Laccase Properties, Physiological Functions, and Evolution
Abstract
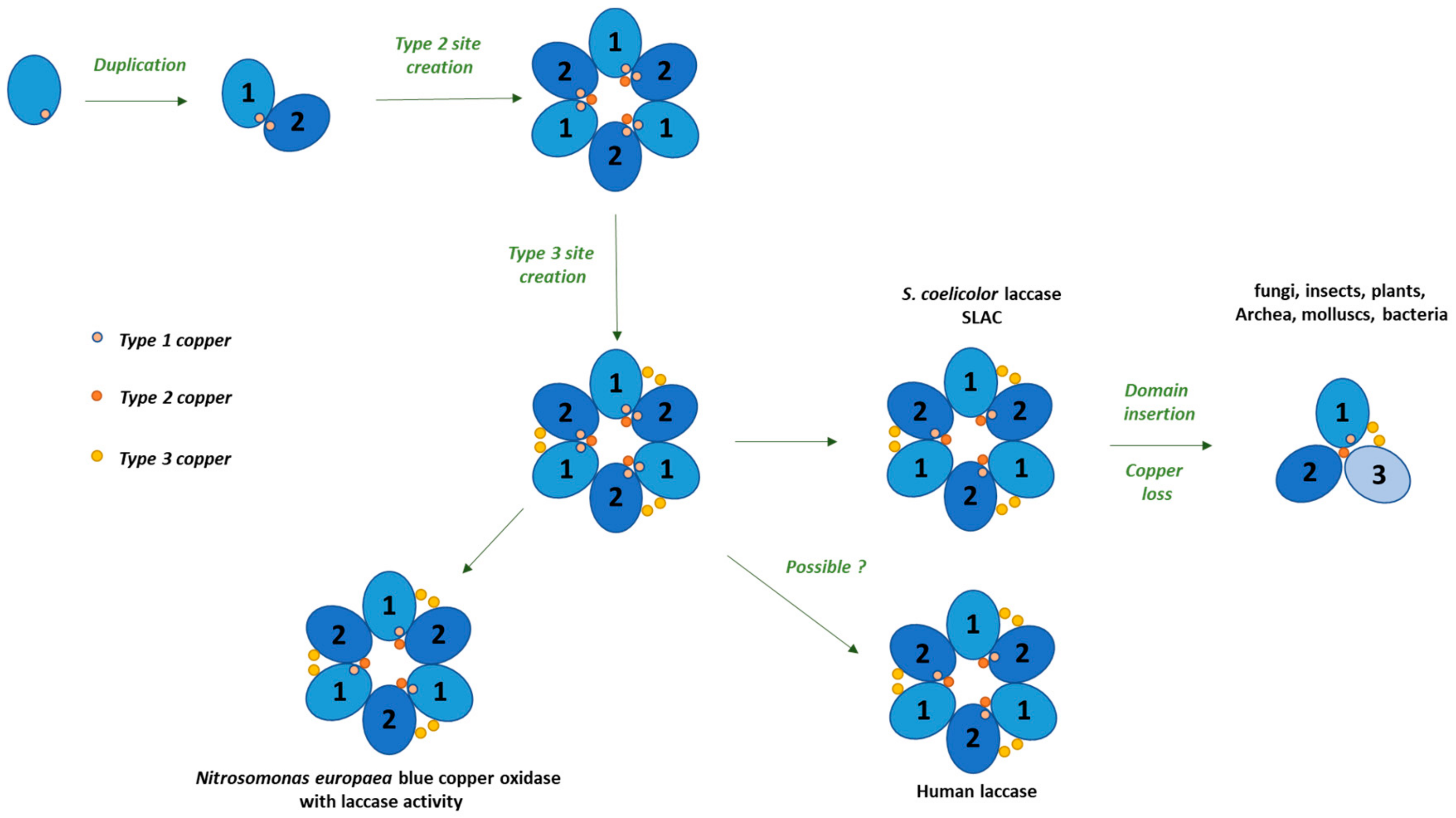
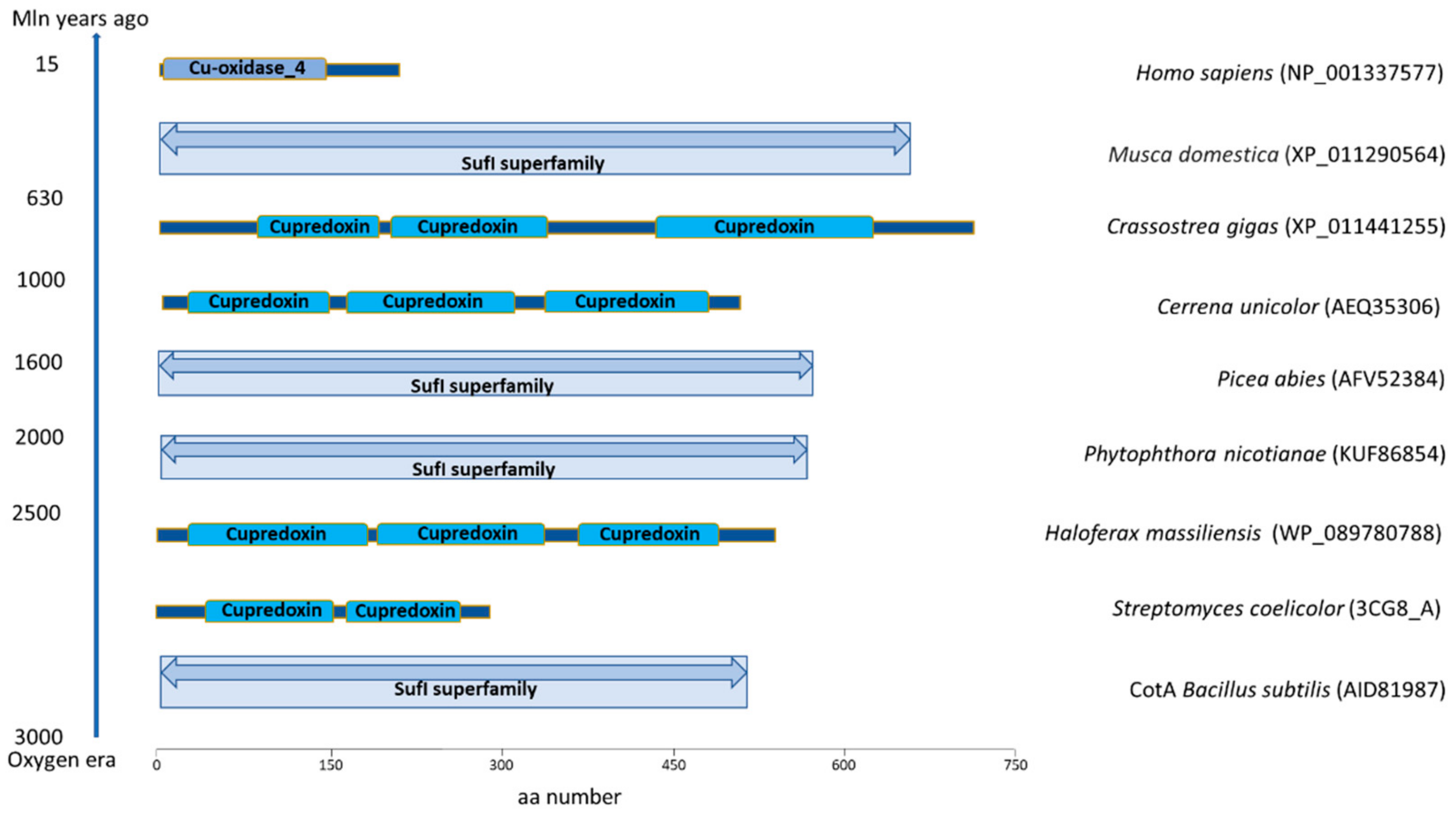
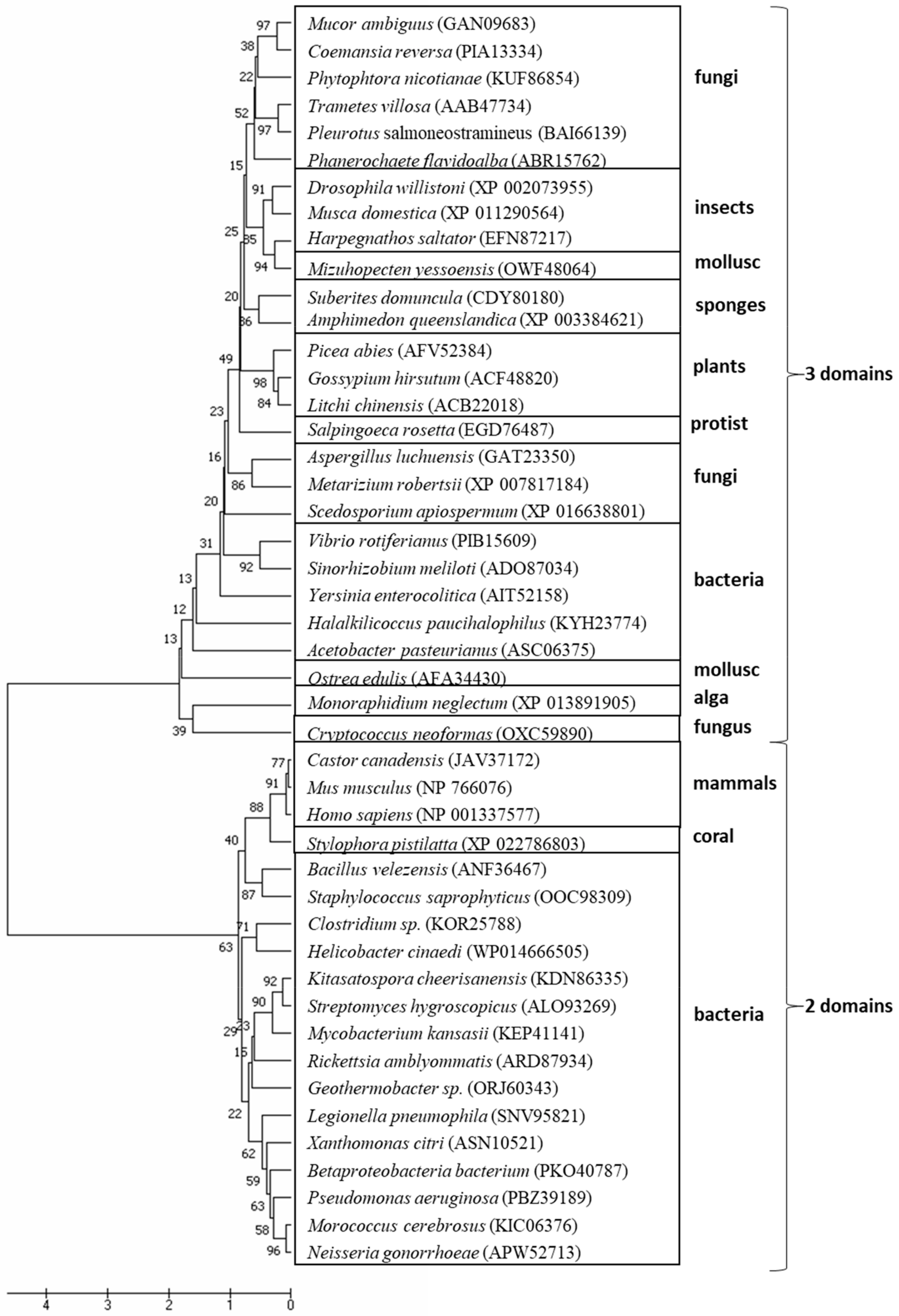
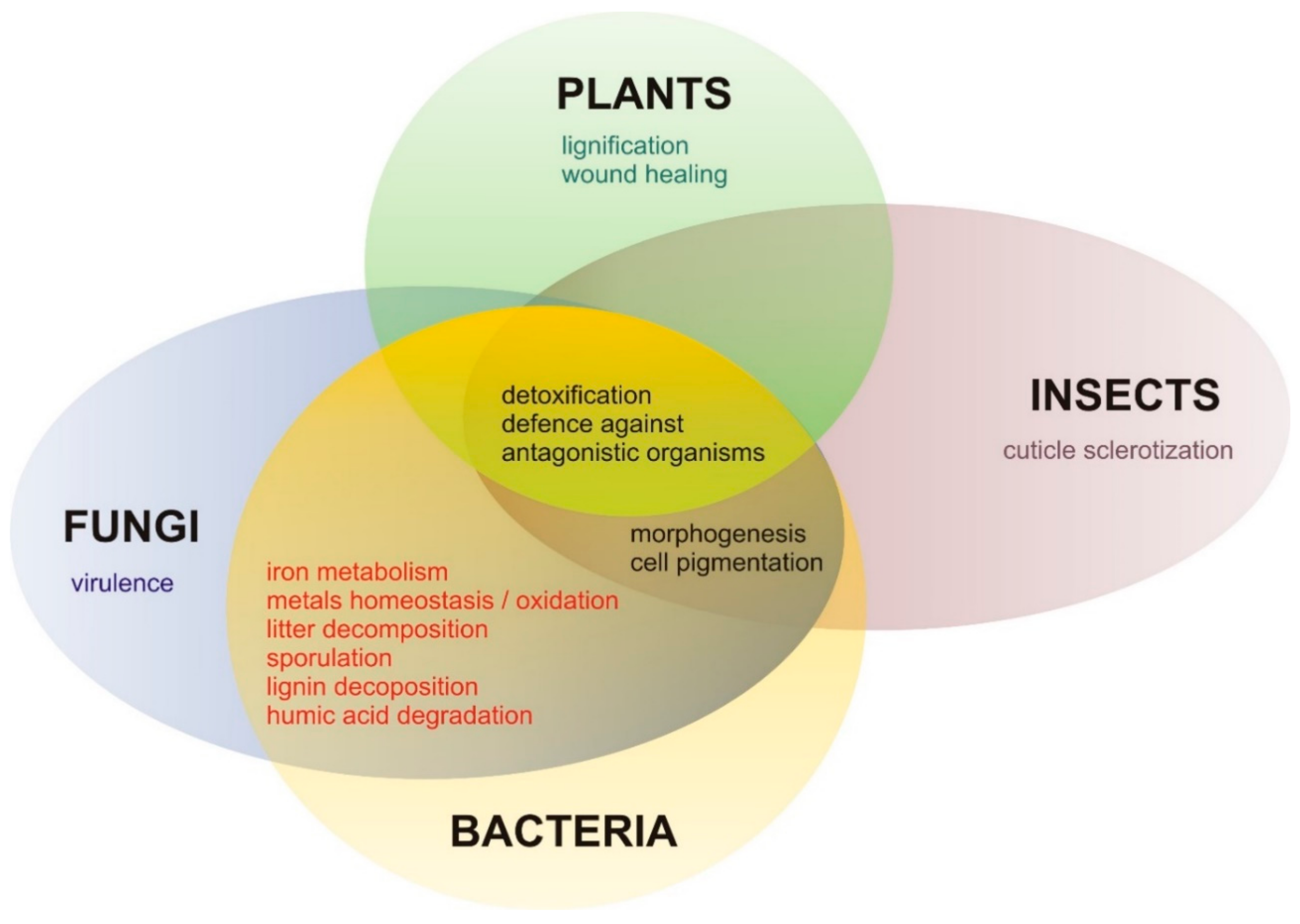
1. Introduction
2. Laccase as a Versatile Biocatalyst
3. Polyphenol oxidase (PPO) Properties and Physiological Functions
4. Bacterial Laccases
5. Plant Laccase—Species Range and Roles
6. Fungal Laccases—Occurrence, Roles, Similarities, and Differences
7. Occurrence and Function of Laccase in Animals
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2,4-DAPG | 2,4-diacetylphloroglucinol |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| DMP | (2,6-dimethoxyphenol) |
| LDA | lignin-degrading auxiliary enzymes |
| LMCO | laccase-like multicopper oxidase |
| MCO | multicopper oxidase |
| NHE | normal hydrogen electrode |
| POD | ligninolytic class II peroxidases |
| PPO | polyphenol oxidase |
| ROS | reactive oxygen species |
| SGZ | syringaldazine |
References
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Crichton, R.R.; Pierre, J.L. Old iron, young copper: From Mars to Venus. Biometals 2001, 14, 99–112. [Google Scholar] [CrossRef]
- Komori, H.; Higuchi, Y. Structural insights into the O-2 reduction mechanism of multicopper oxidase. J. Biochem. 2015, 158, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Davidson, V.L. Cupredoxins-A study of how proteins may evolve to use metals for bioenergetic processes. Metallomics 2011, 3, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure-function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Kallio, J.P.; Gasparetti, C.; Andberg, M.; Boer, H.; Koivula, A.; Kruus, K.; Rouvinen, J.; Hakulinen, N. Crystal structure of an ascomycete fungal laccase from Thielavia arenaria - common structural features of asco-laccases. FEBS J. 2011, 278, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kataoka, K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem. Rec. 2007, 7, 220–229. [Google Scholar] [CrossRef]
- Hakulinen, N.; Rouvinen, J. Three-dimensional structures of laccases. Cell Mol. Life Sci. 2015, 72, 857–868. [Google Scholar] [CrossRef]
- Assadi, G.; Vesterlund, L.; Bonfiglio, F.; Mazzurana, L.; Cordeddu, L.; Schepis, D.; Mjosberg, J.; Ruhrmann, S.; Fabbri, A.; Vukojevic, V.; et al. Functional analyses of the crohn’s disease risk gene LACC1. PLoS ONE 2016, 11, e0168276. [Google Scholar] [CrossRef]
- Komori, H.; Higuchi, Y. Structure and molecular evolution of multicopper blue proteins. Biomol. Concepts 2010, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ruijssenaars, H.J.; Hartmans, S. A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl. Microbiol. Biotechnol. 2004, 65, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci. 2015, 17, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Ihssen, J.; Richter, M.; Eichhorn, E.; Schilling, B.; Thöny-Meyer, L. Laccase versus laccase-like multi-copper oxidase: A comparative study of similar enzymes with diverse substrate spectra. PLoS ONE 2013, 8, e65633. [Google Scholar] [CrossRef]
- Ihssen, J.; Reiss, R.; Luchsinger, R.; Thony-Meyer, L.; Richter, M. Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 2015, 5, 10465. [Google Scholar] [CrossRef]
- Nakamura, K.; Go, N. Function and molecular evolution of multicopper blue proteins. Cell Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef]
- Park, M.; Kim, M.; Kim, S.; Ha, B.; Ro, H.S. Differential expression of laccase genes in Pleurotus ostreatus and biochemical characterization of laccase isozymes produced in Pichia pastoris. Mycobiology 2015, 43, 280–287. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Ng, T.B.; Lin, J.; Ye, X. Laccase Production and Differential Transcription of Laccase Genes in Cerrena sp. in Response to Metal Ions, Aromatic Compounds, and Nutrients. Front. Microbiol. 2015, 6, 1558. [Google Scholar] [CrossRef]
- Yuan, X.; Tian, G.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. Biochemical characteristics of three laccase isoforms from the basidiomycete Pleurotus nebrodensis. Molecules 2016, 21, E203. [Google Scholar] [CrossRef]
- Karp, S.G.; Faraco, V.; Amore, A.; Birolo, L.; Giangrande, C.; Soccol, V.T.; Pandey, A.; Soccol, C.R. Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour. Technol. 2012, 114, 735–739. [Google Scholar] [CrossRef]
