Abstract
Coronary artery disease (CAD), comprising both acute coronary syndromes (ACS) and chronic coronary syndromes (CCS), remains one of the most important killers throughout the entire world. ACS is often quickly diagnosed by either deviation on an electrocardiogram or elevated levels of troponin, but CCS appears to be more complicated. The most used noninvasive strategies to diagnose CCS are coronary computed tomography and perfusion imaging. Although both show reasonable accuracy (80–90%), these modalities are becoming more and more subject of debate due to costs, radiation and increasing inappropriate use in low-risk patients. A reliable, blood-based biomarker is not available for CCS but would be of great clinical importance. Extracellular vesicles (EVs) are lipid-bilayer membrane vesicles containing bioactive contents e.g., proteins, lipids and nucleic acids. EVs are often referred to as the “liquid biopsy” since their contents reflect changes in the condition of the cell they originate from. Although EVs are studied extensively for their role as biomarkers in the cardiovascular field during the last decade, they are still not incorporated into clinical practice in this field. This review provides an overview on EV biomarkers in CCS and discusses the clinical and technological aspects important for successful clinical application of EVs.
1. Introduction
Coronary artery disease (CAD) remains one of the most important killers among the entire world, despite tremendous improvements in diagnostic and therapeutic strategies [1]. CAD comprises acute coronary syndromes (ACS) and chronic coronary syndromes (CCS, e.g., stable angina). The underlying pathophysiology that causes CAD is known as atherosclerosis [1]. This is a longstanding, continuous process of accumulation and progression of plaque material within the vessel wall [2]. Atherosclerotic plaques are often stable for long periods and can eventually cause a diminished oxygen supply to the heart muscle during exertion. This causes ischemia and subsequent chest pain [3]. The resulting clinical syndrome is known as CCS, for which medical or interventional therapies are generally required. Plaque rupture or plaque erosion initiates an acute thrombotic luminal occlusion that can cause acute blockage of one the coronary vessels, resulting in ACS and, subsequently, myocardial infarction [4]. ACS requires immediate revascularization of the affected vessel.
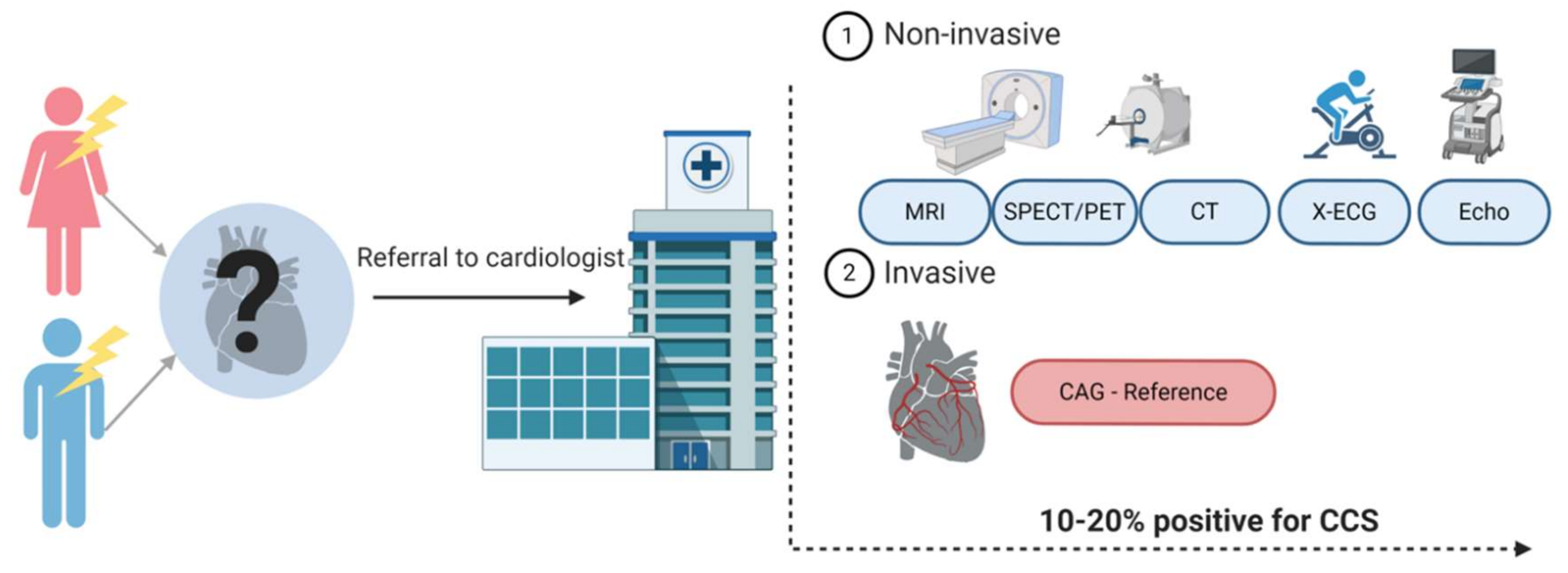
ACS is often quickly diagnosed with either an abnormal electrocardiogram (ECG) or elevated cardiac biomarkers, such as high-sensitive cardiac troponin (hs-cTn), indicating cell damage of the myocardium. Diagnosing CCS appears to be more complicated. Figure 1 provides an overview of the diagnostic workflow of a patient presenting with chest pain at the general practitioner and the diagnostic possibilities once referred to the cardiologist. The reference standard for CCS is still coronary angiography (CAG), but considering its invasive character, this is used with caution [1,5]. Currently, the most used noninvasive strategies are coronary computed tomography (CT) or myocardial perfusion imaging (MPI). The diagnostic accuracy of these different imaging modalities is relatively high (80–90%), but only 10–20% of symptomatic patients turn out to have CCS [6]. The low number of patients suffering from the actual disease are the result of an increasing use of these test modalities in the low-risk population [7,8,9]. They are becoming more and more subject of debate because of unnecessary radiation exposure for the patient and high costs. A reliable, blood-based biomarker would therefore be important to improve the diagnostic strategy around patients suspected for CCS. Until now, no such biomarker exists.
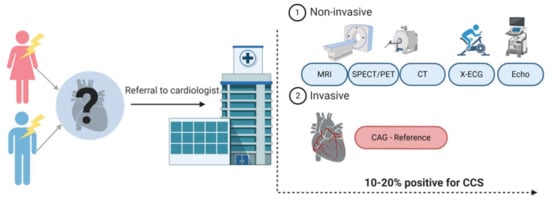
Figure 1.
Diagnostic track of patients with chest pain suspected for a chronic coronary syndrome (CCS). Patients suspected of CCS are often referred to a cardiologist. Patients undergo either noninvasive or invasive tests. The choice for one of the tests is based on the pre-test probability of a patient having CCS and availability in the hospital. Created with BioRender.com.
Since the early 1960s, there is a growing interest for extracellular vesicles (EVs) as potential biomarker sources [10]. EVs are lipid bilayer membrane vesicles containing bioactive contents (e.g., proteins, lipids and nucleic acids) [11]. Almost all cells are able to produce EVs, with their contents changing when the cell of origin changes due to (patho)physiology [12,13]. Due to this, EVs are often referred to as the “liquid biopsy”. The ability to study their (variable) contents makes them an interesting source for future biomarkers.
In this review, we first provide an overview of the performance of existing plasma biomarkers in CCS. Second, we review the existing evidence with regard to the additional value that EV biomarkers might have in diagnosing CCS. Last, despite an increasing number of publications regarding EVs as biomarker, the use of EVs in the cardiovascular field is not yet fully established. The use of EVs were recently incorporated into clinical practice in the cancer field of medicine [14,15,16]. We highlight several clinical aspects that need to be addressed in future studies to accelerate successful clinical implementation of EVs in the cardiovascular field.
2. Current Diagnostic Plasma Biomarkers in CCS
The use of biomarkers to detect CCS are studied extensively. Multiple promising markers were identified using a proteomics or metabolomics approach. However, new markers often fail when applied to an external and/or different population [17,18,19]. The focus of this review is on proteins and their function as biomarker, however, RNA, DNA or other cell particles in theory could also function as biomarkers.
2.1. Single Plasma Biomarker Approach
After the successful implementation of hs-cTn to diagnose ACS, identification of biomarkers with a similar accuracy for other coronary pathologies, such as CCS, received a lot attention. Many different markers for CCS were proposed, with the best known being natriuretic peptides, hs-cTn and C-reactive protein (CRP).
