Percolation Conduction of Carbon Nanocomposites
Abstract
1. Introduction
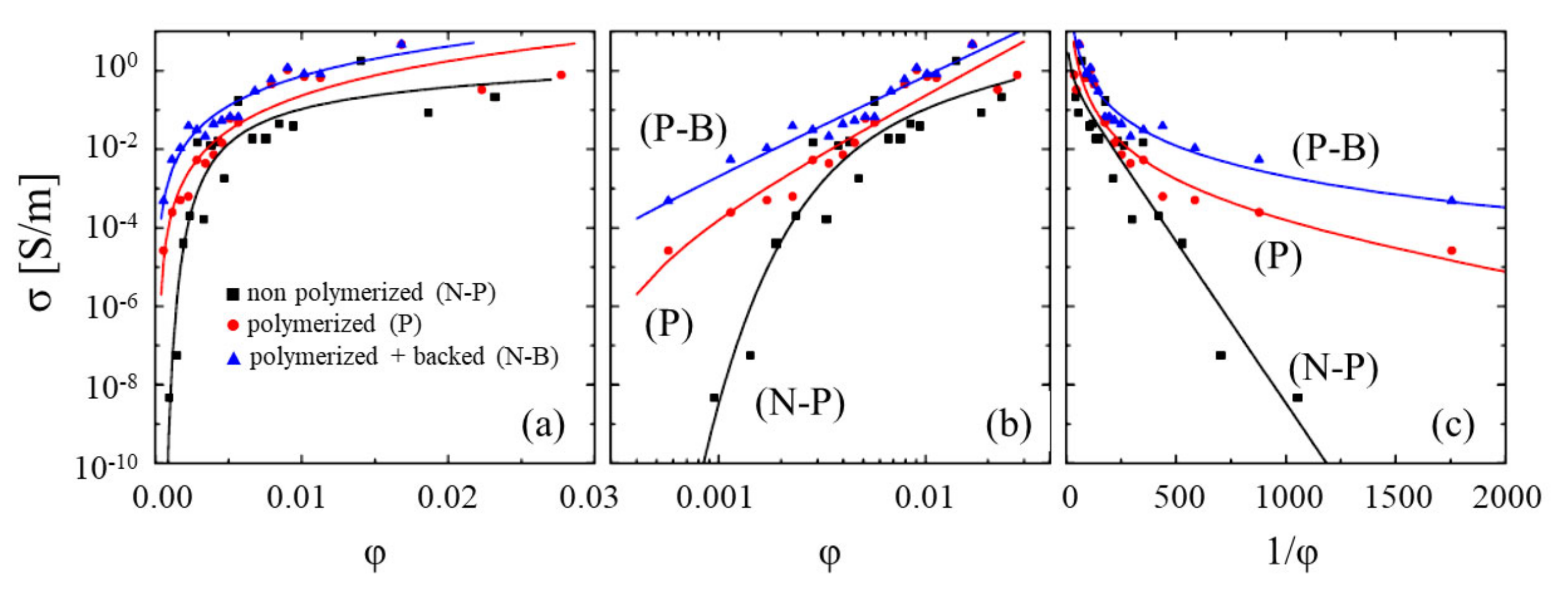
2. Percolation Behavior of Polymer-Based Composites Doped with CNTs
2.1. Model of Charge Transport
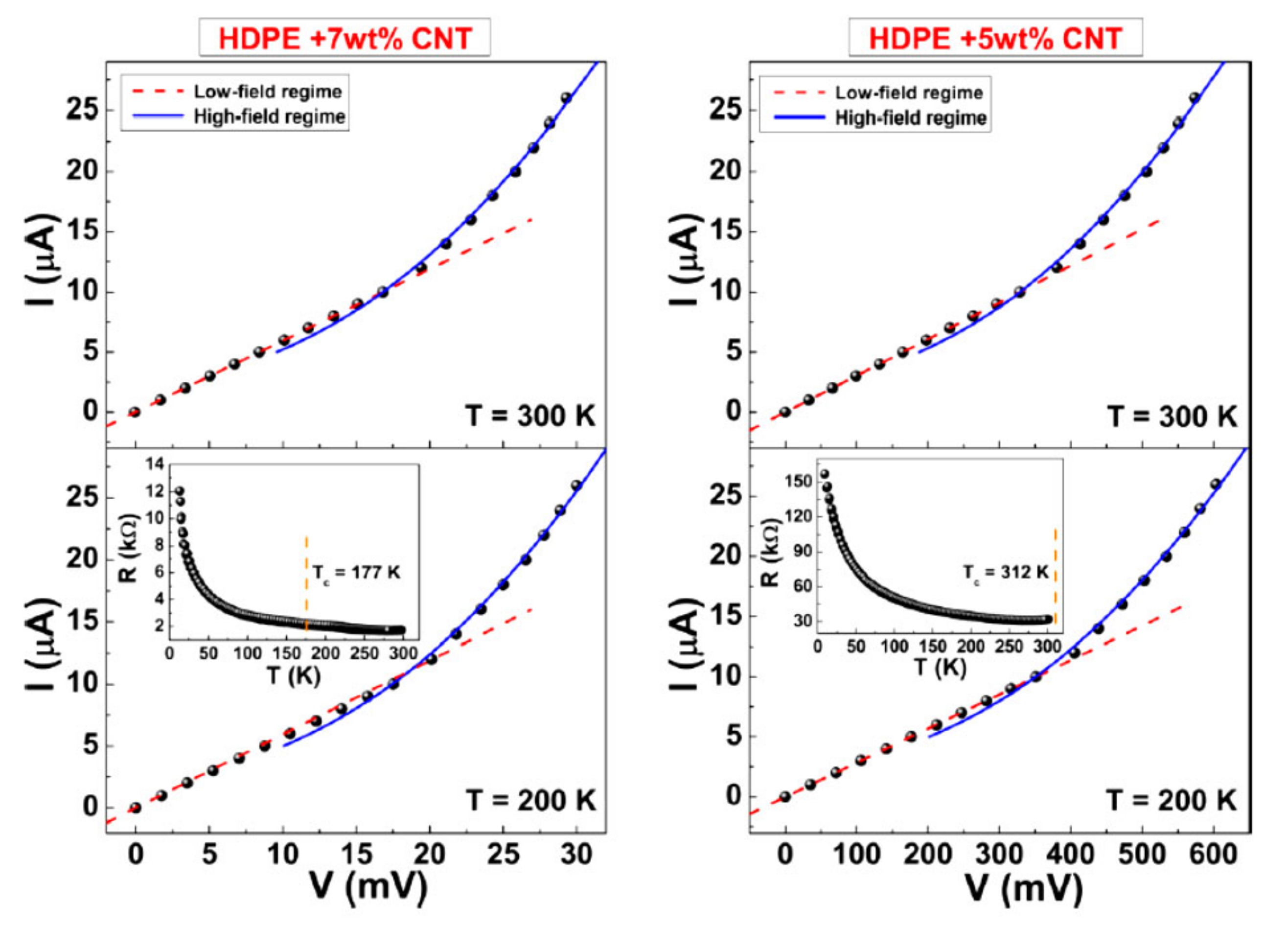
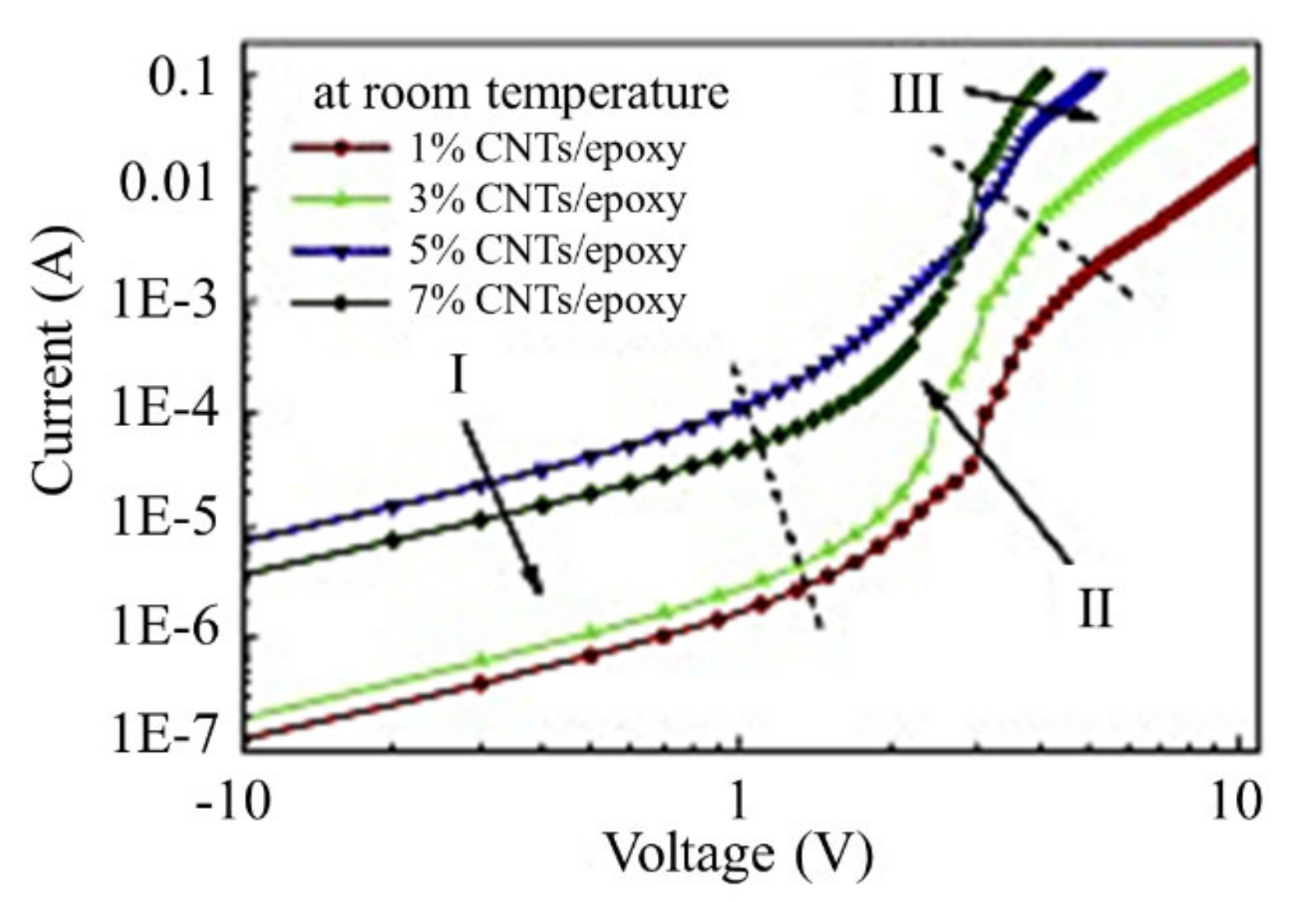
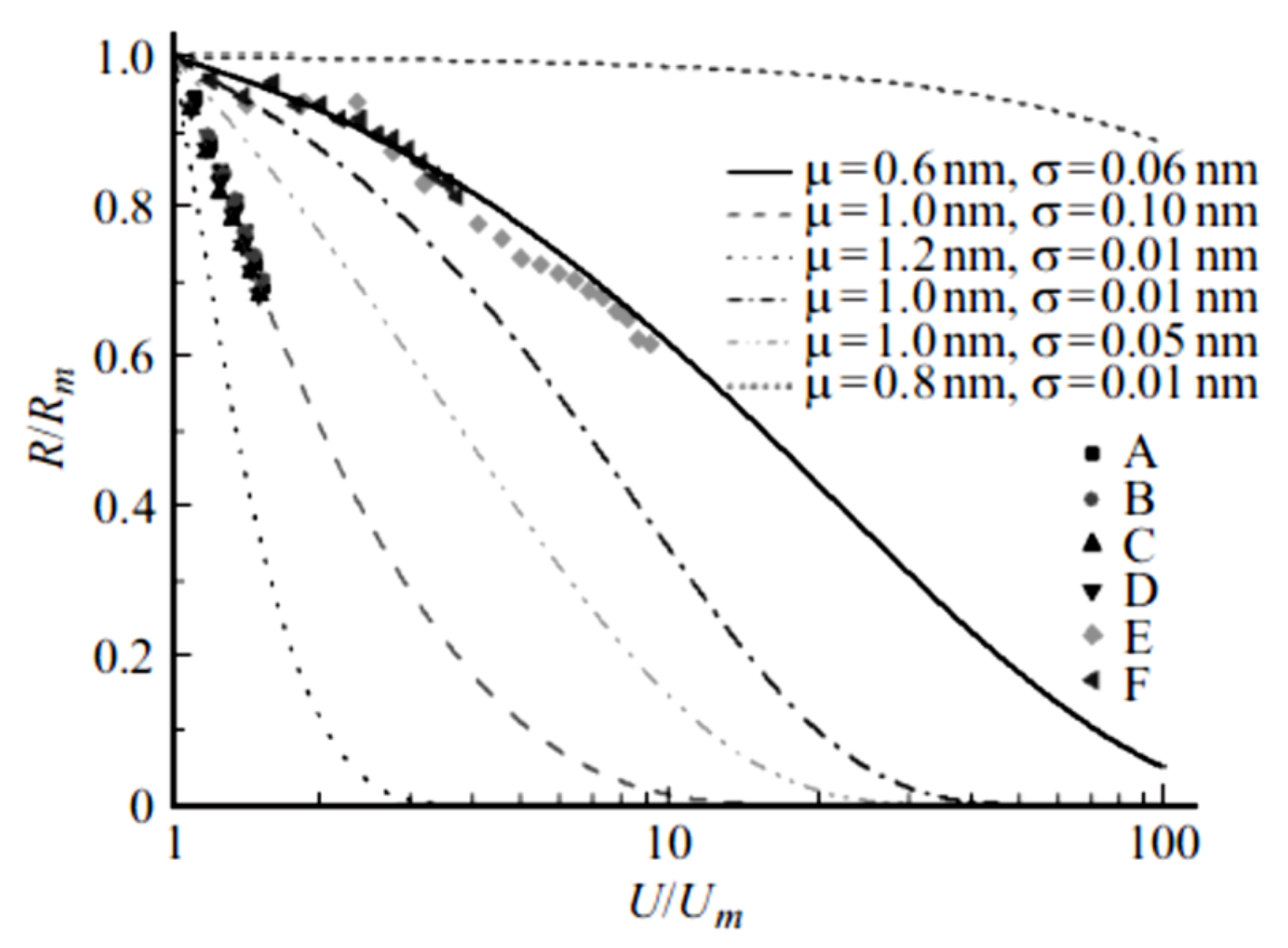
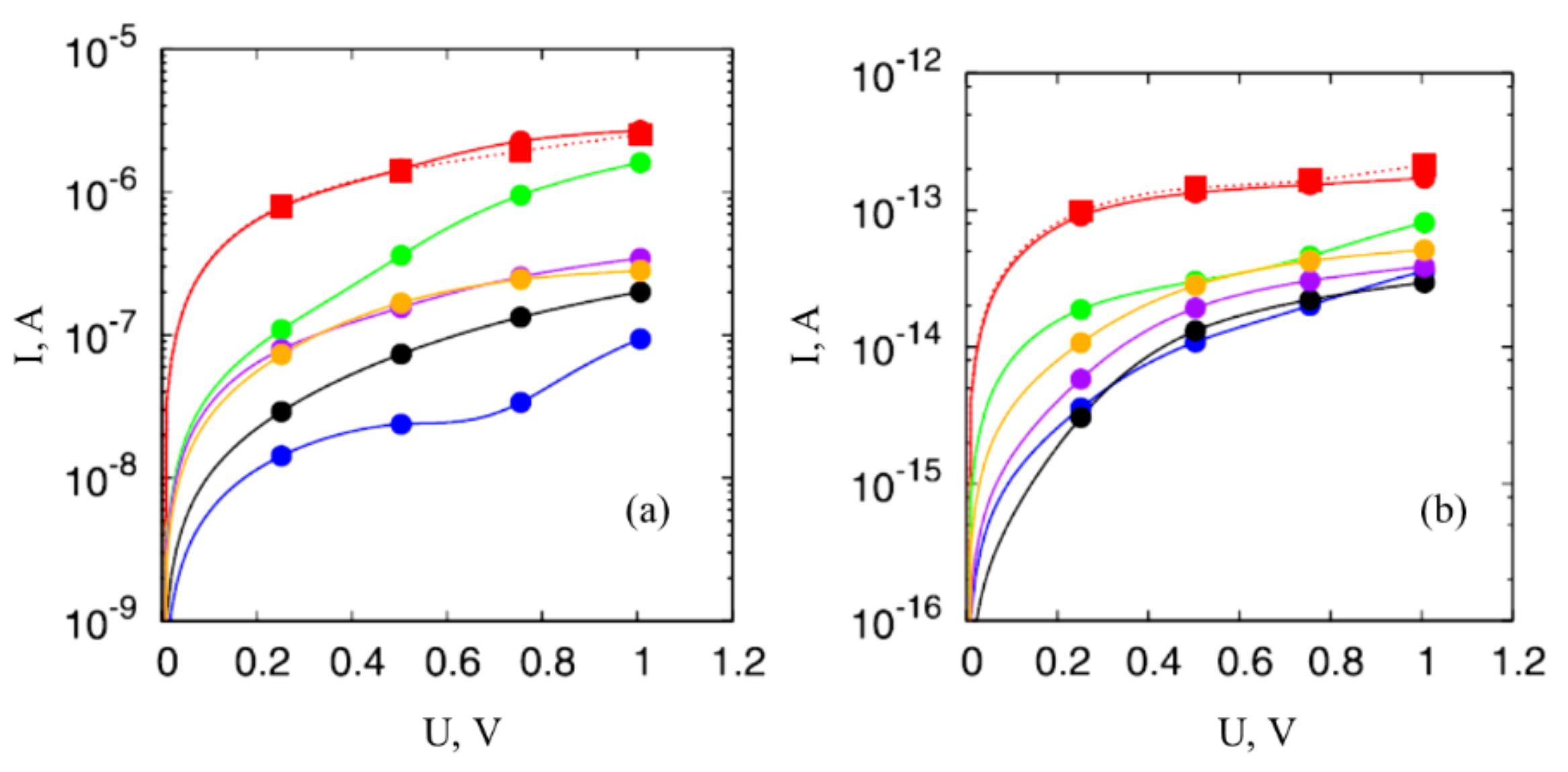
2.2. Non-Ohmic Behavior of Polymer-Based Composited Doped with CNTs
2.3. Dependence of the Contact Resistance on the Intersection Angle
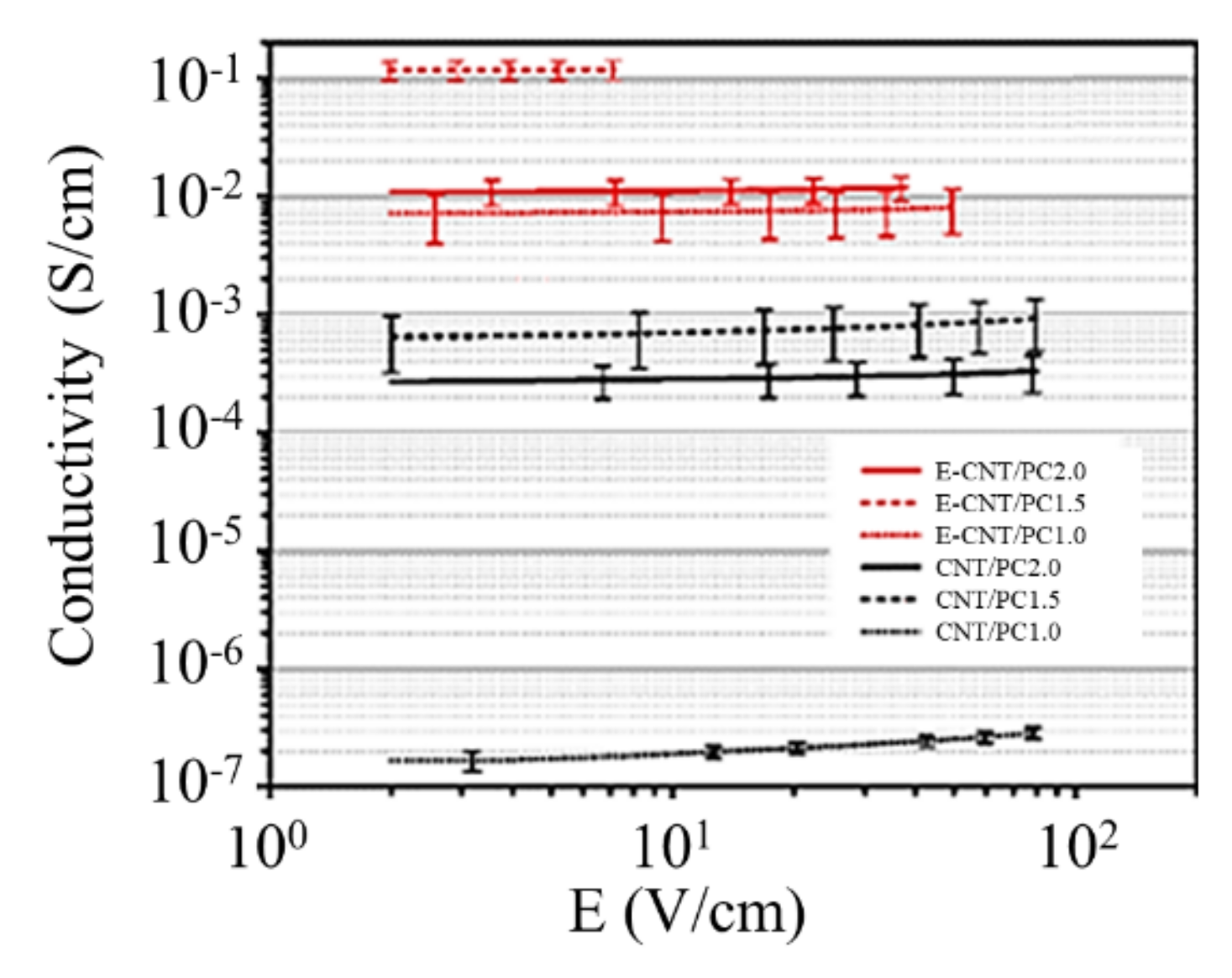
2.4. Polymer-Based Composites with Graphene Filling
3. Percolation Behavior of Reduce Graphene Oxide
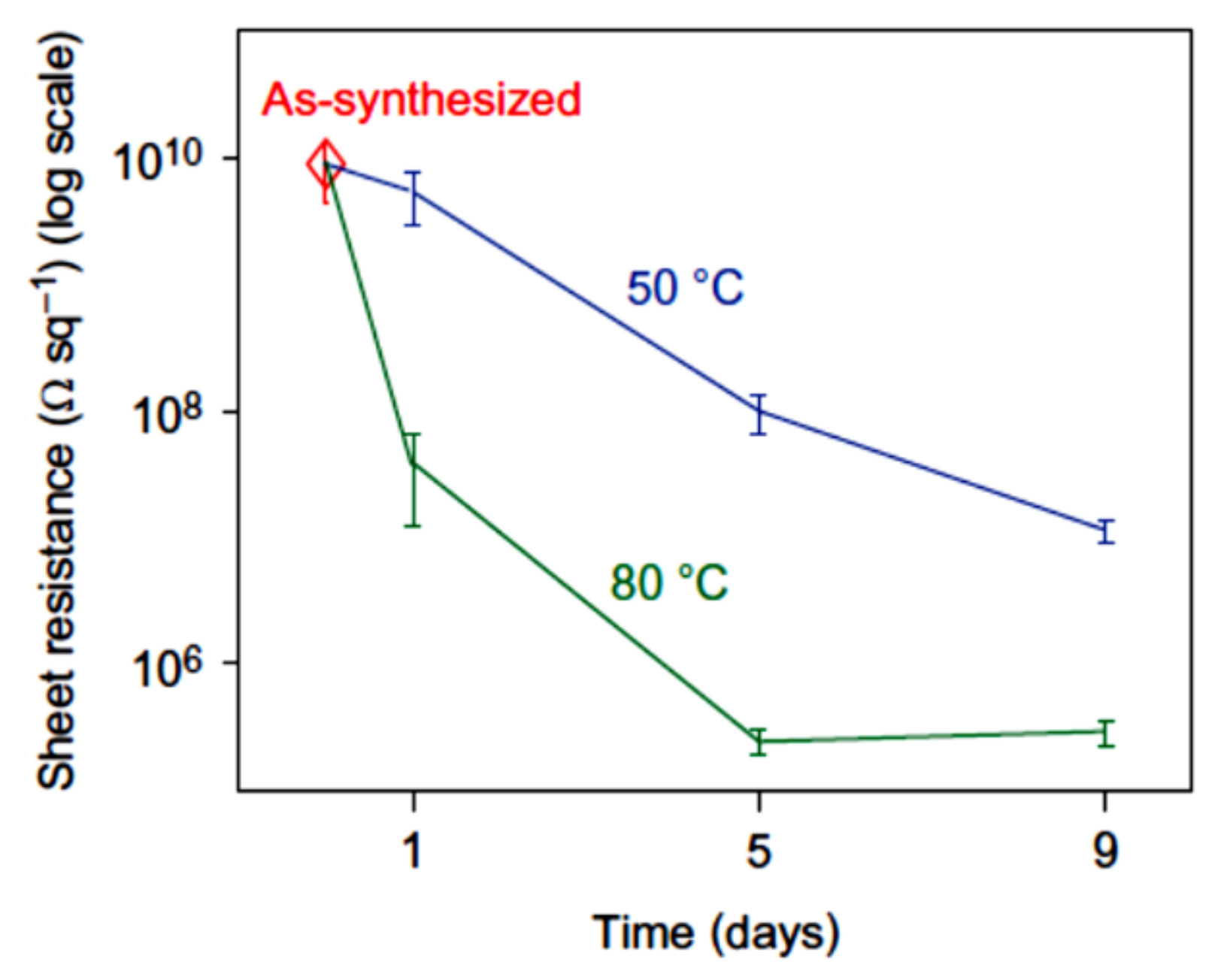
3.1. Chemical Reduction of Graphene Oxide
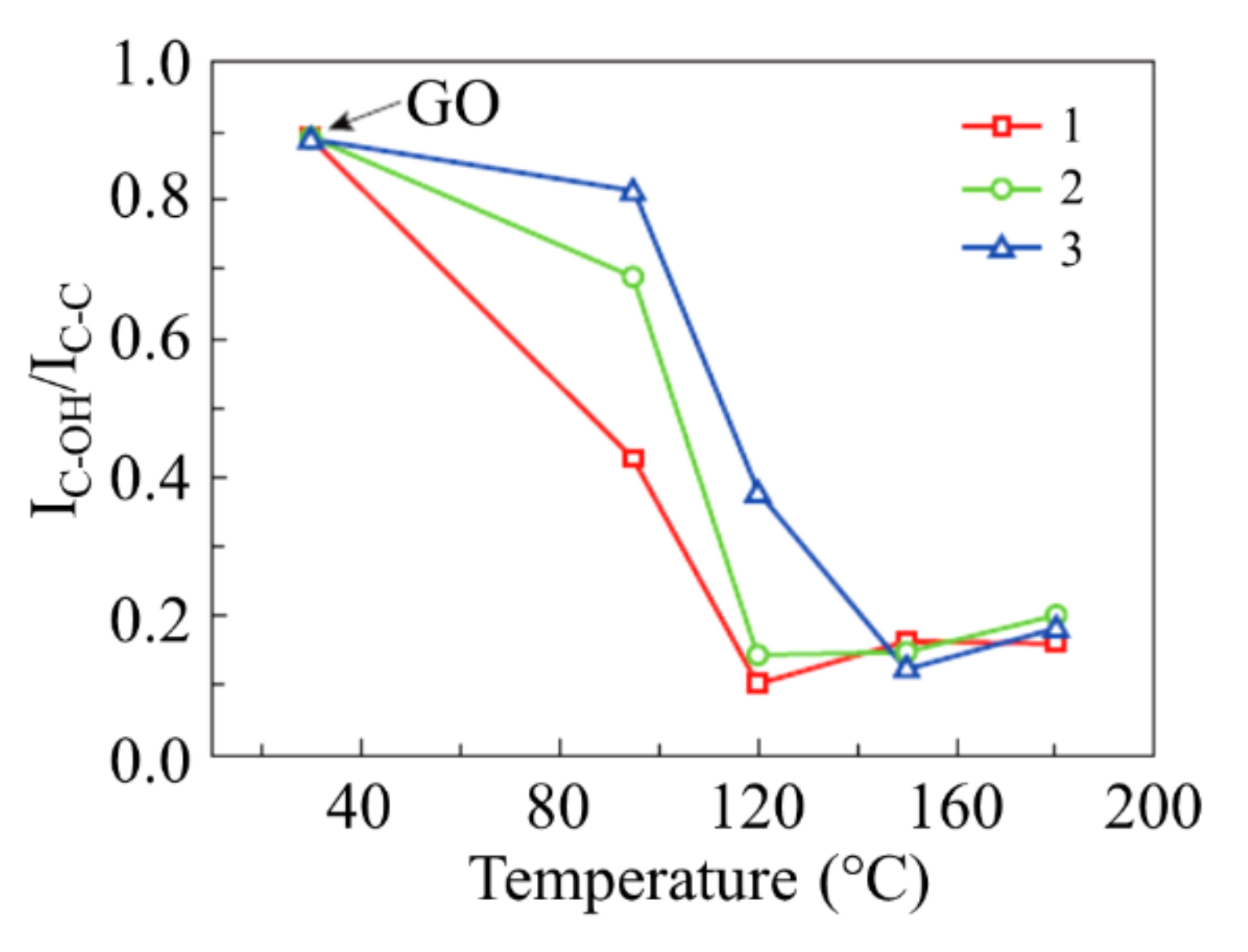
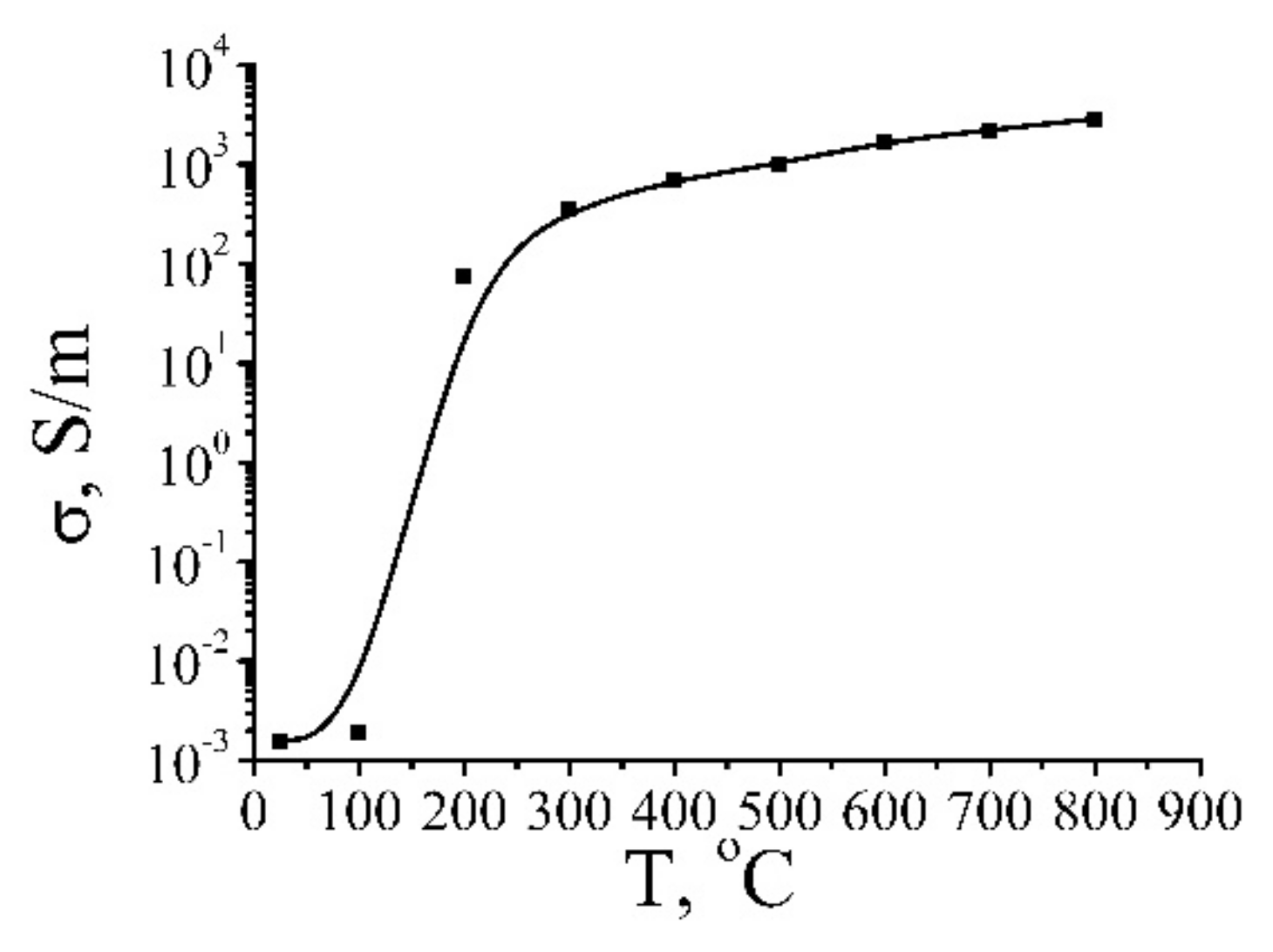
3.2. Thermal Reduction of GO
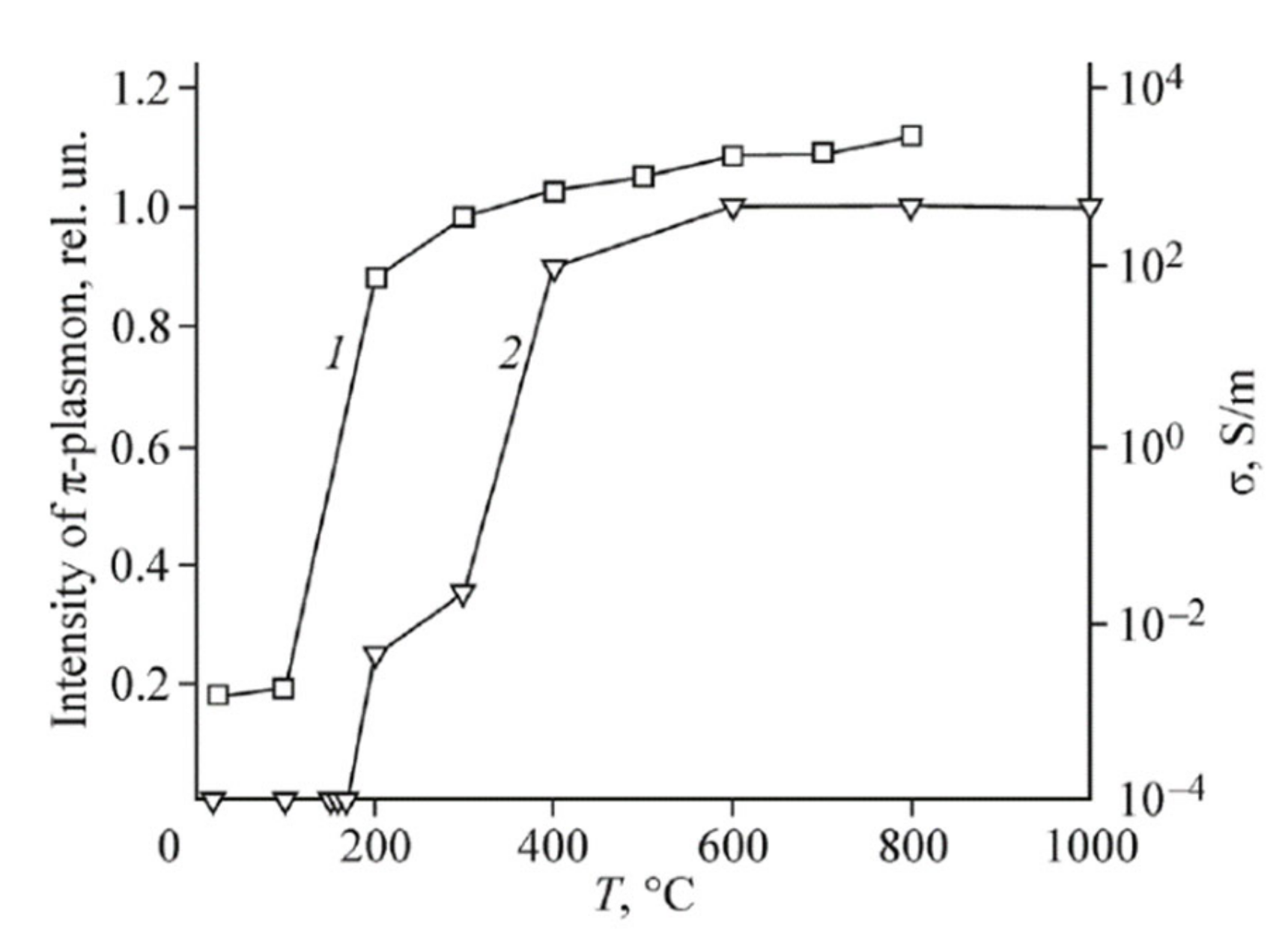
3.3. Electrical Characteristics of rGO
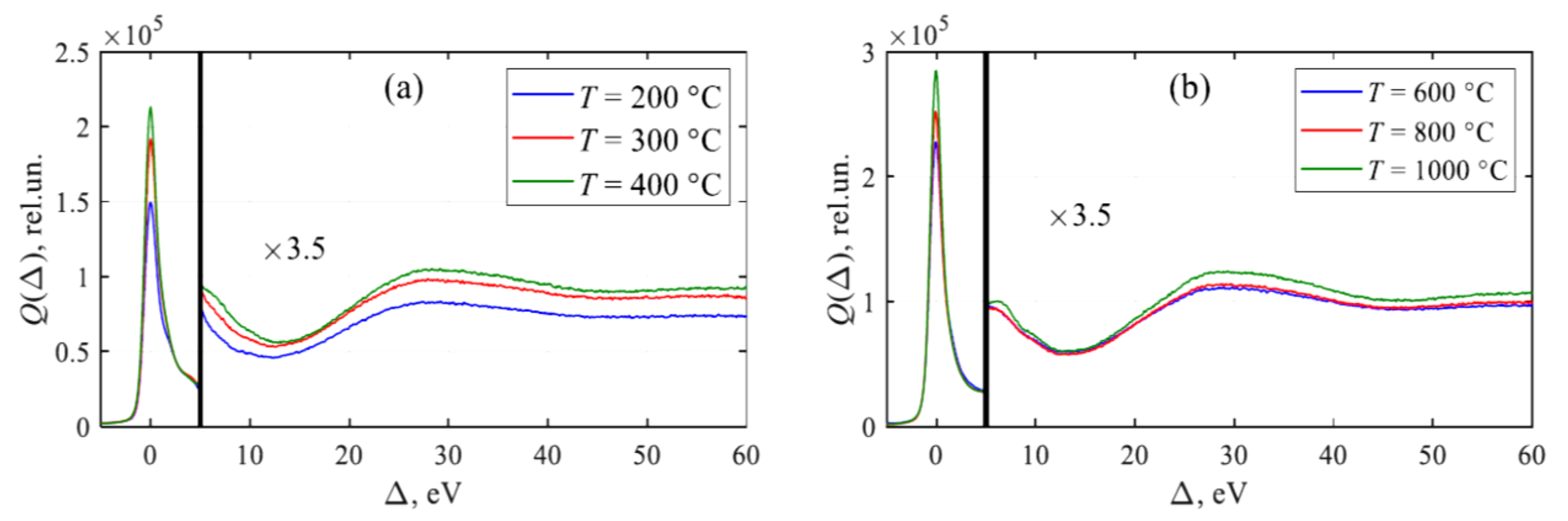
3.4. XPS Spectra of Reduced GO and Plasmons
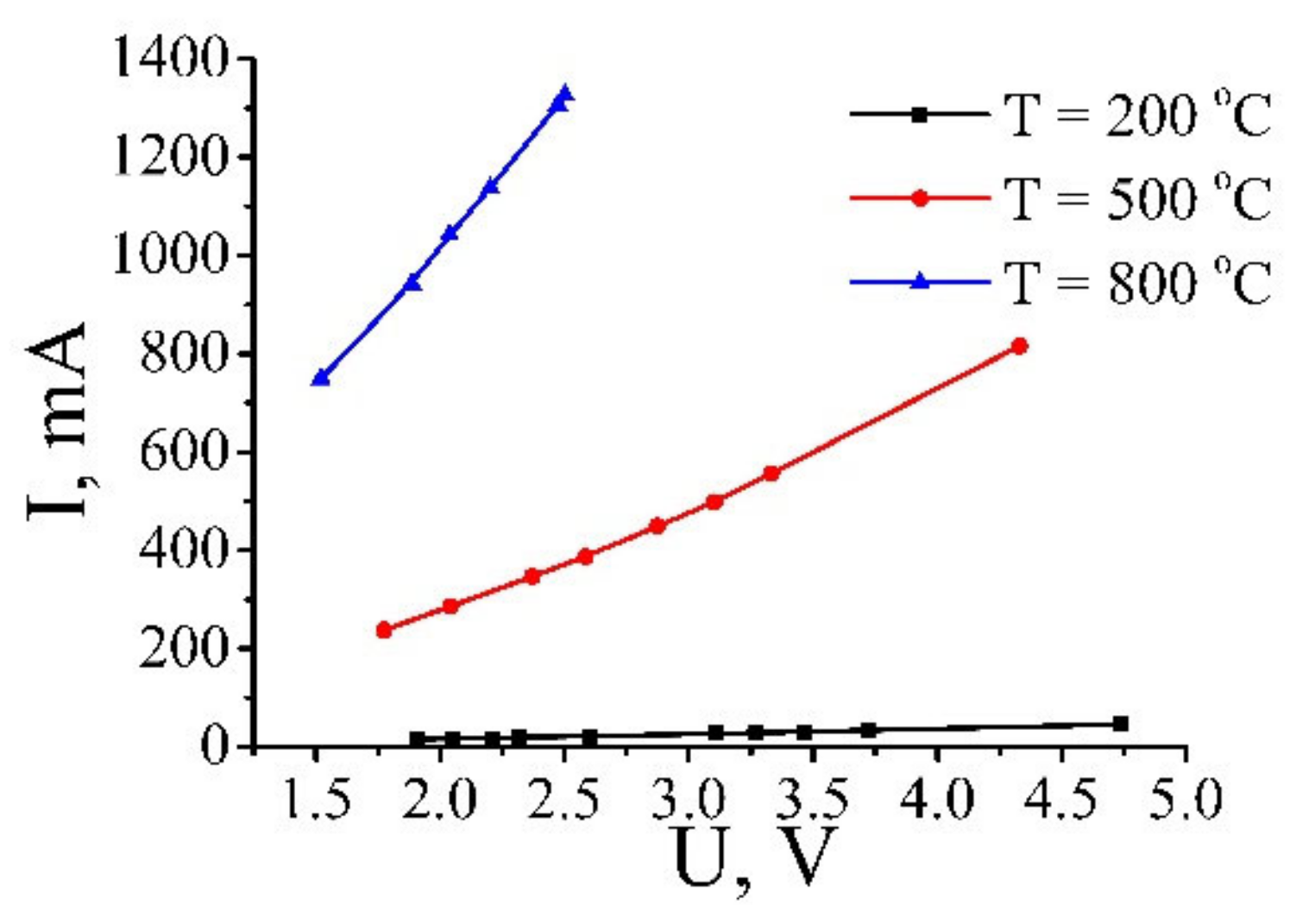
3.5. Non-Ohmic Conduction of Reduced GO
3.6. Model Description on the Non-Ohmic Conduction of GO Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eletskii, A.V.; Knizhnik, A.A.; Potapkin, B.V.; Kenny, J.M. Electrical characteristics of carbon nanotube-doped polymer composites. Phys. Uspech 2015, 50, 209–251. