The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance
Abstract
1. Introduction
2. The Long Non-Coding RNA Repressor GAS5
3. GAS5 in Tumor Therapy-Related Resistance
3.1. GAS5 in Leukemia
3.2. GAS5 in Cervical Cancer
3.3. GAS5 in Breast Cancer
3.4. GAS5 in Ovarian Cancer
3.5. GAS5 in Prostate and Bladder Cancers
3.6. GAS5 in Lung Cancer
3.7. GAS5 in Gastric and Colorectal Cancers
3.8. GAS5 in Liver Cancer
3.9. GAS5 in Brain Tumors
3.10. GAS5 in Osteosarcoma
4. The Special Case of GAS5 and miRNAs
4.1. GAS5 and miRNAs in Leukemia
4.2. GAS5 and miRNAs in Cervical Cancer
4.3. GAS5 and miRNAs in Breast Cancer
4.4. GAS5 and miRNAs in Ovarian Cancer
4.5. GAS5 and miRNAs in Prostate and Bladder Cancers
4.6. GAS5 and miRNAs in Lung Cancer
4.7. GAS5 and miRNAs in Gastric and Colorectal Cancers
4.8. GAS5 and miRNAs in Liver Cancer
4.9. GAS5 and miRNAs in Brain Tumors
4.10. GAS5 and miRNAs in Osteosarcoma
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| AML | Acute Myeloid Leukemia |
| AR | Androgen Receptor |
| GAS5 | Growth Arrest Specific 5 |
| GR | Glucocorticoid Receptor |
| GRE | Glucocorticoid Response Element |
| lncRNAs | long non-coding RNAs |
| miRNA | microRNA |
| MR | Mineralocorticoid Receptor |
| MRE | Mineralocorticoid Response Element |
| ncRNA | non-coding RNA |
| NK | Natural Killer cells |
| piRNAs | piwi-interacting |
| PR | Progesterone Receptor |
| snoRNAs | small non-coding nucleolar RNAs |
| SRA | Steroid Receptor RNA Activator |
| TAD | Transactivation Domain |
References
- Lockhart, D.J.; Winzeler, E.A. Genomics, gene expression and DNA arrays. Nature 2000, 405, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Sherafatian, M.; Mowla, S.J. The origins and evolutionary history of human non-coding rna regulatory networks. J. Bioinform. Comput. Biol. 2017, 15, 1750005. [Google Scholar] [CrossRef] [PubMed]
- Jarroux, J.; Morillon, A.; Pinskaya, M. History, discovery, and classification of lncrnas. Adv. Exp. Med. Biol. 2017, 1008, 1–46. [Google Scholar] [PubMed]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. The functional genomics of noncoding rna. Science 2005, 309, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.C.; Frith, M.C.; Mattick, J.S. Rapid evolution of noncoding rnas: Lack of conservation does not mean lack of function. Trends Genet 2006, 22, 1–5. [Google Scholar] [CrossRef]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding rnas: Regulators of disease. J. Pathol. 2010, 220, 126–139. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding rnas in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Stefani, G.; Slack, F.J. Small non-coding rnas in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding rnas: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Katayama, S.; Tomaru, Y.; Kasukawa, T.; Waki, K.; Nakanishi, M.; Nakamura, M.; Nishida, H.; Yap, C.C.; Suzuki, M.; Kawai, J.; et al. Antisense transcription in the mammalian transcriptome. Science 2005, 309, 1564–1566. [Google Scholar] [PubMed]
- Schneider, C.; King, R.M.; Philipson, L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding rna gas5 is a growth arrest and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [PubMed]
- Garabedian, M.J.; Logan, S.K. Glucocorticoid receptor DNA binding decoy is a gas. Sci. Signal. 2010, 3, pe5. [Google Scholar] [CrossRef]
- Coccia, E.M.; Cicala, C.; Charlesworth, A.; Ciccarelli, C.; Rossi, G.B.; Philipson, L.; Sorrentino, V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biol. 1992, 12, 3514–3521. [Google Scholar] [CrossRef]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a multi-small-nucleolar-rna (snorna) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snorna host genes. Mol. Cell. Biol. 1998, 18, 6897–6909. [Google Scholar] [CrossRef]