- Moiseenko, K.V.; Maloshenok, L.G.; Vasina, D.V.; Bruskin, S.A.; Tyazhelova, T.V.; Koroleva, O.V. Laccase multigene families in Agaricomycetes. J. Basic Microbiol. 2016, 56, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evol. Int. J. Org. Evol. 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Dayhoff, M. Matrices for detecting distant relationships. In Atlas of Protein Sequences; Dayhoff, M., Ed.; The National Biomedical Research Foundation: Silver Spring, MD, USA, 1979; pp. 353–358. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger eatasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, A.K.; Mikkelsen, J.D.; Meyer, A.S. Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit. Rev. Biotechnol. 2016, 36, 70–86. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Canas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, K.H.; Jin, J.; Kim, H.; Kim, J.C.; Yun, S.H.; Lee, Y.W. Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae. Appl. Environ. Microbiol. 2005, 71, 1701–1708. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Kanost, M.R. Insect multicopper oxidases: Diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 2010, 40, 179–188. [Google Scholar] [CrossRef]
- Bao, W.; O’Malley D, M.; Whetten, R.; Sederoff, R.R. A laccase associated with lignification in loblolly pine xylem. Science 1993, 260, 672–674. [Google Scholar] [CrossRef]
- Omalley, D.M.; Whetten, R.; Bao, W.L.; Chen, C.L.; Sederoff, R.R. The role of laccase in lignification. Plant. J. 1993, 4, 751–757. [Google Scholar] [CrossRef]
- Chefetz, B.; Chen, Y.; Hadar, Y. Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl. Environ. Microbiol. 1998, 64, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Mikolasch, A.; Schauer, F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl. Microbiol. Biotechnol. 2009, 82, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Laine, A.C.; Vian, B.; Faye, L. Characterization and localization of laccase forms in stem and cell-cultures of sycamore. Plant J. 1992, 2, 13–24. [Google Scholar] [CrossRef]
- Harakava, R. Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus. Genet. Mol. Biol. 2005, 28, 601–607. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Kerhoas, L.; Caboche, M.; Lepiniec, L.; Debeaujon, I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 2005, 17, 2966–2980. [Google Scholar] [CrossRef]
- Ranocha, P.; Chabannes, M.; Chamayou, S.; Danoun, S.; Jauneau, A.; Boudet, A.M.; Goffner, D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002, 129, 145–155. [Google Scholar] [CrossRef]
- Sugumaran, M.; Giglio, L.; Kundzicz, H.; Saul, S.; Semensi, V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch. Insect. Biochem. Physiol. 1992, 19, 271–283. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Beeman, R.W.; Kanost, M.R.; Kramer, K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA 2005, 102, 11337–11342. [Google Scholar] [CrossRef]
- Kramer, K.J.; Kanost, M.R.; Hopkins, T.L.; Jiang, H.B.; Zhu, Y.C.; Xu, R.D.; Kerwin, J.L.; Turecek, F. Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron 2001, 57, 385–392. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Salazar, A.; Dadachova, E.; Casadevall, A. Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl. Environ. Microbiol. 2007, 73, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Guillen, F.; Gomez-Toribio, V.; Martinez, M.J.; Martinez, A.T. Production of hydroxyl radical by the synergistic action of fungal laccase and aryl alcohol oxidase. Arch. Biochem. Biophys. 2000, 383, 142–147. [Google Scholar] [CrossRef]
- Wei, D.S.; Houtman, C.J.; Kapich, A.N.; Hunt, C.G.; Cullen, D.; Hammel, K.E. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol. 2010, 76, 2091–2097. [Google Scholar] [CrossRef]
- Bajpai, P. Application of enzymes in the pulp and paper industry. Biotechnol. Progr. 1999, 15, 147–157. [Google Scholar] [CrossRef]
- Kawai, S.; Umezawa, T.; Higuchi, T. Degradation mechanisms of phenolic b-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch. Biochem. Biophys. 1988, 262, 99–110. [Google Scholar] [CrossRef]
- Rico, A.; Rencoret, J.; Del Rio, J.C.; Martinez, A.T.; Gutierrez, A. Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol. Biofuels 2014, 7, 6. [Google Scholar] [CrossRef]
- Rochefort, D.; Leech, D.; Bourbonnais, R. Electron transfer mediator systems for bleaching of paper pulp. Green Chem. 2004, 6, 14–24. [Google Scholar] [CrossRef]
- Westereng, B.; Cannella, D.; Agger, J.W.; Jorgensen, H.; Andersen, M.L.; Eijsink, V.G.H.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- D’Acunzo, F.; Galli, C.; Gentili, P.; Sergi, F. Mechanistic and steric issues in the oxidation of phenolic and non-phenolic compounds by laccase or laccase-mediator systems. The case of bifunctional substrates. N. J. Chem. 2006, 30, 583–591. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Masai, E.; Katayama, Y.; Fukuda, M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci. Biotechnol. Biochem. 2007, 71, 1–15. [Google Scholar] [CrossRef]
