Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells
Abstract
1. Introduction
2. Results
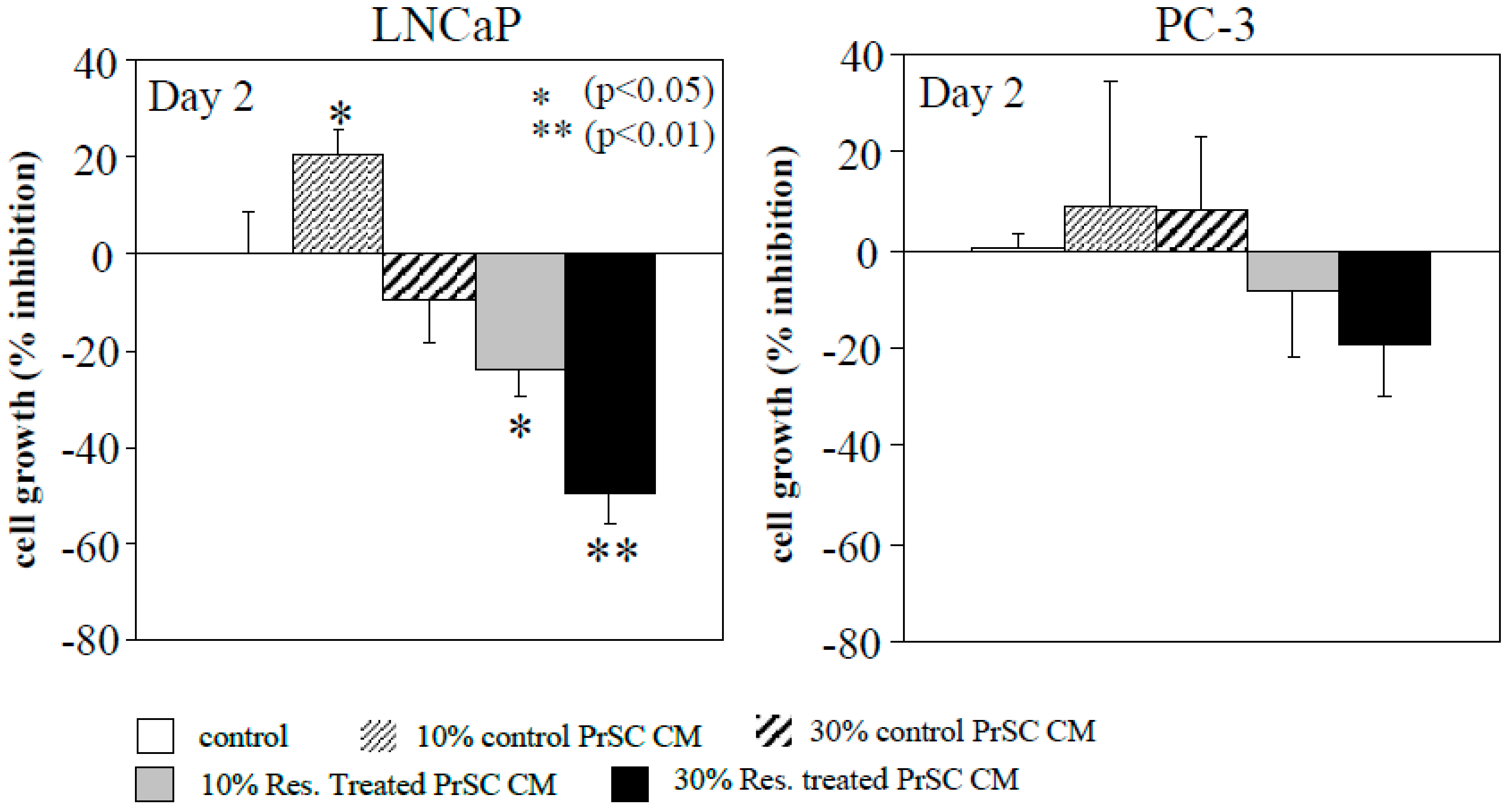
2.1. Conditioned Media (CM) Prepared from Resveratrol-Treated Prostate Stromal Cells (PrSC) Inhibit Epithelial Prostate Cancer Cell Growth
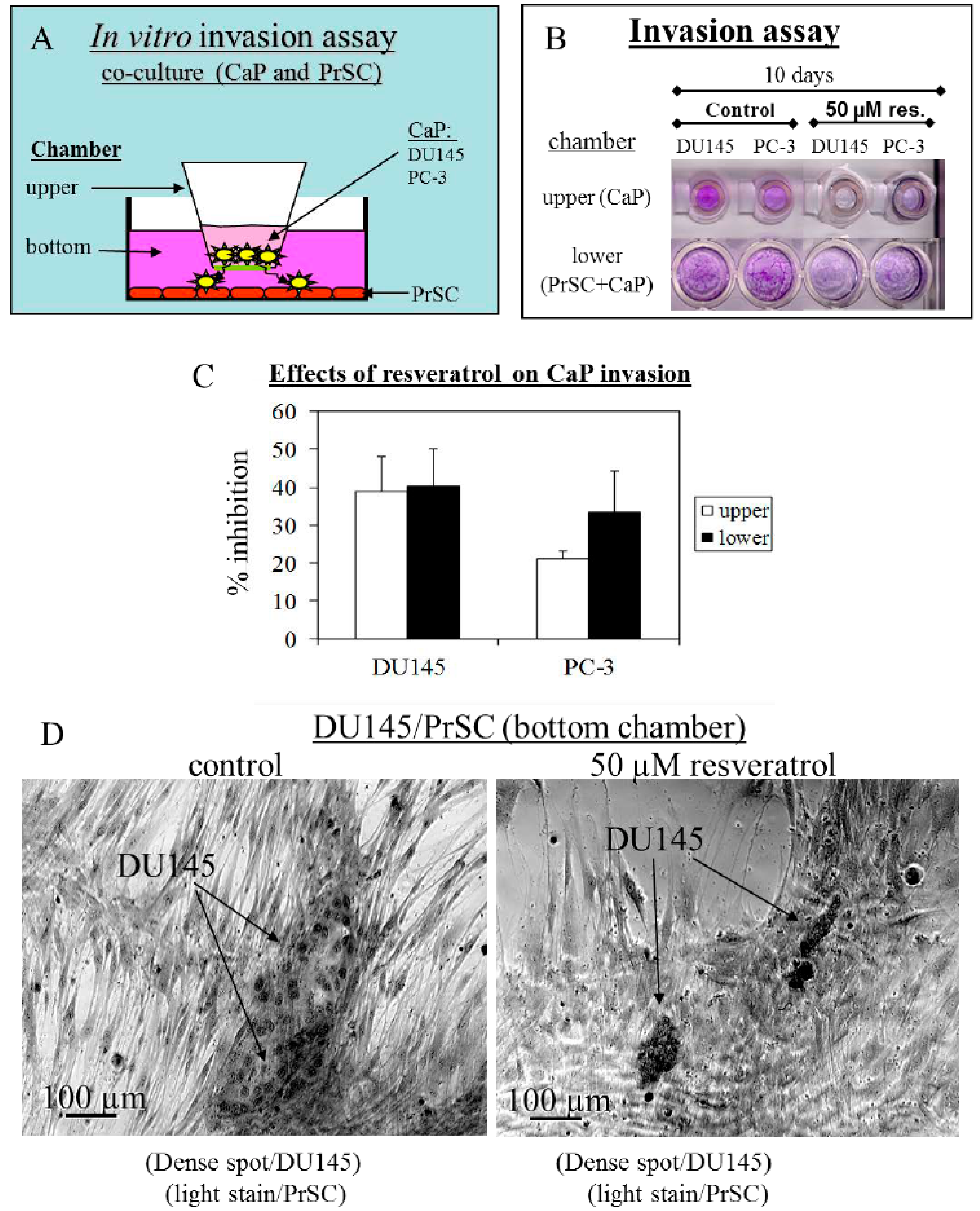
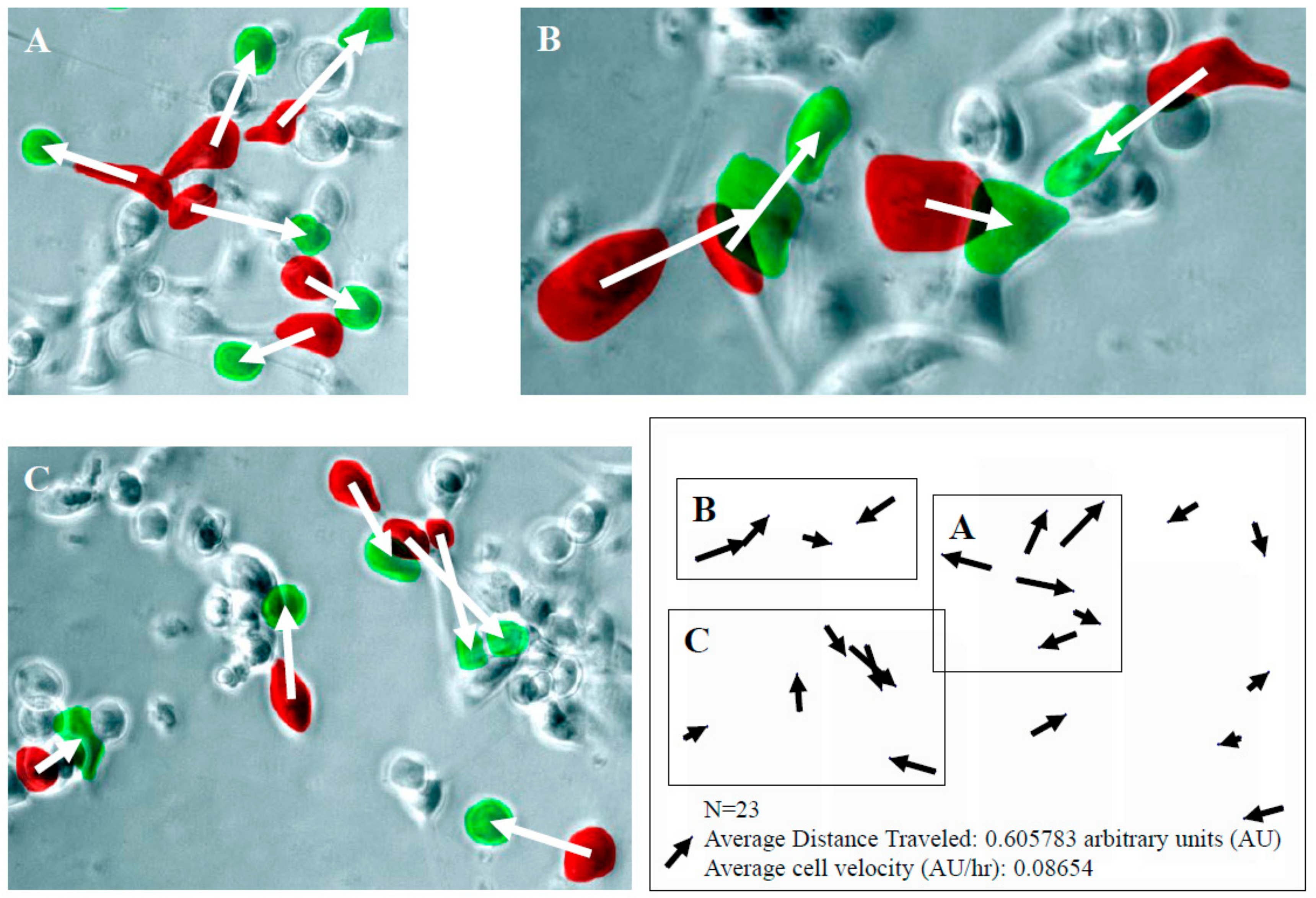
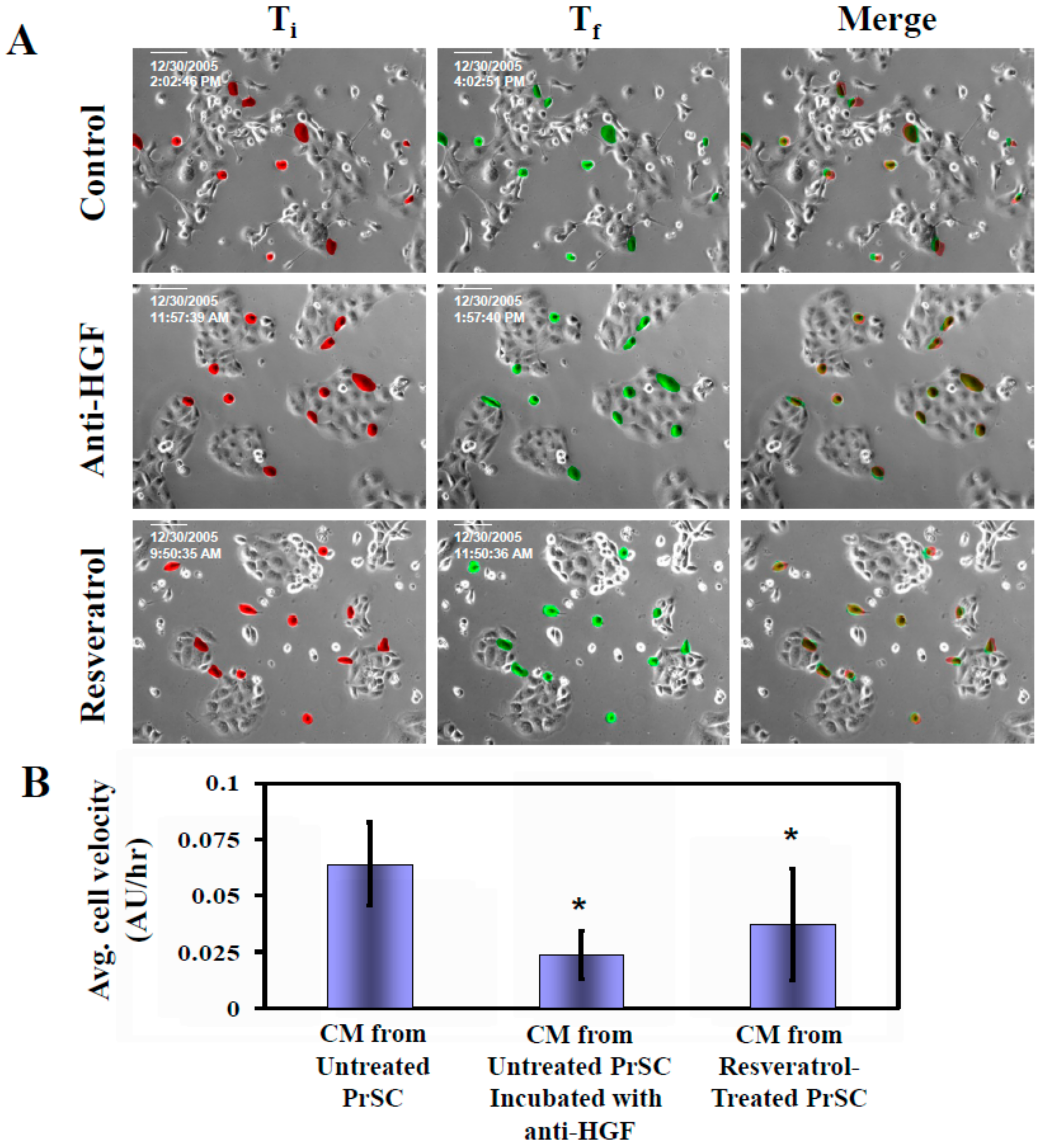
2.2. Tumor Cell Migration/Invasion is Significantly Inhibited by Resveratrol via Attenuation of Bi-Directional Signaling between PrSC and Epithelial Cells
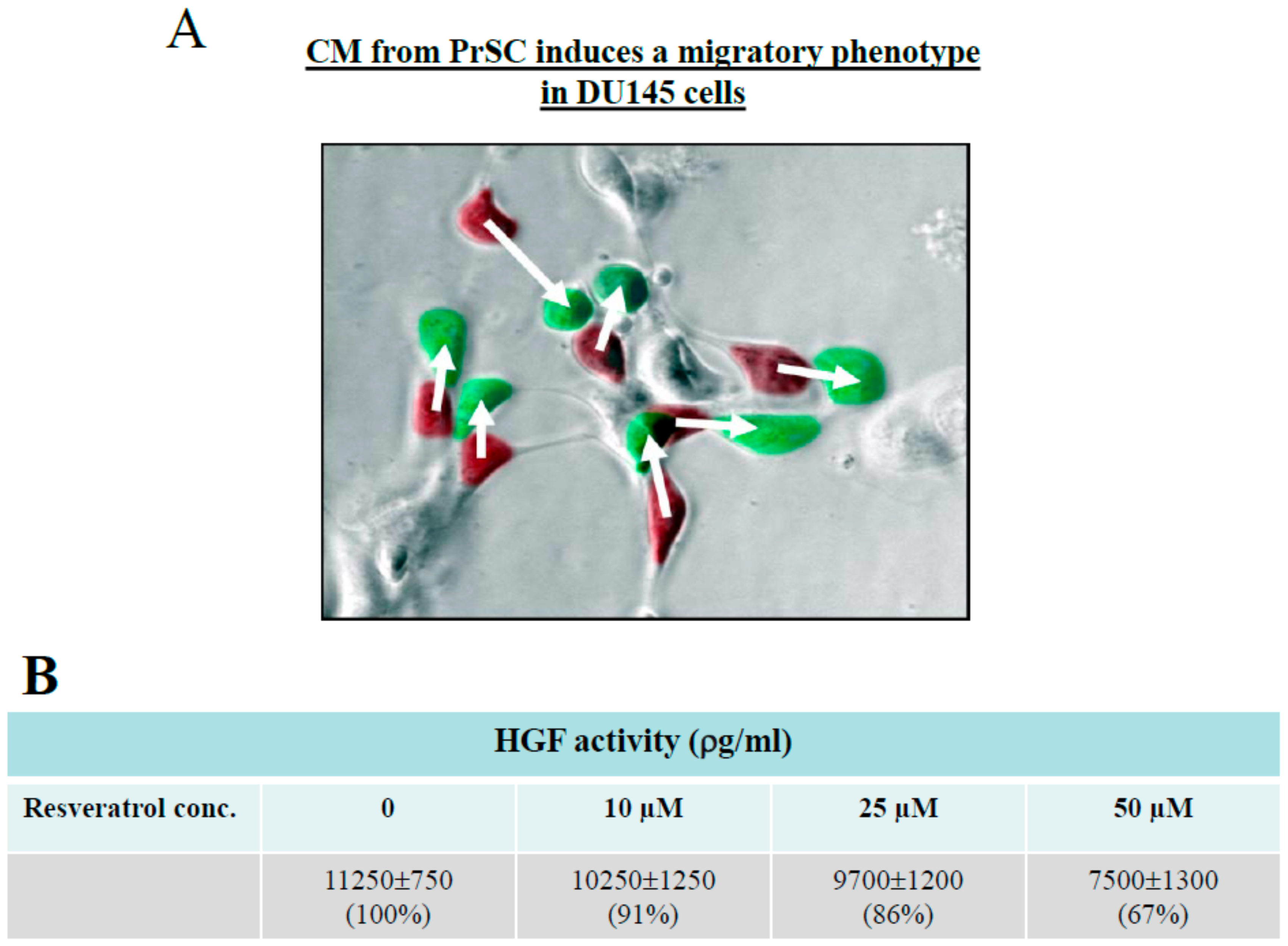
2.3. Control of CaP Cell Migration by PrSC Condition Medium (CM) and Assay of Secreted HGF in CM
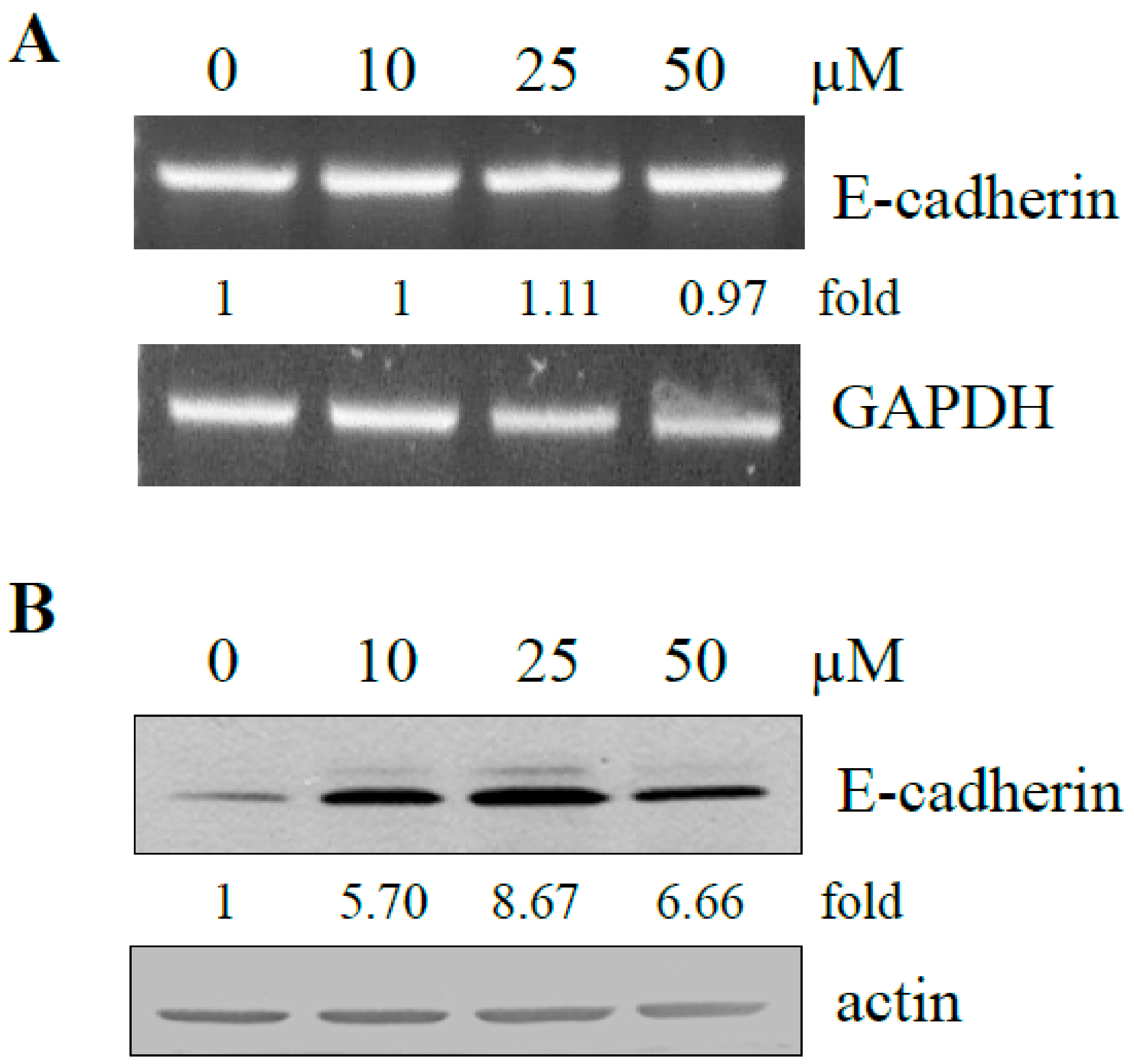
2.4. Effect of Resveratrol on Expression of E-Cadherin in DU145 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Invasion Assay
4.4. Preparation of Conditioned Medium (CM) from PrSC and Assay of Secreted HGF in CM
4.5. Cell Motility/Migration/Scatter Assay
4.6. RT-PCR Analysis and Determination of Gene-Specific mRNA Expression
4.7. Protein Extraction and Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | androgen-dependent |
| AI | androgen-independent |
| ATCC | American-Type Culture Collection |
| AU | arbitrary units |
| CaP | prostate cancer |
| CCD | charge-coupled device |
| CM | conditioned medium |
| ELISA | enzyme-linked immunoassay |
| EMT | epithelial-to-mesenchymal transition |
| HGF | hepatocyte growth factor |
| HRPC | hormone refractory prostate cancer |
| PrEC | prostate epithelium cell |
| PIN | prostatic intraepithelial neoplasia |