- Amaldi, F.; Pierandrei-Amaldi, P. Top genes: A translationally controlled class of genes including those coding for ribosomal proteins. Prog. Mol. Subcell. Biol. 1997, 18, 1–17. [Google Scholar]
- Hudson, W.H.; Pickard, M.R.; de Vera, I.M.; Kuiper, E.G.; Mourtada-Maarabouni, M.; Conn, G.L.; Kojetin, D.J.; Williams, G.T.; Ortlund, E.A. Conserved sequence-specific lincrna-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat. Commun. 2014, 5, 5395. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Z.; Lei, X.; Gan, R. Long non-coding rna gas5 in human cancer. Oncol. Lett. 2020, 20, 2587–2594. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B. Gas5mediated regulation of cell signaling (review). Mol. Med. Rep. 2020, 22, 3049–3056. [Google Scholar] [PubMed]
- Frank, F.; Okafor, C.D.; Ortlund, E.A. The first crystal structure of a DNA-free nuclear receptor DNA binding domain sheds light on DNA-driven allostery in the glucocorticoid receptor. Sci. Rep. 2018, 8, 13497. [Google Scholar] [CrossRef]
- Luisi, B.F.; Xu, W.X.; Otwinowski, Z.; Freedman, L.P.; Yamamoto, K.R.; Sigler, P.B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 1991, 352, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Varani, G.; McClain, W.H. The g x u wobble base pair. A fundamental building block of rna structure crucial to rna function in diverse biological systems. EMBO Rep. 2000, 1, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, R.A.; Zuker, M. Prediction of hybridization and melting for double-stranded nucleic acids. Biophys. J. 2004, 87, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Mayama, T.; Marr, A.K.; Kino, T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm. Metab. Res. 2016, 48, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Goustin, A.S.; Thepsuwan, P.; Kosir, M.A.; Lipovich, L. The growth-arrest-specific (gas)-5 long non-coding rna: A fascinating lncrna widely expressed in cancers. Non-Coding RNA 2019, 5, 46. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G.T. Gas5, a non-protein-coding rna, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takahashi, N.; Kakegawa, E.; Yoshida, K.; Ito, Y.; Kayano, H.; Niitsu, N.; Jinnai, I.; Bessho, M. The gas5 (growth arrest-specific transcript 5) gene fuses to bcl6 as a result of t(1;3)(q25;q27) in a patient with b-cell lymphoma. Cancer Genet. Cytogenet. 2008, 182, 144–149. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hedge, V.L.; Kirkham, L.; Farzaneh, F.; Williams, G.T. Growth arrest in human t-cells is controlled by the non-coding rna growth-arrest-specific transcript 5 (gas5). J. Cell Sci. 2008, 121, 939–946. [Google Scholar] [CrossRef]
- Gasic, V.; Stankovic, B.; Zukic, B.; Janic, D.; Dokmanovic, L.; Krstovski, N.; Lazic, J.; Milosevic, G.; Lucafo, M.; Stocco, G.; et al. Expression pattern of long non-coding rna growth arrest-specific 5 in the remission induction therapy in childhood acute lymphoblastic leukemia. J. Med. Biochem. 2019, 38, 292–298. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.Y.; Li, X.; Yuan, X.Q.; Yang, Y.L.; Zhu, K.W.; Zeng, H.; Li, X.L.; Cao, S.; Zhou, H.H.; et al. Long non-coding rna gas5 polymorphism predicts a poor prognosis of acute myeloid leukemia in chinese patients via affecting hematopoietic reconstitution. Leuk. Lymphoma 2017, 58, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Hasan, A.M.; Farzaneh, F.; Williams, G.T. Inhibition of human t-cell proliferation by mammalian target of rapamycin (mtor) antagonists requires noncoding rna growth-arrest-specific transcript 5 (gas5). Mol. Pharmacol. 2010, 78, 19–28. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Li, G.; Cai, M.; Tan, C.; Han, X.; Han, L. Lncrna gas5 confers the radio sensitivity of cervical cancer cells via regulating mir-106b/ier3 axis. Int. J. Biol. Macromol. 2019, 126, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, Y.; Lyu, H.; Xu, X.; Wu, Q.; Liu, N.; Yin, Q.; Li, J.; Sheng, X. Long noncoding rna gas5, which acts as a tumor suppressor via microrna 21, regulates cisplatin resistance expression in cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 1096–1108. [Google Scholar] [CrossRef]
- Nagini, S. Breast cancer: Current molecular therapeutic targets and new players. Anti-Cancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Arshi, A.; Sharifi, F.S.; Khorramian Ghahfarokhi, M.; Faghih, Z.; Doosti, A.; Ostovari, S.; Mahmoudi Maymand, E.; Ghahramani Seno, M.M. Expression analysis of malat1, gas5, sra, and neat1 lncrnas in breast cancer tissues from young women and women over 45 years of age. Mol. Ther. Nucleic Acids 2018, 12, 751–757. [Google Scholar] [CrossRef]
- Gee, H.E.; Buffa, F.M.; Camps, C.; Ramachandran, A.; Leek, R.; Taylor, M.; Patil, M.; Sheldon, H.; Betts, G.; Homer, J.; et al. The small-nucleolar rnas commonly used for microrna normalisation correlate with tumour pathology and prognosis. Br. J. Cancer 2011, 104, 1168–1177. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative regulation of lncrna gas5 by mir-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef]
- Pickard, M.R.; Williams, G.T. Regulation of apoptosis by long non-coding rna gas5 in breast cancer cells: Implications for chemotherapy. Breast Cancer Res. Treat. 2014, 145, 359–370. [Google Scholar] [CrossRef]
- Li, W.; Zhai, L.; Wang, H.; Liu, C.; Zhang, J.; Chen, W.; Wei, Q. Downregulation of lncrna gas5 causes trastuzumab resistance in breast cancer. Oncotarget 2016, 7, 27778–27786. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, Y.; Wang, X.; Zhou, D.; Shao, C.; Zhou, M.; He, Z. Downregulation of lncrna gas5 confers tamoxifen resistance by activating mir-222 in breast cancer. Cancer Lett. 2018, 434, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Yuan, H.; Huang, X.W.; Xiang, T.; Dai, S. Up-regulated lncrna gas5 promotes chemosensitivity and apoptosis of triple-negative breast cancer cells. Cell Cycle 2019, 18, 1965–1975. [Google Scholar] [CrossRef]
- Guo, L.L.; Wang, S.F. Downregulated long noncoding rna gas5 fails to function as decoy of cebpb, resulting in increased gdf15 expression and rapid ovarian cancer cell proliferation. Cancer Biother. Radiopharm. 2019, 34, 537–546. [Google Scholar] [CrossRef]
- Ma, C.; Wang, W.; Li, P. Lncrna gas5 overexpression downregulates il-18 and induces the apoptosis of fibroblast-like synoviocytes. Clin. Rheumatol. 2019, 38, 3275–3280. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Zheng, J.; Ning, N.; Tang, F.; Yang, Y.; Wang, Y. Lowly-expressed lncrna gas5 facilitates progression of ovarian cancer through targeting mir-196-5p and thereby regulating hoxa5. Gynecol. Oncol. 2018, 151, 345–355. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, C. A novel risk score system for assessment of ovarian cancer based on co-expression network analysis and expression level of five lncrnas. BMC Med. Genet. 2019, 20, 103. [Google Scholar] [CrossRef]
- Song, J.; Zhang, W.; Wang, S.; Liu, K.; Song, F.; Ran, L. A panel of 7 prognosis-related long non-coding rnas to improve platinum-based chemoresistance prediction in ovarian cancer. Int. J. Oncol. 2018, 53, 866–876. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Kino, T. Intracellular glucocorticoid signaling: A formerly simple system turns stochastic. Sci. STKE 2005, 2005, pe48. [Google Scholar] [CrossRef]