- Sharma, K.K.; Kuhad, R.C. An evidence of laccases in archaea. Indian J. Microbiol. 2009, 49, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Liu, L.; Dean, J.F.; Friedman, W.E.; Eriksson, K.E.L. A laccase-like phenoloxidase is correlated with lignin biosynthesis in Zinnia elegans stem tissues. Plant. J. 1994, 6, 213–224. [Google Scholar] [CrossRef]
- Sanchez-Ferrer, A.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F.; Garcia-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Faccio, G.; Kruus, K.; Saloheimo, M.; Thöny-Meyer, L. Bacterial tyrosinases and their applications. Process. Biochem. 2012, 47, 1749–1760. [Google Scholar] [CrossRef]
- Aniszewski, T.; Lieberei, R.; Gulewicz, K. Research on catecholases, laccases and cresolases in plants. Recent progress and future needs. Acta. Biol. Crac. Ser. Bot. 2008, 50, 7–18. [Google Scholar]
- Jiménez, M.; Kameyama, K.; Maloy, W.L.; Tomita, Y.; Hearing, V.J. Mammalian tyrosinase: Biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc. Natl. Acad. Sci. 1988, 85, 3830–3834. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant. Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Molitor, C.; Mauracher, S.G.; Rompel, A. Aurone synthase is a catechol oxidase with hydroxylase activity and provides insights into the mechanism of plant polyphenol oxidases. Proc. Natl. Acad. Sci. 2016, 113, E1806–E1815. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Ferrar, P.H. Diphenol oxidases, enzyme-catalysed browning and plant disease resistance. Biotechnol. Genet. Eng. Rev. 1998, 15, 457–498. [Google Scholar] [CrossRef] [PubMed]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Springer: Berlin, Germany, 2008; pp. 253–270. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Sugumaran, M.; Nellaiappan, K.; Valivittan, K. A new mechanism for the control of phenoloxidase activity: Inhibition and complex formation with quinone isomerase. Arch. Biochem. Biophys. 2000, 379, 252–260. [Google Scholar] [CrossRef]
- Sugumaran, M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment. Cell Res. 2002, 15, 2–9. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Deng, J.; Jiang, H. The structure of a prophenoloxidase (PPO) from Anopheles gambiae provides new insights into the mechanism of PPO activation. BMC Biol. 2016, 14, 2. [Google Scholar] [CrossRef]
- Veluthakkal, R.; Dasgupta, M.G. Pathogenesis-related genes and proteins in forest tree species. Trees 2010, 24, 993–1006. [Google Scholar] [CrossRef]
- Almagro, L.; Ros, L.G.; Belchi-Navarro, S.; Bru, R.; Barceló, A.R.; Pedreño, M. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, E.P.; Okubara, P.A.; Anderson, J.V.; Morris, C.F. Polyphenol oxidase as a biochemical seed defense mechanism. Front. Plant Sci. 2014, 5, 689. [Google Scholar] [CrossRef] [PubMed]
- Selinheimo, E.; NiEidhin, D.; Steffensen, C.; Nielsen, J.; Lomascolo, A.; Halaouli, S.; Record, E.; O’Beirne, D.; Buchert, J.; Kruus, K. Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol. 2007, 130, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—types, structural models, biological functions, and formation routes. N. J. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- McMahon, A.M.; Doyle, E.M.; Brooks, S.; O’Connor, K.E. Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzym. Microb. Technol. 2007, 40, 1435–1441. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Sjoblad, R.; Bollag, J. Oxidative coupling of aromatic compounds by enzymes from soil microorganisms. Soil Biochem. 1981, 5, 113–152. [Google Scholar]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes–ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Alexandre, G.; Zhulin, I.B. Laccases are widespread in bacteria. Trends Biotechnol. 2000, 18, 41–42. [Google Scholar] [CrossRef]
- Claus, H. Laccases and their occurrence in prokaryotes. Arch. Microbiol. 2003, 179, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Givaudan, A.; Effosse, A.; Faure, D.; Potier, P.; Bouillant, M.L.; Bally, R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere - evidence for laccase activity in nonmotile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 1993, 108, 205–210. [Google Scholar] [CrossRef]
- Diamantidis, G.; Effosse, A.; Potier, P.; Bally, R. Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol. Biochem. 2000, 32, 919–927. [Google Scholar] [CrossRef]
- Reiss, R.; Ihssen, J.; Thöny-Meyer, L. Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011, 11, 9. [Google Scholar] [CrossRef]
- Checinska, A.; Burbank, M.; Paszczynski, A.J. Protection of Bacillus pumilus spores by catalases. Appl. Environ. Microbiol. 2012, 78, 6413–6422. [Google Scholar] [CrossRef]
