The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility
Abstract
:1. Introduction
2. Results
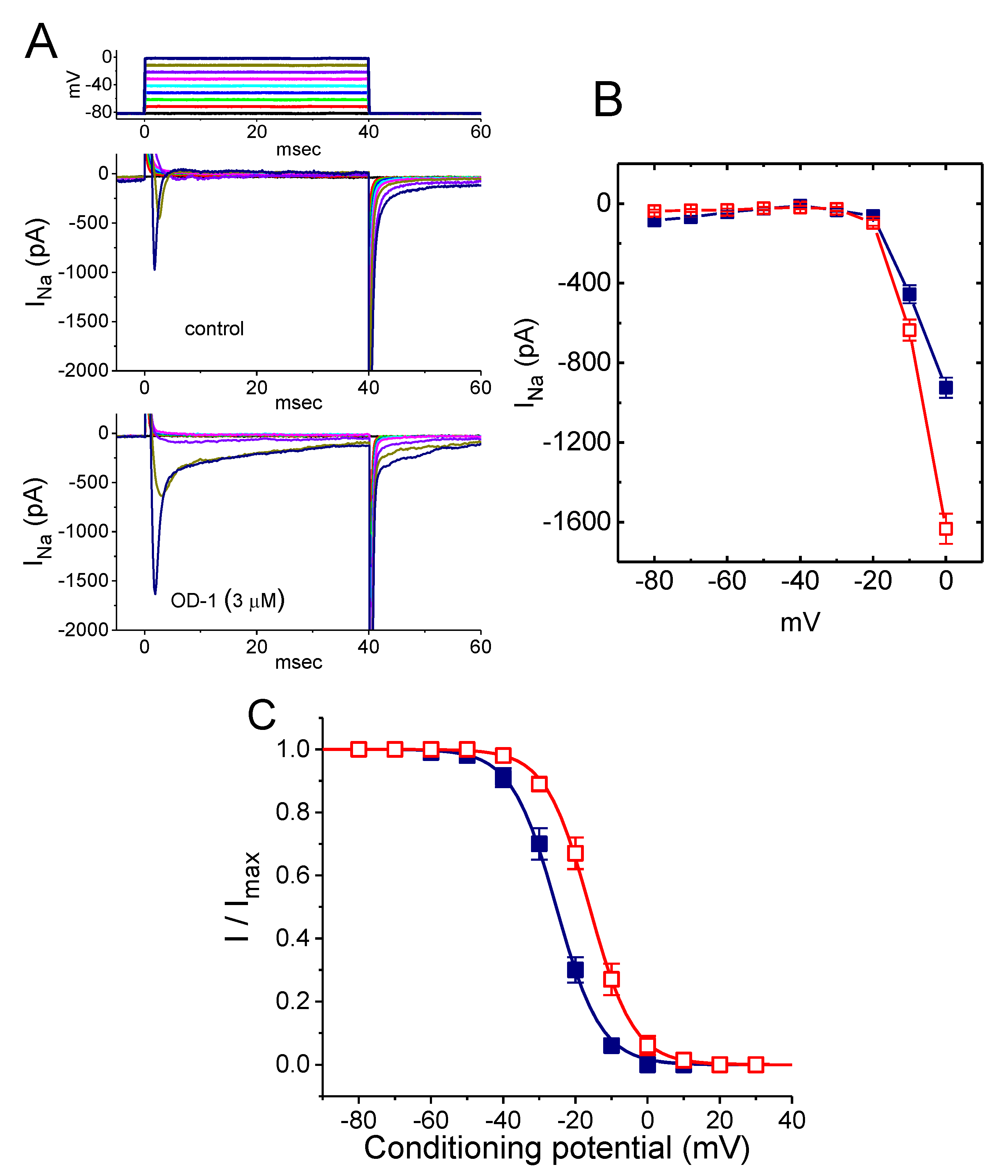
2.1. Effect of OD-1 on the Voltage-Gated Na+ Current (INa)
2.2. Kinetic Study of OD-1-Induced Stimulation of INa
2.3. Stimulatory Effect of OD-1 on the Conductance–Voltage Relationship of Peak INa
2.4. Effect of OD-1 on the Steady-State Inactivation Curve of INa
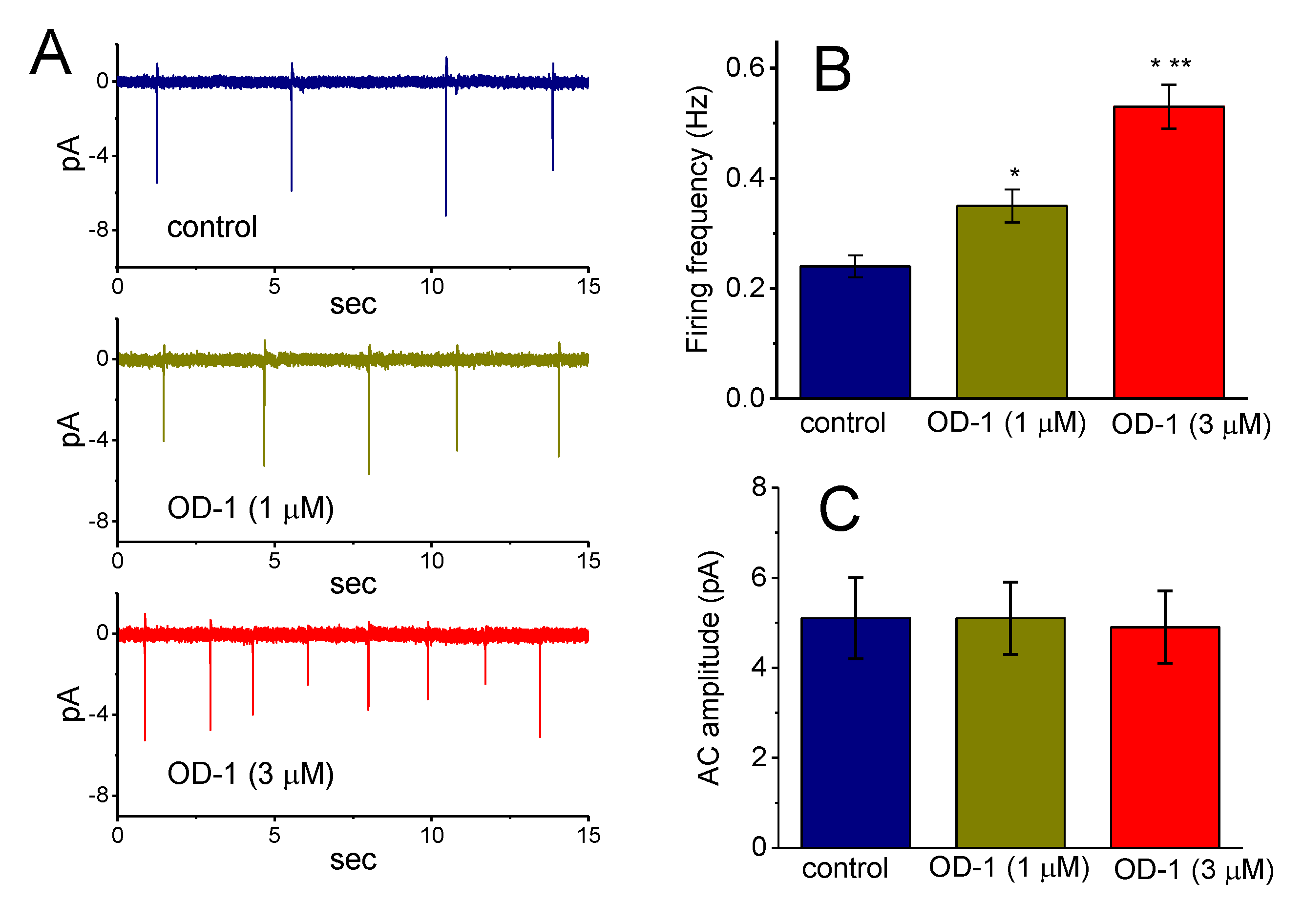
2.5. Effects of OD-1 on Spontaneous ACs
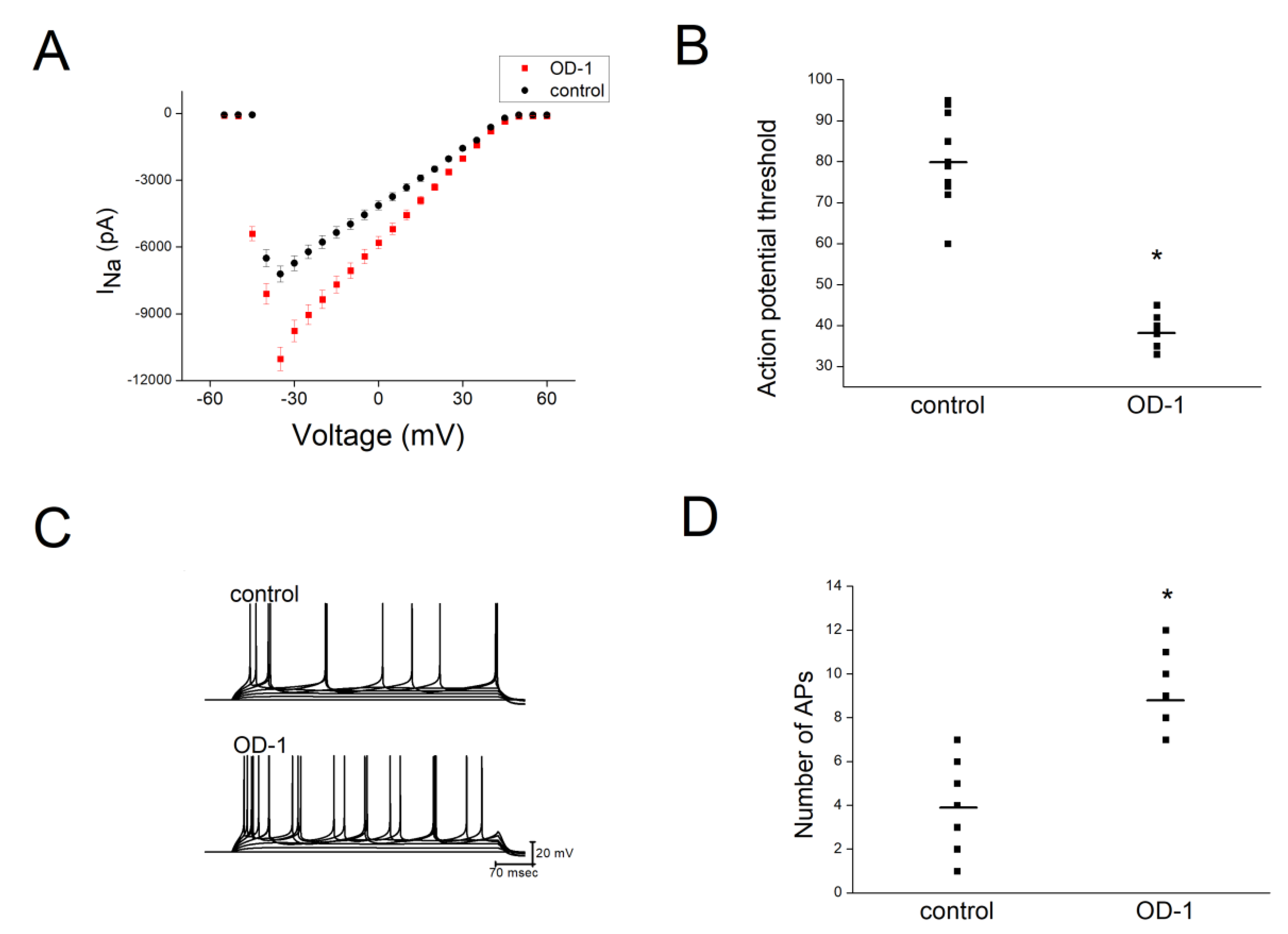
2.6. OD-1 Enhanced Neuronal Excitability in Hippocampal Slices
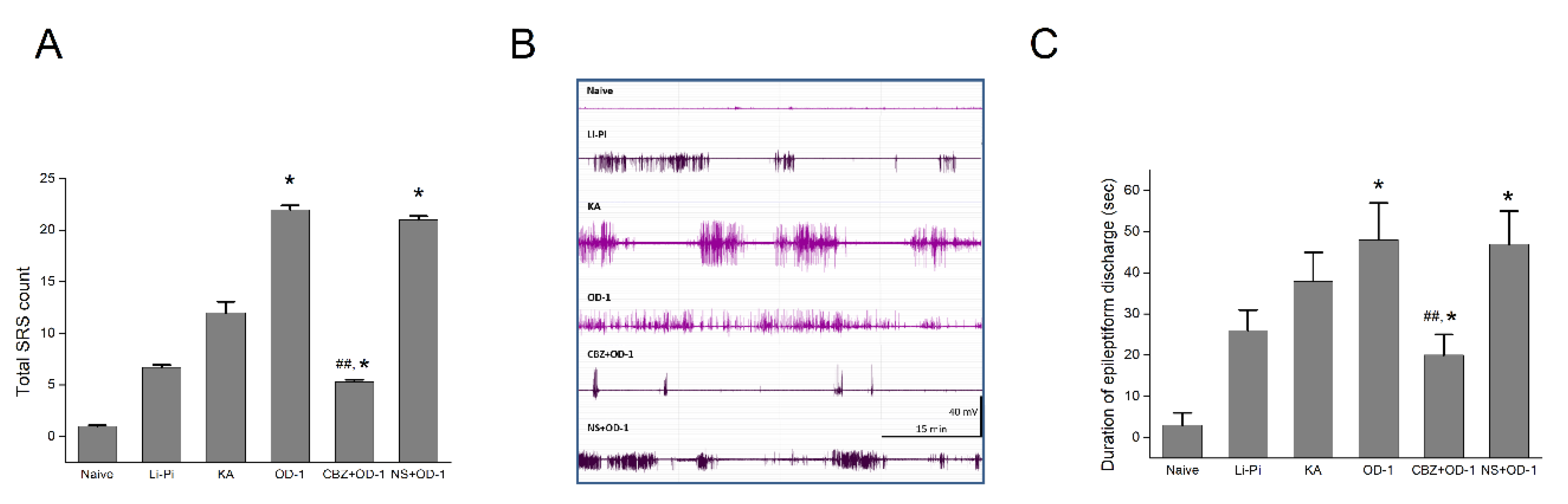
2.7. SRS and Epileptiform Discharges Following a Stereotactic Hippocampal Injection of OD-1
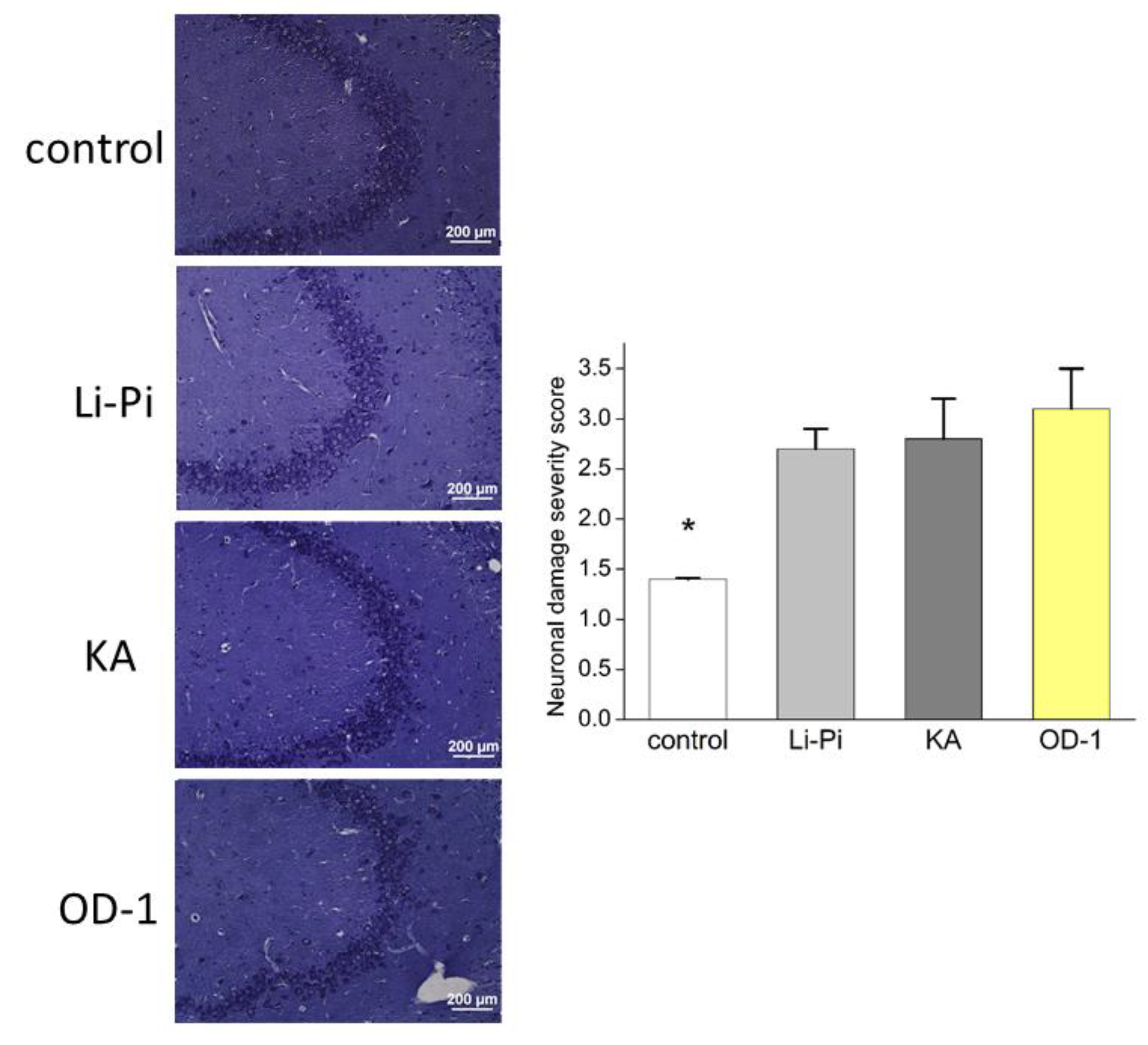
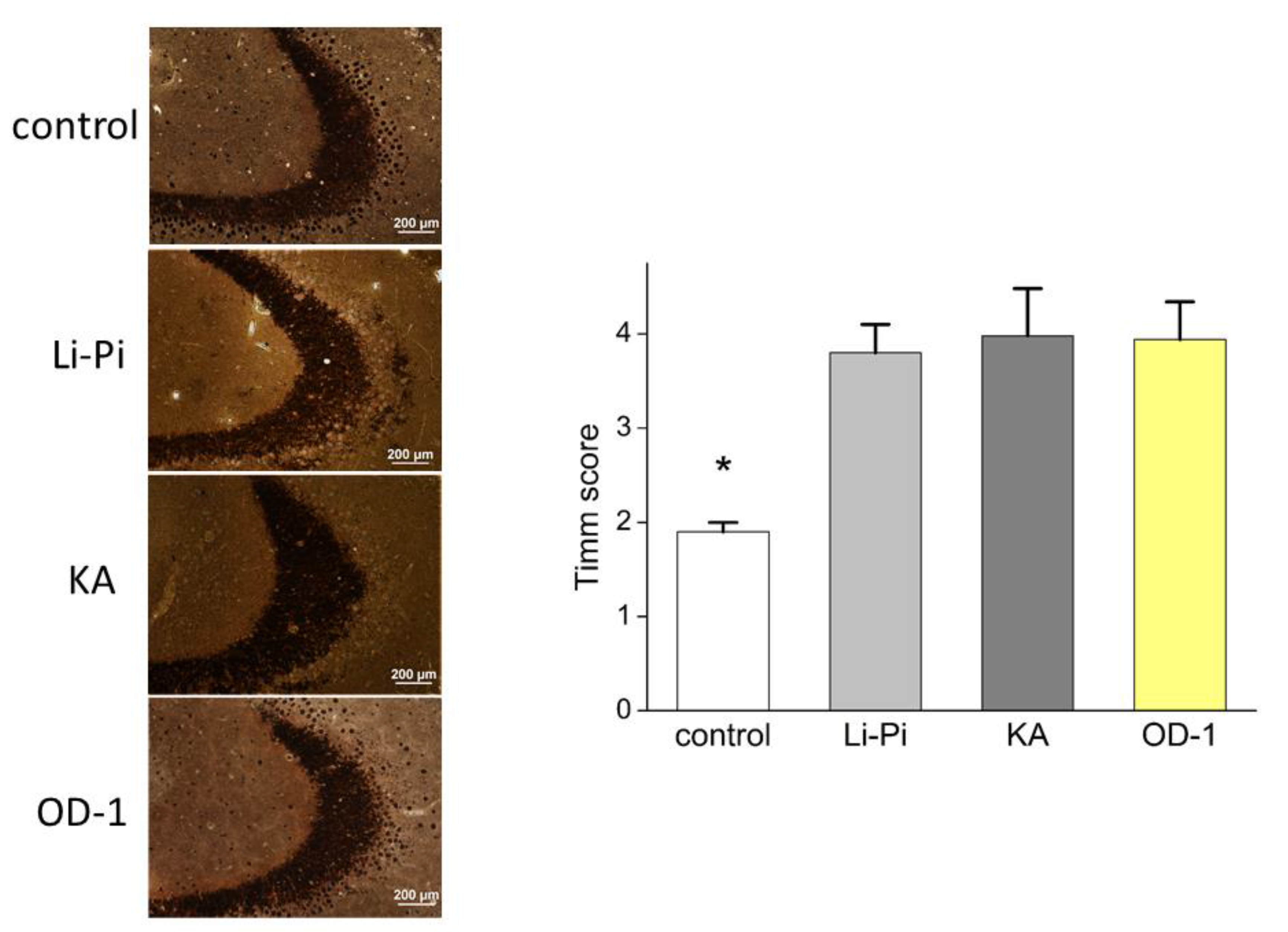
2.8. Hippocampal Neuronal Damage and Aberrant Network Following Stereotactic Hippocampal Injection of OD-1
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Data Analyses
4.5. Animal Experiments
4.6. Preparation of Brain Slices and Patch Clamp Technology
4.7. Animal Seizure Modeling
4.8. Spontaneous Recurrent Seizure (SRS)
4.9. Electroencephalography (EEG)
4.10. Histopathology
4.10.1. Cresyl Violet Staining
4.10.2. Timm’s Staining
4.10.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Action current |
| AP | Action potential |
| EC50 | 50% stimulatory concentration |
| I–V | current versus voltage |