2.1.1. Natriuretic Peptides
Natriuretic peptides, both B-type (BNP), and the N-terminal of the prohormone (NT-proBNP) are secreted as result of myocardial stretch [15]. Two studies investigated the diagnostic potential of natriuretic peptides in patients with stable angina who underwent CAG [20,21]. Weber et al. found NT-proBNP as an independent predictor for obstructive CCS in a small cohort study of 94 patients. They found an area under the curve (AUC) of 0.72 at a cutoff level of 214 pg/mL [20]. Additionally, a larger, comparable study performed in 781 patients found the same association but different cutoff points for men (85 pg/mL), with an AUC of 0.72, and women (165 pg/mL), with an AUC of 0.71 [21]. Both studies excluded patients with known heart failure or left ventricular ejection fraction of <60%. A meta-analysis performed in 2009 included 14 studies with a total of 2784 participants. They found a pooled sensitivity for the detection of stress-induced myocardial ischemia with 71% (NT-pro)BNP, however the pooled specificity was only 52% [22]. The performance of (NT-pro)BNP was consistent throughout different studies but remains limited compared to clinical models [23,24,25,26,27]. Jensen et al. showed an overview of five commonly used clinical risk scores and their performances in a large cohort of 5414 patients [28]. The AUCs of all clinical models varied between 0.68–0.72. Since most BNP studies also showed AUCs of ~0.70, the limited value of BNP on top of clinical models is not surprising. This was also seen when the performance of BNP (AUC 0.66) was compared with a clinical judgement score (AUC 0.66) [29].
2.1.2. High-Sensitive Cardiac Troponin
Hs-cTn is well known for its role in diagnosing ACS, and, since it is a marker of cell damage caused by myocardial ischemia, it might also be helpful in diagnosing CCS. Higher levels of hs-cTn in patients without ACS were observed in patients that were older, had high systolic blood pressure, an increased left ventricular mass and/or renal impairment [30]. Hs-cTn was shown to be associated with the severity of CAD on CAG [31,32]. Moreover, a modest increase in AUC (0.79 to 0.80) to detect CCS in addition to a clinical judgement score was found [33]. However, this finding was not replicated in other large cohorts [29]. Tanglay et al. investigated the incremental value of a single hs-cTn measurement to rule out stress-induced myocardial ischemia and found an AUC to detect stress-induced ischemia with hs-cTn of 0.70 compared to an AUC of 0.69 from their clinical judgement model (p value = not significant) [34].
2.1.3. C-Reactive Protein
CRP is an inflammatory marker, but also an acute-phase protein, and considered to be a nonspecific marker of inflammation [35]. Among all inflammatory biomarkers studied in CAD, CRP requires the most attention; unfortunately, the value of CRP to diagnose CCS appears to be limited [19,36]. The association between CRP and the extend of CAD was studied in a large cohort (>2500 participants) referred for CAG because of typical chest pain [37]. Only very modest correlation coefficients between CRP and CAD severity were found (r: 0.02–0.08). Another study investigating the diagnostic potential of CRP failed to show a statistically significant association between plasma CRP levels and obstructive CAD [38]. Large Mendelian randomization studies analyzing polymorphisms of the CRP gene also did not provide evidence of a causal relationship between CRP and CAD [39,40,41].
2.2. Multimarker Approach
After it was recognized that a single biomarker approach might not be able to improve the accuracy of clinical models to detect CCS, multimarker models were introduced. The idea behind a multimarker approach is the ability to combine different markers, all representing different pathophysiological pathways, thereby providing complementary information. Studies investigating a multimarker approach in diagnosing CCS are limited. One study investigated a dual-biomarker strategy to detect CCS [29], comparing the diagnostic accuracy of a clinical judgement score with BNP and hs-cTn. The addition of hs-cTn to the clinical judgement score significantly improved the diagnostic accuracy (AUC: 0.68 to 0.75), however, a dual marker strategy did not further improve the diagnostic accuracy.
Although multimarker models are studied in more detail regarding the prognosis of CAD patients, until now, the incremental value of multimarker models in future risk stratification was disappointing. Wang et al. studied 3532 patients from the Framingham Offspring Study and found that a high multimarker score was independently associated with both the outcome death as well as major adverse cardiovascular events (MACE) [42]. The multimarker score for death comprised CRP, NT-proBNP, homocysteine, plasma renin and urine albumin-to-creatinine ration. For MACE, two markers were selected: NT-proBNP and urine albumin-to-creatinine ratio. However, no significant differences in C-statistics were found when comparing a model with clinical predictors (death: 0.80, MACE: 0.76) with a multimarker model (death: 0.82, MACE: 0.77). Another study among >5000 patients without known cardiovascular disease (CVD) analyzed the predictive ability of both single and multimarker models on top of clinical predictors [43]. They analyzed two outcomes, namely, coronary events (selected markers were MR-proADM and NT-proBNP) and MACE (selected markers were CRP and NT-proBNP). Only a very modest increase in C-statistic (0.007 for MACE and 0.009 for coronary events) was found when using a multimarker model compared to a model with clinical predictors. Also, no significant reclassification of patients into higher or lower risk categories was found. Comparable results were found in studies with patients with manifest CVD [44,45]. Nevertheless, a multimarker approach could be the solution for a future CCS marker, but perhaps from another, relatively unexplored source, such as EVs.
3. EV Origin
Extracellular vesicles are characterized by a bilayer lipid membrane layer [11]. EVs were reported for the first time in 1946 by Chargaff and West [46], however, they were first recognized by Wolf in 1967 [10]. He observed EVs at that time as “platelet dust”. Following his endeavor, a lot of knowledge on EVs emerged. Almost all different cell types are able to produce and release EVs. EVs are found systemically and in basically all body fluids, including blood, urine, cerebrospinal fluid, milk, tears and saliva [47,48,49,50,51,52,53,54]. Characterization and classification of subpopulations have been subject of debate for the last years, with a consensus still not reached [55,56]. As a common feature, all subpopulations of EVs contain bioactive contents (lipids, proteins and nucleic acids). EV contents originate from the parent cell they are released from [57,58]. Once released into the extracellular space, parts of them can be identified to serve as cell-cell communicators.