| PrSC | Prostate stromal cells |
| PSA | prostate specific antigen |
| RT-PCR | reverse transcribed polymerase chain reaction |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
References
- Kwok, W.K.; Ling, M.T.; Lee, T.W.; Lau, T.C.; Zhou, C.; Zhang, X.; Chua, C.W.; Chan, K.W.; Chan, F.L.; Glackin, C.; et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005, 65, 5153–5162. [Google Scholar] [CrossRef]
- Murray, N.P.; Reyes, E.; Tapia, P.; Badinez, L.; Orellana, N.; Fuentealba, C.; Olivares, R.; Porcell, J.; Dueñas, R. Redefining micrometastasis in prostate cancer—A comparison of circulating prostate cells, bone marrow disseminated tumor cells and micrometastasis: Implications in determining local or systemic treatment for biochemical failure after radical prostatectomy. Int. J. Mol. Med. 2012, 30, 896–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrett, J.M.; Mangold, K.A.; Jilling, T.; Kaul, K.L. Bi-directional interactions of prostate cancer cells and bone marrow endothelial cells in three-dimensional culture. Prostate 2005, 64, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.K.; Dayyani, F.; Gallick, G.E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 2011, 128, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Bogenrieder, T.; Herlyn, M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene 2003, 22, 6524–6536. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Hida, M.; Shimbo, T.; Nakamura, K.; Yamagata, J.; Satoh, T. Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer 1984, 54, 3078–3084. [Google Scholar] [CrossRef]