- Hullo, M.F.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430. [Google Scholar] [CrossRef]
- Koschorreck, K.; Richter, S.M.; Ene, A.B.; Roduner, E.; Schmid, R.D.; Urlacher, V.B. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl. Microbiol. Biotechnol. 2008, 79, 217–224. [Google Scholar] [CrossRef]
- Suzuki, T.; Endo, K.; Ito, M.; Tsujibo, H.; Miyamoto, K.; Inamori, Y. A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Biosci. Biotechnol. Biochem. 2003, 67, 2167–2175. [Google Scholar] [CrossRef]
- Endo, K.; Hayashi, Y.; Hibi, T.; Hosono, K.; Beppu, T.; Ueda, K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J. Biochem. 2003, 133, 671–677. [Google Scholar] [CrossRef]
- Solano, F.; Lucas-Elío, P.; López-Serrano, D.; Fernández, E.; Sanchez-Amat, A. Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. FEMS Microbiol. Lett. 2001, 204, 175–181. [Google Scholar] [CrossRef]
- Miyazaki, K. A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 2005, 9, 415–425. [Google Scholar] [CrossRef]
- Castro-Sowinski, S.; Martinez-Drets, G.; Okon, Y. Laccase activity in melanin-producing strains of Sinorhizobium meliloti. FEMS Microbiol. Lett. 2002, 209, 119–125. [Google Scholar] [CrossRef]
- Palanisami, S.; Saha, S.K.; Lakshmanan, U. Laccase and polyphenol oxidase activities of marine cyanobacteria: A study with Poly R-478 decolourization. World J. Microbiol. Biotechnol. 2010, 26, 63–69. [Google Scholar] [CrossRef]
- Uthandi, S.; Saad, B.; Humbard, M.A.; Maupin-Furlow, J.A. LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferax volcanii. Appl. Environ. Microbiol. 2010, 76, 733–743. [Google Scholar] [CrossRef]
- Sanchez-Amat, A.; Solano, F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp shares catalytic capabilities of tyrosinases and laccases. Biochem. Biophys. Res. Commun. 1997, 240, 787–792. [Google Scholar] [CrossRef]
- Fernandes, A.T.; Soares, C.M.; Pereira, M.M.; Huber, R.; Grass, G.; Martins, L.O. A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J. 2007, 274, 2683–2694. [Google Scholar] [CrossRef]
- Bains, J.; Capalash, N.; Sharma, P. Laccase from a non-melanogenic, alkalotolerant gamma-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol. Lett. 2003, 25, 1155–1159. [Google Scholar] [CrossRef]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Alexandre, G.; Bally, R.; Taylor, B.L.; Zhulin, I.B. Loss of cytochrome c oxidase activity and acquisition of resistance to quinone analogs in a laccase-positive variant of Azospirillum lipoferum. J. Bacteriol. 1999, 181, 6730–6738. [Google Scholar] [CrossRef]
- Dubé, E.; Shareck, F.; Hurtubise, Y.; Daneault, C.; Beauregard, M. Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl. Microbiol. Biotechnol. 2008, 79, 597–603. [Google Scholar] [CrossRef]
- Sondhi, S.; Sharma, P.; Saini, S.; Puri, N.; Gupta, N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE 2014, 9, e96951. [Google Scholar] [CrossRef]
- Claus, H. Copper-containing oxidases: Occurrence in soil microorganisms, properties, and applications. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 281–313. [Google Scholar]
- Machczynski, M.C.; Vijgenboom, E.; Samyn, B.; Canters, G.W. Characterization of SLAC: A small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci. 2004, 13, 2388–2397. [Google Scholar] [CrossRef]
- Niladevi, K.; Sheejadevi, P.; Prema, P. Strategies for enhancing laccase yield from Streptomyces psammoticus and its role in mediator-based decolorization of azo dyes. Appl. Biochem. Biotechnol. 2008, 151, 9–19. [Google Scholar] [CrossRef]
- Gunne, M.; Hoppner, A.; Hagedoorn, P.L.; Urlacher, V.B. Structural and redox properties of the small laccase Ssl1 from Streptomyces sviceus. FEBS J. 2014, 281, 4307–4318. [Google Scholar] [CrossRef]
- Skalova, T.; Dohnalek, J.; Ostergaard, L.H.; Ostergaard, P.R.; Kolenko, P.; Duskova, J.; Stepankova, A.; Hasek, J. The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. J. Mol. Biol. 2009, 385, 1165–1178. [Google Scholar] [CrossRef]
- Nothaft, H.; Szymanski, C.M. Protein glycosylation in bacteria: Sweeter than ever. Nat. Rev. Microbiol. 2010, 8, 765–778. [Google Scholar] [CrossRef]
- Pawlik, A.; Wojcik, M.; Rulka, K.; Motyl-Gorzel, K.; Osinska-Jaroszuk, M.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A.; Rogalski, J.; Janusz, G. Purification and characterization of laccase from Sinorhizobium meliloti and analysis of the lacc gene. Int. J. Biol. Macromol. 2016, 92, 138–147. [Google Scholar] [CrossRef]
- Fernandes, A.T.; Damas, J.M.; Todorovic, S.; Huber, R.; Baratto, M.C.; Pogni, R.; Soares, C.M.; Martins, L.O. The multicopper oxidase from the archaeon Pyrobaculum aerophilum shows nitrous oxide reductase activity. Febs. J. 2010, 277, 3176–3189. [Google Scholar] [CrossRef]
