Multi-Walled Carbon Nanotubes Can Promote Brassica napus L. and Arabidopsis thaliana L. Root Hair Development through Nitric Oxide and Ethylene Pathways
Abstract
1. Introduction
2. Results
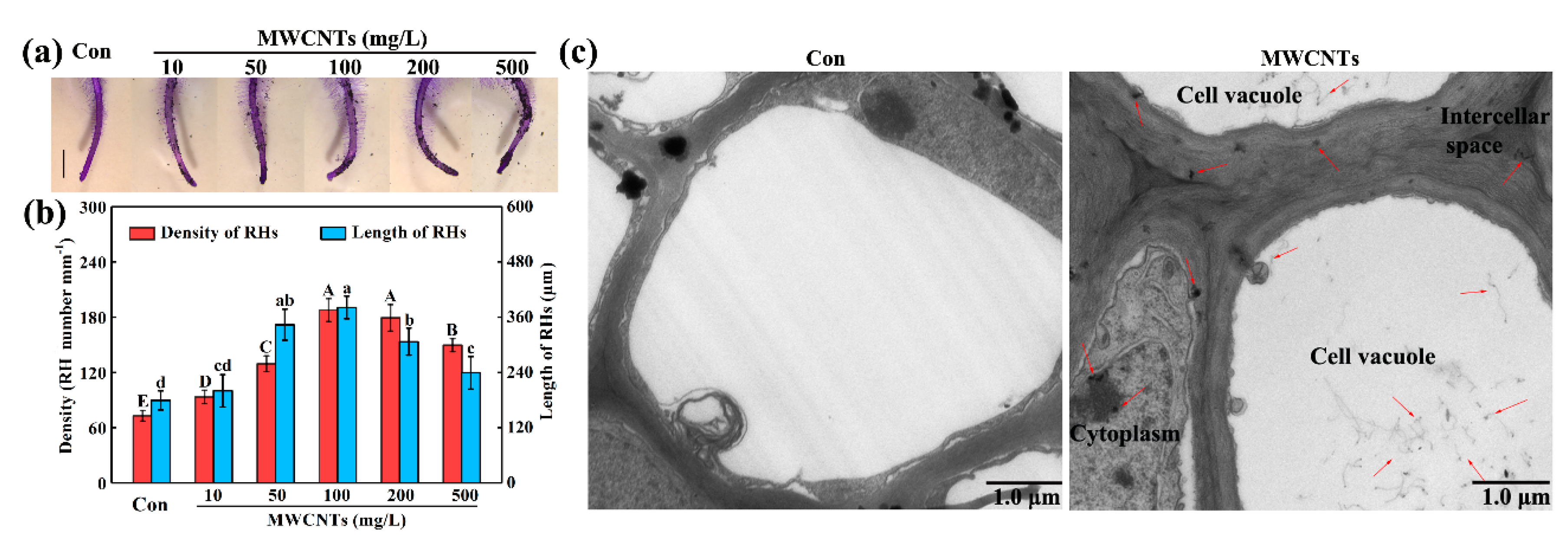
2.1. MWCNTs-Stimulated Root Hair Growth and the Distribution of MWCNTs
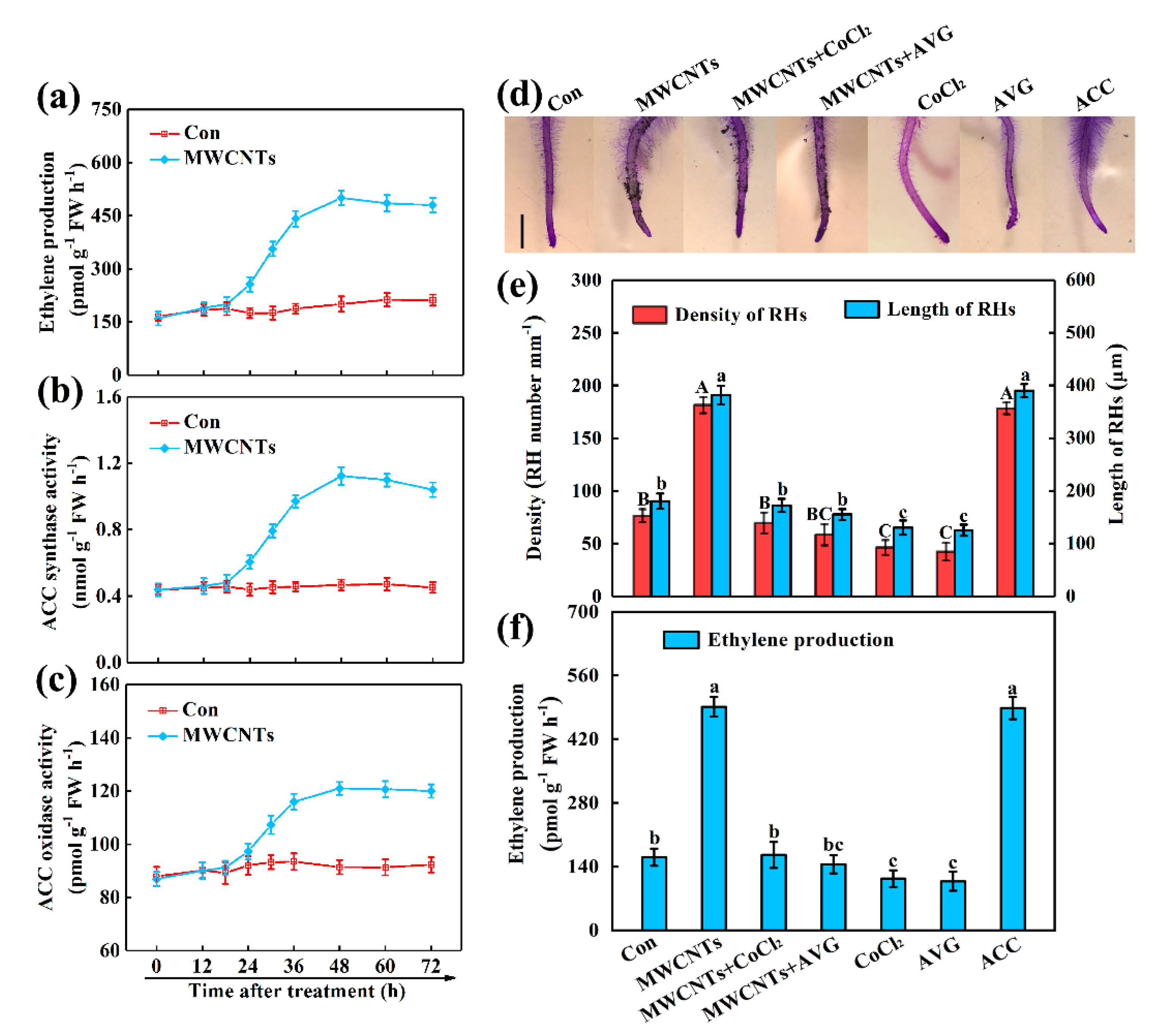
2.2. Ethylene Was Involved in MWCNTs-Induced Root Hair Development
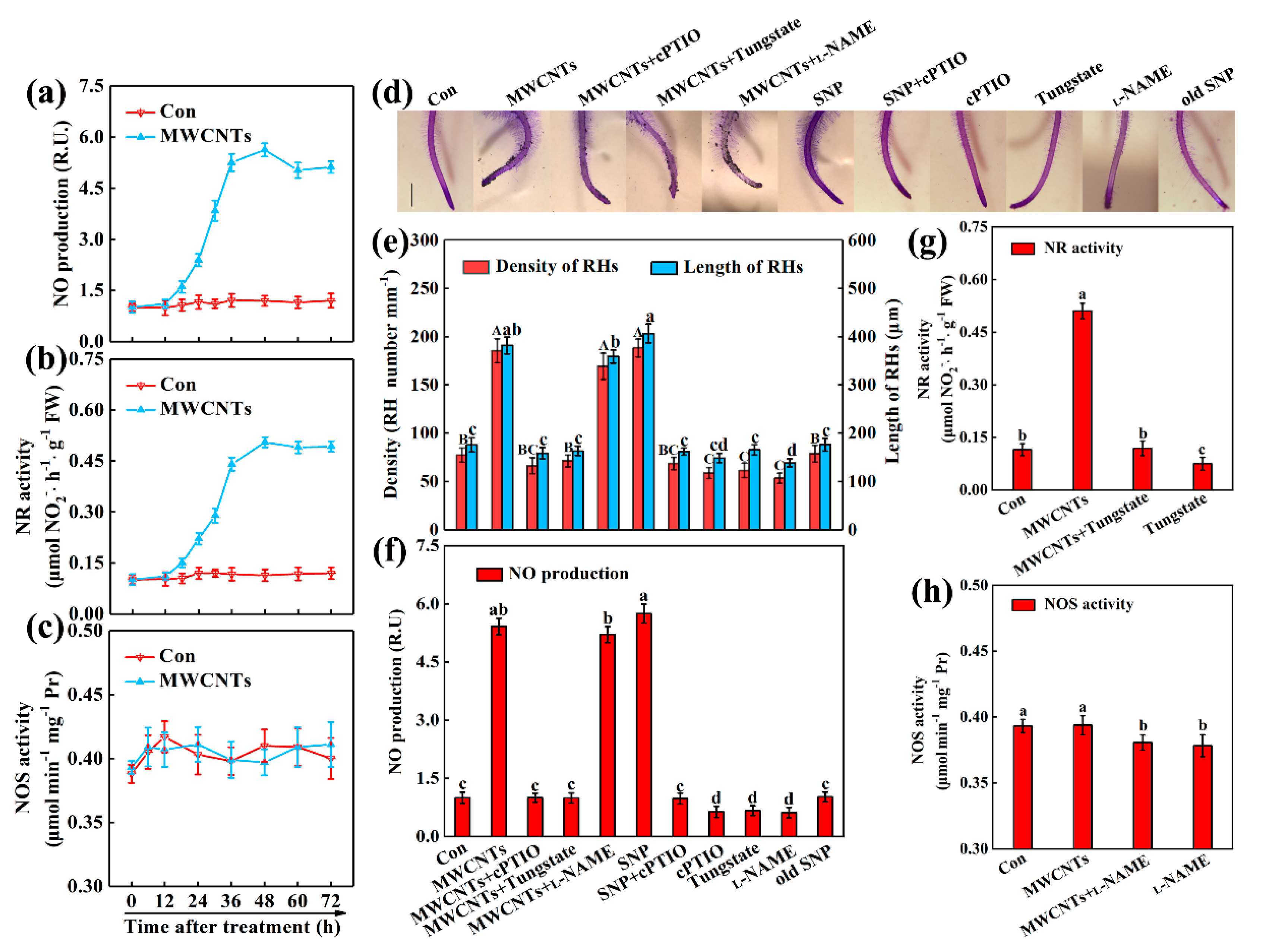
2.3. NR-Dependent NO Was Associated with MWCNTs-Induced Root Hair Development
2.4. Ethylene Acts Downstream of NO in MWCNTs-Induced Root Hair Growth
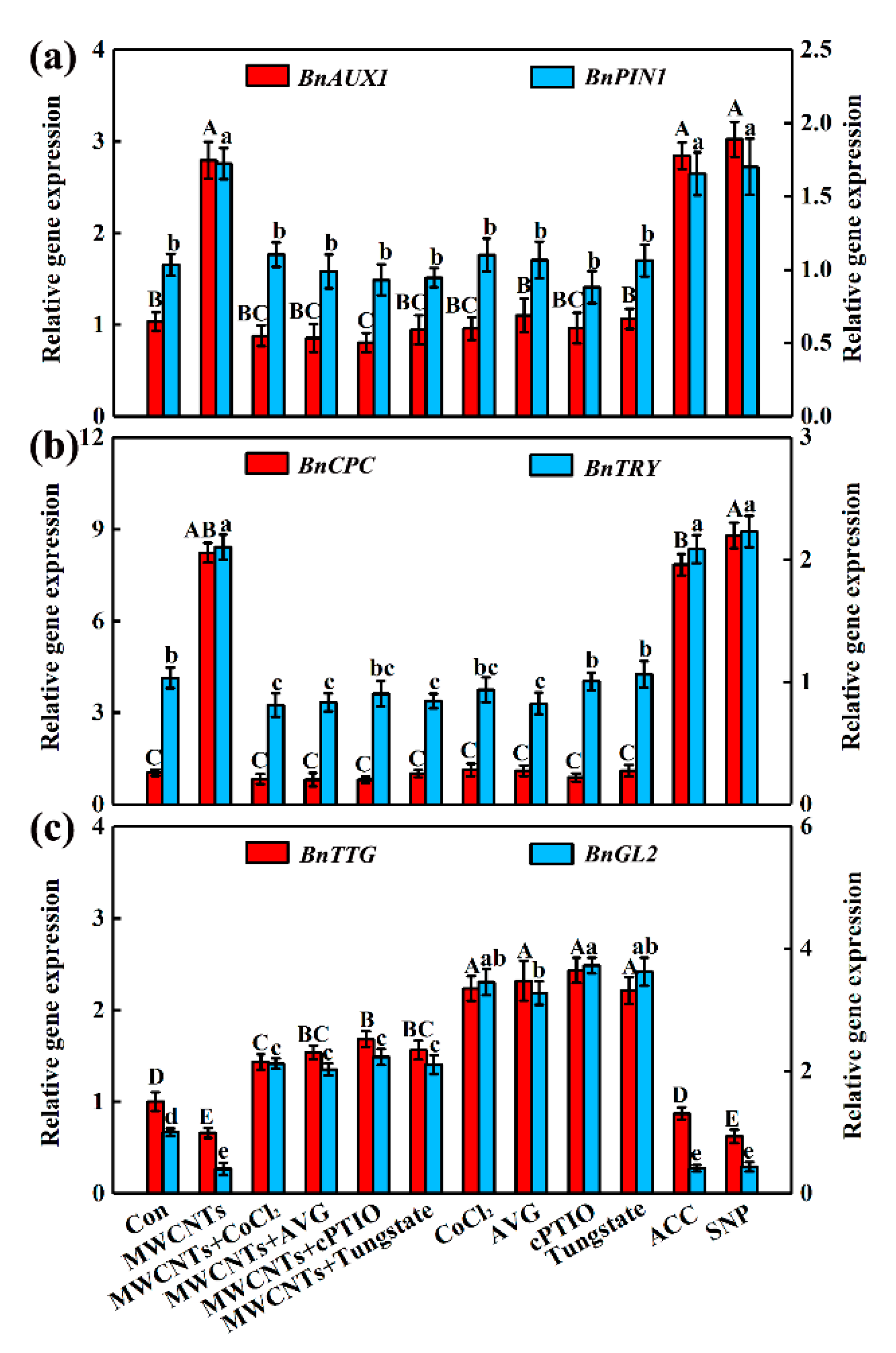
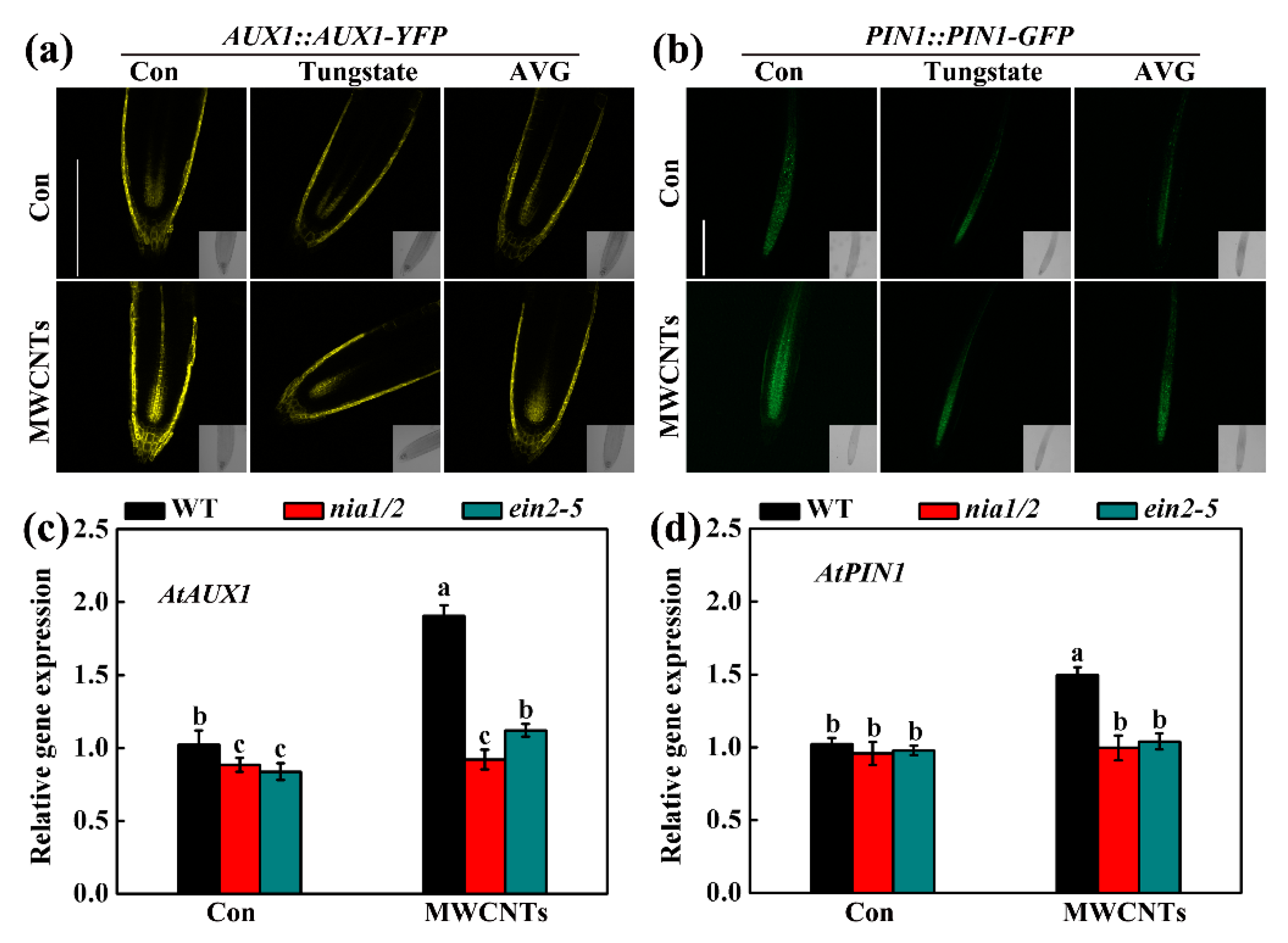
2.5. MWCNTs-Modulated Transcripts Related to Root Hair Development were Dependent on NO and Ethylene Synthesis
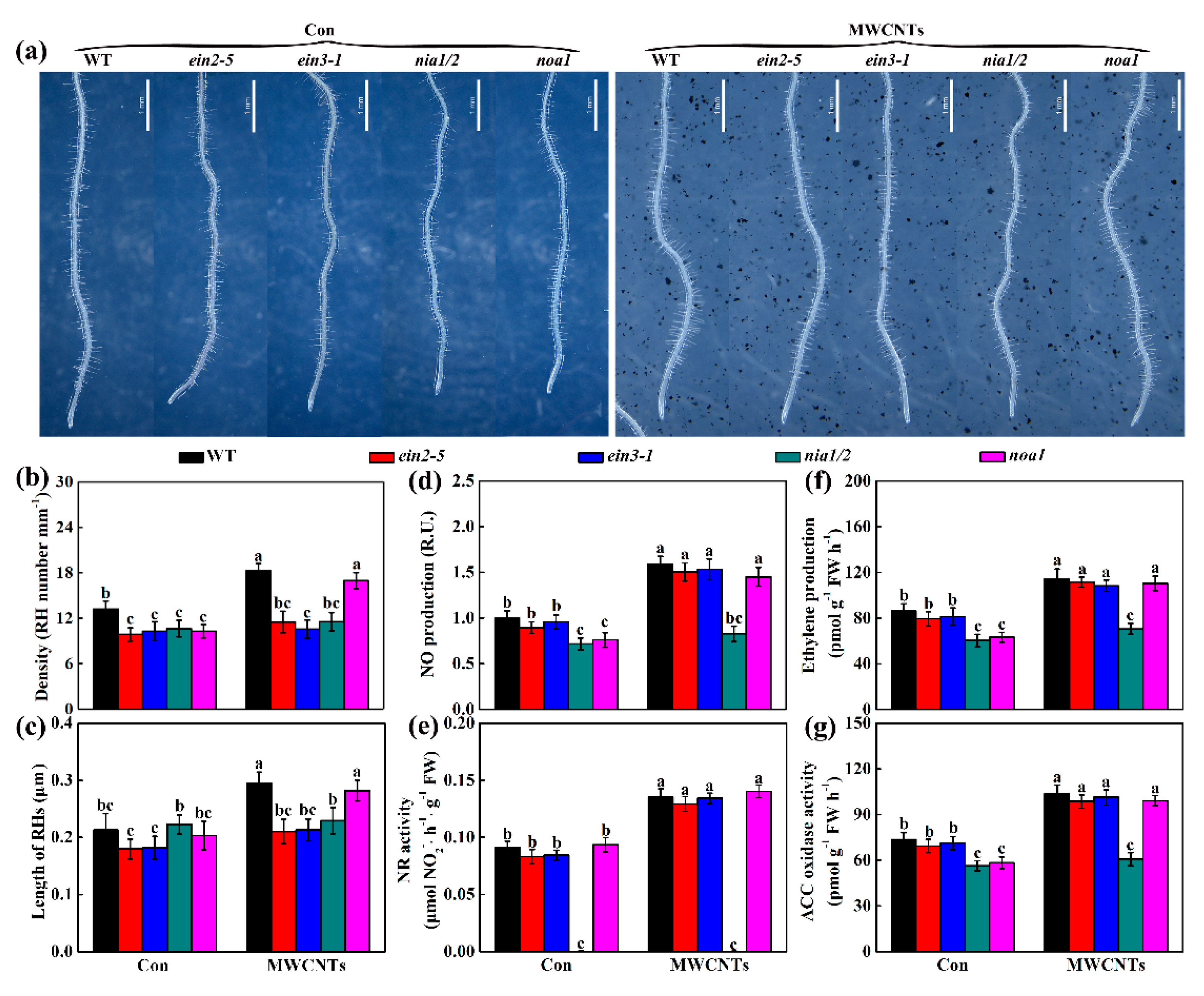
2.6. Genetic Evidence Revealed that Ethylene and NR-Dependent NO Were Associated with MWCNTs-Induced Root Hair Development
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Growth Conditions
4.3. Measurement of Root Hairs
4.4. Detection of MWCNTs Distribution
4.5. Measurement of Ethylene Production, ACC Oxidase, and ACC Synthase Activities
4.6. Determination of NO Content, Nitrate Reductase (NR), and NO Synthase (NOS) Activities
4.7. Analysis of Gene Transcription
4.8. Experimental Design
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNTs | carbon nanotubes |
| MWCNTs | multi-walled carbon nanotube |
| NR | nitrate reductase |
| NO | nitric oxide |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| AVG | aminoethoxyvinylglycine |
| NOS | nitric oxide synthase |
