Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α2-Adrenergic Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Cell Preparations
2.3. Electrophysiological Measurements
2.4. Data Recordings
2.5. Data Analyses

2.6. Statistical Analyses
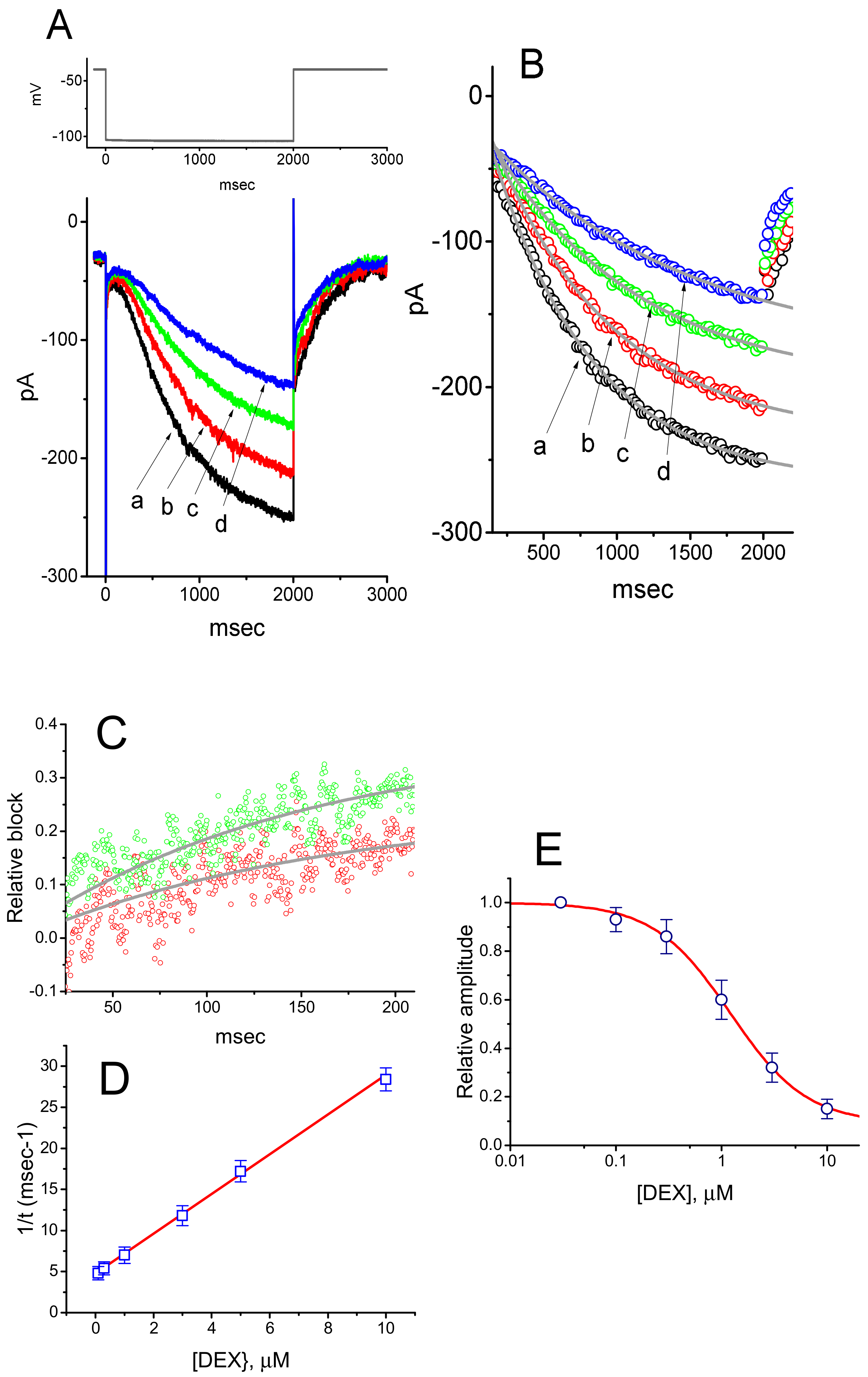
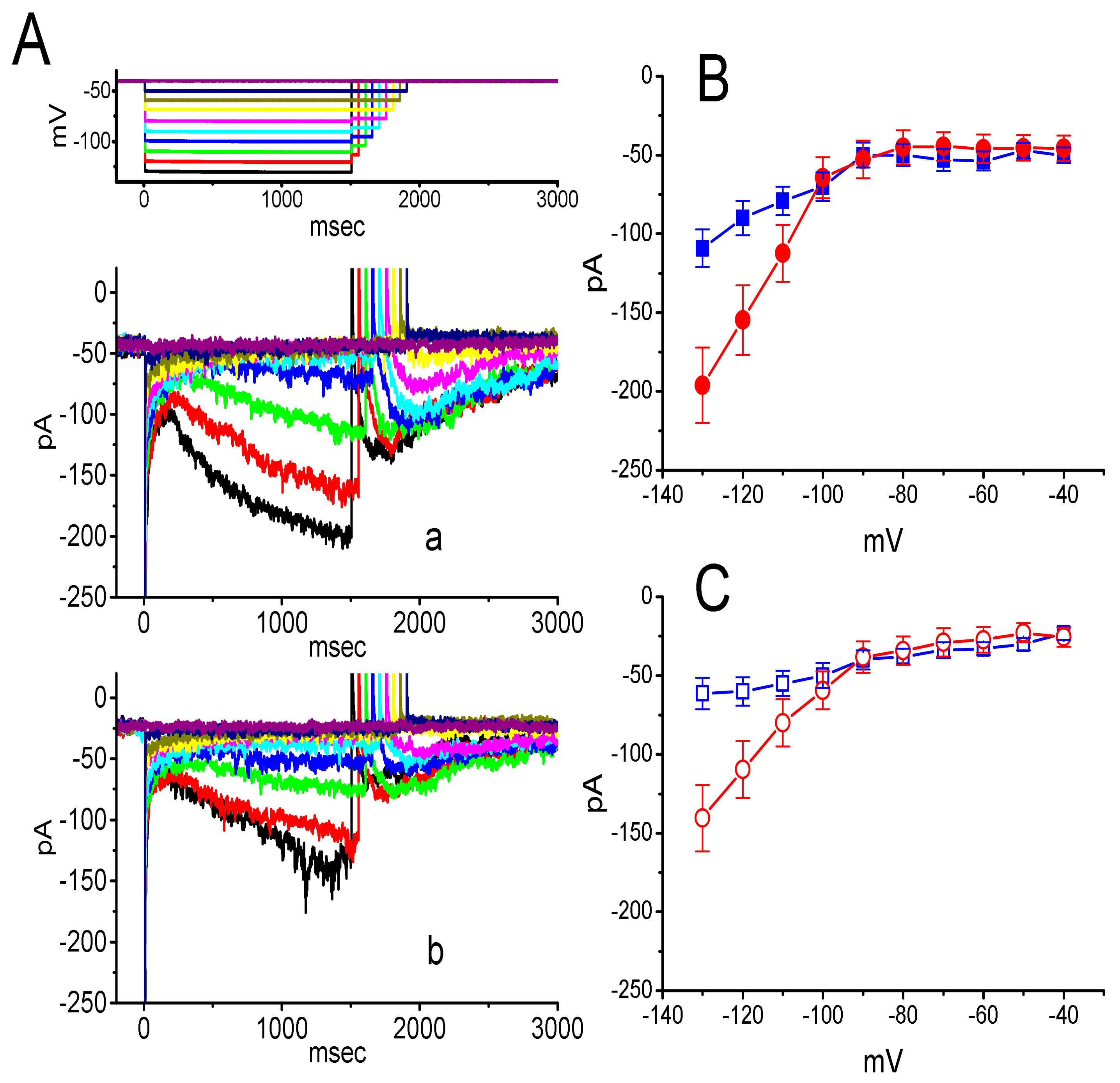
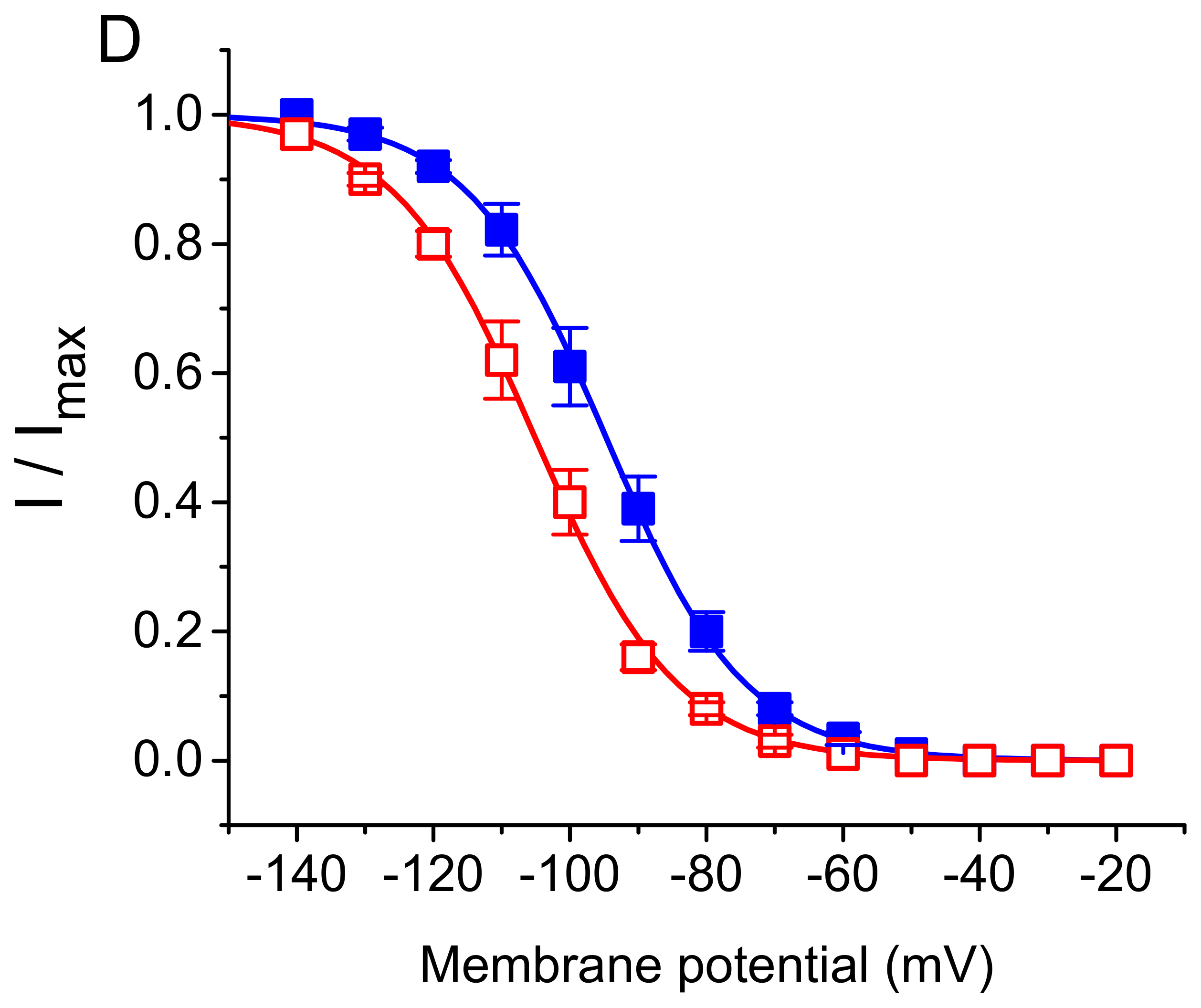
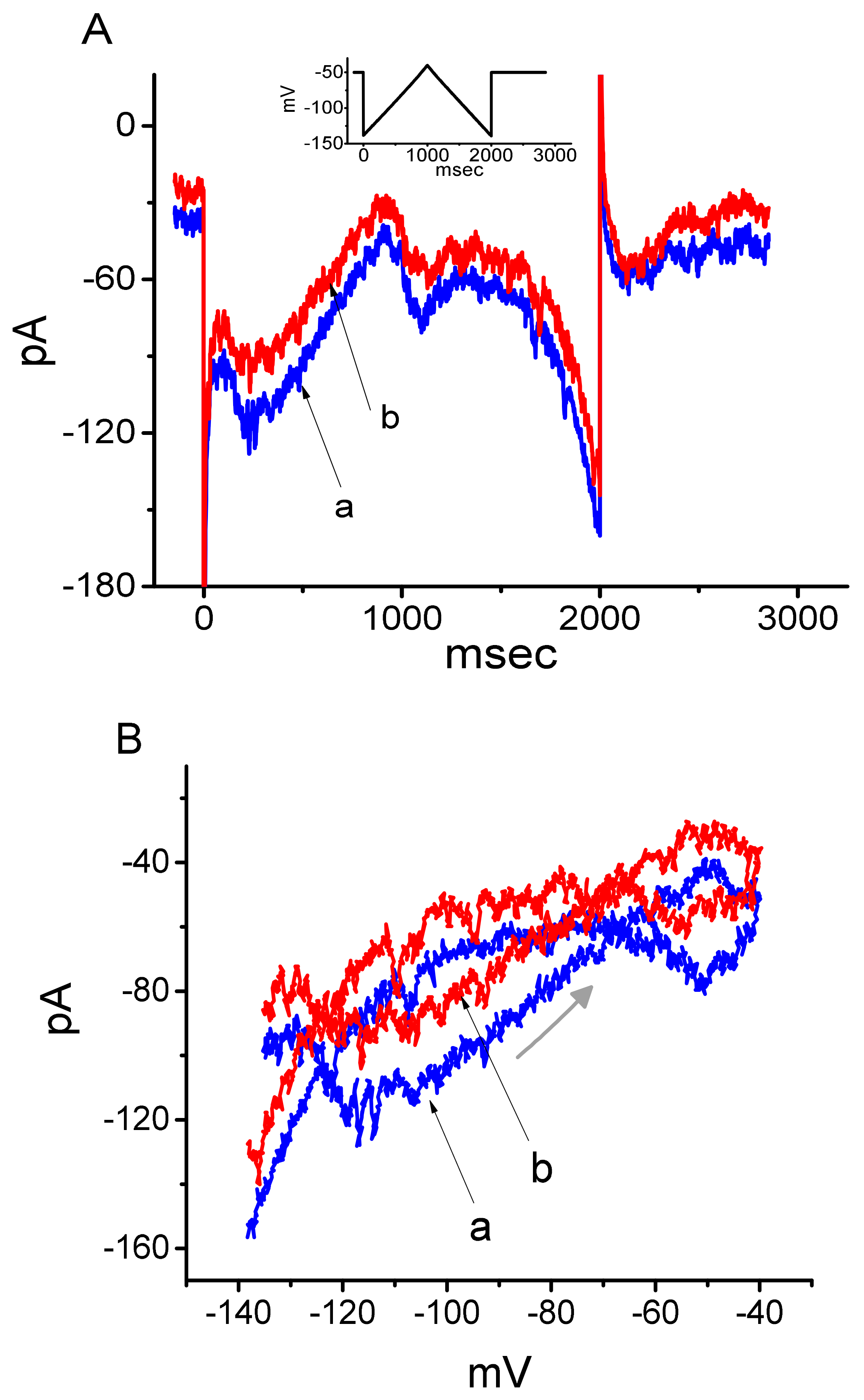
3. Results
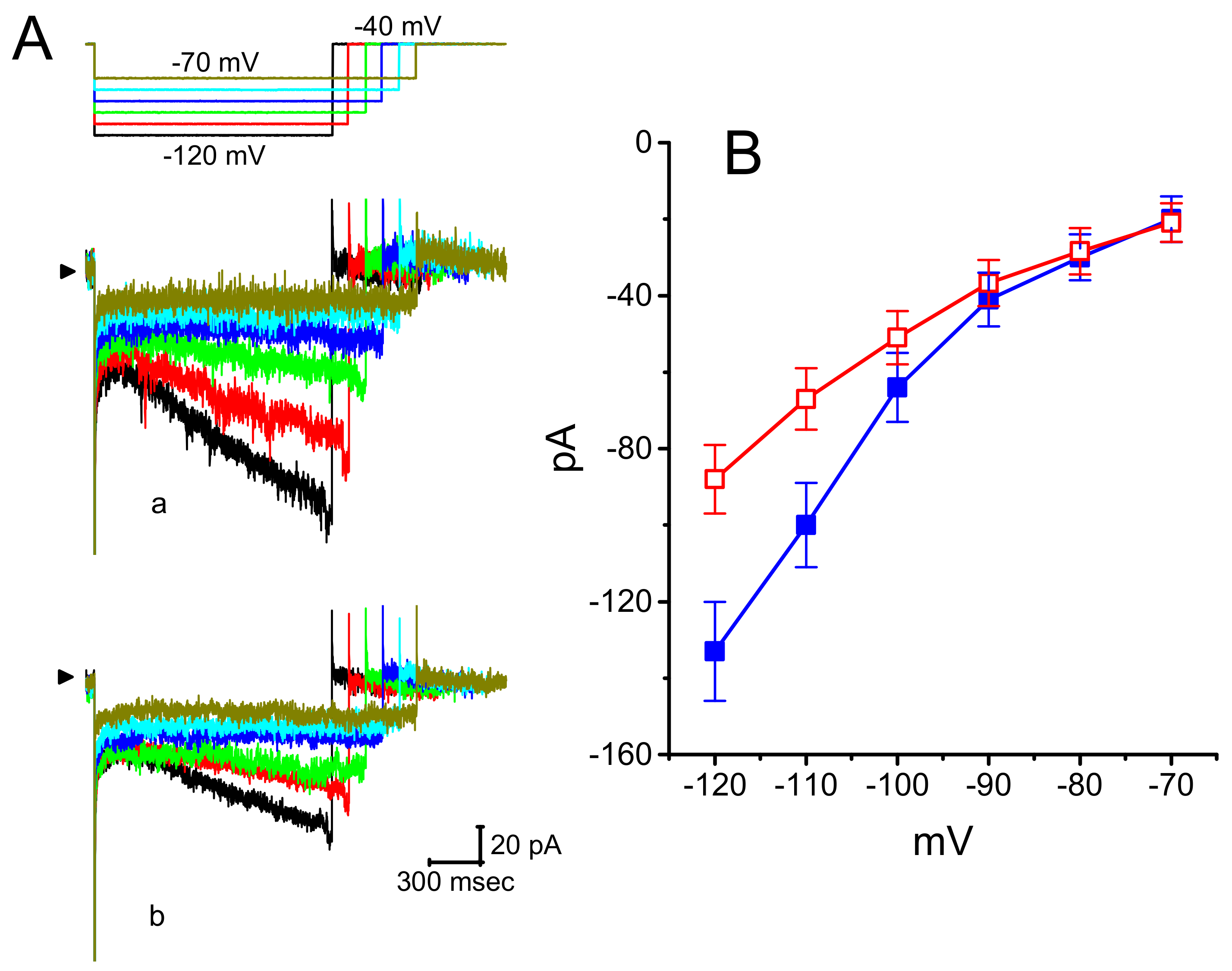
3.1. Effect of DEX on Hyperpolarization-Activated Cation Current (Ih) Recorded from GH3 Cells
3.2. Effect of DEX on the Steady-State Activation Curve of ih
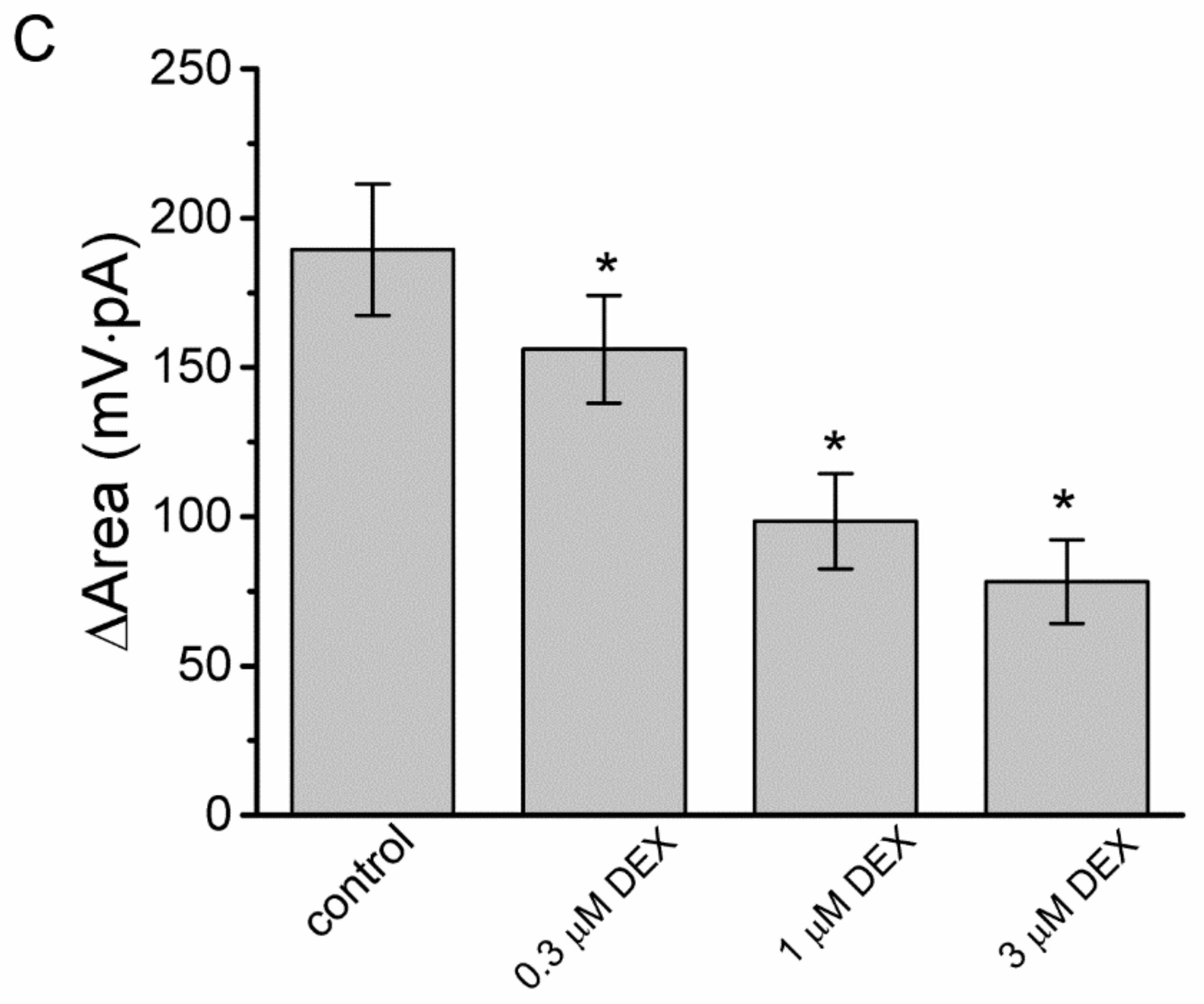
3.3. Effect of DEX on the Voltage-Dependent Hysteresis Elicited in Response to Long-Lasting Triangular Ramp Pulse
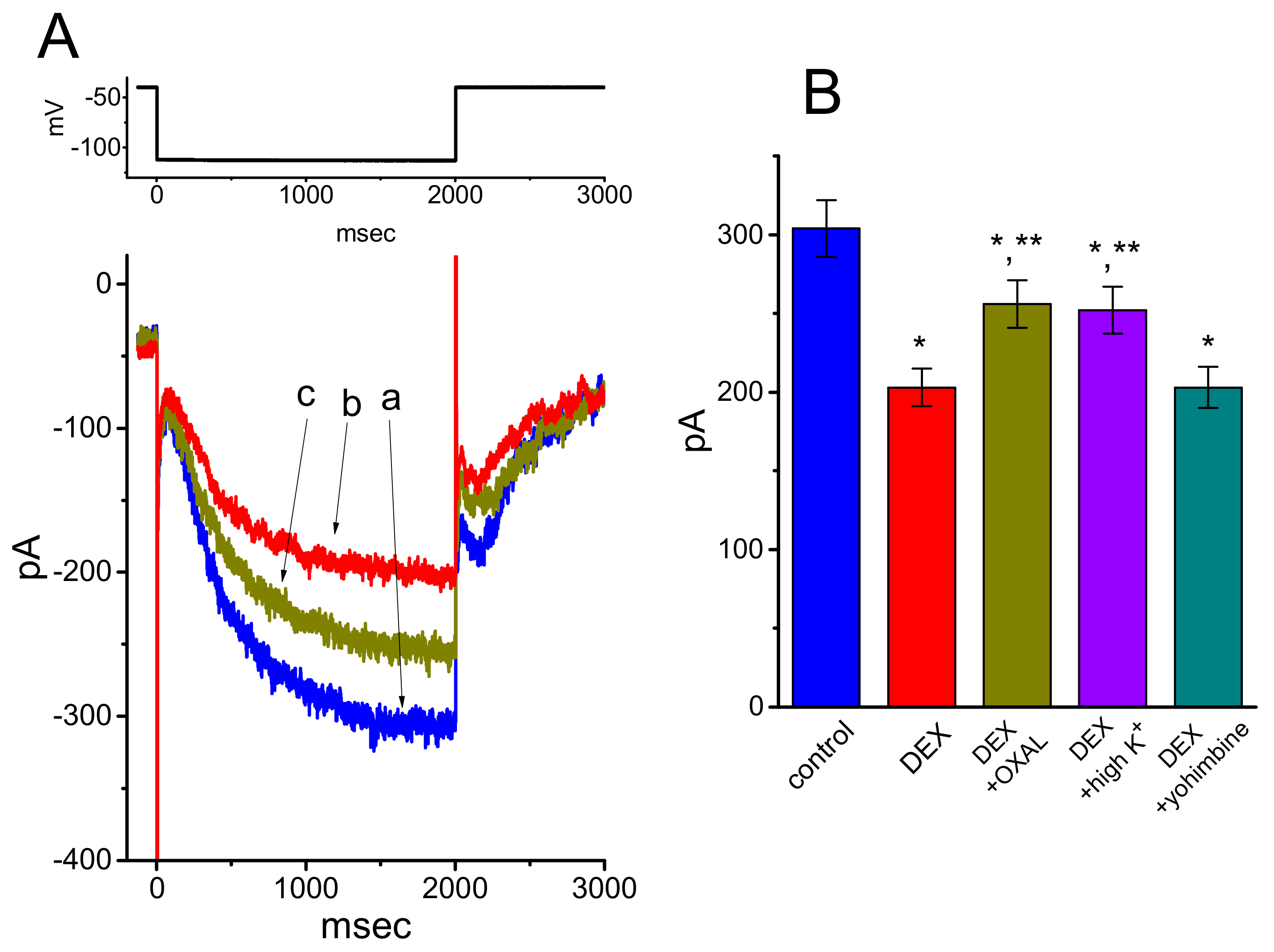
3.4. Effects of DEX, DEX Plus Oxaliplatin (OXAL), DEX Plus High K, and DEX Plus Yohimbine on the Amplitude of Ih in GH3 Cells
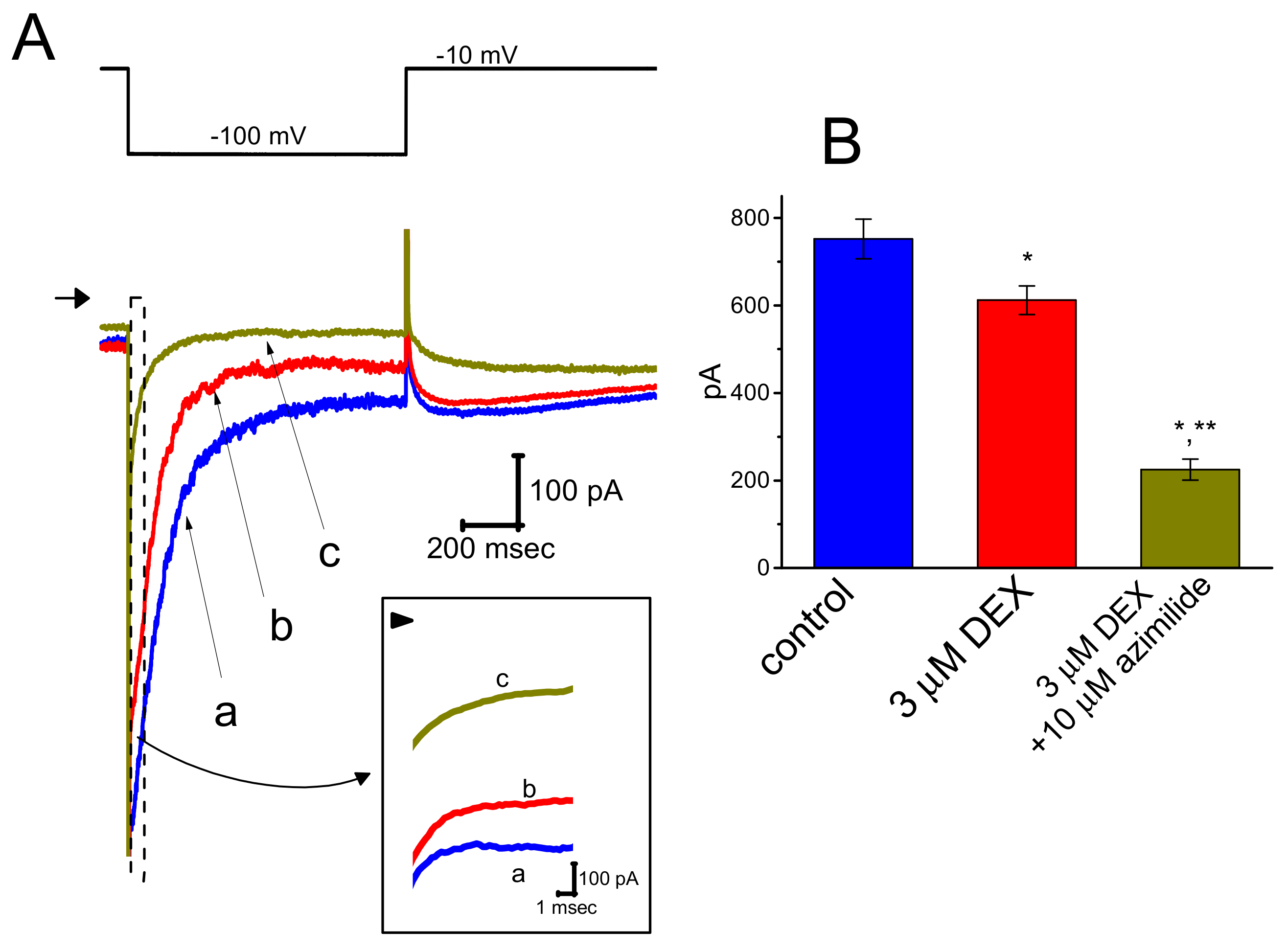
3.5. Effect of DEX on Erg-Mediated K+ Current (IK(erg)) in GH3 Cells
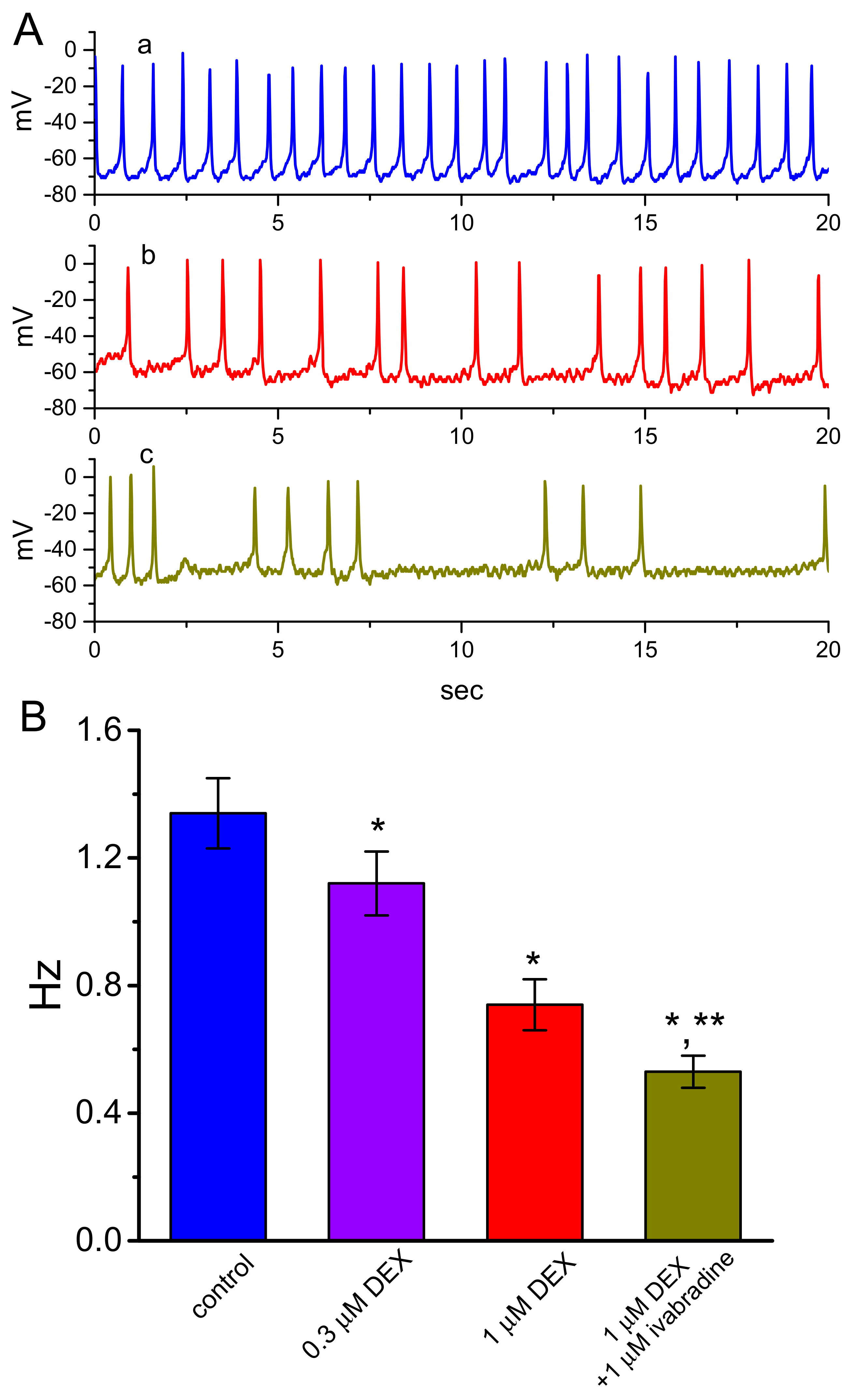
3.6. Effect of DEX on Spontaneous Action Potentials (APs) Recorded from GH3 Cells