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovas, J. A review and analysis of electrical percolation in carbon nanotube polymer composites 1486. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Chiolerio, A.; Castellino, M.; Jagdale, P.; Giorcelli, M.; Bianco, S.; Tagliaferro, A. Carbon Nanotubes–Polymer Nanocomposites; Yellampalli, S., Ed.; InTech Open access Publisher: London, UK, 2011; pp. 215–233. [Google Scholar] [CrossRef]
- Bocharov, G.S.; Eletskii, A.V.; Knizhnik, A.A.; Potapkin, B.V. Percolation phenomena in polymer nanocomposites doped with carbon nanotubes. In Synthesis, Structure and Properties of Metal/Semiconductor Containing Nanocomposites; Trachtenberg, L.I., Melnikov, M.Y., Eds.; Tehcnospera Publ.: Moscow, Russia, 2016; pp. 54–84. (In Russian) [Google Scholar]
- Shulga, Y.M.; Martynenko, V.M.; Muradyan, V.E.; Baskakov, S.A.; Smirnov, V.A.; Gutsev, G.L. Gaseous products of thermo- and photo-reduction of graphite oxide. Chem. Phys. Lett. 2010, 498, 287–291. [Google Scholar] [CrossRef]
- Bocharov, G.S.; Eletskii, A.V.; Mel’nikov, V.P. Electrical properties of thermally reduced graphene oxide. Nanosyst. Phys. Chem. Math. 2018, 9, 98–101. [Google Scholar] [CrossRef]
- Bocharov, G.S.; Eletskii, A.V.; Knizhnik, A.A. Nonlinear resistance of polymer composites with carbon nanotube additives in the percolation state. Tech. Phys. 2016, 61, 1506–1510. [Google Scholar] [CrossRef]