- Melo, E.P.; Fernandes, A.T.; Durao, P.; Martins, L.O. Insight into stability of CotA laccase from the spore coat of Bacillus subtilis. Biochem. Soc. Trans. 2007, 35, 1579–1582. [Google Scholar] [CrossRef]
- Gunne, M.; Urlacher, V.B. Characterization of the alkaline laccase Ssl1 from Streptomyces sviceus with unusual properties discovered by genome mining. PLoS ONE 2012, 7, e52360. [Google Scholar] [CrossRef]
- Molina-Guijarro, J.M.; Perez, J.; Munoz-Dorado, J.; Guillen, F.; Moya, R.; Hernandez, M.; Arias, M.E. Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2009, 12, 13–21. [Google Scholar] [CrossRef]
- Sharma, K.K.; Kuhad, R.C. Laccase: Enzyme revisited and function redefined. Indian J. Microbiol. 2008, 48, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.O.; Durao, P.; Brissos, V.; Lindley, P.F. Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cell Mol. Life Sci. 2015, 72, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goel, R.; Capalash, N. Bacterial laccases. World J. Microb. Biot. 2007, 23, 823–832. [Google Scholar] [CrossRef]
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from prokaryotes: A new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases - occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Brown, M.E.; Chang, M.C.Y. Exploring bacterial lignin degradation. Curr. Opin. Chem. Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Faure, D.; Bouillant, M.L.; Jacoud, C.; Bally, R. Phenolic derivatives related to lignin metabolism as substrates for Azospirillum laccase activity. Phytochemistry 1996, 42, 357–359. [Google Scholar] [CrossRef]
- Endo, K.; Hosono, K.; Beppu, T.; Ueda, K. A novel extracytoplasmic phenol oxidase of Streptomyces: Its possible involvement in the onset of morphogenesis. Microbiology 2002, 148, 1767–1776. [Google Scholar] [CrossRef]
- Arias, M.E.; Arenas, M.; Rodriguez, J.; Soliveri, J.; Ball, A.S.; Hernandez, M. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl. Environ. Microbiol. 2003, 69, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.C.; Nayar, P.G.; Begley, T.P.; Villafranca, J.J. Stoichiometry and spectroscopic identity of copper centers in phenoxazinone synthase: A new addition to the blue copper oxidase family. Biochemistry 1993, 32, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Fathi-Roudsari, M.; Mollania, N.; Badoei-Dalfard, A.; Khajeh, K. Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: Purification and biochemical characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.K.; Aronson, A.I. Properties of the Bacillus subtilis spore coat. J. Bacteriol. 1979, 137, 1208–1218. [Google Scholar] [CrossRef]
- Francis, C.A.; Tebo, B.M. cumA multicopper oxidase genes from diverse Mn(II)-oxidizing and non-Mn(II)-oxidizing Pseudomonas strains. Appl. Environ. Microbiol. 2001, 67, 4272–4278. [Google Scholar] [CrossRef]
- Kim, C.; Lorenz, W.W.; Hoopes, J.T.; Dean, J.F.D. Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK Gene. J. Bacteriol. 2001, 183, 4866–4875. [Google Scholar] [CrossRef]
- Roberts, S.A.; Weichsel, A.; Grass, G.; Thakali, K.; Hazzard, J.T.; Tollin, G.; Rensing, C.; Montfort, W.R. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl Acad. Sci. USA 2002, 99, 2766–2771. [Google Scholar] [CrossRef]
- Mellano, M.A.; Cooksey, D.A. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J. Bacteriol. 1988, 170, 2879–2883. [Google Scholar] [CrossRef]
- Ansari, M.K.A.; Khatib, U.M.; Owens, G.; Fatma, T. Evaluation of methyl red tolerant cyanobacteria for simultaneous laccase production and dye decolorization. Int. J. Waste Resour. 2016, 6, 2252–5211. [Google Scholar] [CrossRef]
- Afreen, S.; Anwer, R.; Singh, R.K.; Fatma, T. Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J. Biol. Sci. 2018, 25, 1446–1453. [Google Scholar] [CrossRef]
- Otto, B.; Schlosser, D. First laccase in green algae: Purification and characterization of an extracellular phenol oxidase from Tetracystis aeria. Planta 2014, 240, 1225–1236. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Jia, W.; Chang, S.; Li, S.; Li, Y. Lignin engineering through laccase modification: A promising field for energy plant improvement. Biotechnol. Biofuels 2015, 8, 145. [Google Scholar] [CrossRef]
- Harvey, B.M. Laccases in Higher Plants. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 1997. [Google Scholar]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Bligny, R.; Douce, R. Excretion of laccase by sycamore (Acer pseudoplatanus L) cells.1. purification and properties of the enzyme. Biochem. J. 1983, 209, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Bao, W.L.; Sederoff, R.; Whetten, R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda). J. Plant Res. 2001, 114, 147–155. [Google Scholar] [CrossRef]