EV Subpopulations
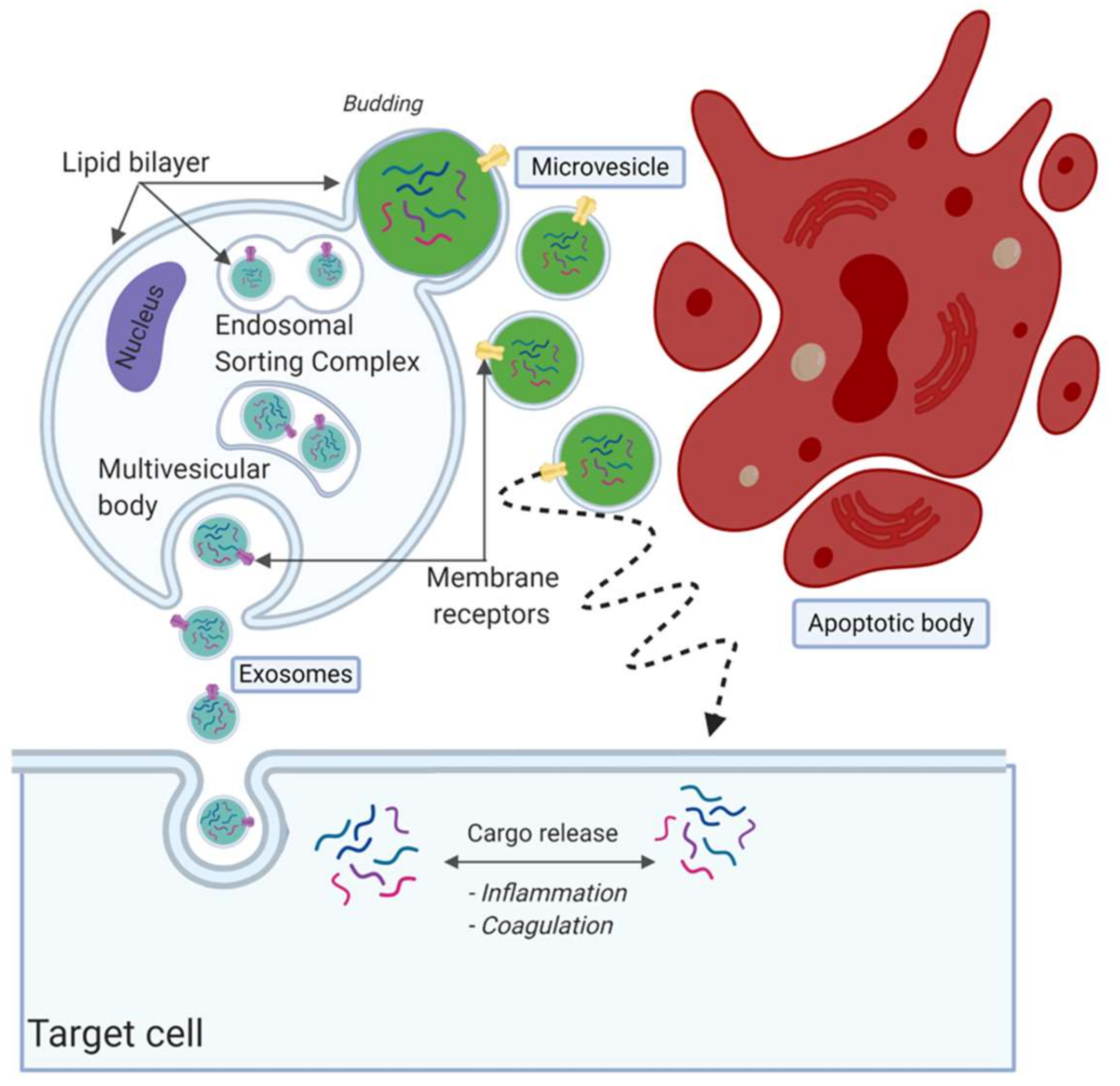
Although it is an ongoing debate regarding how to classify the EV subpopulations, EVs are often divided in three subtypes based on their size and formation route, namely, apoptotic bodies, microvesicles and exosomes [59,60,61] (Figure 2). There is no consensus on specific identifying protein markers to distinguish between the three subpopulations [62,63,64]. Exosomes are considered as the smallest particles in the EV family, with a size of 30–150 nm [65]. The release and formation of exosomes is via the endosomal sorting complex release transport (ESCRT) pathway [66]. They are formed as intraluminal vesicles and mature into multivesicular bodies (MVBs) [57]. MVBs fuse with the outer plasma membrane to be released within the extracellular space [67]. It was suggested that multiple subpopulations of exosomes exist, potentially providing additional information on their origin and role [65]. Microvesicles are EVs that form by outward budding, sometimes called blubbing, of the cell membrane. Their size is approximately between 100 nm and 1000 nm [59,60,61,68,69]. The last subpopulation of EVs are the apoptotic bodies, which are released after cell death. They are >1000 nm in size and relatively large compared to exosomes and microvesicles.
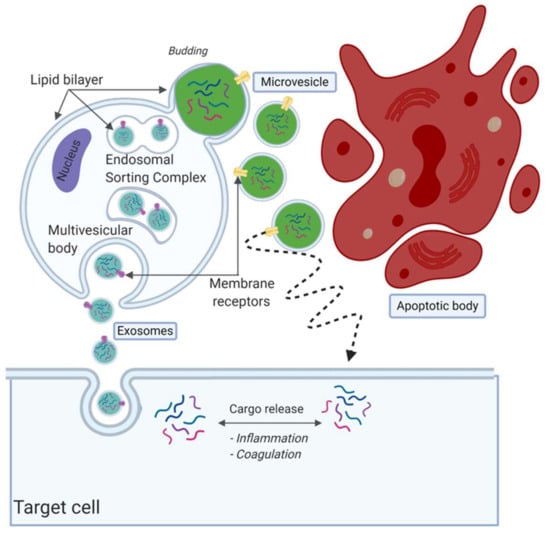
Figure 2.
Overview of extracellular vesicle (EV) subpopulations and formation routes. EVs are often divided into three subpopulations, namely, exosomes, microvesicles and apoptotic bodies. Exosomes are considered the smallest population, released by fusion with the plasma membrane. Microvesicles are secreted by blubbing, as can be seen in green. Lastly, apoptotic bodies are fragments released from cells during apoptosis, considered to be the largest in size. Created with BioRender.com.
4. EVs as Diagnostic Biomarkers in Atherosclerosis
Atherosclerosis is considered to be the underlying syndrome of cardiovascular disease. EVs are considered to be key mediators in both the atherosclerotic plaque formation and its progression. EVs are thought to be involved in inflammation and thrombus formation and are therefore thought to carry useful information to serve as biomarkers [70,71]. Clinical risk factors associated with CAD are diabetes mellitus, hypertension, metabolic syndrome, hypercholesterolemia and smoking [72,73]. Several studies showed higher levels of circulation EVs in plasma to be associated with some of these risk factors [74,75], including diabetes [76,77], hypertension [78,79], hypercholesterolemia [80,81] and smoking [82,83,84]. Moreover, associations between EVs and subclinical atherosclerosis (diagnosed with ultrasound of the femoral artery, carotid artery or abdominal aorta) were also found [85,86]. Within CVD, EVs are the most studied as prognostic markers in CAD [87,88,89]. However, less is known about their diagnostic potential for CCS.
4.1. Extracellular Vesicle Count in CCS
One way to analyze plasma EV levels is to measure the number of circulating EVs, also described as count. There is an increasing number of publications on EVs in the cardiovascular field, however the subject of this review is the presumed role of EVs specifically in CCS. We focus specifically on their diagnostic potential in CCS patients. Table 1 provides a preselected overview of studies that investigated the role of EV counts of different subpopulations based on cellular origin with regard to CCS. Some of the studies are described below in more detail. Chironi et al. showed that the number of circulating leukocyte-derived EVs (LDEVs) were independently associated with subclinical atherosclerosis [85]. A study among 33 postmenopausal women undergoing coronary calcium scoring on coronary CT showed a positive association between the number of circulating EVs and both the Framingham risk score (FRS) as well as coronary calcium scores [86]. The effect of our circadian rhythm on the levels of circulating EVs was studied by a Scandinavian group in 30 patients, of which 10 had CCS and 20 patients were healthy controls [90]. They found a slight variation in total circulating EV count and the circadian rhythm, but no effect was seen for platelet-derived EVs.

Table 1.
Overview of preselected publications on extracellular vesicle count in chronic coronary syndrome patients, including details on subpopulations.
CCS is characterized by stress-induced ischemia. Augustine et al. showed an increase in circulating EVs after dobutamine stress echocardiography, except for the patients with signs suggestive for stress-induced ischemia [91]. Sinning et al. [92] found a similar result of diminished EV release after stress-test imaging in patients with significant CCS, emphasizing a dynamic process of EV release. Several studies showed differences in the number of circulating (subpopulations of) EVs between patients with CCS and healthy controls, but also between patients with CCS and ACS [93,94,95,96,97,98]. These were however, mainly studies in small cohorts and were often cross-sectional. Mirachi et al. compared levels of two species of endothelial-derived EVs (EDEVs) (CD31+ and CD51+) between 84 patients with CAD (64 ACS and 20 CCS) and 42 healthy controls [93]. Levels of CD31+ EDEVs differed significantly between the ACS, CCS and controls, whereas CD51+ EDEVs only differed between CAD versus control, however no differences between ACS and CCS were observed. Additionally, this study also investigated levels of platelet-derived EVs (PDEVs), showing only elevated levels in patients with ACS. No differences were seen between CCS and ACS or CCS and controls [93]. Another study performed by Biasucci et al. compared levels of EDEVs, PDEVs and circulating EVs (cEVs) in 76 patients [97]. In this study population, 33 patients were diagnosed with CCS and 43 with ACS. All EV subpopulations were found in significantly higher levels in patients with ACS compared to patients with SA. They also investigated whether the levels changed over time, which was seen only for the total amount of circulating EVs [97]. There are contradicting results regarding circulating EV levels and the degree of luminal stenosis. Werner et al. showed a significant (adjusted) correlation between levels of circulating EVs and luminal stenosis [99], whereas two other studies did not [98,100]. Only a few studies investigated the diagnostic or prognostic properties of the number of circulating EVs in CCS patients. The largest study was performed by Nozaki et al. [88], showing in 378 CCS patients that endothelial-derived EVs were an independent predictor for MACE (hazard ratio (HR): 1.35 95%; confidence interval (CI): 1.09–1.65). Their prognostic model had an AUC of 0.73 and included the FRS as a clinical prediction rule and plasma biomarkers (CRP and BNP). After addition of the total count of endothelial-derived EVs, this increased to an AUC of 0.76. These findings were in line with other comparable studies showing the same results [87,89,101].