- Long, X.; Song, K.; Hu, H.; Tian, Q.; Wang, W.; Dong, Q.; Yin, X.; Di, W. Long non-coding rna gas5 inhibits ddp-resistance and tumor progression of epithelial ovarian cancer via gas5-e2f4-parp1-mapk axis. J. Exp. Clin. Cancer Res. CR 2019, 38, 345. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The epidemiology of prostate cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hao, T.; Sun, J.; Wei, P.; Zhang, H. Long noncoding RNA GAS5 modulates α-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed. Pharmacother. 2019, 112, 108656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Y.; Song, Y.; Shang, C. Long noncoding rna gas5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother. Pharmacol. 2017, 79, 49–55. [Google Scholar] [CrossRef]
- De Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, G.Q.; Shao, X.M.; Wei, L. Gas5 modulated autophagy is a mechanism modulating cisplatin sensitivity in nsclc cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2271–2277. [Google Scholar] [PubMed]
- Xue, Y.; Ni, T.; Jiang, Y.; Li, Y. Long noncoding rna gas5 inhibits tumorigenesis and enhances radiosensitivity by suppressing mir-135b expression in non-small cell lung cancer. Oncol. Res. 2017, 25, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, J.; Ou, B.; Liu, C.; Zou, Y.; Chen, Q. Gas5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (nsclc) cell to cisplatin (ddp) through regulating mir-21/pten axis. Biomed. Pharmacother. 2017, 93, 570–579. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Yang, T.; Zhang, N.; Liu, Z.; Jiang, Y. Low long noncoding rna growth arrest-specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J. Oncol. 2019, 2019, 2476175. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Aminian, K.; Mashayekhi, F.; Mirzanejad, L.; Salehi, Z. A functional genetic variant in gas5 lncrna (rs145204276) modulates p27(kip1) expression and confers risk for gastric cancer. Br. J. Biomed. Sci. 2019, 76, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Q.; Zhu, M.; Liu, K.; Zhang, Z. Integrated analysis reveals potential long non-coding rna biomarkers and their potential biological functions for disease free survival in gastric cancer patients. Cancer Cell Int. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, X.; Liu, D. Overexpression of long noncoding rna gas5 suppresses tumorigenesis and development of gastric cancer by sponging mir-106a-5p through the akt/mtor pathway. Biol. Open 2019, 8, bio.041343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, L.; Chen, C.; Zhang, X.; Wang, S. Long non-coding RNA GAS5 inhibits migration and invasion in gastric cancer via interacting with p53 protein. Dig. Liver Dis. 2020, 52, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, A.-Y.; Wang, X.; Sun, X.-M.; Xue, H.-Z. Gas5 is downregulated in gastric cancer cells by promoter hypermethylation and regulates adriamycin sensitivity. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3199–3205. [Google Scholar] [PubMed]
- Abel, G.A.; Wochnik, G.M.; Ruegg, J.; Rouyer, A.; Holsboer, F.; Rein, T. Activity of the gr in g2 and mitosis. Mol. Endocrinol. 2002, 16, 1352–1366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, J.; Shu, H.; Zhang, L.; Xiong, J. Long noncoding RNA GAS5 inhibits angiogenesis and metastasis of colorectal cancer through the Wnt/β-catenin signaling pathway. J. Cell. Biochem. 2019, 120, 6937–6951. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Su, Z.; Fu, W.; Cui, Z.; Jiang, X.; Tai, S. Altered expression of long non-coding RNA GAS5 in digestive tumors. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ghouri, Y.A.; Mian, I. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding rnas are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Wei, J.; Zhang, K.; Shi, Z.; Duan, R.; Li, S.; Zhou, X.; Pu, P.; Zhang, J.; et al. Snord76, a box c/d snorna, acts as a tumor suppressor in glioblastoma. Sci. Rep. 2015, 5, 8588. [Google Scholar] [CrossRef]