- Ranocha, P.; McDougall, G.; Hawkins, S.; Sterjiades, R.; Borderies, G.; Stewart, D.; Cabanes-Macheteau, M.; Boudet, A.M.; Goffner, D. Biochemical characterization, molecular cloning and expression of laccases - a divergent gene family - in poplar. Eur. J. Biochem. 1999, 259, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; McDougall, G.J. A laccase-type polyphenol oxidase from lignifying xylem of tobacco. Phytochemistry 1997, 44, 229–235. [Google Scholar] [CrossRef]
- Wosilait, W.D.; Nason, A.; Terrell, A.J. Pyridine nucleotide-quinone reductase.2. role in electron transport. J. Biol. Chem. 1954, 206, 271–282. [Google Scholar]
- McCaig, B.C.; Meagher, R.B.; Dean, J.F. Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana. Planta 2005, 221, 619–636. [Google Scholar] [CrossRef]
- Berthet, S.; Thevenin, J.; Baratiny, D.; Demont-Caulet, N.; Debeaujon, I.; Bidzinski, P.; Leple, J.C.; Huis, R.; Hawkins, S.; Gomez, L.D.; et al. Role of plant laccases in lignin polymerization. Adv. Bot. Res. 2012, 61, 145. [Google Scholar] [CrossRef]
- Polak, J.; Jarosz-Wilkołazka, A. Reakcje katalizowane przez lakazę–mechanizm i zastosowanie w biotechnologii. Biotechnologia 2007, 4, 82–94. [Google Scholar]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Hoopes, J.T.; Dean, J.F. Ferroxidase activity in a laccase-like multicopper oxidase from Liriodendron tulipifera. Plant. Physiol. Biochem. 2004, 42, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Purification and physico-chemical properties of laccase. Biochim. Biophys. Acta. 1958, 30, 44–52. [Google Scholar] [CrossRef]
- Sterjiades, R.; Dean, J.F.; Eriksson, K.E. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 1992, 99, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Hoegger, P.J.; Kilaru, S.; James, T.Y.; Thacker, J.R.; Kues, U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006, 273, 2308–2326. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.T.; Speranza, M.; Ruiz-Duenas, F.J.; Ferreira, P.; Camarero, S.; Guillen, F.; Martinez, M.J.; Gutierrez, A.; del Rio, J.C. Biodegradation of lignocellulosics: Microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Ruiz-Duenas, F.J.; Martinez, A.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A useful group of oxidoreductive enzymes. Bioremediat. J. 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Voriskova, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Barlocher, F.; Boddy, L. Aquatic fungal ecology - How does it differ from terrestrial? Fungal Ecol. 2016, 19, 5–13. [Google Scholar] [CrossRef]
- Blackwood, C.B.; Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L. Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ. Microbiol. 2007, 9, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Sole, M.; Kellner, H.; Brock, S.; Buscot, F.; Schlosser, D. Extracellular laccase activity and transcript levels of putative laccase genes during removal of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica. FEMS Microbiol. Lett. 2008, 288, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Corvini, P.F.; Vinken, R.; Junghanns, C.; Krauss, G.; Schlosser, D. Quantification of the influence of extracellular laccase and intracellular reactions on the isomer-specific biotransformation of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica. Appl. Environ. Microbiol. 2009, 75, 4398–4409. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raheem, A.M. Laccase activity of lignicolous aquatic hyphomycetes isolated from the River Nile in Egypt. Mycopathologia 1997, 139, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, C.; Parra, R.; Keshavarz, T.; Schlosser, D. Towards higher laccase activities produced by aquatic ascomycetous fungi through combination of elicitors and an alternative substrate. Eng. Life Sci. 2008, 8, 277–285. [Google Scholar] [CrossRef]
- Shevchenko, S.M.; Bailey, G.W. Life after death: Lignin-humic relationships reexamined. Crit. Rev. Environ. Sci. Technol. 1996, 26, 95–153. [Google Scholar] [CrossRef]