- Mamoune, A.; Kassis, J.; Kharait, S.; Kloeker, S.; Manos, E.; Jones, D.A.; Wells, A. DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Exp. Cell Res. 2004, 299, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.M.; Kyprianou, N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl. Androl. Urol. 2013, 2, 202–211. [Google Scholar] [CrossRef]
- Sistigu, A.; Di Modugno, F.; Manic, G.; Nisticò, P. Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine Growth Factor Rev. 2017, 36, 67–77. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Ohnishi, T.; Daikuhara, Y. Hepatocyte growth factor/scatter factor in development, inflammation and carcinogenesis: Its expression and role in oral tissues. Arch. Oral Biol. 2003, 48, 797–804. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Y.; Wang, Y.; Chen, Y.; Li, M.; Jiang, Y. Hepatocyte growth factor increases the invasive potential of PC-3 human prostate cancer cells via an ERK/MAPK and Zeb-1 signaling pathway. Oncol. Lett. 2016, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Aldahl, J.; Mi, J.; Pineda, A.; Kim, W.K.; Olson, A.; Hooker, E.; He, Y.; Yu, E.J.; Le, V.; Lee, D.H.; et al. Aberrant activation of hepatocyte growth factor/MET signaling promotes β-catenin-mediated prostatic tumorigenesis. J. Biol. Chem. 2020, 295, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.Y.; Childs, E.E.; Xi, S.; Coppelli, F.M.; Gooding, W.E.; Wells, A.; Ferris, R.L.; Grandis, J.R. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene 2005, 24, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Nakashiro, K.; Okamoto, M.; Hayashi, Y.; Oyasu, R. Hepatocyte growth factor secreted by prostate-derived stromal cells stimulates growth of androgen-independent human prostatic carcinoma cells. Am. J. Pathol. 2000, 157, 795–803. [Google Scholar] [CrossRef]