A different way to analyze subpopulations of EVs is to divide them based on density. This concept of EV separation was derived from a study showing a reduced amount of EVs in patients with familial hypercholesterolemia who underwent LDL apheresis [102]. EV subpopulations are still relatively unexplored and need to be studied in more detail to provide answers on the biological and pathophysiological functions [103]. They could, however, reflect different origins and a better signal-to-noise ratio, thereby providing additional information.
4.2. Extracellular Vesicle Content
When the (patho)physiological circumstances of a cell change, not only the number of EVs secreted by this cell changes, but also their content [11,104]. Compared with the count, data on the role of EV content in CCS is limited. Both EV nucleotides as well as protein content were described in CVD [104]. RNA quantification relies on the very sensitive and established technology of qPCR [105]. RNA has, however, the disadvantage of rapid degeneration by RNAse, which is present at high levels in blood. Further, mRNA levels often do not correlate with encoded proteins or reflect the ongoing biological process. Protein levels reflect much closer the ongoing process, and quantification is done by using immunoassays that are commonly used in clinical laboratories. Therefore, we think that proteins have the largest potential in diagnosing CCS [11,104].
One of the first studies that looked into the EV proteome was performed by Vélez et al. The main focus of the study was to explore whether they could study the proteome of EVs by comparing 10 ST-elevation myocardial infarction (STEMI) patients with 10 CCS patients [106]. They found 117 differentially regulated proteins between the two groups, indicating a potential source for protein markers. Another study compared EV-protein levels between STEMI patients and healthy controls [107]. Protein differences were analyzed with a proximity extension assay (Olink, CVD-II panel, N = 92) on the EV lysates and plasma. They identified three proteins (chyotripsin C, tyrosine-protein kinase (SRC) and C-C chemokine ligand 17) that showed differences in levels in EVs but not in plasma. Validation in another set of STEMI patients, CCS patients and healthy controls exposed CRS to be significantly associated with the degree of CAD. This finding was not found in plasma, indicating the additional diagnostic value of EVs.
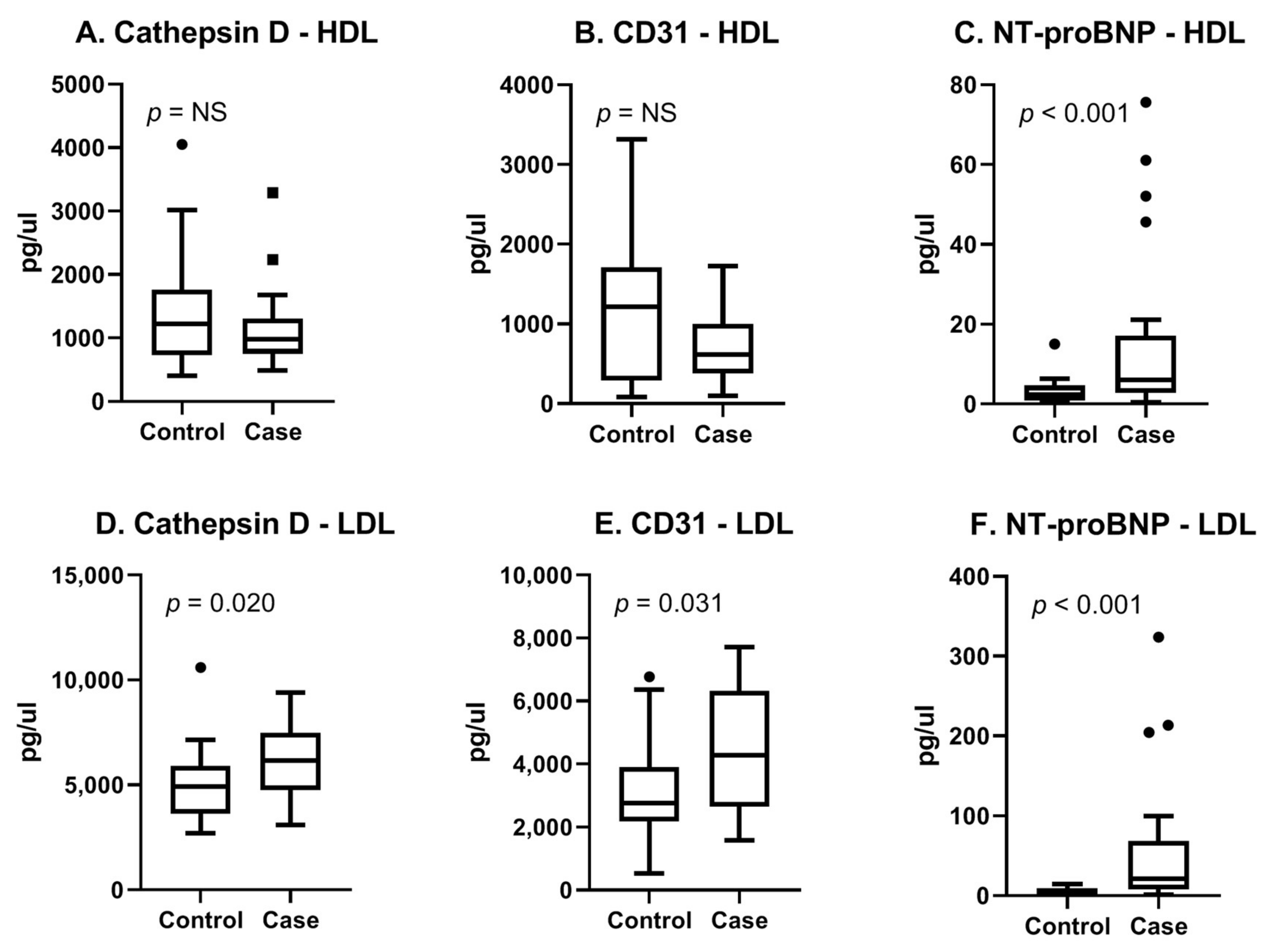
The myomarker study cohort consisted of consecutive patients presenting with stable chest pain at the outpatient clinic of the Meander Medical Centre in the Netherlands. Details on the study design and study population can be found in a previous publication [108]. For this study, a case control analysis of 44 men suspected of CCS was performed. Cases were defined as patients with stress-induced ischemia determined with MPI. Controls were matched based on age and general cardiovascular risk factors (Supplementary Table S1). In this cohort, we performed proteomics on EV subpopulations (rather than a total EV population) based on density since we hypothesized that this would provide a more detailed view of the cell condition. For this, we separated two subpopulations (called the HDL subpopulation and LDL subpopulation, respectively), as described in the study of Wang et al. [109]. We analyzed in the HDL- and LDL subpopulations using both the cardiometabolic panel as well as the cardiovascular III panel (Olink, Proteomics, Uppsala University Sweden). Each panel consisted of 92 proteins known for their associations with CVD. We identified the three most promising proteins (Cathepsin D, CD31 and NT-proBNP) based on literature, their diagnostic properties, and the availability of antibodies (Supplementary Table S2). Using the Meso Scale Discovery (MSD, Rockville, MD, USA) immunoassay, we confirmed our findings. Figure 3A–C shows boxplots of the MSD results for the selected three proteins in the EV-HDL subpopulation. The results for the LDL subpopulation are summarized in Figure 3D–F. It can be appreciated that in the EV-LDL subpopulation, protein levels of Cathepsin D, CD31 and NT-proBNP were significantly higher in cases compared with controls. In the HDL subpopulation, only NT-proBNP-protein levels were found to be significantly different between cases and controls. Our results therefore show the potential of using the Olink technology for the enrichment of EV proteins in EV subpopulations, followed by confirmation in an established immunoassay.
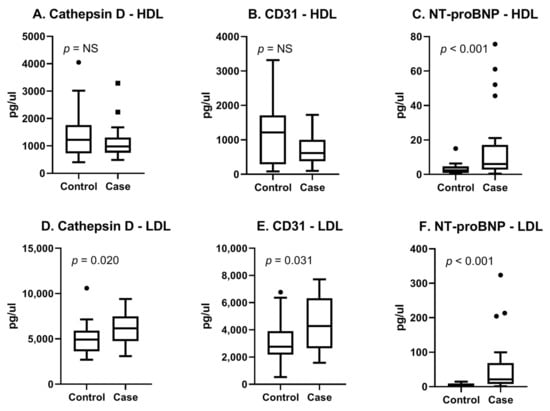
Figure 3.