- Watts, P.C.P.; Hsu, W.; Randall, D.P.; Kroto, H.; Walton, D.R.M. Non-linear current-voltage characteristics of electrically conducting carbon nanotube-polystyrene composites. Phys. Chem. Chem. Phys. 2002, 4, 5655–5662. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Luo, S.; Chu, B.; Sun, R.; Wong, C.P. Investigation of nonlinear I–V behavior of CNTs filled polymer composites. Mater. Sci. Eng. B 2016, 206, 55–60. [Google Scholar] [CrossRef]
- Ounaies, Z.; Park, C.; Wise, K.E.; Siochi, E.J.; Harrison, J.S. Electrical properties of single wall carbon nanotube reinforced polyimide composites. Compos. Sci. Technol. 2003, 63, 1637–1646. [Google Scholar] [CrossRef]
- Liu, C.H.; Fan, S.S. Nonlinear electrical conducting behavior of carbon nanotube networks in silicone elastomer. Appl. Phys. Lett. 2007, 90, 041905. [Google Scholar] [CrossRef]
- Hu, C.H.; Liua, C.H.; Chen, L.Z.; Fan, S.S. Semiconductor behaviors of low loading multiwall carbon nanotube/poly(dimethylsiloxane) composites. Appl. Phys. Lett. 2009, 95, 103103. [Google Scholar] [CrossRef]
- Barone, C.; Pagano, S.; Neitzert, H.C. Transport and noise spectroscopy of MWCNT/HDPE composites with different nanotube concentrations. J. Appl. Phys. 2011, 110, 113716. [Google Scholar] [CrossRef]
- Grimaldi, C.; Mionić, M.; Gaal, R.; László Forró, L.; Magrez, A. Electrical conductivity of multi-walled carbon nanotubes-SU8 epoxy composites. Appl. Phys. Lett. 2013, 102, 223114. [Google Scholar] [CrossRef]