- Nakashiro, K.; Hayashi, Y.; Oyasu, R. Immunohistochemical expression of hepatocyte growth factor and c-Met/HGF receptor in benign and malignant human prostate tissue. Oncol. Rep. 2003, 10, 1149–1153. [Google Scholar] [CrossRef]
- Nakashiro, K.; Hara, S.; Shinohara, Y.; Oyasu, M.; Kawamata, H.; Shintani, S.; Hamakawa, H.; Oyasu, R. Phenotypic switch from paracrine to autocrine role of hepatocyte growth factor in an androgen-independent human prostatic carcinoma cell line, CWR22R. Am. J. Pathol. 2004, 165, 533–540. [Google Scholar] [CrossRef]
- Knudsen, B.S.; Edlund, M. Prostate cancer and the met hepatocyte growth factor receptor. Adv. Cancer Res. 2004, 91, 31–67. [Google Scholar] [CrossRef]
- Jeffers, M.; Rong, S.; Vande Woude, G.F. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J. Mol. Med. (Berl) 1996, 74, 505–513. [Google Scholar] [CrossRef]
- Stuart, K.A.; Riordan, S.M.; Lidder, S.; Crostella, L.; Williams, R.; Skouteris, G.G. Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int. J. Exp. Pathol. 2000, 81, 17–30. [Google Scholar] [CrossRef]
- Ridley, A.J.; Comoglio, P.M.; Hall, A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell Biol. 1995, 15, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Abounader, R.; Ranganathan, S.; Lal, B.; Fielding, K.; Book, A.; Dietz, H.; Burger, P.; Laterra, J. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J. Natl. Cancer Inst. 1999, 91, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Watkins, G.; Mason, M.D.; Jiang, W.G. Targeting the HGF/SF receptor c-met using a hammerhead ribozyme transgene reduces in vitro invasion and migration in prostate cancer cells. Prostate 2004, 60, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp. Cell Res. 1999, 249, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res. 2000, 20, 225–228. [Google Scholar]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar] [CrossRef]
- Chung, L.W.; Huang, W.C.; Sung, S.Y.; Wu, D.; Odero-Marah, V.; Nomura, T.; Shigemura, K.; Miyagi, T.; Seo, S.; Shi, C.; et al. Stromal-epithelial interaction in prostate cancer progression. Clin. Genitourin. Cancer 2006, 5, 162–170. [Google Scholar] [CrossRef]
- Josson, S.; Matsuoka, Y.; Chung, L.W.; Zhau, H.E.; Wang, R. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell Dev. Biol. 2010, 21, 26–32. [Google Scholar] [CrossRef]
- Tyekucheva, S.; Bowden, M.; Bango, C.; Giunchi, F.; Huang, Y.; Zhou, C.; Bondi, A.; Lis, R.; Van Hemelrijck, M.; Andrén, O.; et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat. Commun. 2017, 8, 420. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- De Groot, A.E.; Roy, S.; Brown, J.S.; Pienta, K.J.; Amend, S.R. Revisiting Seed and Soil: Examining the Primary Tumor and Cancer Cell Foraging in Metastasis. Mol. Cancer Res. 2017, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Tuxhorn, J.A.; Wheeler, T.M.; Frolov, A.; Scardino, P.T.; Ohori, M.; Wheeler, M.; Spitler, J.; Rowley, D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 4792–4801. [Google Scholar] [PubMed]