| INa | voltage-gated Na+ current |
| KD | dissociation constant |
| NaV | voltage-gated Na+ channel |
| SEM | standard error of mean |
| τ | time constant |
References
- Jalali, A.; Bosmans, F.; Amininasab, M.; Clynen, E.; Cuypers, E.; Zaremirakabadi, A.; Sarbolouki, M.N.; Schoofs, L.; Vatanpour, H.; Tytgat, J. OD1, the first toxin isolated from the venom of the scorpion Odonthobuthus doriae active on voltage-gated Na+ channels. FEBS Lett. 2005, 579, 4181–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maertens, C.; Cuypers, E.; Amininasab, M.; Jalali, A.; Vatanpour, H.; Tytgat, J. Potent modulation of the voltage-gated sodium channel Nav1.7 by OD1, a toxin from the scorpion Odonthobuthus doriae. Mol. Pharmacol. 2006, 70, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, A.H.; Xiao, Y.; Scales, J.; Linse, K.D.; Rowe, M.P.; Cummins, T.R.; Zakon, H.H. Isolation and characterization of CvIV4: A pain inducing alpha-scorpion toxin. PLoS ONE 2011, 6, e23520. [Google Scholar] [CrossRef] [PubMed]
- Motin, L.; Durek, T.; Adams, D.J. Modulation of human Nav1.7 channel gating by synthetic alpha-scorpion toxin OD1 and its analogs. Channels (Austin) 2016, 10, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durek, T.; Vetter, I.; Wang, C.I.; Motin, L.; Knapp, O.; Adams, D.J.; Lewis, R.J.; Alewood, P.F. Chemical engineering and structural and pharmacological characterization of the alpha-scorpion toxin OD1. ACS Chem. Biol. 2013, 8, 1215–1222. [Google Scholar] [CrossRef]
