PCa risk (probable) | Decrease in estradiol, testosterone, and androgen receptors | ||
| Protein | Total protein | Animal meat, soy, dairy product, | No change in PCa risk | N/A | [45,46,47,48,49,50,51,52,53,54,55,56,57,58] |
| Heterocyclic amine | Well-cooked meat | Increased PCa risk | Mast cell, Macrophage infiltration | ||
| Dairy product | Milk, cheese | Increased PCa risk (probable) | N/A | ||
| Carbohydrate | Fruit, rice, potato, sugar | Increased PCa risk (probable) | IGF-I signaling pathway | [28,60,61,62,63,64,65,66] | |
| Vitamin | VitaminA (Lycopene) | Tomato | Decreased PCa risk | Decrease in androgen metabolism Antioxidative effect | [69,70,71,72,73,74,75,76] |
| VitaminD (Calcitoriol) | Fish, dairy product, mushroom | Decreased PCa risk (controversial) | Promotion of immune cell differentiation | [78,79,80,81,82,83,84,85] | |
| VitaminE (Tocopherol) | Vegetable oil | No change in PCa risk (controversial) | Anti-inflammatory effects Endoplasmic reticulum stress Antioxidative effect | [88,89,90,91,92,93,94,95,96,97] | |
| Polyphenol | Catechin | Green tea | Decreased PCa risk | IGF-I signaling pathway COX-2-mediated anti-inflammatory effects | [99,100,101,102,103,104,105,106,107,108] |
| Isoflavone | Legume | Decreased PCa risk (probable) | Estrogenic effects Antioxidative effect Inhibition of tyrosine kinase Suppression of NFκB | [109,110,111,112,113,114,115,116,117,118,119,120,121] | |
| Dietary pattern | Western pattern | Meat, potato, dairy product | Increased PCa risk (controversial) | N/A | [123,124,125,126,127,128,129] |
| Prudent pattern | Vegetable, fruit, legume, fish | Decreased PCa risk (controversial) | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. https://doi.org/10.3390/ijms21041447
Matsushita M, Fujita K, Nonomura N. Influence of Diet and Nutrition on Prostate Cancer. International Journal of Molecular Sciences. 2020; 21(4):1447. https://doi.org/10.3390/ijms21041447
Chicago/Turabian StyleMatsushita, Makoto, Kazutoshi Fujita, and Norio Nonomura. 2020. "Influence of Diet and Nutrition on Prostate Cancer" International Journal of Molecular Sciences 21, no. 4: 1447. https://doi.org/10.3390/ijms21041447
APA StyleMatsushita, M., Fujita, K., & Nonomura, N. (2020). Influence of Diet and Nutrition on Prostate Cancer. International Journal of Molecular Sciences, 21(4), 1447. https://doi.org/10.3390/ijms21041447