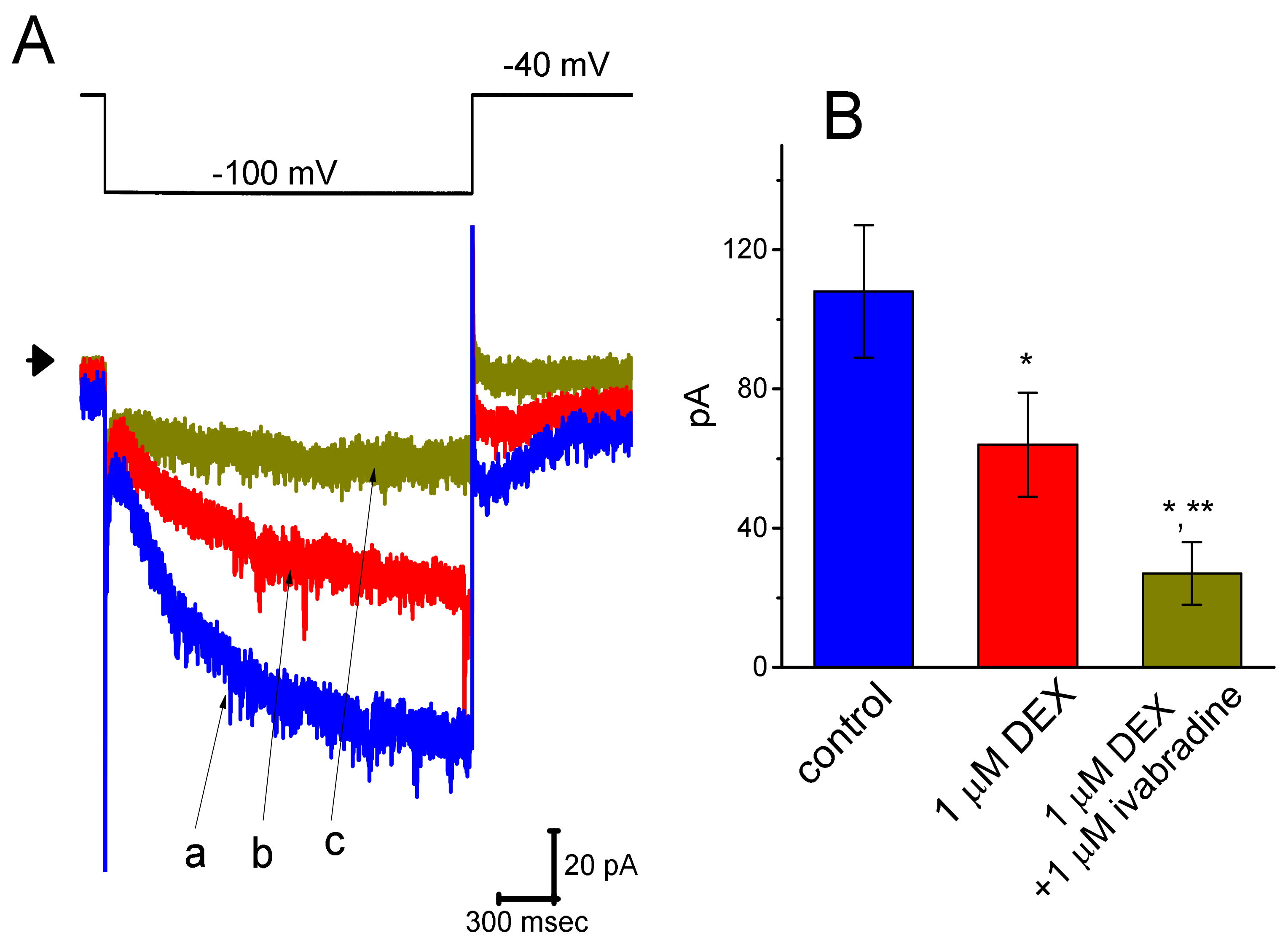
3.7. Effect of DEX and DEX Plus Ivabradine on Ih Recorded from Pheochromocytoma PC12 Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Martin, E.; Ramsay, G.; Mantz, J.; Sum-Ping, S.T. The role of the a2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J. Intensive Care Med. 2003, 18, 29–41. [Google Scholar]
- Shehabi, Y.; Howe, B.D.; Bellomo, R.; Arabi, Y.M.; Bailey, M.; Bass, F.E.; Bin Kadiman, S.; McArthur, C.J.; Murray, L.; Reade, M.C.; et al. ANZUCS Clinical Trials Group: SPICE III Investigators: ANZICS Clinical Trials Group and the SPICE III Investigators. N. Engl. J. Med. 2019, 380, 2506–2517. [Google Scholar] [PubMed]
- Chen, B.S.; Peng, H.; Wu, S.N. Dexmedetomidine, an α2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br. J. Anaesth. 2009, 103, 244–254. [Google Scholar] [PubMed]
- Kosugi, T.; Mizuta, K.; Fujita, T.; Nakashima, M.; Kumamoto, E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without a2-adrenoceptor activation. Br. J. Pharmacol. 2010, 160, 1662–1676. [Google Scholar] [PubMed]