- Wells, C.M.; Ahmed, T.; Masters, J.R.; Jones, G.E. Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil. Cytoskeleton 2005, 62, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Nishimura, K.; Tsujimura, A.; Matsumiya, K.; Matsumoto, K.; Nakamura, T.; Okuyama, A. Effects of hepatocyte growth factor on E-cadherin-mediated cell-cell adhesion in DU145 prostate cancer cells. Urology 2001, 58, 1064–1069. [Google Scholar] [CrossRef]
- Nishimura, K.; Kitamura, M.; Miura, H.; Nonomura, N.; Takada, S.; Takahara, S.; Matsumoto, K.; Nakamura, T.; Matsumiya, K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate 1999, 41, 145–153. [Google Scholar] [CrossRef]
- Davies, G.; Jiang, W.G.; Mason, M.D. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J. Urol. 2000, 163, 985–992. [Google Scholar] [CrossRef]
- Duhon, D.; Bigelow, R.L.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef]
- Chang, H.Y.; Kao, M.C.; Way, T.D.; Ho, C.T.; Fu, E. Diosgenin suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition by down-regulation of Mdm2 and vimentin. J. Agric. Food Chem. 2011, 59, 5357–5363. [Google Scholar] [CrossRef]
- Hu, H.J.; Lin, X.L.; Liu, M.H.; Fan, X.J.; Zou, W.W. Curcumin mediates reversion of HGF-induced epithelial-mesenchymal transition via inhibition of c-Met expression in DU145 cells. Oncol. Lett. 2016, 11, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Geleta, B.; Leck, L.Y.W.; Paluncic, J.; Chiang, S.; Jansson, P.J.; Kovacevic, Z.; Richardson, D.R. Thiosemicarbazones suppress expression of the c-Met oncogene by mechanisms involving lysosomal degradation and intracellular shedding. J. Biol. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ireland, S.K.; Pham, T.; Temple, B.; Chen, R.; Raj, M.H.; Biliran, H. TLE1 promotes EMT in A549 lung cancer cells through suppression of E-cadherin. Biochem. Biophys. Res. Commun. 2014, 455, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ye, M.; Zhou, J.; Wang, Z.W.; Zhu, X. Restoring E-cadherin Expression by Natural Compounds for Anticancer Therapies in Genital and Urinary Cancers. Mol. Ther. Oncolytics. 2019, 14, 130–138. [Google Scholar] [CrossRef]
- Cunha, G.R.; Hayward, S.W.; Wang, Y.Z. Role of stroma in carcinogenesis of the prostate. Differentiation 2002, 70, 473–485. [Google Scholar] [CrossRef]
- Cunha, G.R.; Hayward, S.W.; Wang, Y.Z.; Ricke, W.A. Role of the stromal microenvironment in carcinogenesis of the prostate. Int. J. Cancer 2003, 107, 1–10. [Google Scholar] [CrossRef]
- Cunha, G.R.; Ricke, W.; Thomson, A.; Marker, P.C.; Risbridger, G.; Hayward, S.W.; Wang, Y.Z.; Donjacour, A.A.; Kurita, T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 2004, 92, 221–236. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive stroma in prostate cancer progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002, 8, 2912–2923. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Rowley, D.R. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998, 17, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.; Perryman, M. An epithelial scatter factor released by embryo fibroblasts. J. Cell Sci. 1985, 77, 209–223. [Google Scholar] [PubMed]