- Hofrichter, M.; Fritsche, W. Depolymerization of low rank coal by extracellular fungal enzyme systems.1. Screening for low rank-coal-depolymerizing activities. Appl. Microbiol. Biotechnol. 1996, 46, 220–225. [Google Scholar] [CrossRef]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and transformation of humic substances by saprotrophic fungi: Processes and mechanisms. Fungal Biol. Rev. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Feng, S.Z.; Su, Y.R.; Dong, M.Z.; He, X.Y.; Kumaresan, D.; O’Donnell, A.; Wu, J.S.; Chen, X.B. Laccase activity is proportional to the abundance of bacterial laccase-like genes in soil from subtropical arable land. World J. Microbiol. Biotechnol. 2015, 31, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Polak, J. Structure/Redox potential relationship of simple organic compounds as potential precursors of dyes for laccase-mediated transformation. Biotechnol. Progr. 2012, 28, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Savoie, J.M.; Mata, G. Antagonistic action of Trichoderma sp. hyphae to Lentinula edodes hyphae changes lignocellulolytic activities during cultivation in wheat straw. World J. Microb. Biotechnol. 1999, 15, 369–373. [Google Scholar] [CrossRef]
- Velazquez-Cedeno, M.; Farnet, A.M.; Billette, C.; Mata, G.; Savoie, J.M. Interspecific interactions with Trichoderma longibrachiatum induce Pleurotus ostreatus defence reactions based on the production of laccase isozymes. Biotechnol. Lett. 2007, 29, 1583–1590. [Google Scholar] [CrossRef]
- Flores, C.; Vidal, C.; Trejo-Hernandez, M.R.; Galindo, E.; Serrano-Carre, L. Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J. Appl. Microbiol. 2009, 106, 249–257. [Google Scholar] [CrossRef]
- Sjaarda, C.P.; Abubaker, K.S.; Castle, A.J. Induction of lcc2 expression and activity by Agaricus bisporus provides defence against Trichoderma aggressivum toxic extracts. Microb. Biotechnol. 2015, 8, 918–929. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Sadasivan, C. Trichoderma viride laccase plays a crucial role in defense mechanism against antagonistic organisms. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Schouten, A.; Maksimova, O.; Cuesta-Arenas, Y.; van den Berg, G.; Raaijmakers, J.M. Involvement of the ABC transporter BcAtrB and the laccase BcLCC2 in defence of Botrytis cinerea against the broad-spectrum antibiotic 2,4-diacetylphloroglucinol. Environ. Microbiol. 2008, 10, 1145–1157. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Dean, J.F.D.; Eriksson, K.E.L. Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett. 1995, 376, 202–206. [Google Scholar] [CrossRef]
- Eggert, C. Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol. Res. 1997, 152, 315–318. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.; Niang, R.; Casadevall, A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001, 107, E66. [Google Scholar] [CrossRef]
- Erickson, T.; Liu, L.; Gueyikian, A.; Zhu, X.; Gibbons, J.; Williamson, P.R. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol. Microbiol. 2001, 42, 1121–1131. [Google Scholar] [CrossRef]
- Jacobson, E.S.; Emery, H.S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J. Bacteriol. 1991, 173, 401–403. [Google Scholar] [CrossRef]
- Polacheck, I.; Hearing, V.J.; Kwon-Chung, K.J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J. Bacteriol. 1982, 150, 1212–1220. [Google Scholar] [CrossRef]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef]
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J. Bacteriol. 1994, 176, 656–664. [Google Scholar] [CrossRef]
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572. [Google Scholar] [CrossRef]
- Lee, S.C.; Dickson, D.W.; Casadevall, A. Pathology of cryptococcal meningoencephalitis: Analysis of 27 patients with pathogenetic implications. Hum. Pathol. 1996, 27, 839–847. [Google Scholar] [CrossRef]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Gibbons, J.; Garcia-Rivera, J.; Casadevall, A.; Williamson, P.R. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect. Immun. 2001, 69, 5589–5596. [Google Scholar] [CrossRef] [PubMed]
- Sapmak, A.; Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Andrianopoulos, A.; Pruksaphon, K.; Youngchim, S. Talaromyces marneffei laccase modifies THP-1 macrophage responses. Virulence 2016, 7, 702–717. [Google Scholar] [CrossRef]
- Sapmak, A.; Boyce, K.J.; Andrianopoulos, A.; Vanittanakom, N. The pbrB gene encodes a laccase required for DHN-melanin synthesis in conidia of Talaromyces (Penicillium) marneffei. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Williamson, P.R. Role of laccase in the virulence of Talaromyces marneffei: A common link between AIDS-related fungal pathogens? Virulence 2016, 7, 627–629. [Google Scholar] [CrossRef]
- Moreno, L.F.; Feng, P.; Weiss, V.A.; Vicente, V.A.; Stielow, J.B.; de Hoog, S. Phylogenomic analyses reveal the diversity of laccase-coding genes in Fonsecaea genomes. PLoS ONE 2017, 12, e0171291. [Google Scholar] [CrossRef]
- Wei, Y.X.; Pu, J.J.; Zhang, H.; Liu, Y.N.; Zhou, F.X.; Zhang, K.L.; Liu, X.M. The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol. Mol. Plant Pathol. 2017, 99, 55–64. [Google Scholar] [CrossRef]