| SNP | sodium nitroprusside |
| c-PTIO | 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide |
| L-NAME | NG-Nitro-L-Arginine Methyl Ester Hydrochloride |
| WT | wild type |
References
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nat. Cell Biol. 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Liné, C.; LaRue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, C.; Chen, H.; Duoni; Yu, X.; Qian, W. The Application of Carbon Nanotube/Graphene-Based Nanomaterials in Wastewater Treatment. Small 2019, 16, e1902301. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Baba, Y. Design for DNA Separation Medium Using Bacterial Cellulose Fibrils. Anal. Chem. 2005, 77, 7090–7093. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Zhao, J.; Zhao, Q.; Xing, B. Uptake of Engineered Nanoparticles by Food Crops: Characterization, Mechanisms, and Implications. Annu. Rev. Food Sci. Technol. 2018, 9, 129–153. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Panigrahy, M.; Sahoo, P.K.; Panigrahi, K.C.S. Carbon nanoparticles influence photomorphogenesis and flowering time in Arabidopsis thaliana. Plant Cell Rep. 2018, 37, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Wang, R.; Guan, R.; Pu, H.; Shen, W. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale 2019, 11, 10511–10523. [Google Scholar] [CrossRef]
- Zaytseva, O.; Wang, Z.; Neumann, G. Phytotoxicity of carbon nanotubes in soybean as determined by interactions with micronutrients. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Das, K.K.; You, Y.; Torres, M.; Barrios-Masias, F.H.; Wang, X.; Tao, S.; Xing, B.; Yang, Y. Development and application of a digestion-Raman analysis approach for studying multiwall carbon nanotube uptake in lettuce. Environ. Sci. Nano 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Fan, X.; Xu, J.; Lavoie, M.; Peijnenburg, W.; Zhu, Y.; Lu, T.; Ota, T.; Zhu, T.; Qian, H. Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana. Environ. Pollut. 2018, 233, 633–641. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Kumar, V.; Sachdev, D.; Pasricha, R.; Maheshwari, P.H.; Taneja, N.K. Zinc-Supported Multiwalled Carbon Nanotube Nanocomposite: A Synergism to Micronutrient Release and a Smart Distributor to Promote the Growth of Onion Seeds in Arid Conditions. ACS Appl. Mater. Interfaces 2018, 10, 36733–36745. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Chen, J.; Tian, Y.; Shi, J.; Li, D.; Guo, C.; Ma, Q. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J. Hazard. Mater. 2014, 274, 404–412. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Xu, P.; Zhang, C.; Cheng, M.; Xue, W.; Chen, S. Roles of multiwall carbon nanotubes in phytoremediation: Cadmium uptake and oxidative burst in Boehmeria nivea (L.) Gaudich. Environ. Sci. Nano 2019, 6, 851–862. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ketelaar, T. The actin cytoskeleton in root hairs: All is fine at the tip. Curr. Opin. Plant Biol. 2013, 16, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Roberts, K.J.; Dolan, L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 1995, 8, 943–948. [Google Scholar] [CrossRef]