- Huo, J.F.; Chen, X.B. Long noncoding rna growth arrest-specific 5 facilitates glioma cell sensitivity to cisplatin by suppressing excessive autophagy in an mtor-dependent manner. J. Cell. Biochem. 2019, 120, 6127–6136. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, W.; Zhu, S.; Cheng, K.; Xu, H.; Lv, Y.; Long, X.; Ma, L.; Huang, J.; Sun, S.; et al. Long noncoding rna gas5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating mir-18a-5p. J. Cell. Physiol. 2018, 234, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hodges, T.R.; Song, R.; Gong, Y.; Calin, G.A.; Heimberger, A.B.; Zhao, H. Serum hotair and gas5 levels as predictors of survival in patients with glioblastoma. Mol. Carcinog. 2018, 57, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, S.; Zhang, K.; Shi, R.; Bao, X. Low gas5 levels as a predictor of poor survival in patients with lower-grade gliomas. J. Oncol. 2019, 2019, 1785042. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Zhang, X.; Du, K.; Lou, Z.; Ding, K.; Zhang, F.; Zhu, J.; Chang, Z. The ctbp1-hdac1/2-irf1 transcriptional complex represses the expression of the long noncoding rna gas5 in human osteosarcoma cells. Int. J. Biol. Sci. 2019, 15, 1460–1471. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhang, L.; Xia, X.; Chao, Y.; He, R.; Han, C.; Zhao, W. Zbtb7a, a mir-663a target gene, protects osteosarcoma from endoplasmic reticulum stress-induced apoptosis by suppressing lncrna gas5 expression. Cancer Lett. 2019, 448, 105–116. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D. Lncrna gas5 represses osteosarcoma cells growth and metastasis via sponging mir-203a. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 844–855. [Google Scholar] [CrossRef]
- Ye, K.; Wang, S.; Zhang, H.; Han, H.; Ma, B.; Nan, W. Long noncoding rna gas5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma by regulating the mir-221/arhi pathway. J. Cell. Biochem. 2017, 118, 4772–4781. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Gao, L.; Wang, H.; Chen, J.; Nie, B.; Hong, Q. Long non-coding rna gas5 regulates human b lymphocytic leukaemia tumourigenesis and metastasis by sponging mir-222. Cancer Biomark. Sect. A Dis. Mark. 2019, 26, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ruoyu, L.; Xing, L.; Hua, L.; Jun, Z.; Yaqin, P.; Lu, W.; Aili, T.; Yuzi, Z.; Lin, M.; et al. Long non-coding rna gas5 regulates the growth and metastasis of human cervical cancer cells via induction of apoptosis and cell cycle arrest. Arch. Biochem. Biophys. 2020, 684, 108320. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Lu, R.; Zhang, J.; Fang, X.; Fan, L.; Huang, C.; Lin, R.; Lin, Z. Growth arrest-specific 5 attenuates cisplatin-induced apoptosis in cervical cancer by regulating stat3 signaling via mir-21. J. Cell. Physiol. 2019, 234, 9605–9615. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hong, L.; Xu, X.; Wang, Q.; Huang, J.; Jiang, L. Lncrna gas5 suppresses the tumorigenesis of cervical cancer by downregulating mir-196a and mir-205. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317711315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pan, T.; Jiang, D.; Jin, L.; Geng, Y.; Feng, X.; Shen, A.; Zhang, L. The lncrna-gas5/mir-221-3p/dkk2 axis modulates abcb1-mediated adriamycin resistance of breast cancer via the wnt/beta-catenin signaling pathway. Mol. Ther. Nucleic Acids 2020, 19, 1434–1448. [Google Scholar] [CrossRef]