- Won, J.; Lee, P.R.; Oh, S.B. Alpha 2 adrenoceptor agonist guanabenz directly inhibits hyperpolarization-activated, cyclic nucleotide-modulated (HCN) channels in mesencephalic trigeminal nucleus neurons. Eur. J. Pharmacol. 2019, 854, 320–327. [Google Scholar]
- Dahmani, S.; Paris, A.; Jannier, V.; Hein, L.; Rouelle, D.; Scholz, J.; Gressens, P.; Mantz, J. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an α2-adrenoceptor-independent mechanism: Evidence for the involvement of imidazoline I1 receptors. Anesthesiology 2008, 108, 457–466. [Google Scholar]
- Colthorpe, K.L.; Nalliah, J.; Anderson, S.T.; Curlewis, J.D. Adrenoceptor subtype involvement in suppression of prolactin secretion by noradrenaline. J. Neuroendocrinol. 2000, 12, 297–302. [Google Scholar]
- Shirasaka, T.; Kannan, H.; Takasaki, M. Activation of a G protein-coupled inwardly rectifying K+ current and suppression of Ih contribute to dexemedetomidine-induced inhibition of rat hypothalamic paraventricular nucleus neurons. Anesthesiology 2007, 107, 605–615. [Google Scholar]
- Chiu, K.M.; Lin, T.Y.; Lee, M.Y.; Lu, C.W.; Wang, M.J.; Wang, S.J. Dexmedetomidine protects neurons from kainic acid-induced excitotoxicity by activating BDNF signaling. Neurochem. Int. 2019, 129, 104493. [Google Scholar]