- Deuis, J.R.; Wingerd, J.S.; Winter, Z.; Durek, T.; Dekan, Z.; Sousa, S.R.; Zimmermann, K.; Hoffmann, T.; Weidner, C.; Nassar, M.A.; et al. Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of NaV1.7-mediated pain. Toxin 2016, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory features of the novel spider toxin μ-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International union of pharmacology: XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Morinville, A.; Fundin, B.; Meury, L.; Jureus, A.; Sandberg, K.; Krupp, J.; Ahmad, S.; O’Donnell, D. Distribution of the voltage-gated sodium channel Nav1.7 in the rat: Expression in the autonomic and endocrine systems. J. Comp. Neurol. 2007, 504, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Lin, M.W.; Lin, A.A.; Wu, S.N. Riluzole-induced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life Sci. 2008, 82, 11–20. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.; Yoo, J.; Gong, X.; Liu, D.; Han, Q.; Luo, X.; Chang, W.; Chen, G.; Im, S.T.; Kim, Y.H.; et al. Differential Inhibition of Nav1.7 and Neuropathic Pain by Hybridoma-Produced and Recombinant Monoclonal Antibodies that Target Nav1.7: Differential activities of Nav1.7-targeting monoclonal antibodies. Neurosci. Bull. 2018, 34, 22–41. [Google Scholar] [CrossRef]