Boxplots of three selected proteins measured with MSD. Assessment of reproducibility of Olink results with a clinically available immunoassay. (A–C) HDL and (D–F) LDL indicate EV-subpopulations. Cases were 22 male patients with proven CCS and controls were 22 age- and risk-factor-matched patients who were symptomatic without CCS. Original assay units are pg/uL.
For EV-based diagnosis of CCS, not many data exist. A recent study investigated whether a selected group of EV-proteins were associated with CCS [108]. EV-Serpin C1, EV-CD14, EV-Serpin G1, EV-Serpin F2 and EV-Cystatin C (mostly in the HDL-subpopulation) were shown to be independently associated with the presence of stress-induced ischemia. The prognostic value of EV-protein content in a large CVD cohort was for described for the first time by Kanhai et al. [110]. They found EV-Cystatin C, EV-Serpin F2 and EV-CD14 protein levels to be independently associated with future cardiovascular events. A different study found an independent association between the extent of CVD and the levels of EV-CD14 [111]. Several other studies investigated the role of EV content in ACS, heart failure, unstable angina and manifest CVD [112,113,114].
5. Clinical Aspects of CCS Diagnosis Using (EV) Blood Tests
The population suspected of CCS is very heterogenous, ranging from patients presenting with clear symptoms and obstructive CAD to patients with nonspecific chest pain without obstructive CAD and everything in between. The current ongoing search towards a biomarker for more accurate detection of CCS is being developed to apply to all patients suspected for CCS but, considering the heterogeneity in this population, this should raise questions. A study performed by Ouellete et al. found clear differences in the clinical profile of patients with respectively normal, near normal, nonobstructive CAD and obstructive CAD [115]. These differences in clinical profiles between the groups seem obvious but are important in the development of a future biomarker. A more patient-tailored search for a future biomarker focusing on specific subgroups seems reasonable. Moreover, considering the fact that EV content enable us to look at cellular level, one could imagine the EV content of a patient with a known history of CAD is not comparable to a patient with new-onset disease.
An important subject within this heterogeneity that merits consideration is sex. Although sex differences are established and acknowledged regarding clinical symptoms and pathophysiology, the exact underlying mechanisms are barely understood [116]. Evolving knowledge supports the differences in pathophysiology, diagnostic test performance and also prognosis [117,118,119]. Women tend to have less obstructive CAD and more often a preserved ejection fraction, yet higher mortality rates and more extensive myocardial ischemia [118]. Women often present with more complex signs and symptoms. It was suggested that this is due to a more complex and multifactorial pathophysiological process compared to men [119]. A large study investigating biomarkers within CVD showed a difference in protein profile between men and women of almost 85% [120]. Research performed in EVs also showed differences in the associations of both EV count as well as content with clinical outcomes stratified on clinical factors [103,108,112].
These data and hypotheses raise the question whether future studies on biomarkers should focus on predefined subgroups of patients rather than the entire “suspected CCS” group. It emphasizes the need to incorporate clinical aspects associated with CCS into future studies with EVs. From our point of view, the most important clinical aspects that merit attention are sex, age and the cardiovascular status of a patient. This cardiovascular status refers to whether or not a patient is already known with atherosclerotic disease or if a patient previously received (invasive) treatment. Until now, EV studies in CCS did not have enough power to perform reliable subanalyses to reveal different associations within this heterogenous group. It might be possible that we need to develop different biomarkers, or cut-offs, within the entire group of patients suspected of CCS.
6. Future Perspectives
Despite great efforts of the internation society of extracellular vesicles (ISEV) to standardize EV research and improve reproducibility, it remains difficult to compare results between studies [56]. This is mainly because studies still use numerous different techniques for isolation and quantification [121]. There are various protocols for sample preparation, processing and centrifugation, which are known to cause different results [122]. Currently, flow cytometry is the most used method to quantify EVs (see also Table 1). This method is standardized and accepted for the identification and detection of different cell types, however, is most reliable for particles >200 nm [123]. Considering the fact that most EVs are around 100–120 nm in the blood on average, it is questionable whether this is the best method to count circulating EVs. Also, it does not enable measuring EV contents besides proteins stained on the EV membrane.
6.1. Automation
One reason why the use of EVs in clinical practice is hampered is the inability to use high-throughput isolation techniques [122]. Currently, ultracentrifugation is often used to isolate EVs from whole plasma, however, this is time-consuming, labor intensive and requires many manual steps [107]. Before clinical implementation of EVs is considered, large confirmatory trials are needed [104]. Considering the current time effort, costs and the amount of precious clinical blood used for the isolation and quantification of EVs, this is a disillusion. Future studies should therefore focus on development of an automated method for EV isolation, purification and downstream analysis [124], ideally using very small sample volumes to improve chances for clinical implementation.
6.2. Internal Standard
The use of a reliable internal standard would also increase the chances for clinical implementation. As rightly opposed by Loyer et al., despite efforts to identify specific subpopulations of EVs with specific membrane markers, very few studies report the purity of their obtained subpopulations [13]. Improvement can be obtained with an internal standard for the number of EVs per milliliter plasma in a sample. For this, a housekeeping protein present in all EVs (e.g., beta-actin) might be a way of developing such a standard. Alongside this, an internal control to visualize the loss of EVs during isolation is needed. Labeled synthetic beads or liposomes might be used for this. Already, these two standards could improve reproducibility and accuracy of EV count and content and measurements in precious clinical samples.
6.3. Future Directions
Future studies should focus on clinical applicability by developing internal standards and introduce automation and standardization of EV isolation and quantification [56]. Larger cohorts are warranted in order to derive valid clinical prediction models that enable the added value of EV contents as biomarkers to be shown, particularly when taking the heterogeneity within CCS patients into account.
Since EV protein content are based on established immunoassays and are increasingly showing merit in the diagnosis and prognosis of CVD, including the potential for automation and standardization, we expect this to prevail in this field in the next few years.
Although technical challenges still have to be resolved, we anticipate that EVs will be used as a reliable source for research into the diagnosis and prognosis of CCS in the next few years. This could potentially contribute to more personalized medicine and a more efficient use of our healthcare system.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/23/9128/s1.
Author Contributions
M.D.: Conceptualization, formal analysis, investigation, data curation and drafting original article. F.W.: Conceptualization, formal analysis, review and editing. N.T.: Formal analysis, review and editing, visualization. M.J.M.S.: Resources, review and editing, visualization. L.T.: Writing, review and editing, resources, funding acquisition and supervision. D.P.V.d.K.: Conceptualization, writing, review and editing, project administration, funding acquisition. All authors read and agreed to the published version of the manuscript.
Funding
This study was funded by research grants from the Dutch Heart Foundation (CVON 2017-05 pERSUASIVE.
Conflicts of Interest
The authors have nothing to declare.