- Zheng, S.; Li, M.; Miao, K.; Xu, H. Lncrna gas5-promoted apoptosis in triple-negative breast cancer by targeting mir-378a-5p/sufu signaling. J. Cell. Biochem. 2020, 121, 2225–2235. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, Y.; Sun, X.; Xu, T.; Cheng, X.; Qin, Y. Mir-221/222 promote tumor growth and suppress apoptosis by targeting lncrna gas5 in breast cancer. Biosci. Rep. 2019, 39, BSR20181859. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Y.; Wang, X.; Zhou, D.; Wang, X.; Zhou, M.; He, Z. Effect of the LncRNA GAS5-MiR-23a-ATG3 Axis in Regulating Autophagy in Patients with Breast Cancer. Cell. Physiol. Biochem. 2018, 48, 194–207. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Wang, Z.; Wang, P.; Gao, X.; Wang, Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. Pharmacother. 2018, 104, 451–457. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Xie, S.; Cheung, E. Long non-coding rna gas5 and zfas1 are prognostic markers involved in translation targeted by mir-940 in prostate cancer. Oncotarget 2018, 9, 1048–1062. [Google Scholar] [CrossRef]
- Chen, L.; Ren, P.; Zhang, Y.; Gong, B.; Yu, D.; Sun, X. Long noncoding rna gas5 increases the radiosensitivity of a549 cells through interaction with the mir21/pten/akt axis. Oncol. Rep. 2020, 43, 897–907. [Google Scholar] [PubMed]
- Dong, L.; Li, G.; Li, Y.; Zhu, Z. Upregulation of long noncoding rna gas5 inhibits lung cancer cell proliferation and metastasis via mir-205/pten axis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Si, J.; Wang, Y.; Huang, Z.; Zhu, H.; Feng, S.; Wu, X.; Wu, L. Long noncoding rna gas5 suppresses tumorigenesis by inhibiting mir-23a expression in non-small cell lung cancer. Oncol. Res. 2017, 25, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.F.; Gu, Z.S.; Zheng, L.L.; Zhao, M.X.; Wang, X.J. Long non-coding rna gas5 promotes natural killer cell cytotoxicity against gastric cancer by regulating mir-18a. Neoplasma 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, J.; Lu, H. The gas5/mir-222 axis regulates proliferation of gastric cancer cells through the pten/akt/mtor pathway. Dig. Dis. Sci. 2017, 62, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, Z.; Wang, G.; Wang, J.; Zhu, W. Lncrna gas5 inhibits colorectal cancer cell proliferation via the mir1825p/foxo3a axis. Oncol. Rep. 2018, 40, 2371–2380. [Google Scholar]
- Liu, L.; Meng, T.; Yang, X.H.; Sayim, P.; Lei, C.; Jin, B.; Ge, L.; Wang, H.J. Prognostic and predictive value of long non-coding rna gas5 and mircorna-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. Sect. A Dis. Markers 2018, 22, 283–299. [Google Scholar] [CrossRef]
- Zhao, P.; Cui, X.; Zhao, L.; Liu, L.; Wang, D. Overexpression of growth-arrest-specific transcript 5 improved cisplatin sensitivity in hepatocellular carcinoma through sponging mir-222. DNA Cell Biol. 2020, 39, 724–732. [Google Scholar] [CrossRef]
- Wang, C.; Ke, S.; Li, M.; Lin, C.; Liu, X.; Pan, Q. Downregulation of lncrna gas5 promotes liver cancer proliferation and drug resistance by decreasing pten expression. Mol. Genet. Genom. 2020, 295, 251–260. [Google Scholar] [CrossRef]