- Chang, W.; Berta, T.; Kim, Y.H.; Lee, S.; Lee, S.Y.; Ji, R.R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018, 34, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yu, F.H.; Sharp, E.M.; Beacham, D.; Scheuer, T.; Catterall, W.A. Functional properties and differential neuromodulation of Nav1.6 channels. Mol. Cell. Neurosci. 2008, 38, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.; Cannon, S.C. Cold-induced defects of sodium channel gating in atypical periodic paralysis plus myotonia. Neurology 2008, 70, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Abdelsayed, M.; Sokolov, S. Voltage-gated sodium channels: Pharmacological targets via anticonvulsants to treat epileptic syndromes. Channels 2013, 7, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Fruscione, F.; Valente, P.; Sterlini, B.; Romei, A.; Baldassari, S.; Fadda, M.; Prestigio, C.; Giansante, G.; Sartorelli, J.; Rossi, P.; et al. PPRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain 2018, 141, 1000–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchi, N.; Fan, Q.; Ghosh, C.; Fazio, V.; Bertolini, F.; Betto, G.; Batra, A.; Carlton, E.; Najm, I.; Granata, T.; et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis. 2009, 33, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Vezzani, A.; Balosso, S.; Ravizza, T. The role of cytokines in the pathophysiology of epilepsy. Brain. Behav. Immun. 2008, 22, 797–803. [Google Scholar] [CrossRef]