References
- Knuuti, J.; Wijns, W.; Achenbach, S.; Agewall, S.; Barbato, E.; Bax, J.J.; Capodanno, D.; Cuisset, T.; Deaton, C.; Dickstein, K.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The vascular biology of atherosclerosis. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Libby, P. History of Discovery: Inflammation in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Gili, S.; De Filippo, O.; D’Amico, S.; Gagliardi, M.; Bertaina, M.; Mazzilli, S.; Rettegno, S.; Bongiovanni, F.; Gatti, P.; et al. Diagnostic accuracy of functional, imaging and biochemical tests for patients presenting with chest pain to the emergency department: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, V.; Bellelli, S.; Caselli, C.; Knuuti, J.; Underwood, S.R.; Neglia, D.; Turchetti, G. Cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable coronary artery disease: Results from the EVINCI study. Eur. J. Health Econ. 2019. [Google Scholar] [CrossRef]
- Brenner, D.J. Medical Imaging in the 21st Century—Getting the Best Bang for the Rad. N. Engl. J. Med. 2010, 362, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Ladapo, J.A.; Blecker, S.; Douglas, P.S. Physician Decision Making and Trends in the Use of Cardiac Stress Testing in the United States. Ann. Intern. Med. 2014, 161, 482. [Google Scholar] [CrossRef]
- Rozanski, A.; Gransar, H.; Wong, N.D.; Shaw, L.J.; Miranda-Peats, R.; Polk, D.; Hayes, S.W.; Friedman, J.D.; Berman, D.S. Clinical Outcomes After Both Coronary Calcium Scanning and Exercise Myocardial Perfusion Scintigraphy. J. Am. Coll. Cardiol. 2007, 49, 1352–1361. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Reviews. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Martinez, M.C.; Tual-Chalot, S.; Leonetti, D.; Andriantsitohaina, R. Microparticles: Targets and tools in cardiovascular disease. Trends Pharmacol. Sci. 2011, 32, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.-C.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell–Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J. Am. Coll. Cardiol. 2014, 64. [Google Scholar] [CrossRef]
- McCarthy, C.P.; McEvoy, J.W.; Januzzi, J.L. Biomarkers in stable coronary artery disease. Am. Heart J. 2018, 196, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Tutrone, R.; Donovan, M.J.; Torkler, P.; Tadigotla, V.; McLain, T.; Noerholm, M.; Skog, J.; McKiernan, J. Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef]
- Yin, X.; Subramanian, S.; Hwang, S.J.; O’Donnell, C.J.; Fox, C.S.; Courchesne, P.; Muntendam, P.; Gordon, N.; Adourian, A.; Juhasz, P.; et al. Protein biomarkers of new-onset cardiovascular disease: Prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 939–945. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N.R. Statins: New American guidelines for prevention of cardiovascular disease. Lancet 2013, 382, 1762–1765. [Google Scholar] [CrossRef]
- Ho, J.E.; Lyass, A.; Courchesne, P.; Chen, G.; Liu, C.; Yin, X.; Hwang, S.J.; Massaro, J.M.; Larson, M.G.; Levy, D. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Weber, M.; Dill, T.; Arnold, R.; Rau, M.; Ekinci, O.; Müller, K.D.; Berkovitsch, A.; Mitrovic, V.; Hamm, C. N-terminal B-type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am. Heart J. 2004, 148. [Google Scholar] [CrossRef]
- Wolber, T.; Maeder, M.; Rickli, H.; Riesen, W.; Binggeli, C.; Duru, F.; Ammann, P. N-terminal pro-brain natriuretic peptide used for the prediction of coronary artery stenosis. Eur. J. Clin. Investig. 2007, 37. [Google Scholar] [CrossRef]
- Nadir, M.A.; Witham, M.D.; Szwejkowski, B.R.; Struthers, A.D. Meta-analysis of B-type natriuretic peptide’s ability to identify stress induced myocardial ischemia. Am. J. Cardiol. 2011, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Jander, N.; Trenk, D.; Neumann, F.-J.; Mueller, C. The use of B-type natriuretic peptides in the detection of myocardial ischemia in settings with rapid access to coronary angiography. Int. J. Cardiol. 2007, 119, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Staub, D.; Jonas, N.; Zellweger, M.J.; Nusbaumer, C.; Wild, D.; Pfisterer, M.E.; Mueller-Brand, J.; Perruchoud, A.P.; Mueller, C. Use of N-terminal pro-B-type natriuretic peptide to detect myocardial ischemia. Am. J. Med. 2005, 118, 1287.e9–1287.e16. [Google Scholar] [CrossRef]
- Staub, D.; Nusbaumer, C.; Zellweger, M.J.; Jonas, N.; Wild, D.; Pfisterer, M.E.; Mueller-Brand, J.; Perruchoud, A.P.; Mueller, C. Use of B-type natriuretic peptide in the detection of myocardial ischemia. Am. Heart J. 2006, 151, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, J.; Staub, D.; Laule-Kilian, K.; Nusbaumer, C.; Breidthardt, T.; Hochholzer, W.; Christ, M.; Mueller, C. Neurohormonal activation and left ventricular ejection fraction in patients with suspected myocardial ischemia. Int. J. Cardiol. 2007, 120. [Google Scholar] [CrossRef]
- Lee, G.; Sou, S.M.; Twerenbold, R.; Reichlin, T.; Oshima, S.; Hochgruber, T.; Zürcher, S.; Matter, D.; Tanglay, Y.; Freese, M.; et al. B-type natriuretic peptide and clinical judgment in the detection of exercise-induced myocardial ischemia. Am. J. Med. 2014, 127. [Google Scholar] [CrossRef]
- Jensen, J.M.; Voss, M.; Hansen, V.B.; Andersen, L.K.; Johansen, P.B.; Munkholm, H.; Nørgaard, B.L. Risk stratification of patients suspected of coronary artery disease: Comparison of five different models. Atherosclerosis 2012, 220. [Google Scholar] [CrossRef]
- Puelacher, C.; Wagener, M.; Honegger, U.; Assadian, M.; Schaerli, N.; Mueller, D.; Strebel, I.; Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; et al. Combining high-sensitivity cardiac troponin and B-type natriuretic peptide in the detection of inducible myocardial ischemia. Clin. Biochem. 2018, 52, 33–40. [Google Scholar] [CrossRef]
- McKie, P.M.; Heublein, D.M.; Scott, C.G.; Gantzer, M.L.; Mehta, R.A.; Rodeheffer, R.J.; Redfield, M.M.; Burnett, J.C.; Jaffe, A.S. Defining high-sensitivity cardiac troponin concentrations in the community. Clin. Chem. 2013, 59. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Braun, S.; Schulz, S.; Mehilli, J.; Schömig, A.; Kastrati, A. High-sensitivity troponin T level and angiographic severity of coronary artery disease. Am. J. Cardiol. 2011, 108. [Google Scholar] [CrossRef]
- Yamazaki, K.; Iijima, R.; Nakamura, M.; Sugi, K. High-sensitivity cardiac troponin T level is associated with angiographic complexity of coronary artery disease: A cross-sectional study. Heart Vessel. 2016, 31. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.D.; Hunter, A.; Madsen, D.M.; Shah, A.S.V.; McAllister, D.A.; Pawade, T.A.; Williams, M.C.; Berry, C.; Boon, N.A.; Flather, M.; et al. High-Sensitivity Cardiac Troponin i and the Diagnosis of Coronary Artery Disease in Patients with Suspected Angina Pectoris. Circ. Cardiovasc. Qual. Outcomes 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanglay, Y.; Twerenbold, R.; Lee, G.; Wagener, M.; Honegger, U.; Puelacher, C.; Reichlin, T.; Sou, S.M.; Druey, S.; Hochgruber, T.; et al. Incremental value of a single high-sensitivity cardiac troponin i Measurement to rule out myocardial ischemia. Am. J. Med. 2015, 128. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Marchesi, P.; Pulakazhi Venu, V.K.; Pasqualini, F.; Anselmo, A.; Moalli, F.; Pizzitola, I.; Garlanda, C.; Mantovani, A.; Catapano, A.L. Deficiency of the long pentraxin ptx3 promotes vascular inflammation and atherosclerosis. Circulation 2009, 120. [Google Scholar] [CrossRef]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link? J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef]
- Zebrack, J.S.; Muhlestein, J.B.; Horne, B.D.; Anderson, J.L. C-reactive protein and angiographic coronary artery disease: Independent and additive predictors of risk in subjects with angina. J. Am. Coll. Cardiol. 2002, 39. [Google Scholar] [CrossRef]
- Ho, J.S.; Cannaday, J.J.; Barlow, C.E.; Reinhardt, D.B.; Wade, W.A.; Ellis, J.R. Utility of high-sensitivity C-reactive protein versus coronary artery calcium for the detection of obstructive stenoses in stable patients. Am. J. Cardiol. 2013, 111. [Google Scholar] [CrossRef]