- Dolan, L. The role of ethylene in root hair growth in Arabidopsis. J. Plant Nutr. Soil Sci. 2001, 164, 141–145. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.X.; He, X.L.; Liu, L.J.; Wu, H.; Tang, C.X.; Zhang, Y.S.; Jin, C.W. Ethylene and nitric oxide interact to regulate the magnesium deficiency-induced root hair development in Arabidopsis. New Phytol. 2017, 213, 1242–1256. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, P.; Li, B.; Li, P.; Wen, X.; An, F.; Gong, Y.; Xin, Y.; Zhu, Z.; Wang, Y.; et al. Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 13834–13839. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant. Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Prasad, M.E.; Schofield, A.; Lyzenga, W.; Liu, H.; Stone, S.L. Arabidopsis RING E3 Ligase XBAT32 Regulates Lateral Root Production through Its Role in Ethylene Biosynthesis. Plant. Physiol. 2010, 153, 1587–1596. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; LaMattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant. Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.C.; LaMattina, L. Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J. Exp. Bot. 2012, 63, 4875–4885. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.F.; Gaspar, M.; Da Silva, F.R.; Pattathil, S.; Hahn, M.G.; Salgado, I.; Braga, M.R. S-nitrosoglutathione promotes cell wall remodelling, alters the transcriptional profile and induces root hair formation in the hairless root hair defective 6 (rhd6) mutant of Arabidopsis thaliana. New Phytol. 2016, 213, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.; Fang, X.; Zhang, Y.S.; Jin, C. Auxin Acts Downstream of Ethylene and Nitric Oxide to Regulate Magnesium Deficiency-Induced Root Hair Development in Arabidopsis thaliana. Plant. Cell Physiol. 