- Vezzani, A. Pilocarpine-induced seizures revisited: What does the model mimic? Epilepsy Curr. 2009, 9, 146–148. [Google Scholar] [CrossRef] [Green Version]
- Swann, J.W.; Al-Noori, S.; Jiang, M.; Lee, C.L. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 2000, 10, 617–625. [Google Scholar] [CrossRef]
- Blume, W.T. The progression of epilepsy. Epilepsia 2006, 47, s71–s78. [Google Scholar] [CrossRef]
- Becker, A.J. Review: Animal models of acquired epilepsy: Insights into mechanisms of human epileptogenesis. Neuropathol. Appl. Neurobiol. 2018, 44, 112–129. [Google Scholar] [CrossRef]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative, on voltage-gated Na+ and Ca2+-activated K+ currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.; Vatanpour, H.; Hosseininasab, Z.; Rowan, E.G.; Harvey, A.L. The effect of the venom of the yellow Iranian scorpion Odontobuthus doriae on skeletal muscle preparations in vitro. Toxicon 2007, 50, 1019–1026. [Google Scholar] [CrossRef]
- Neto, E.B.; Mariano, D.O.C.; Freitas, L.A.; Dorce, A.L.C.; Martins, A.N.; Pimenta, D.C.; Portaro, F.C.V.; Cajado-Carvalho, D.; Dorce, V.A.C.; Nencioni, A.L.A. Tb II-I, a fraction isolated from Tityus bahiensis scorpion venom, alters cytokines’ level and induces seizures when intrahippocampally injected in rats. Toxins 2018, 10, 250. [Google