- Gu, X.Y.; Liu, B.L.; Zang, K.K.; Yang, L.; Xu, H.; Pan, H.L.; Zhao, Z.Q.; Zhang, Y.Q. Dexmedetomidine inhibits tetrodotoxin-resistant NaV1.8 sodium channel activity through Gi/o-dependent pathway in rat dorsal root ganglion neurons. Mol. Brain 2015, 8, 15. [Google Scholar]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [PubMed]
- McCormick, D.A.; Pape, H.C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J. Physiol. 1990, 431, 291–318. [Google Scholar] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [PubMed]
- Liu, Y.C.; Wang, Y.J.; Wu, P.Y.; Wu, S.N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn-Schmiedbergs Arch. Pharmacol. 2009, 379, 127–135. [Google Scholar]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels from cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar]
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar]
- Kuruma, A.; Hirayama, Y.; Hartzell, H.C. A hyperpolarization- and acid-activated nonselective cation currents in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 2000, 279, C1401–C1413. [Google Scholar]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose-response curves. J. Pharmacol. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Vivaudou, M. eeFit: A Microsoft Excel-embedded program for interactive analysis and fitting of experimental dose-response data. Biotechniques 2019, 66, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. Endocrinol. Metab. 1997, 272, E405–E414. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef]
- Männikö, R.; Pandey, S.; Larsson, P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef]
- Barthel, L.; Reetz, O.; Strauss, U. Use dependent attenuation of rat HCN1-mediated Ih in intact HEK293 cells. Cell Physiol. Biochem. 2016, 38, 2079–2093. [Google Scholar] [CrossRef]
- Resta, F.; Micheli, L.; Laurino, A.; Spinelli, V.; Mello, T.; Sartiani, L.; Di Cesare Mannelli, L.; Cerbai, E.; Ghelardini, C.; Romanelli, M.N.; et al. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018, 131, 403–413. [Google Scholar] [CrossRef]
- Hu, R.; Ferguson, K.A.; Whiteus, C.B.; Meijer, D.H.; Araneda, R.C. Hyperpolarization-activated currents and subthreshold resonance in granule cells of the olfactory bulb. eNouro 2016, 3. [Google Scholar] [CrossRef]