- Wensley, F.; Gao, P.; Burgess, S.; Kaptoge, S.; Di Angelantonio, E.; Shah, T.; Engert, J.C.; Clarke, R.; Davey-Smith, G.; Nordestgaard, B.G.; et al. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011, 342, d548. [Google Scholar]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D.; et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA J. Am. Med Assoc. 2009, 302. [Google Scholar] [CrossRef]
- Zacho, J.; Tybjærg-Hansen, A.; Jensen, J.S.; Grande, P.; Sillesen, H.; Nordestgaard, B.G. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 2008, 359. [Google Scholar] [CrossRef]
- Wang, T.J.; Gona, P.; Larson, M.G.; Tofler, G.H.; Levy, D.; Newton-Cheh, C.; Jacques, P.F.; Rifai, N.; Selhub, J.; Robins, S.J.; et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N. Engl. J. Med. 2006, 355. [Google Scholar] [CrossRef] [PubMed]
- Melander, O.; Newton-Cheh, C.; Almgren, P.; Hedblad, B.; Berglund, G.; Engström, G.; Persson, M.; Smith, J.G.; Magnusson, M.; Christensson, A.; et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA J. Am. Med Assoc. 2009, 302. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; McQueen, M.J.; Smieja, M.; Pogue, J.; Balion, C.; Lonn, E.; Rupprecht, H.J.; Bickel, C.; Tiret, L.; Cambien, F.; et al. Comparative impact of multiple biomarkers and N-terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2006, 114. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Schulz, A.; Messow, C.M.; Lubos, E.; Wild, P.S.; Zeller, T.; Sinning, C.R.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; et al. Multiple marker approach to risk stratification in patients with stable coronary artery disease. Eur. Heart J. 2010, 31, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 166. [Google Scholar]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; Di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Simeone, P.; Bologna, G.; Pieragostino, D.; Cufaro, M.C.; Graziano, V.; Peri, M.; Di Marino, P.; De Tursi, M.; et al. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Lanuti, P.; Marchisio, M.; De Laurenzi, V.; Del Boccio, P.; Pieragostino, D. Multi-omics approach for studying tears in treatment-naïve glaucoma patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; Van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204. [Google Scholar] [CrossRef]
- Ciccocioppo, F.; Lanuti, P.; Centonze, D.; Miscia, S.; Marchisio, M. The Link Among Neurological Diseases: Extracellular Vesicles as a Possible Brain Injury Footprint. Neuro-Signals 2019, 27. [Google Scholar] [CrossRef]
- Grande, R.; Dovizio, M.; Marcone, S.; Szklanna, P.B.; Bruno, A.; Ebhardt, H.A.; Cassidy, H.; Ní Áinle, F.; Caprodossi, A.; Lanuti, P.; et al. Platelet-derived microparticles from obese individuals: Characterization of number, size, proteomics, and crosstalk with cancer and endothelial cells. Front. Pharmacol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Santilli, F.; Marchisio, M.; Pierdomenico, L.; Vitacolonna, E.; Santavenere, E.; Iacone, A.; Davì, G.; Romano, M.; Miscia, S. A novel flow cytometric approach to distinguish circulating endothelial cells from endothelial microparticles: Relevance for the evaluation of endothelial dysfunction. J. Immunol. Methods 2012, 380. [Google Scholar] [CrossRef] [PubMed]
- Pipino, C.; Mandatori, D.; Buccella, F.; Lanuti, P.; Preziuso, A.; Castellani, F.; Grotta, L.; Di Tomo, P.; Marchetti, S.; Di Pietro, N.; et al. Identification and Characterization of a Stem Cell-Like Population in Bovine Milk: A Potential New Source for Regenerative Medicine in Veterinary. Stem Cells Dev. 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Torrano, V.; Royo, F.; Peinado, H.; Loizaga-Iriarte, A.; Unda, M.; Falcón-Perez, J.M.; Carracedo, A. Vesicle-MaNiA: Extracellular vesicles in liquid biopsy and cancer. Curr. Opin. Pharmacol. 2016, 29, 47–53. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Wollert, T.; Hurley, J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010, 464. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes-vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Rautou, P.E.; Leroyer, A.S.; Ramkhelawon, B.; Devue, C.; Duflaut, D.; Vion, A.C.; Nalbone, G.; Castier, Y.; Leseche, G.; Lehoux, S.; et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 2011, 108. [Google Scholar] [CrossRef]
- Rautou, P.-E.; Vion, A.-C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M. Microparticles, Vascular Function, and Atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef]
- Iqbal, R.; Anand, S.; Ounpuu, S.; Islam, S.; Zhang, X.; Rangarajan, S.; Chifamba, J.; Al-Hinai, A.; Keltai, M.; Yusuf, S. Dietary patterns and the risk of acute myocardial infarction in 52 countries: Results of the INTERHEART study. Circulation 2008, 118. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, P.S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364. [Google Scholar] [CrossRef]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef]
- Arteaga, R.B.; Chirinos, J.A.; Soriano, A.O.; Jy, W.; Horstman, L.; Jimenez, J.J.; Mendez, A.; Ferreira, A.; De Marchena, E.; Ahn, Y.S. Endothelial Microparticles and Platelet and Leukocyte Activation in Patients With the Metabolic Syndrome. Am. J. Cardiol. 2006, 98. [Google Scholar] [CrossRef] [PubMed]
- Diamant, M.; Nieuwland, R.; Pablo, R.F.; Sturk, A.; Smit, J.W.A.; Radder, J.K. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 2002, 106. [Google Scholar] [CrossRef]
- Sabatier, F.; Darmon, P.; Hugel, B.; Combes, V.; Sanmarco, M.; Velut, J.G.; Arnoux, D.; Charpiot, P.; Freyssinet, J.M.; Oliver, C.; et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 2002, 51. [Google Scholar] [CrossRef]
- Preston, R.A.; Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Valle, M.; Aime, G.; Ahn, Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003, 41. [Google Scholar] [CrossRef]
- Nomura, S.; Shouzu, A.; Omoto, S.; Nishikawa, M.; Iwasaka, T. Effects of Losartan and Simvastatin on Monocyte-Derived Microparticles in Hypertensive Patients with and Without Type 2 Diabetes Mellitus. Clin. Appl. Thromb. Hemost. 2004, 10. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Peter, A.A.; Mendez, A.J.; Jimenez, J.J.; Mauro, L.M.; Chirinos, J.A.; Ghany, R.; Virani, S.; Garcia, S.; Horstman, L.L.; et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 2004, 110. [Google Scholar] [CrossRef]
- Koga, H.; Sugiyama, S.; Kugiyama, K.; Fukushima, H.; Watanabe, K.; Sakamoto, T.; Yoshimura, M.; Jinnouchi, H.; Ogawa, H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur. Heart J. 2006, 27. [Google Scholar] [CrossRef]
- Gordon, C.; Gudi, K.; Krause, A.; Sackrowitz, R.; Harvey, B.G.; Strulovici-Barel, Y.; Mezey, J.G.; Crystal, R.G. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am. J. Respir. Crit. Care Med. 2011, 184. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Amabile, N.; Lee, A.C.; Real, W.M.; Schick, S.F.; Lao, D.; Wong, M.L.; Jahn, S.; Angeli, F.S.; Minasi, P.; et al. Brief Secondhand Smoke Exposure Depresses Endothelial Progenitor Cells Activity and Endothelial Function. Sustained Vascular Injury and Blunted Nitric Oxide Production. J. Am. Coll. Cardiol. 2008, 51. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, Y.; Chen, Y.; Yu, D.; Williams, K.J.; Liu, M.L. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am. J. Pathol. 2013, 182. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.; Simon, A.; Hugel, B.; Del Pino, M.; Gariepy, J.; Freyssinet, J.-M.; Tedgui, A. Circulating Leukocyte-Derived Microparticles Predict Subclinical Atherosclerosis Burden in Asymptomatic Subjects. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2775–2780. [Google Scholar] [CrossRef]
- Jayachandran, M.; Litwiller, R.D.; Owen, W.G.; Heit, J.A.; Behrenbeck, T.; Mulvagh, S.L.; Araoz, P.A.; Budoff, M.J.; Harman, S.M.; Miller, V.M. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am. J. Physiol. Heart Circ. Physiol. 2008, 295. [Google Scholar] [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31 +/Annexin V + microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll. Cardiol. 2009, 54, 601–608. [Google Scholar] [CrossRef]
- Koga, H.; Sugiyama, S.; Kugiyama, K.; Watanabe, K.; Fukushima, H.; Tanaka, T.; Sakamoto, T.; Yoshimura, M.; Jinnouchi, H.; Ogawa, H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 2005, 45, 1622–1630. [Google Scholar] [CrossRef]