Scholar] [CrossRef] [Green Version]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, I.; Scamps, F.; Chabbert, C.; Sans, A.; Valmier, J. Molecular diversity of voltage-gated sodium channel alpha subunits expressed in neuronal and non-neuronal excitable cells. Neuroscience 2005, 130, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Solé, L.; Johnson, B.; Beheiry, M.E.; Masson, J.B.; Krapf, D.; Tamkun, M.M. Single-imaging of Nav1.6 on the surface of hippocampal neurons reveals somatic nanoclusters. Biophys. J. 2016, 111, 1235–1247. [Google Scholar] [CrossRef]
- Sikora, G.; Wylomańska, A.; Gajda, J.; Solé, L.; Akin, E.J.; Tamkun, M.M.; Krapf, D. Elucidating distinct ion channel populations on the surface of hippocampal neurons via single-particle tracking recurrence analysis. Phys. Rev. E. 2017, 96, 062404. [Google Scholar] [CrossRef] [Green Version]
- Sojo, L.E.; Kwan, R.; Dang, C.; Tung, M.; Li, J. On the feasibility of quantifying sodium channel Nav 1.6 protein in mouse brain using targeted ultra-high-performance/electrospray ionization multiple reaction monitoring mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 683–696. [Google Scholar]
- McKerrall, S.J.; Sutherlin, D.P. Na(v)1.7 inhibitors for the treatment of chronic pain. Bioorg. Med. Chem. Lett. 2018, 28, 3141–3149. [Google Scholar] [CrossRef]