2018, 59, 1452–1465. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhu, C.Q.; Wang, C.; Dong, X.Y.; Shen, R.F. Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice. J. Exp. Bot. 2017, 68, 753–760. [Google Scholar] [CrossRef]
- Xu, X.-T.; Jin, X.; Liao, W.-B.; Dawuda, M.M.; Li, X.-P.; Wang, M.; Niu, L.-J.; Ren, P.-J.; Zhu, Y. Nitric oxide is involved in ethylene-induced adventitious root development in cucumber (Cucumis sativus L.) explants. Sci. Hortic. 2017, 215, 65–71. [Google Scholar] [CrossRef]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant. Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef]
- Gale, M.D. Plant Comparative Genetics after 10 Years. Science 1998, 282, 656–659. [Google Scholar] [CrossRef]
- Ledon, T.; Valle, E.; Valmaseda, T.; Cedré, B.; Campos, J.; Rodríguez, B.L.; Marrero, K.; García, H.; García, L.; Fando, R.; et al. Construction and characterisation of O139 cholera vaccine candidates. Vaccine 2003, 21, 1282–1291. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2016, 40, 462–472. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, H.; Kong, L.; Li, L.; Wang, R.; Shen, W. A Novel Mechanism Underlying Multi-walled Carbon Nanotube-Triggered Tomato Lateral Root Formation: The Involvement of Nitric Oxide. Nanoscale Res. Lett. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Zheng, T.; Shen, W. Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J. Exp. Bot. 2013, 64, 3045–3060. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; LaMattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric Oxide Acts Downstream of Auxin to Trigger Root Ferric-Chelate Reductase Activity in Response to Iron Deficiency in Arabidopsis. Plant. Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef]