- Stoker, M. Effect of scatter factor on motility of epithelial cells and fibroblasts. J. Cell Physiol. 1989, 139, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.L.; Apodaca, G.; Mostov, K.E. Hepatocyte growth factor induces MDCK cell morphogenesis without causing loss of tight junction functional integrity. Am. J. Physiol. Cell Physiol. 2004, 286, C482–C494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsieh, T.C. Uptake of resveratrol and role of resveratrol-targeting protein, quinone reductase 2, in normally cultured human prostate cells. Asian J. Androl. 2009, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, N.; Tanaka, T.; Toda, K.; Imamura, S. Identification of novel cadherins expressed in human melanoma cells. J. Invest. Dermatol. 1997, 108, 908–913. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-c.; Wu, J.M. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1760. https://doi.org/10.3390/ijms21051760
Hsieh T-c, Wu JM. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. International Journal of Molecular Sciences. 2020; 21(5):1760. https://doi.org/10.3390/ijms21051760
Chicago/Turabian StyleHsieh, Tze-chen, and Joseph M Wu. 2020. "Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells" International Journal of Molecular Sciences 21, no. 5: 1760. https://doi.org/10.3390/ijms21051760
APA StyleHsieh, T.-c., & Wu, J. M. (2020). Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. International Journal of Molecular Sciences, 21(5), 1760. https://doi.org/10.3390/ijms21051760