- Pitkanen, A.; Schwartzkroin, P.A.; Moshe, S.M. Models of Seizures and Epilepsy; Elsevier Academic Press: Burlington, MA, USA, 2006. [Google Scholar]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptors alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Lai, M.C.; Hung, T.Y.; Lin, K.M.; Huang, C.W.; Wu, S.N. Pioglitazone, a PPAR-γ activator, stimulates BKCa but suppresses IKM in hippocampal neurons. Front. Pharmacol. 2018, 9, 977. [Google Scholar] [CrossRef]
- Lai, M.C.; Tzeng, R.C.; Huang, C.W.; Wu, S.N. The Novel Direct Modulatory Effects of Perampanel, an Antagonist of AMPA Receptors, on Voltage-Gated Sodium and M-type Potassium Currents. Biomolecules 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3400–3421. [Google Scholar] [CrossRef]
- Yang, C.S.; Lai, M.C.; Liu, P.Y.; Lo, Y.C.; Huang, C.W.; Wu, S.N. Characterization of the Inhibitory Effect of Gastrodigenin and Gastrodin on M-type K(+) Currents in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 21, 117. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, H.T.; Lee, Y.C.; Liu, Y.C.; Kuo, P.C.; Wu, S.N. Differential suppression of delayed-rectifier and inwardly rectifier K+ currents by a group of ent-kaurane-type diterpenoids from Croton tonkinensis, in microglial cells. Eur. J. Pharmacol. 2019, 856, 172414. [Google Scholar] [CrossRef]
- Hung, T.Y.; Chu, F.L.; Wu, D.C.; Wu, S.N.; Huang, C.W. The protective role of peroxisome proliferator-activated receptor-gamma in seizure and neuronal excitotoxicity. Mol. Neurobiol. 2019, 56, 5497–5506. [Google Scholar] [CrossRef]