- Christersson, C.; Lindahl, B.; Siegbahn, A. The composition and daily variation of microparticles in whole blood in stable coronary artery disease. Scand. J. Clin. Lab. Investig. 2016, 76, 25–32. [Google Scholar] [CrossRef]
- Augustine, D.; Ayers, L.; Lima, E.; Newton, L.; Lewandowski, A.J.; Davis, E.F.; Ferry, B.; Leeson, P. Dynamic release and clearance of circulating microparticles during cardiac stress. Circ. Res. 2014, 114. [Google Scholar] [CrossRef]
- Sinning, J.M.; Jansen, F.; Hammerstingl, C.; Meier, A.; Losch, J.; Rohwer, K.; Schmitz, T.; Paul, K.; Sedaghat, A.; Schueler, R.; et al. Circulating Microparticles Decrease after Cardiac Stress in Patients with Significant Coronary Artery Stenosis. Clin. Cardiol. 2016, 39, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; De Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Sansone, R.; Baaken, M.; Horn, P.; Schuler, D.; Westenfeld, R.; Amabile, N.; Kelm, M.; Heiss, C. Release of endothelial microparticles in patients with arterial hypertension, hypertensive emergencies and catheter-related injury. Atherosclerosis 2018, 273, 67–74. [Google Scholar] [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.-M.; Tedgui, A. Elevated Levels of Shed Membrane Microparticles With Procoagulant Potential in the Peripheral Circulating Blood of Patients With Acute Coronary Syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Steogonekpień, E.; Stankiewicz, E.; Zalewski, J.; Godlewski, J.; Zmudka, K.; Wybrańska, I. Number of Microparticles Generated During Acute Myocardial Infarction and Stable Angina Correlates with Platelet Activation. Arch. Med. Res. 2012, 43, 31–35. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Porto, I.; Di Vito, L.; De Maria, G.L.; Leone, A.M.; Tinelli, G.; Tritarelli, A.; Di Rocco, G.; Snider, F.; Capogrossi, M.C.; et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 2012, 76, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Tayebjee, M.H.; Macfadyen, R.J.; Lip, G.Y.H.; Blann, A.D. Elevated platelet microparticles in stable coronary artery disease are unrelated to disease severity or to indices of inflammation. Platelets 2005, 16, 368–371. [Google Scholar] [CrossRef]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26. [Google Scholar] [CrossRef]
- Song, R.; Chou, Y.I.S.; Kong, J.; Li, J.; Pan, B.; Cui, M.; Zhou, E.; Zhang, Y.; Zheng, L. Association of endothelial microparticle with NO, eNOS, ET-1, and fractional flow reserve in patients with coronary intermediate lesions. Biomarkers 2015, 20, 429–435. [Google Scholar] [CrossRef]
- Hu, S.S.; Zhang, H.G.; Zhang, Q.J.; Xiu, R.J. Small-size circulating endothelial microparticles in coronary artery disease. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Connolly, K.D.; Willis, G.R.; Datta, D.B.N.; Ellins, E.A.; Ladell, K.; Price, D.A.; Guschina, I.A.; Rees, D.A.; James, P.E. Lipoprotein-apheresis reduces circulating microparticles in individuals with familial hypercholesterolemia. J. Lipid Res. 2014, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Vernooij, F.; Ibrahim, I.; Ooi, S.; Gijsberts, C.M.; Schoneveld, A.H.; Sen, K.W.; Den Ruijter, H.M.; Timmers, L.; Richards, A.M.; et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: An observational study in a dyspnoea cohort. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular vesicles in cardiovascular disease. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.G.; Percy, A.J.; Simon, R.; Borchers, C.H. MRM for the verification of cancer biomarker proteins: Recent applications to human plasma and serum. Expert Rev. Proteom. 2014, 11, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Vélez, P.; Parguiña, A.F.; Ocaranza-Sánchez, R.; Grigorian-Shamagian, L.; Rosa, I.; Alonso-Orgaz, S.; De la Cuesta, F.; Guitián, E.; Moreu, J.; Barderas, M.G.; et al. Identification of a circulating microvesicle protein network involved in ST-elevation myocardial infarction. Thromb. Haemost. 2014, 112. [Google Scholar] [CrossRef] [PubMed]
- Gidlöf, O.; Evander, M.; Rezeli, M.; Marko-Varga, G.; Laurell, T.; Erlinge, D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.; Waissi, F.; Van Bennekom, J.; Silvis, M.J.M.; Timmerman, N.; Bank, I.E.M.; Walter, J.E.; Mueller, C.; Schoneveld, A.H.; Schiffelers, R.M.; et al. Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, Y.N.; Sze, S.K.; Van de Weg, S.M.; Vernooij, F.; Schoneveld, A.H.; Tan, S.H.; Versteeg, H.H.; Timmers, L.; Lam, C.S.P.; et al. Lowering low-density lipoprotein particles in plasma using dextran sulphate co-precipitates procoagulant extracellular vesicles. Int. J. Mol. Sci. 2018, 19, 94. [Google Scholar] [CrossRef]
- Kanhai, D.A.; Visseren, F.L.J.; Van Der Graaf, Y.; Schoneveld, A.H.; Catanzariti, L.M.; Timmers, L.; Kappelle, L.J.; Uiterwaal, C.S.P.M.; Lim, S.K.; Sze, S.K.; et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int. J. Cardiol. 2013, 168, 2358–2363. [Google Scholar] [CrossRef]
- Vrijenhoek, J.E.; Pasterkamp, G.; Moll, F.L.; De Borst, G.J.; Bots, M.L.; Catanzariti, L.; Van De Weg, S.M.; De Kleijn, D.P.V.; Visseren, F.L.; Ruijter, H.M.D. Extracellular vesicle-derived CD14 is independently associated with the extent of cardiovascular disease burden in patients with manifest vascular disease. Eur. J. Prev. Cardiol. 2015, 22, 451–457. [Google Scholar] [CrossRef]
- De Hoog, V.C.; Timmers, L.; Schoneveld, A.H.; Wang, J.W.; Van De Weg, S.M.; Sze, S.K.; Van Keulen, J.K.; Hoes, A.W.; Den Ruijter, H.M.; De Kleijn, D.P.; et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.; Waissi, F.; Van Bennekom, J.; Silvis, M.J.M.; Timmerman, N.; Schoneveld, A.H.; Grobbee, D.E.; De Winter, R.J.; Mosterd, A.; Timmers, L.; et al. Extracellular Vesicle cystatin c is associated with unstable angina in troponin negative patients with acute chest pain. PLoS ONE 2020, 15, e0237036. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.E.G.; De Kleijn, D.P.V.; Kalkhoven, E.; Kanhai, D.A.; Uiterwaal, C.S.P.M.; Van Der Graaf, Y.; Pasterkamp, G.; Visseren, F.L.J.; Doevendans, P.A.; Algra, A.; et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc. Diabetol. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.L.; Löffler, A.I.; Beller, G.A.; Workman, V.K.; Holland, E.; Bourque, J.M. Clinical characteristics, sex differences, and outcomes in patients with normal or near-normal coronary arteries, non-obstructive or obstructive coronary artery disease. J. Am. Heart Assoc. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Bugiardini, R.; Bairey Merz, C.N. Women and Ischemic Heart Disease: Evolving Knowledge Leslee. J. Am. Coll. Cardiol. 2009, 54, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Shaw, R.E.; Merz, C.N.B.; Brindis, R.G.; Klein, L.W.; Nallamothu, B.; Douglas, P.S.; Krone, R.J.; McKay, C.R.; Block, P.C.; et al. Impact of Ethnicity and Gender Differences on Angiographic Coronary Artery Disease Prevalence and In-Hospital Mortality in the American College of Cardiology–National Cardiovascular Data Registry. Circulation 2008, 117, 1787–1801. [Google Scholar] [CrossRef]
- Shaw, L.J.; Bairey Merz, C.N.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular cor. J. Am. Coll. Cardiol. 2006, 47, S21–S29. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Guseh, J.S.; Bhambhani, V.; Zanni, M.V.; Courchesne, P.; Lyass, A.; Larson, M.G.; Levy, D.; Ho, J.E. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, 1543–1553. [Google Scholar] [CrossRef]
- Bank, I.E.M.; Timmers, L.; Gijsberts, C.M.; Zhang, Y.N.; Mosterd, A.; Wang, J.W.; Chan, M.Y.; De Hoog, V.; Lim, S.K.; Sze, S.K.; et al. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev. Mol. Diagn. 2015, 15, 1577–1588. [Google Scholar] [CrossRef]
- Lacroix, R.; Judicone, C.; Poncelet, P.; Robert, S.; Arnaud, L.; Sampol, J.; Dignat-George, F. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Kormelink, T.G.; Arkesteijn, G.J.A.; Nauwelaers, F.A.; Van den Engh, G.; Nolte-’t Hoen, E.N.M.; Wauben, M.H.M. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytom. Part A 2016, 89. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).