- Chien, A.-T.; Cho, S.; Joshi, Y.; Kumar, S. Electrical conductivity and Joule heating of polyacrylonitrile/carbon nanotube composite fibers. Polymer 2014, 55, 6896–6905. [Google Scholar] [CrossRef]
- Ventura, I.A.; Zhou, J.; Lubineau, G. Investigating the inter-tube conduction mechanism in polycarbonate nanocomposites prepared with conductive polymer-coated carbon nanotubes. Nanoscale Res. Lett. 2015, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Castellino, M.; Rovere, M.; Shahzad, M.I.; Tagliaferro, A. Conductivity in carbon nanotube polymer composites: A comparison between model and experiment. Compos. Part. A Appl. Sci. Manuf. 2016, 87, 237–242. [Google Scholar] [CrossRef]
- Huh, S.H. Physics and Applications of Graphene Experiments. 2011. Available online: https://www.intechopen.com/books/physics (accessed on 22 March 2011).
- Bocharov, G.S.; Eletskii, A.V. Percolation transition under thermal reduction of graphene oxide. J. Struct. Chem. 2018, 59, 806–814. [Google Scholar] [CrossRef]
- Terenzi, A.; Natali, M.; Petrucci, R.M.; Rallini, M.; Peponi, L.; Beaumont, M.; Eletskii, A.; Knizhnik, A.; Potapkin, B.; Kenny, J.M. Analysis and simulation of the electrical properties of CNTs/epoxy nanocomposites for high performance composite matrices. Polym. Compos. 2017, 38, 105–115. [Google Scholar] [CrossRef]
- Bocharov, G.S.; Eletskii, A.V. Percolation phenomena in nanocarbon composites. Fuller. Nanotub. Carbon Nanostruct. 2019, 28, 104–111. [Google Scholar] [CrossRef]