- Mello, L.E.; Cavalheiro, E.A.; Tan, A.M.; Kupfer, W.R.; Pretorius, J.K.; Babb, T.L.; Finch, D.M. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: Cell loss and mossy fiber sprouting. Epilepsia 1993, 34, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Huang, A.M.; Kuo, Y.M.; Wang, S.T.; Chang, Y.Y.; Huang, C.C. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann. Neurol. 2003, 54, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Hung, T.Y.; Lin, K.M.; Sung, P.S.; Wu, S.J.; Yang, C.S.; Wu, Y.J.; Tsai, J.J.; Wu, S.N.; Huang, C.W. Sodium Metabisulfite: Effects on Ionic Currents and Excitotoxicity. Neurotox. Res. 2018, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.-C.; Wu, S.-N.; Huang, C.-W. The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility. Int. J. Mol. Sci. 2020, 21, 8254. https://doi.org/10.3390/ijms21218254
Lai M-C, Wu S-N, Huang C-W. The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility. International Journal of Molecular Sciences. 2020; 21(21):8254. https://doi.org/10.3390/ijms21218254
Chicago/Turabian StyleLai, Ming-Chi, Sheng-Nan Wu, and Chin-Wei Huang. 2020. "The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility" International Journal of Molecular Sciences 21, no. 21: 8254. https://doi.org/10.3390/ijms21218254