- Chang, W.T.; Gao, Z.H.; Li, S.W.; Liu, P.Y.; Lo, Y.C.; Wu, S.N. Characterization in dual activation by oxaliplatin, a platinum-based chemotherapeutic agent of hyperpolarization-activated cation and electroporation-induced currents. Int. J. Mol. Sci. 2020, 21, 396. [Google Scholar] [CrossRef]
- Lalchandani, S.G.; Lei, L.; Zheng, W.; Suni, M.M.; Moore, B.M.; Liggett, S.B.; Miller, D.D.; Feller, D.R. Yohimbine Dimers Exhibiting Selectivity for the Human α2C-Adrenoceptor Subtype. J. Pharmacol. Exp. Ther. 2002, 303, 979. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Bi, X.; Wang, Q.; Li, Y.; Zhang, N.; Lao, J.; Liu, X. Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity via downregulation of Stathmin 1. Drug Des. Dev. Ther. 2019, 13, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Chang, W.T.; Chan, C.H.; Wu, S.N. Evidence for effective multiple K+-current inhibitions by tolvaptan, a non-peptide antagonist of vasopressin V2 receptor. Front. Pharmacol. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Aman, T.K.; DiMaio, F.; Zagotta, W.N. The HCN channel voltage sensor undergoes a large downward motion during hyperpolarization. Nat. Struct. Mol. Biol. 2019, 26, 686–694. [Google Scholar] [CrossRef]
- Venn, R.M.; Karol, M.D.; Grounds, R.M. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br. J. Anaesth. 2002, 88, 669–675. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Hirata, N.; Kawaguichi, R.; Tokinaga, Y.; Yamakage, M. Dexmedetomidine maintains its direct cardioprotective effect against ischemia/reperfusion injury in hypertensive hypertrophied myocardium. Anesth. Analg. 2018, 126, 443–452. [Google Scholar] [CrossRef]
- Ortmann, L.A.; Keshary, M.; Bisseiou, K.S.; Kutty, S.; Affolter, J.T. Association between postoperative dexmedetomidine use and arrhythmias in infants after cardiac surgery. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 440–445. [Google Scholar] [CrossRef]
- Moura, E.; Afonso, J.; Hein, L.; Vieira-Coelho, M.A. α2-Adrenoceptor subtypes involved in the regulation of catecholamine release from the adrenal medulla of mice. Br. J. Pharmacol. 2006, 149, 1049–1058. [Google Scholar] [CrossRef]
- Lehto, J.; Scheinin, A.; Johansson, J.; Marjamäki, P.; Arponen, E.; Scheinin, H.; Scheinin, M. Detecting a dexmedetomidine-evoked reduction of noradrenaline release in the human brain with the alpha2C-adrenoceptor PET ligand [11C]ORM-13070. Synapse 2016, 70, 57–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.-L.; Lu, T.-J.; Wu, S.-N. Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α2-Adrenergic Receptors. Int. J. Mol. Sci. 2020, 21, 9110. https://doi.org/10.3390/ijms21239110
Lu T-L, Lu T-J, Wu S-N. Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α2-Adrenergic Receptors. International Journal of Molecular Sciences. 2020; 21(23):9110. https://doi.org/10.3390/ijms21239110
Chicago/Turabian StyleLu, Te-Ling, Te-Jung Lu, and Sheng-Nan Wu. 2020. "Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α2-Adrenergic Receptors" International Journal of Molecular Sciences 21, no. 23: 9110. https://doi.org/10.3390/ijms21239110
APA StyleLu, T.-L., Lu, T.-J., & Wu, S.-N. (2020). Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α2-Adrenergic Receptors. International Journal of Molecular Sciences, 21(23), 9110. https://doi.org/10.3390/ijms21239110