- Eletskii, A.V. Carbon nanotube-based electron field emitters. Phys. Uspekhi 2010, 53, 863–892. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Quantum Mechanics: Non-Relativistic Theory, 3rd ed.; Pergamon: Oxford, UK, 1977. [Google Scholar]
- Sheng, P. Fluctuation-induced tunneling conduction in disordered materials. Phys. Rev. B 1980, 21, 2180. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Ivanov, E.; Bychanok, D.; Paddubskaya, A.; Demidenko, M.; Macutkevic, J.; Maksimenko, S.; Kuzhir, P. Effects of sonochemical modification of carbon nanotubes on electrical and electromagnetic shielding properties of epoxy composites. Compos. Sci. Technol. 2015, 106, 85–92. [Google Scholar] [CrossRef]
- Khromov, K.Y.; Knizhnik, A.A.; Potapkin, B.V.; Kenny, J.M. Multiscale modeling of electrical conductivity of carbon nanotubes based polymer nanocomposites. J. Appl. Phys. 2017, 121, 225102. [Google Scholar] [CrossRef]
- Iskandarova, I.; Khromov, K.; Knizhnik, A.; Potapkin, B. The role of neutral point defects in carrier mobility degradation in bulk 4H-SiC and at 4H-SiC/SiO2 interface: First-principles investigation using Green’s functions. J. Appl. Phys. 2015, 117, 175703. [Google Scholar] [CrossRef]
- A Software Package for Nano-Scale Material Simulations for OpenMX (Open Source Package for Material eXplorer). Available online: http://www.openmx-square.org (accessed on 21 March 2020).