- Kuo, H.C.; Detry, N.; Choi, J.; Lee, Y.H. Potential roles of laccases on virulence of Heterobasidion annosum s.s. Microb. Pathog. 2015, 81, 16–21. [Google Scholar] [CrossRef]
- Holker, U.; Dohse, J.; Hofer, M. Extracellular laccases in ascomycetes Trichoderma atroviride and Trichoderma harzianum. Folia Microbiol. 2002, 47, 423–427. [Google Scholar] [CrossRef]
- Xu, F. Laccase. In Encyclopedia of Bioprocess Technology; Flickinger, M.C., Drew, S.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Arimoto, M.; Yamagishi, K.; Wang, J.; Tanaka, K.; Miyoshi, T.; Kamei, I.; Kondo, R.; Mori, T.; Kawagishi, H.; Hirai, H. Molecular breeding of lignin-degrading brown-rot fungus Gloeophyllum trabeum by homologous expression of laccase gene. AMB Express 2015, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, N.T.; Suderman, R.J.; Jiang, H.; Zhu, Y.C.; Gorman, M.J.; Kramer, K.J.; Kanost, M.R. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. 2004, 34, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.L.; Shen, W.F.; Liu, Y.; Weng, H.B.; He, L.H.; Mu, J.J.; Wu, Z.L.; Jiang, P.; Tao, Y.Z.; Meng, Z.Q. Cloning and RNAi-mediated functional characterization of MaLac2 of the pine sawyer, Monochamus alternatus. Insect Mol. Biol. 2008, 17, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Barrett, F.M. Phenoloxidases from larval cuticle of the sheep blowfly, Lucilia cuprina - characterization, developmental-changes, and Inhibition by antiphenoloxidase antibodies. Arch. Insect Biochem. Physiol. 1987, 5, 99–118. [Google Scholar] [CrossRef]
- Yamazaki, H.I. Cuticular phenoloxidase in Drosophila virilis. J. Insect Physiol. 1969, 15, 2203–2211. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Korzhev, M.; Schroder, H.C.; Link, T.; Tahir, M.N.; Diehl-Seifert, B.; Muller, W.E. Potential biological role of laccase from the sponge Suberites domuncula as an antibacterial defense component. Biochim. Biophys. Acta 2015, 1850, 118–128. [Google Scholar] [CrossRef]
- Tartar, A.; Wheeler, M.M.; Zhou, X.; Coy, M.R.; Boucias, D.G.; Scharf, M.E. Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes. Biotechnol. Biofuels 2009, 2, 25. [Google Scholar] [CrossRef]
- Hongoh, Y. Toward the functional analysis of uncultivable, symbiotic microorganisms in the termite gut. Cell Mol. Life Sci. 2011, 68, 1311–1325. [Google Scholar] [CrossRef]
- Thomas, B.R.; Yonekura, M.; Morgan, T.D.; Czapla, T.H.; Hopkins, T.L.; Kramer, K.J. A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989, 19, 611. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Gorman, M.J.; Kanost, M.R. Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2009, 39, 596–606. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticular sclerotization in insects. Comp. Biochem. Phys. B 1996, 113, 689–705. [Google Scholar] [CrossRef]
- Hattori, M.; Tsuchihara, K.; Noda, H.; Konishi, H.; Tamura, Y.; Shinoda, T.; Nakamura, M.; Hasegawa, T. Molecular characterization and expression of laccase genes in the salivary glands of the green rice leafhopper, Nephotettix cincticeps (Hemiptera: Cicadellidae). Insect Biochem. Mol. Biol. 2010, 40, 331–338. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Spellman, P.T.; Rubin, G.M.; Lemaitre, B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA 2001, 98, 12590–12595. [Google Scholar] [CrossRef]
- Yatsu, J.; Asano, T. Cuticle laccase of the silkworm, Bombyx mori: Purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem. Mol. Biol. 2009, 39, 254–262. [Google Scholar] [CrossRef]
- Hattori, M.; Konishi, H.; Tamura, Y.; Konno, K.; Sogawa, K. Laccase-type phenoloxidase in salivary glands and watery saliva of the green rice leafhopper, Nephotettix cincticeps. J. Insect Physiol. 2005, 51, 1359–1365. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. https://doi.org/10.3390/ijms21030966
Janusz G, Pawlik A, Świderska-Burek U, Polak J, Sulej J, Jarosz-Wilkołazka A, Paszczyński A. Laccase Properties, Physiological Functions, and Evolution. International Journal of Molecular Sciences. 2020; 21(3):966. https://doi.org/10.3390/ijms21030966
Chicago/Turabian StyleJanusz, Grzegorz, Anna Pawlik, Urszula Świderska-Burek, Jolanta Polak, Justyna Sulej, Anna Jarosz-Wilkołazka, and Andrzej Paszczyński. 2020. "Laccase Properties, Physiological Functions, and Evolution" International Journal of Molecular Sciences 21, no. 3: 966. https://doi.org/10.3390/ijms21030966
APA StyleJanusz, G., Pawlik, A., Świderska-Burek, U., Polak, J., Sulej, J., Jarosz-Wilkołazka, A., & Paszczyński, A. (2020). Laccase Properties, Physiological Functions, and Evolution. International Journal of Molecular Sciences, 21(3), 966. https://doi.org/10.3390/ijms21030966