- Hu, L.; Ye, H.; Huang, G.; Luo, F.; Liu, Y.; Liu, Y.; Yang, X.; Shen, J.; Liu, Q.; Zhang, J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumor Biol. 2015, 37, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Xiang, L.; Chen, W.; Li, S.; Huang, S.; Li, J.; Zhuge, L.; Jin, L.; Feng, W.; Chen, Y.; et al. Lncrna gas5 enhanced the killing effect of nk cell on liver cancer through regulating mir-544/runx3. Innate Immun. 2019, 25, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J. Gas5 regulates reck expression and inhibits invasion potential of hcc cells by sponging mir-135b. BioMed Res. Int. 2019, 2019, 2973289. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Alghamdi, S.A.; El-Wazir, A.; Hosny, M.M.; Hussein, M.H.; Khashana, M.S.; Fawzy, M.S. Dual biomarkers long non-coding rna gas5 and microrna-34a co-expression signature in common solid tumors. PLoS ONE 2018, 13, e0198231. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Han, B.; Wang, Q.; Zhang, B.; Qi, C.; Liu, F. Lncrna gas5-as1 inhibits glioma proliferation, migration, and invasion via mir-106b-5p/tusc2 axis. Hum. Cell 2020, 33, 416–426. [Google Scholar] [CrossRef]
- Willingham, A.T.; Orth, A.P.; Batalov, S.; Peters, E.C.; Wen, B.G.; Aza-Blanc, P.; Hogenesch, J.B.; Schultz, P.G. A strategy for probing the function of noncoding rnas finds a repressor of nfat. Science 2005, 309, 1570–1573. [Google Scholar] [CrossRef]
- Barrandon, C.; Spiluttini, B.; Bensaude, O. Non-coding rnas regulating the transcriptional machinery. Biol. Cell 2008, 100, 83–95. [Google Scholar] [CrossRef]
- Willkomm, D.K.; Hartmann, R.K. 6s rna—An ancient regulator of bacterial rna polymerase rediscovered. Biol. Chem. 2005, 386, 1273–1277. [Google Scholar] [CrossRef]
- Lanz, R.B.; McKenna, N.J.; Onate, S.A.; Albrecht, U.; Wong, J.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. A steroid receptor coactivator, sra, functions as an rna and is present in an src-1 complex. Cell 1999, 97, 17–27. [Google Scholar] [CrossRef]
- Leygue, E. Steroid Receptor RNA Activator (SRA1): Unusual Bifaceted Gene Products with Suspected Relevance to Breast Cancer. Nucl. Recept. Signal. 2007, 5, e006. [Google Scholar] [CrossRef]
- Ghaforui-Fard, S.; Taheri, M. Growth arrest specific transcript 5 in tumorigenesis process: An update on the expression pattern and genomic variants. Biomed. Pharmacother. 2019, 112, 108723. [Google Scholar] [CrossRef]
- Ma, C.; Shi, X.; Zhu, Q.; Li, Q.; Liu, Y.; Yao, Y.; Song, Y. The growth arrest-specific transcript 5 (GAS5): A pivotal tumor suppressor long noncoding RNA in human cancers. Tumor Biol. 2015, 37, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Williams, G.T. Molecular and cellular mechanisms of action of tumour suppressor gas5 lncrna. Genes 2015, 6, 484–499. [Google Scholar] [CrossRef] [PubMed]
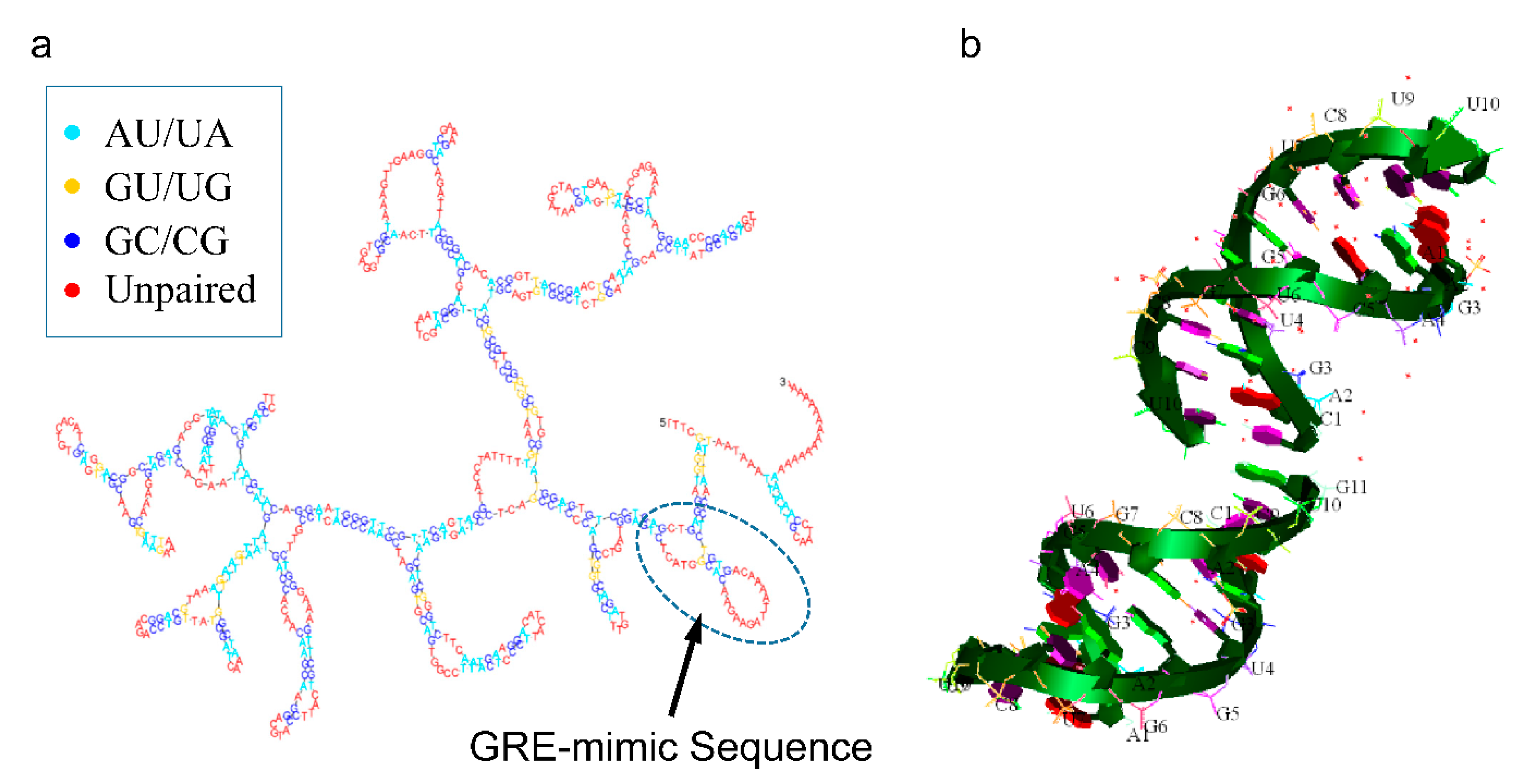
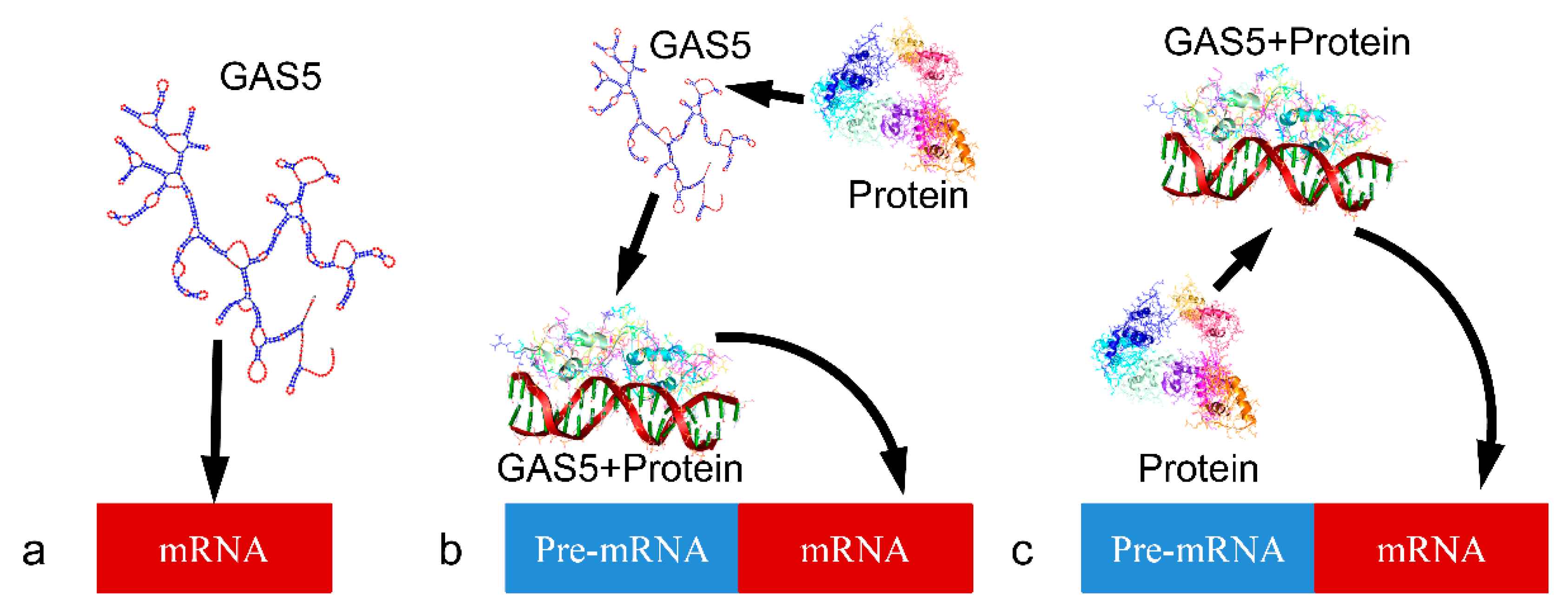
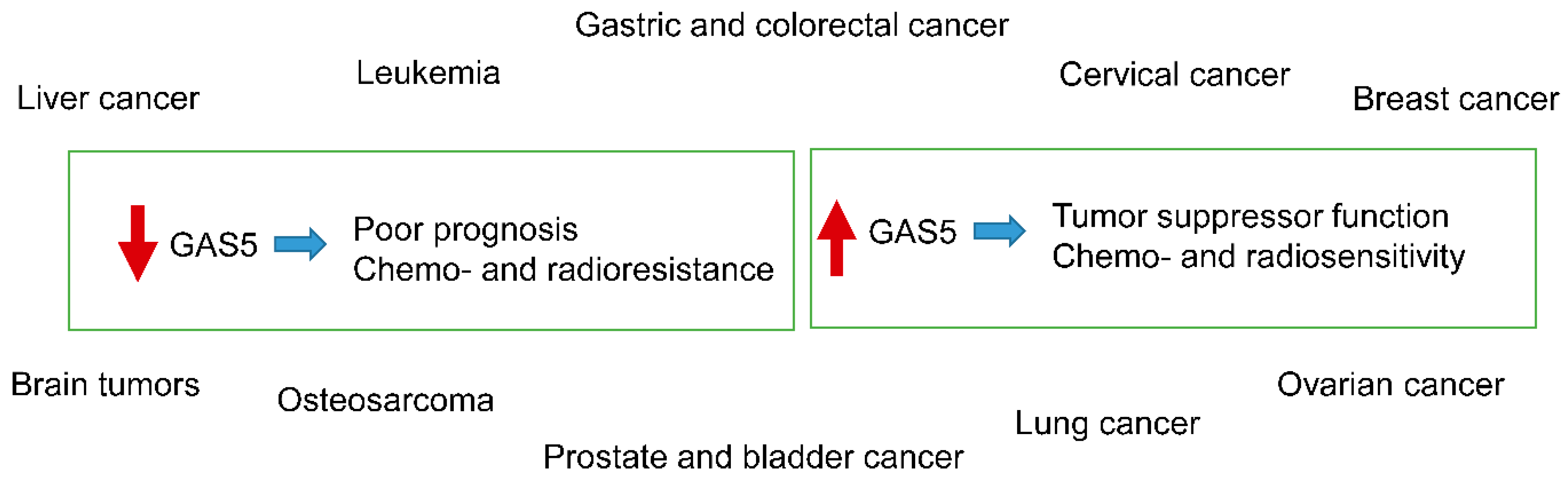
| Tumor | miRNA | Relation between GAS5 and miRNA | GAS5 Effect on Tumor | Effect on Therapy-Related Resistance | Citation |
|---|---|---|---|---|---|
| Leukemia | miR-222 | Direct Suppression | Tumor suppressor | Unknown | Jing et al. (2019) [82] |
| Cervical Cancer | miR-106b | Direct Suppression/Sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Gao et al. (2019) [33] |
| miR-135a | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Yan et al. (2020) [83] | |
| miR-21 | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Yao et al. (2019), Li (2016) [41,84] | |
| miR-205 | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Yang et al. (2017), Wen et al. (2017) [34,85] | |
| Breast Cancer | miR-221-3p | Direct Suppression/Sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Chen et al. (2020) [86] |
| miR-378-5p | Indirect Suppression/Sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Zheng et al. (2020) [87] | |
| miR-221/222 | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Zong et al. (2019), Gu et al. (2018) [42,88] | |
| miR-23a | Direct Suppression/sponge | Tumor suppressor/induces autophagy | Unknown | Gu et al. (2018) [89] | |
| miR-196a-5p | Direct Suppression/sponge | Induces autophagy | Unknown | Li et al. (2018) [90] | |