- Ozaki, T.; Kino, H. Efficient projector expansion for the ab initio LCAO method. Phys. Rev. B 2005, 72, 045121. [Google Scholar] [CrossRef]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.-Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal properties of the binary-filler hybrid composites with graphene and copper nanoparticles. Adv. Funct. Mater. 2019, 30, 1904008. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Magana, A.S.; Kargar, F.; Balandin, A.A. Thermal and electrical conductivity control in hybrid composites with graphene and boron nitride fillers. Mater. Res. Express 2019, 6, 085325. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Balinskiy, M.; Magana, A.S.; Lewis, J.S. Balandin, A.A. Dual-functional graphene composites for electromagnetic shielding and thermal management. Adv. Electron. Mater. 2019, 5, 1800558. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Kotsilkova, R.; Tabakova, S.; Ivanova, R.; Angelova, P.; Angelov, V.; Ivanov, E.; Di Maio, R.; et al. Morphological, rheological and electromagnetic properties of nanocarbon/poly(lactic) acid for 3d printing: Solution blending vs. melt mixing. Materials 2018, 11, 2256. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevic, J.; Kuzhir, P.; Banys, J.; Bychanok, D.Z.; Lambin, P.H.; Bistarelli, S.; Cataldo, A.; Micciulla, F.; Bellucci, S. Electromagnetic properties of graphene nanoplatelets/epoxy composites. Compos. Sci. Technol. 2016, 128, 75. [Google Scholar] [CrossRef]
- Barani, Z.; Kargar, F.; Godziszewski, K.; Rehman, A.; Yashchyshyn, Y.; Rumyantsev, S.; Cywiński, G.; Knap, W.; Balandin, A.A. Graphene epoxy-based composites as efficient electromagnetic absorbers in the extremely high-frequency band. ACS Appl. Mater. Interfaces 2020, 12, 28635–28644. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Petrova-Doycheva, I.; Menseidov, D.; Ivanov, E.; Paddubskaya, A.; Kuzhir, P. Exploring thermal annealing and graphene-carbon nanotube additives to enhance crystallinity, thermal, electrical and tensile properties of aged poly(lactic) acid-based filament for 3D printing. Compos. Sci. Technol. 2019, 181, 107712. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.; Kohlhaas, K.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.; Ruoff, R. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Eda, G.; Mattevi, C.; Kim, H.; Chhowalla, M. Highly uniform 300 mm wafer-scale deposition of single and multilayered chemically derived graphene thin films. ACS Nano 2010, 4, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.; Gilje, S.; Kaner, R.B.; Wallace, G.C. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically conductive alkylated graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Okhay, O.; Gonçalves, G.; Tkach, A.; Dias, C.; Ventura, J.; da Silva, M.F.R.; Gonçalves, L.M.V.; Titus, E. Thin film versus paper-like reduced graphene oxide: Comparative study of structural, electrical, and thermoelectrical properties. J. Appl. Phys. 2016, 120, 051706. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yin, K.; Li, H.; Xia, Y.; Bi, H.; Sun, J.; Liu, Z.; Sun, L. Thermodynamic and kinetic analysis of low temperature thermal reduction of graphene oxide. Nano Micro Lett. 2011, 3, 51–55. [Google Scholar] [CrossRef]
- Tu, N.D.K.; Choi, J.; Park, C.R.; Kim, H. Remarkable conversion between n-and p-type reduced graphene oxide on varying the thermal annealing temperature. Chem. Mater. 2015, 27, 7362. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A.; Enzelberger, M.; Müller, P. Formation and decomposition of CO2 intercalated graphene oxide. Chem. Mater. 2012, 24, 1276–1282. [Google Scholar] [CrossRef]
- Cui, P.; Lee, J.; Hwang, E.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, V.P.; Bocharov, G.S.; Eletskii, A.V.; Ridzel, O.Y.; Kaplya, P.S.; Köppen, M. Evolution of photoelectron spectra at thermal reduction of graphene oxide. J. Vacuum Sci. Technol. B 2017, 35, 041804. [Google Scholar] [CrossRef]
- Afanas’ev, V.P.; Bocharov, G.S.; Gryazev, A.S.; Eletskii, A.V.; Kaplya, P.S.; Ridzel, O.Y. Reduced graphene oxide studied by X-ray photoelectron spectroscopy: Evolution of plasmon mode. J. Phys. Conf. Ser. 2018, 1121, 012001. [Google Scholar] [CrossRef]
- Afanas’ev, V.P.; Fedorovich, S.D.; Lubenchenko, A.V.; Esimov, M.S. Kilovolt electron backscattering. Z. Phys. B Condens. Matter 1994, 96, 253–259. [Google Scholar] [CrossRef]
- Werner, W.S.M. Partial intensity analysis (PIA) for quantitative electron spectroscopy. Surf. Interface Anal. 1995, 23, 737. [Google Scholar] [CrossRef]