- Lozano-Juste, J.; León, J. Enhanced Abscisic Acid-Mediated Responses in nia1nia2noa1-2 Triple Mutant Impaired in NIA/NR- and AtNOA1-Dependent Nitric Oxide Biosynthesis in Arabidopsis. Plant. Physiol. 2010, 152, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Desikan, R.; Hancock, J.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant. J. 2005, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Huang, J.-J.; Bi, Y.-R. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant. Sci. 2010, 179, 281–288. [Google Scholar] [CrossRef]
- Hu, Y.; You, J.; Liang, X. Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant. Cell Rep. 2014, 34, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Schiefelbein, J. Cell-fate specification in the epidermis: A common patterning mechanism in the root and shoot. Curr. Opin. Plant. Biol. 2003, 6, 74–78. [Google Scholar] [CrossRef]
- Schiefelbein, J. Constructing a Plant Cell. The Genetic Control of Root Hair Development. Plant. Physiol. 2000, 124, 1525–1531. [Google Scholar] [CrossRef]
- Molendijk, A.J.; Bischoff, F.; Rajendrakumar, C.S.; Friml, J.; Braun, M.; Gilroy, S.; Palme, K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001, 20, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Shen, J.-J.; Fu, Y.; Li, H.; Yang, Z.; Grierson, C. The Arabidopsis Rop2 GTPase Is a Positive Regulator of Both Root Hair Initiation and Tip Growth. Plant Cell 2002, 14, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene Regulates Root Growth through Effects on Auxin Biosynthesis and Transport-Dependent Auxin Distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Jones, A.R.; Kramer, E.M.; Knox, K.; Swarup, R.; Bennett, M.J.; Lazarus, C.M.; Leyser, O.; Grierson, C.S. Auxin transport through non-hair cells sustains root-hair development. Nat. Cell Biol. 2009, 11, 78–84. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Kong, D.-D.; Gu, X.-X.; Gao, H.-B.; Wang, J.-Z.; Xia, M.; Gao, Q.; Tian, L.-L.; Xu, Z.-H.; Bao, F.; et al. Cytokinins can act as suppressors of nitric oxide inArabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1548–1553. [Google Scholar] [CrossRef]
- Binder, B.; Mortimore, L.A.; Stepanova, A.N.; Ecker, J.R.; Bleecker, A.B. Short-Term Growth Responses to Ethylene in Arabidopsis Seedlings Are EIN3/EIL1 Independent. Plant. Physiol. 2004, 136, 2921–2927. [Google Scholar] [CrossRef]
- Qiao, H.; Chang, K.N.; Yazaki, J.; Ecker, J.R. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 2009, 23, 512–521. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.-S.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and Subcellular Trafficking of ER-Tethered EIN2 Control Response to Ethylene Gas. Sci. 2012, 338, 390–393. [Google Scholar] [CrossRef]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-Induced Stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 Is Mediated by Proteasomal Degradation of EIN3 Binding F-Box 1 and 2 That Requires EIN2 in Arabidopsis. Plant. Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Guo, K.; Kong, W.W.; Yang, Z.M. Carbon monoxide promotes root hair development in tomato. Plant Cell Environ. 2009, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.D.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 456–477. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Zhao, Y.; Lou, W.; Abdalmegeed, D.; Guan, R.; Shen, W. Multi-Walled Carbon Nanotubes Can Promote Brassica napus L. and Arabidopsis thaliana L. Root Hair Development through Nitric Oxide and Ethylene Pathways. Int. J. Mol. Sci. 2020, 21, 9109. https://doi.org/10.3390/ijms21239109
Zhao G, Zhao Y, Lou W, Abdalmegeed D, Guan R, Shen W. Multi-Walled Carbon Nanotubes Can Promote Brassica napus L. and Arabidopsis thaliana L. Root Hair Development through Nitric Oxide and Ethylene Pathways. International Journal of Molecular Sciences. 2020; 21(23):9109. https://doi.org/10.3390/ijms21239109
Chicago/Turabian StyleZhao, Gan, Yingying Zhao, Wang Lou, Dyaaaldin Abdalmegeed, Rongzhan Guan, and Wenbiao Shen. 2020. "Multi-Walled Carbon Nanotubes Can Promote Brassica napus L. and Arabidopsis thaliana L. Root Hair Development through Nitric Oxide and Ethylene Pathways" International Journal of Molecular Sciences 21, no. 23: 9109. https://doi.org/10.3390/ijms21239109
APA StyleZhao, G., Zhao, Y., Lou, W., Abdalmegeed, D., Guan, R., & Shen, W. (2020). Multi-Walled Carbon Nanotubes Can Promote Brassica napus L. and Arabidopsis thaliana L. Root Hair Development through Nitric Oxide and Ethylene Pathways. International Journal of Molecular Sciences, 21(23), 9109. https://doi.org/10.3390/ijms21239109