| miR-196a-5p | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Zheng et al. (2020) [87] | |
| miR-21 | Direct Suppression/Sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Li (2016) [41] | |
| Ovarian Cancer | miR-196a-5p | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Zhao et al. (2018) [46] |
| Prostate Cancer | miR-940 | Indirect Suppression | Tumor suppressor | Unknown | Chen et al. (2017) [91] |
| miR-18a | Indirect Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Yang et al. (2019) [53] | |
| Lung Cancer | miR-21 | Indirect Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Chen et al. (2020) [92] |
| miR-29-3p | Indirect Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Cheng et al. (2019) [59] | |
| miR-21 | Indirect Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Cao et al. (2017) [58] | |
| miR-205 | Direct Suppression | Tumor suppressor | Unknown | Dong et al. (2019) [93] | |
| miR-135b | Direct Suppression | Tumor suppressor | Induces chemo- and radiosensitivity | Xue et al. (2017) [57] | |
| miR-23a | Indirect Suppression | Tumor suppressor | Unknown | Mei et al. (2017) [94] | |
| Gastric Cancer | miR-18a | Direct Suppression | Tumor suppressor | Unknown | Wei et al. (2020) [95] |
| miR-106a-5p | Indirect Suppression | Tumor suppressor | Unknown | Dong et al. (2019) [63] | |
| miR-222 | Direct Suppression/sponge | Tumor suppressor | Unknown | Li et al. (2017) [96] | |
| Colorectal Cancer | miR-182-5p | Direct Suppression/sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Cheng et al. (2018) [97] |
| miR-221 | Indirect Suppression | Tumor suppressor | Unknown | Liu et al. (2018) [98] | |
| Liver Cancer | miR-222 | Direct Suppression/sponge | Tumor suppressor | Induces chemo- and radiosensitivity | Zhao et al. (2020) [99] |
| miR-21 | Direct Suppression/sponge | Tumor suppressor | Unknown | Wang et al. (2018), Hu et al. (2016) [100,101] | |
| miR-544 | Indirect Suppression | Tumor suppressor | Unknown | Fang et al. (2019) [102] | |
| miR-135b | Indirect Suppression | Tumor suppressor | Unknown | Yang et al. (2019) [103] | |
| miR-34a | Indirect Suppression | Tumor suppressor/sponge | Unknown | Toraih et al. (2018) [104] | |
| Glioma | miR-106b | Indirect Suppression | Tumor suppressor/sponge | Unknown | Huang et al. (2020) [105] |
| miR-18a-3p | Indirect Suppression | Tumor suppressor/sponge | Unknown | Liu et al. (2018) [73] | |
| Osteo-sarcoma | miR-663a | Indirect Suppression | Tumor suppressor/sponge | Unknown | Zhang et al. (2019) [79] |
| miR-203a | Indirect Suppression | Tumor suppressor/sponge | Unknown | Wang et al. (2018) [80] | |
| miR-221 | Direct Suppression | Tumor suppressor/sponge | Unknown | Ye et al. (2017) [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrou, G.I.; Hatziagapiou, K.; Zaravinos, A. The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance. Int. J. Mol. Sci. 2020, 21, 7633. https://doi.org/10.3390/ijms21207633
Lambrou GI, Hatziagapiou K, Zaravinos A. The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance. International Journal of Molecular Sciences. 2020; 21(20):7633. https://doi.org/10.3390/ijms21207633
Chicago/Turabian StyleLambrou, George I., Kyriaki Hatziagapiou, and Apostolos Zaravinos. 2020. "The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance" International Journal of Molecular Sciences 21, no. 20: 7633. https://doi.org/10.3390/ijms21207633
APA StyleLambrou, G. I., Hatziagapiou, K., & Zaravinos, A. (2020). The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance. International Journal of Molecular Sciences, 21(20), 7633. https://doi.org/10.3390/ijms21207633