- Afanas’ev, V.P.; Golovina, O.Y.; Gryazev, A.S. Photoelectron spectra of finite-thickness layers. J. Vac. Sci. Technol. B 2015, 33, 03D101. [Google Scholar] [CrossRef]
- Eletskii, A.; Fedorov, G.; Ryzhikov, I.; Kurochkin, I.; Egin, M.; Gaiduchenko, I.; Bocharov, G.; Boginskaya, I.; Sarychev, A.; Ivanov, A. Amplification of the signal of raman scattering by carbon nanotubes. Doklady Akademii Nauk 2018, 483, 502–505. [Google Scholar] [CrossRef]
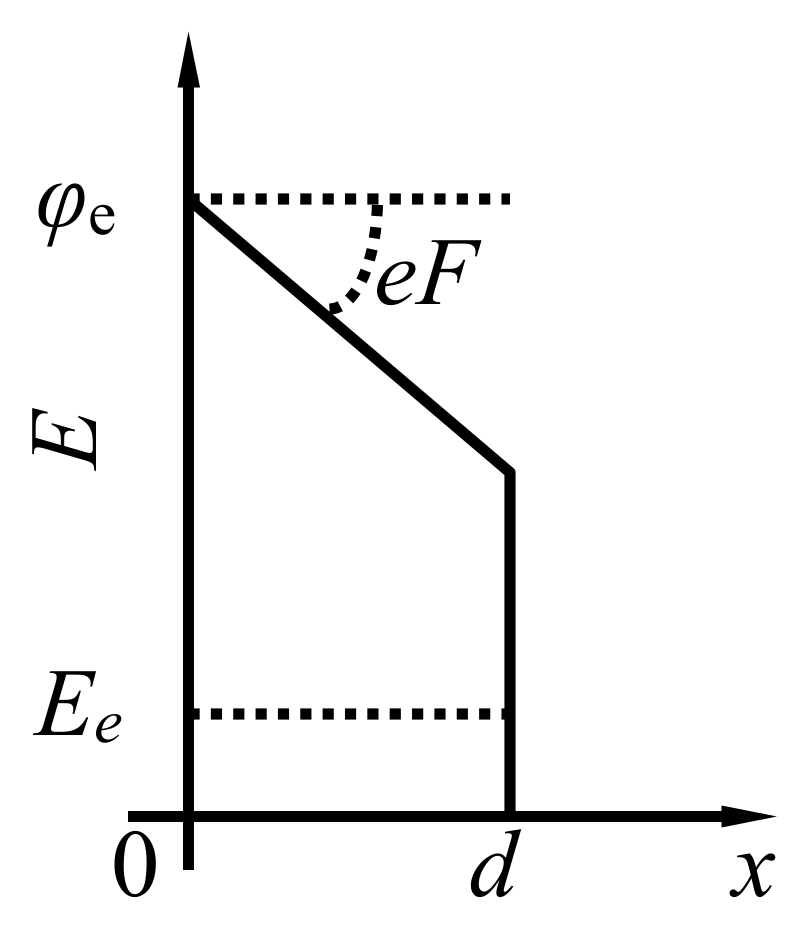
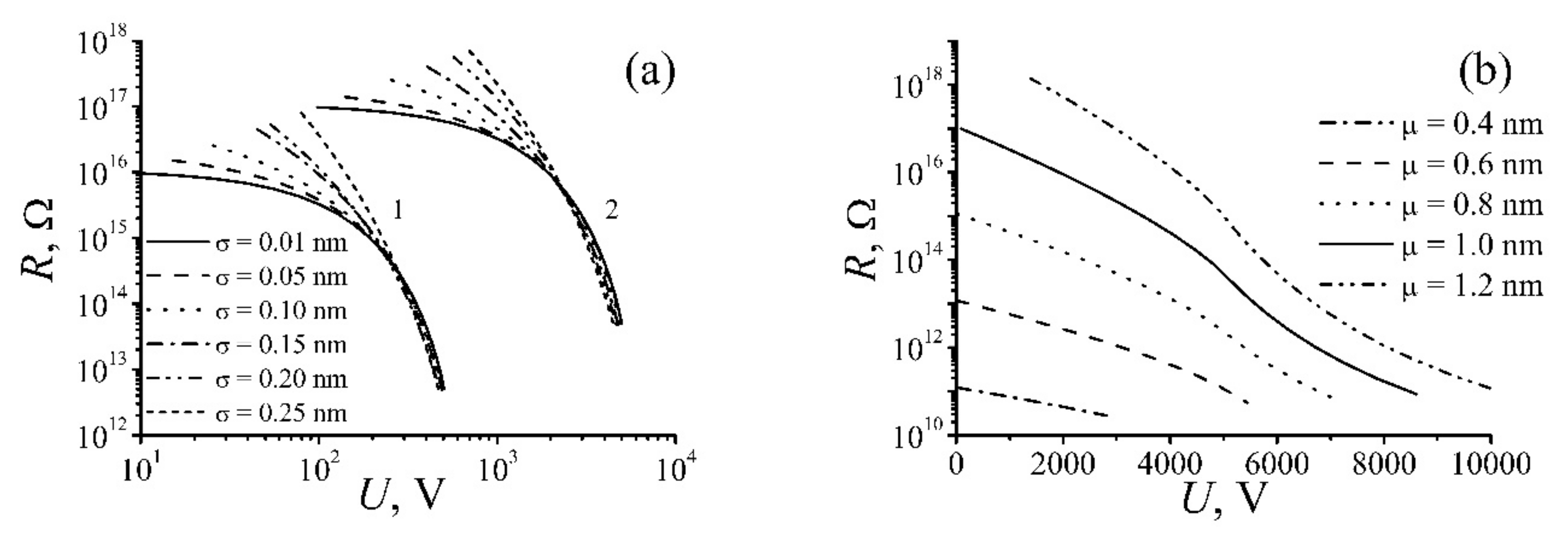
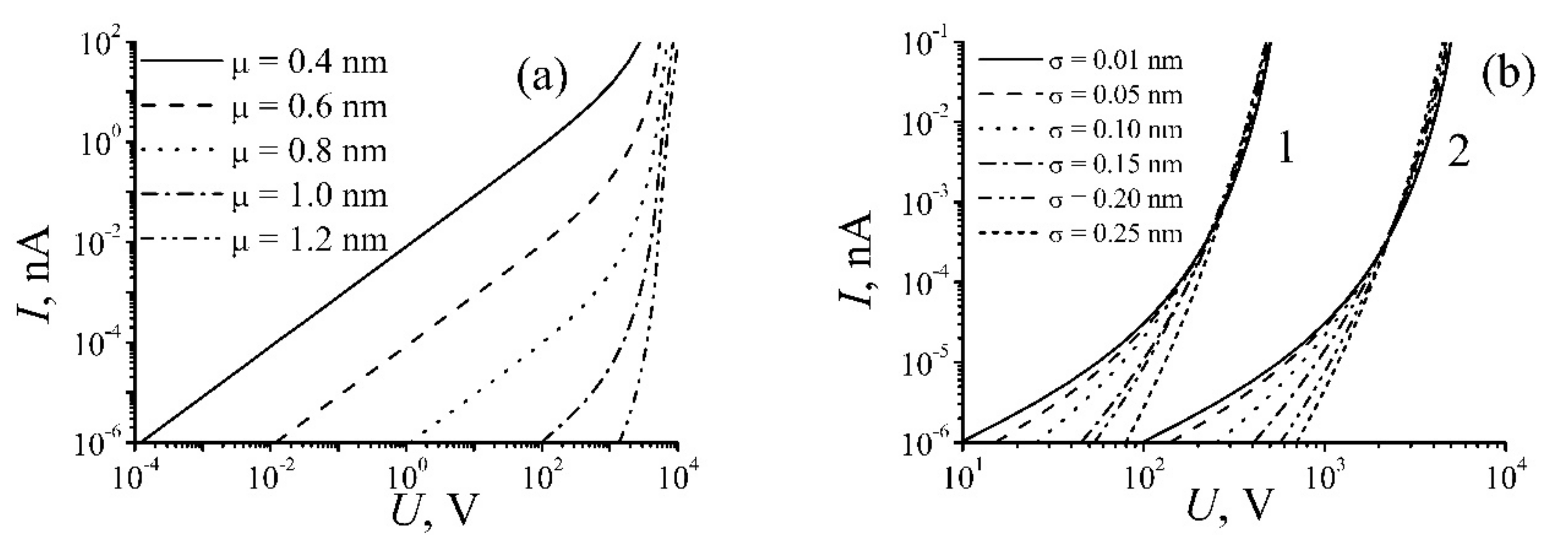

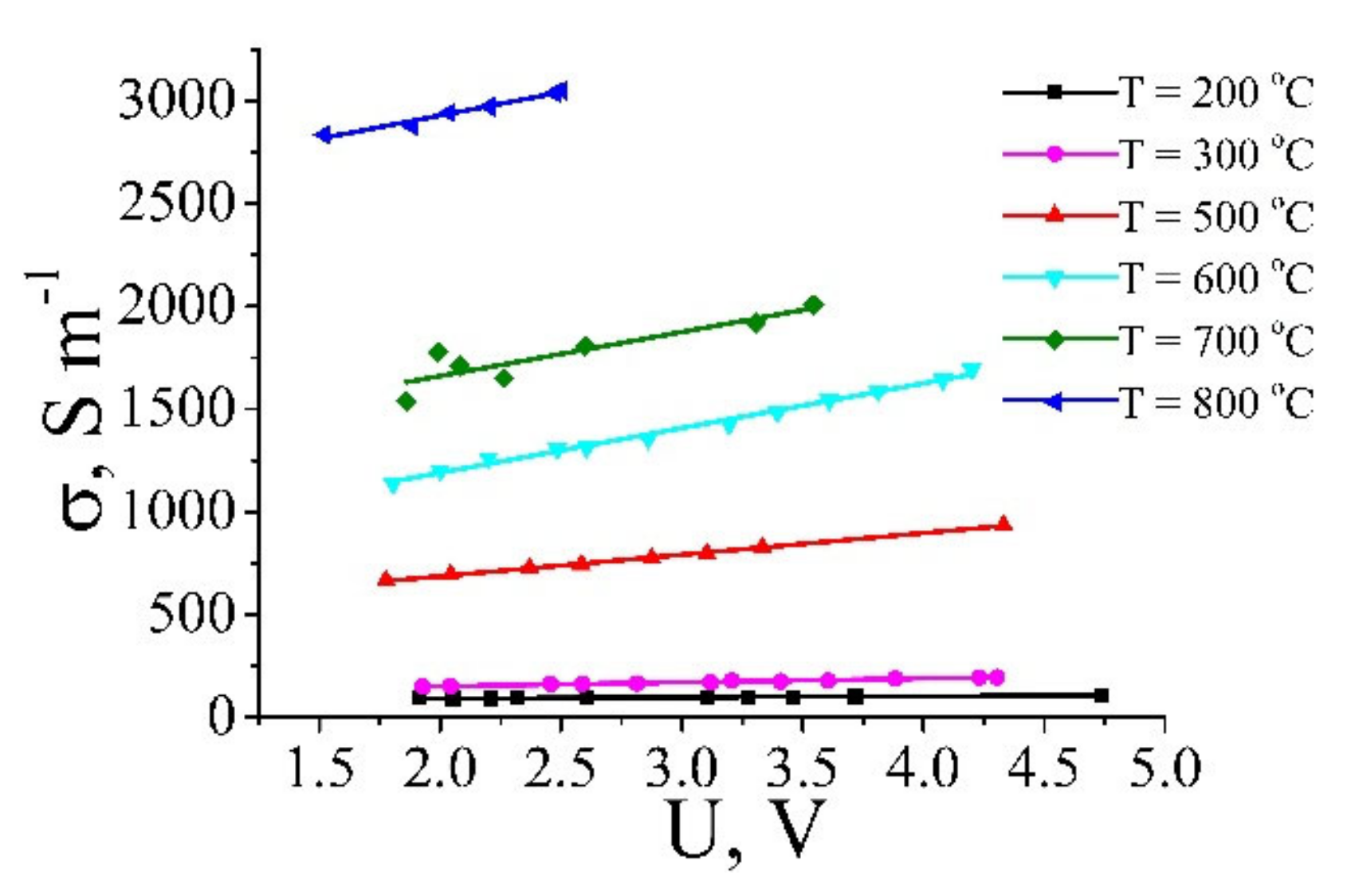
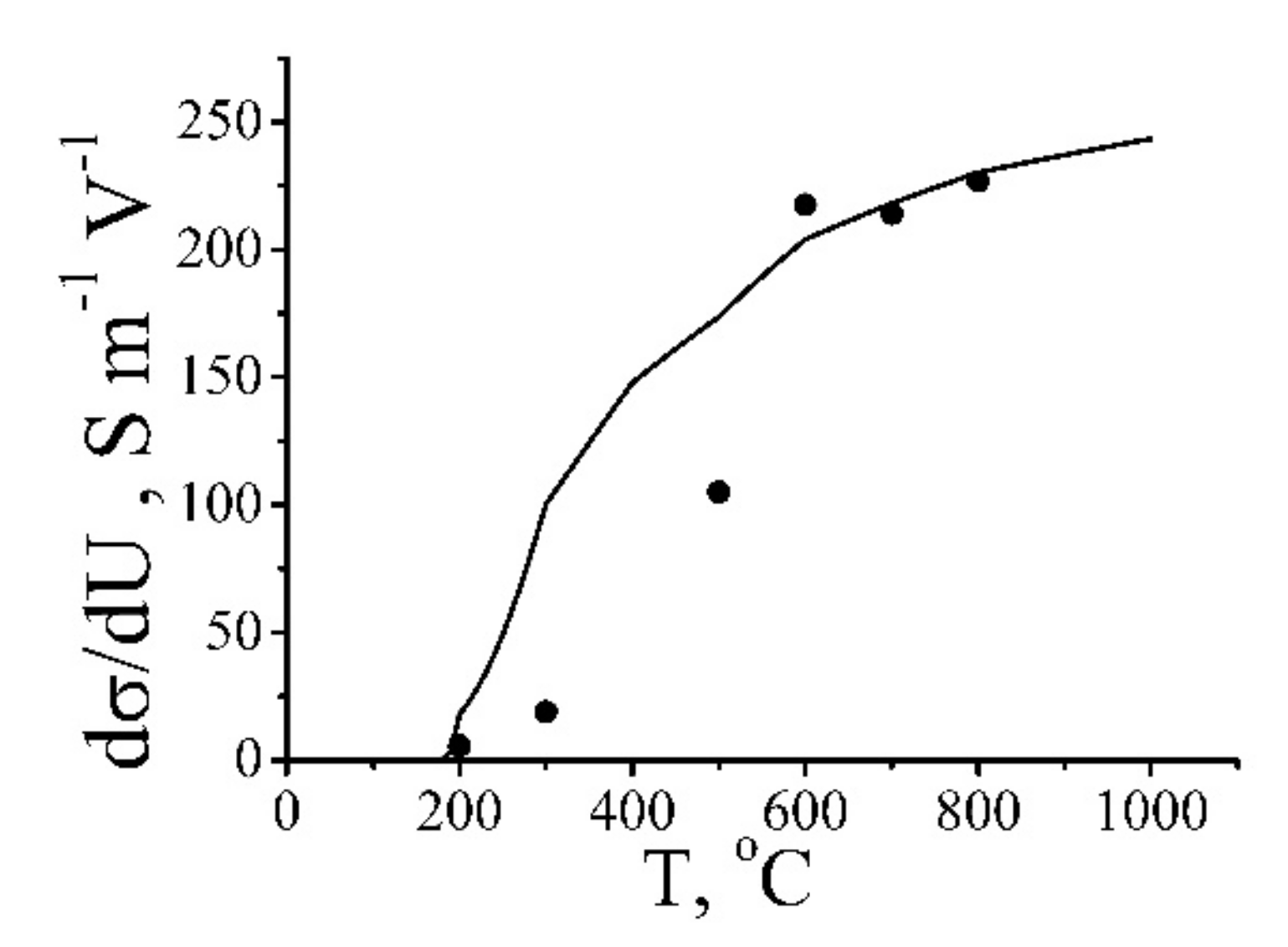
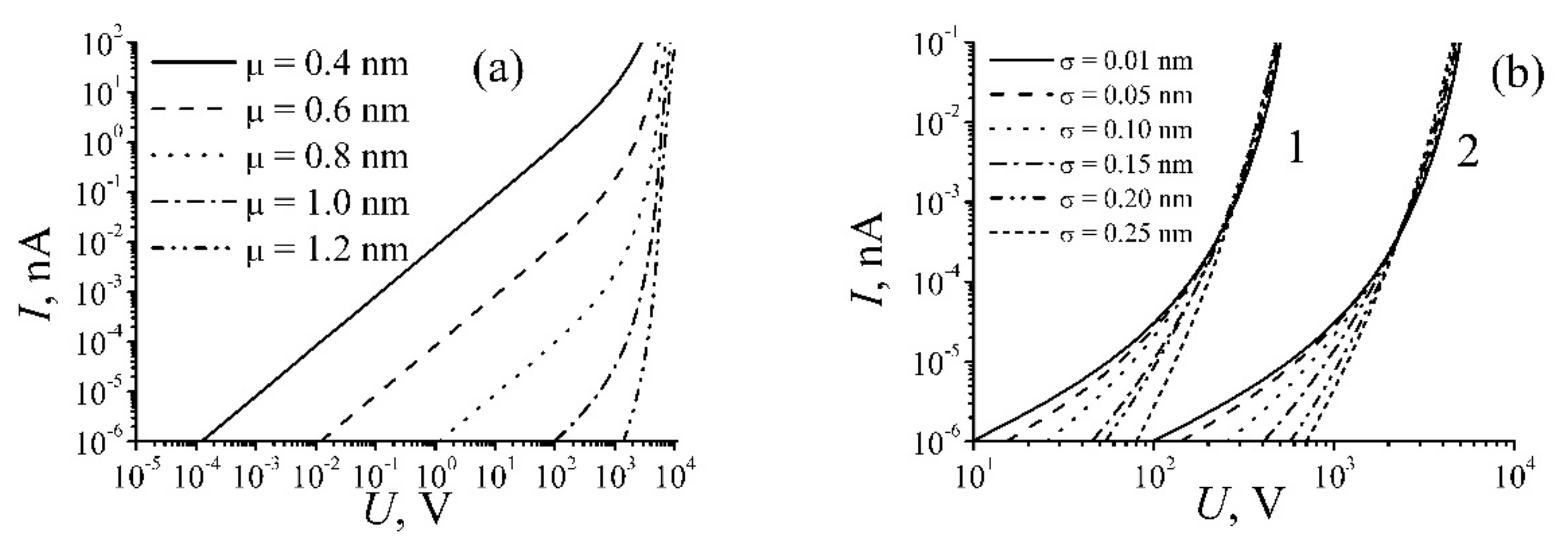
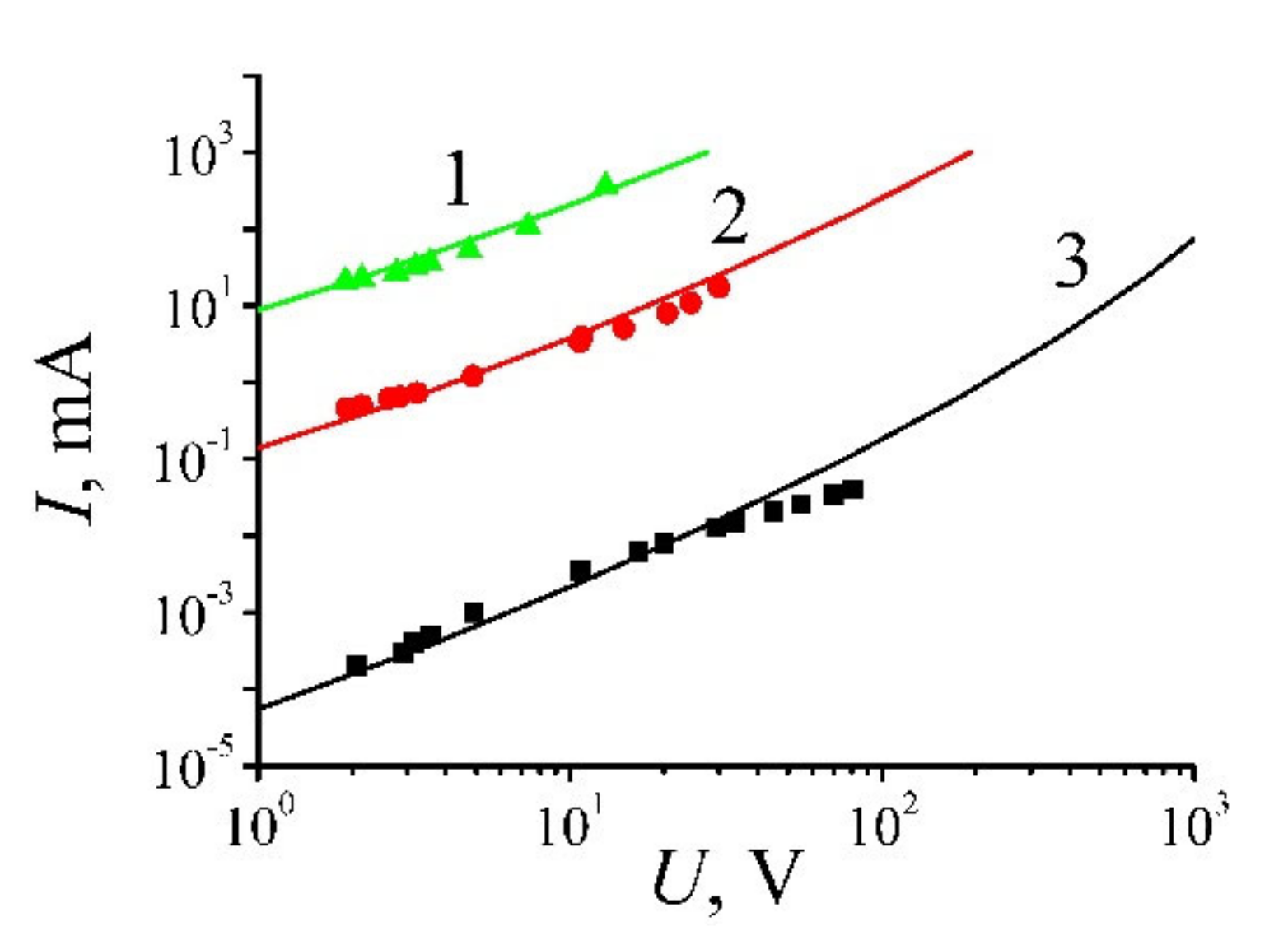
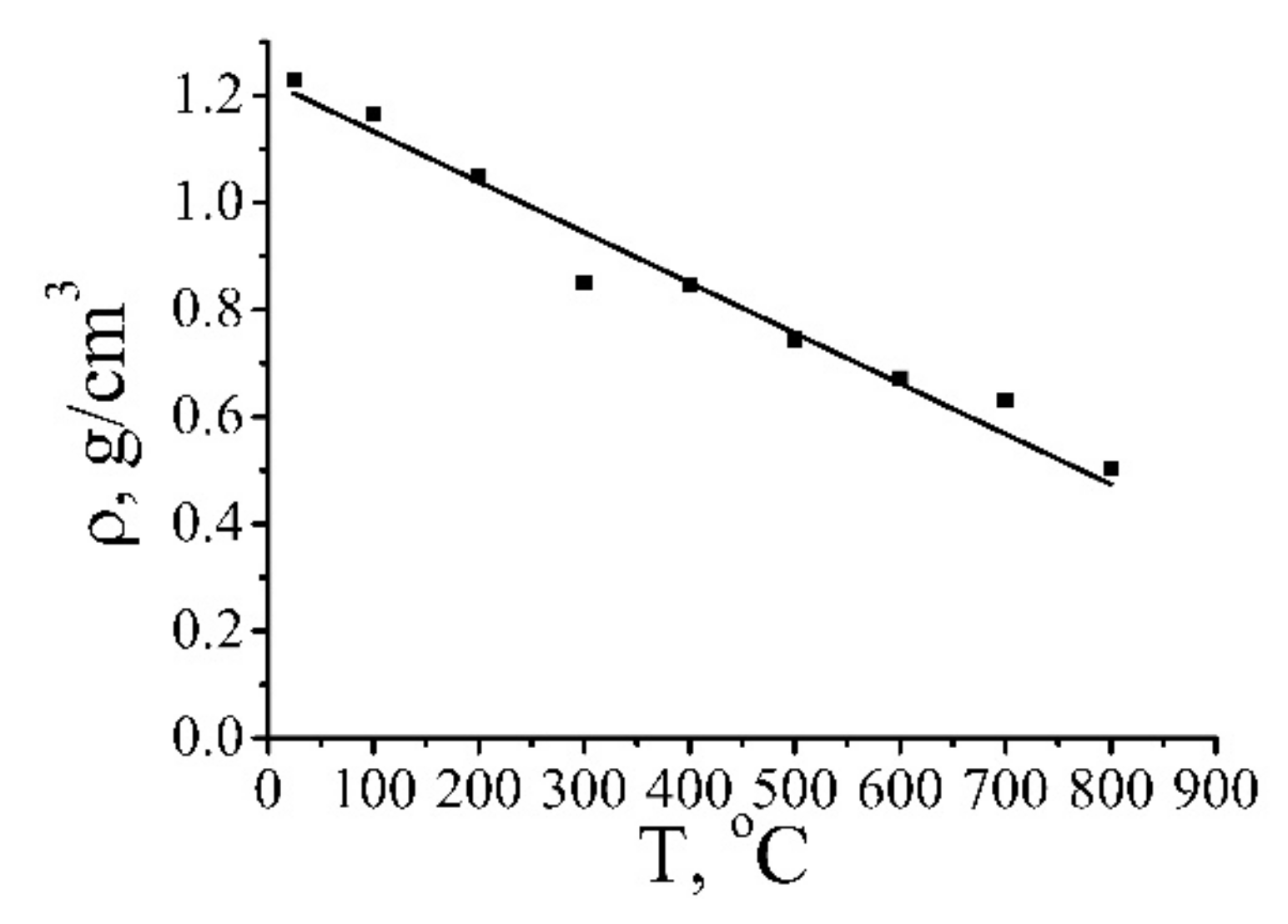
| Annealing Temperature, °C | 250 | 350 | 700 | 1000 |
|---|---|---|---|---|
| Concentration and sign of current curriers, 1016 cm−3 | 1.99 | −29.5 | 19.4 | −60.1 |
| Mobility of current carriers, cm2∙V−1∙s−1 | 2.56 | 5.48 | 62.8 | 188.0 |
| Calculated conductivity, S/cm | 0.05 | 0.12 | 1.75 | 9.44 |
| Measured conductivity, S/cm | 0.002 | 0.07 | 1.75 | 9.44 |
| Annealing Temperature, °C | C,% | O,% | C/O | N,% | S,% | Si,% |
|---|---|---|---|---|---|---|
| 25 | 74.7 | 23.0 | 3,25 | 1.3 | 0.5 | 0.4 |
| 150 | 73.6 | 25.1 | 2,93 | 0.7 | 0.5 | - |
| 200 | 82.0 | 15.2 | 5,39 | 1.6 | 0.5 | 0.7 |
| 600 | 90.6 | 8.1 | 11,2 | 0.5 | - | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocharov, G.S.; Eletskii, A.V. Percolation Conduction of Carbon Nanocomposites. Int. J. Mol. Sci. 2020, 21, 7634. https://doi.org/10.3390/ijms21207634
Bocharov GS, Eletskii AV. Percolation Conduction of Carbon Nanocomposites. International Journal of Molecular Sciences. 2020; 21(20):7634. https://doi.org/10.3390/ijms21207634
Chicago/Turabian StyleBocharov, Grigorii S., and Alexander V. Eletskii. 2020. "Percolation Conduction of Carbon Nanocomposites" International Journal of Molecular Sciences 21, no. 20: 7634. https://doi.org/10.3390/ijms21207634
APA StyleBocharov, G. S., & Eletskii, A. V. (2020). Percolation Conduction of Carbon Nanocomposites. International Journal of Molecular Sciences, 21(20), 7634. https://doi.org/10.3390/ijms21207634